Abstract
The gene coding interleukin 6 (IL‐6) is a promising candidate in predisposition to type 2 diabetes mellitus (T2DM). This study aimed to meta‐analytically examine the association of IL‐6 gene −174G/C polymorphism with T2DM and circulating IL‐6 changes across −174G/C genotypes. Odds ratio (OR) and standard mean difference (SMD) with 95% confidence interval (CI) were calculated. Twenty‐five articles were meta‐analysed, with 20 articles for T2DM risk and 9 articles for circulating IL‐6 changes. Overall, there was no detectable significance for the association between −174G/C polymorphism and T2DM, and this association was relatively obvious under dominant model (OR: 0.82, 95% CI: 0.56‐1.21). Improved heterogeneity was seen in some subgroups, with statistical significance found in studies involving subjects of mixed races (OR: 0.63, 95% CI: 0.46‐0.86). Begg's and filled funnel plots, along with Egger's tests revealed week evidence of publication bias. In genotype‐phenotype analyses, carriers of −174CC and −174CG genotypes separately had 0.10 and 0.03 lower concentrations (pg/mL) of circulating IL‐6 than −174GG carriers. Albeit no detectable significance for the association of −174G/C with T2DM, our findings provided suggestive evidence on a dose‐dependent relation between −174G/C mutant alleles and circulating IL‐6 concentrations, indicating possible implication of this polymorphism in the pathogenesis of T2DM.
Keywords: interleukin 6, polymorphism, risk, type 2 diabetes mellitus
1. INTRODUCTION
Diabetes is a chronic metabolic disorder, and globally an estimated 422 million persons are affected by diabetes, mainly in low‐ and middle‐income countries. 1 The most common is type 2 diabetes mellitus (T2DM), which accounts for 90% to 95% of all diabetes. T2DM is a complex, multifactorial disease, attributing to the interaction between genetic defects and environmental factors. 2 , 3 As a risk factor of nearly all‐cause mortality, T2DM can affect people across different life stages. 4 So, early identification of persons at a higher risk for T2DM is of great clinical and public health importance.
It is well known that T2DM is a polygenic disease. Extensive efforts have been made to decipher the genetic basis of T2DM, especially with the advent of genome‐wide association studies (GWASs). 5 , 6 , 7 Although over a hundred genetic variants in predisposition to T2DM have been characterized, only a modest portion of T2DM heritability can be interpreted. 8 , 9 One of the major challenges facing global geneticists is the inconsistent replication of candidate genes with biological implications across different populations. 10 , 11 The gene coding interleukin 6 (IL‐6) is one such gene.
Biologically speaking, IL‐6 can induce the development of insulin resistance and pathogenesis of T2DM via regulating inflammatory responses. 12 , 13 A promoter polymorphism in IL‐6 gene, −174G/C or rs1800795, has been extensively studied in association with T2DM, yet the results of most prior studies are poorly replicated. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 The underlying reasons are manifold, likely involving differences in genetic backgrounds, study designs and statistical power, as well as baseline characteristics of diverse populations.
To shed some light upon these reasons and yield more information for future investigations, we here prepared a systematic review of published studies to meta‐analytically examine the association of IL‐6 gene −174G/C polymorphism with T2DM, as well as the changes of circulating IL‐6 concentrations across −174G/C genotypes. Meanwhile, the possible sources for between‐study heterogeneity attributed to inconsistent observations were also interrogated.
2. METHODS
This meta‐analysis was proceeded in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) statement. 25 The PRISMA checklist is presented in Table S1.
2.1. Search strategy
Public databases including Medline/PubMed, EMBASE (Excerpta Medica database) and Web of Science were reviewed to seek potentially qualified articles published prior to 8 September 2020. Key terms for literature search were (‘interleukin 6’ OR ‘IL‐6’ OR ‘inflamma*’ OR ‘cytokine*’) [Title and Abstract] AND (‘diabet*’) [Title] AND (‘SNP’ OR ‘polymorphism’ OR ‘varia*’ OR ‘mutation*’) [Title and Abstract]. Only articles written in the English language and conducted among human participants were retrieved.
In addition, the reference lists of major articles or reviews were scanned for potential missing articles. Search process was independently completed by two of us (Hao Cheng and Wenbin Zhu), by using same key terms aforementioned, and any conflicts were adjudicated by a third author (Chunjing Zhang). The results were integrated, and duplicates were removed from the final reference set.
2.2. Eligibility criteria
Eligible articles needed to meet the following three criteria: (i) available genotype or allele counts of IL‐6 gene −174G/C polymorphism in both T2DM patients and controls or available circulating IL‐6 concentrations across the genotypes of −174G/C polymorphism; (ii) clear definition of T2DM according to official guidelines; (iii) the adoption of validated assaying methods to determine three −174G/C genotypes.
If the retrieved publication was a narrative or quantitative review, was an animal study, focused on diabetic complications, did not have valid control groups, lacked necessary genotype information or was published in the languages other than the English, this publication was excluded from this meta‐analysis.
2.3. Data extraction
From each qualified article, extracted data included first author's name, year of publication, race or ethnicity, disease status, T2DM diagnosis, control source, study design, matched condition, age, gender and body mass index, as well as, if available, haemoglobin A1c (HbA1c), fasting plasma glucose (FPG), postprandial glucose (PPG), total cholesterol (TC), triglyceride (TG), high‐density lipoprotein cholesterol (HDLC) and low‐density lipoprotein cholesterol (LDLC). Data extraction process was independently finished by two of us (Hao Cheng and Wenbin Zhu), and disagreement was solved by a third author (Chunjing Zhang).
2.4. Statistical analyses
All statistical analyses were performed with the use of STATA software Release 14.1 (StataCorp, College Station, TX, USA).
Weighted odds ratio (OR) and its 95% confidence interval (95% CI) were calculated to assess the association between IL‐6 gene −174G/C polymorphism and T2DM. In addition, changes in circulating IL‐6 and the other laboratory biomarkers across −174G/C genotypes were expressed as standard mean difference (SMD) and 95% CI. Pooled OR and SMD were derived under the random‐effects model. The inconsistency index (I 2) was adopted to appraise between‐study heterogeneity, which meant that the percentage of observed variability between studies that was due to heterogeneity instead of a chance finding. If the I2 is over 50.0%, statistically significant heterogeneity is recorded. Subsidiary analyses were done to interrogate underlying sources for between‐study heterogeneity.
Cumulative analyses and sensitivity analyses were carried out to appraise the risk of bias. The former measured the impact of the first publication on subsequent publications and the evolution of cumulative estimates over time. The latter removed one publication at a time to appraise the influence of a single publication on pooled estimates.
Both Begg's plots and filled funnel plots were depicted to appraise the probability of publication bias. If the funnel shape was symmetric and the probability of Egger's tests was over 10%, a low probability of publication bias was recorded.
3. RESULTS
3.1. Retrieved articles
Figure 1 shows the detailed search process for eligible articles. Our initial search of three public databases retrieved a total of 186 articles, and only 25 of them met our pre‐specified inclusion and exclusion criteria. Twenty articles 10 , 11 , 40 including 26 studies with 4,688 patients and 10,700 controls provided data on the association between IL‐6 gene −174G/C polymorphism and T2DM. Nine articles 10 , 18 , 21 , 26 , 27 , 41 , 42 , 43 , 44 including 12 studies with 4,090 subjects provided data on the changes of circulating IL‐6 concentrations across −174G/C genotypes.
FIGURE 1.
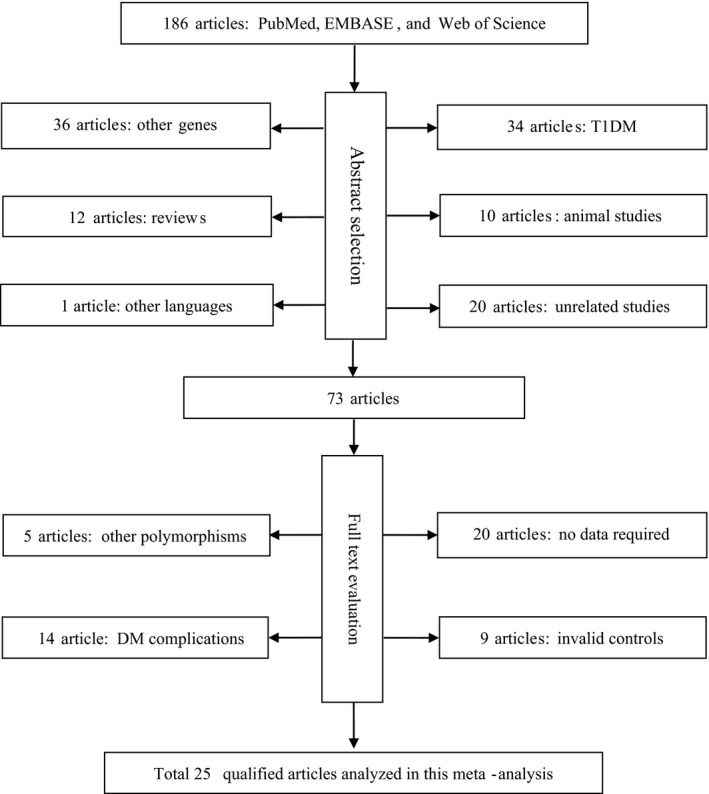
Flow diagram of selection process in the current meta‐analysis
3.2. Baseline characteristics of eligible studies
Table 1 provides the baseline characteristics of all eligible studies. All included articles were published during the years between 2003 and 2019. Total sample sizes ranged from 40 to 5840. T2DM was doctors’ diagnosed or according to the ADA (American Diabetes Association) or WHO (World Health Organization) 1999 guidelines.
TABLE 1.
The baseline characteristics of all involved studies in the current meta‐analysis
| First Author | Year | Country | Ethnicity | Disease | Matched | Control status | Diagnosis | Study design | Age (yrs) in cases | Age (yrs) in controls |
|---|---|---|---|---|---|---|---|---|---|---|
| Fathy, SA (T2DM w/t DKD) | 2019 | Kuwait | Caucasian | T2DM without DKD | NA | Healthy | Doctor's diagnosis | Retrospective | 58.5 | 53.8 |
| Fathy, SA (T2DM w/o DKD) | 2019 | Kuwait | Caucasian | T2DM with DKD | NA | Healthy | Doctor's diagnosis | Retrospective | 61.0 | 53.8 |
| Lara‐Gómez, RE | 2019 | Mexico | Mixed | T2DM | NA | Healthy | Doctor's diagnosis | Cross‐sectional | 59.1 | 36.2 |
| Saxena, M. | 2018 | India | Indian | T2DM | NA | Healthy | NA | Prospective | ||
| Plataki, MN | 2018 | Greece | Caucasian | T2DM | NA | Controls (Normal Glucose) | ADA | Retrospective | 68.3 | 74.9 |
| Hameed, I. (T2DM w/t DKD) | 2018 | India | Indian | T2DM without DKD | NA | Healthy | Doctor's diagnosis | Prospective | 54.1 | |
| Hameed, I. (T2DM w/o DKD) | 2018 | India | Indian | T2DM with DKD | NA | Healthy | Doctor's diagnosis | Prospective | 57.9 | |
| Rodrigues, KF | 2017 | Brasil | Mixed | T2DM | YES | Healthy | ADA | Cross‐sectional | 56.0 | 53.0 |
| Ponnana, M. | 2017 | India | Indian | T2DM | YES | Healthy | Doctor's diagnosis | Prospective | 33.3 | 30.2 |
| Neelofar, K. (T2DM w/t DKD) | 2017 | India | Indian | T2DM without DKD | YES | Hospital, Healthy | ADA | Prospective | 52.8 | 50.1 |
| Neelofar, K. (T2DM w/o DKD) | 2017 | India | Indian | T2DM with DKD | YES | Hospital, Healthy | ADA | Prospective | 51.3 | 50.1 |
| Kavitha, L. | 2017 | India | Mixed | T2DM | YES | Hospital, Healthy | Doctor's diagnosis | Retrospective | ||
| Ghavimi, R. | 2016 | Iran | Middle Eastern | T2DM | YES | Healthy, Transfusion Organization | Doctor's diagnosis | Retrospective | 51.3 | 50.2 |
| Eze, I. C. | 2016 | Switzerland | Caucasian | DM | YES | Healthy | ADA | Cross‐sectional | ||
| Buraczynska, M. (T2DM w/t CVD) | 2016 | Poland | Caucasian | T2DM without CVD | NA | Volunteers, Healthy | ADA | Retrospective | 54.3 | |
| Buraczynska, M. (T2DM w/o CVD) | 2016 | Poland | Caucasian | T2DM with CVD | NA | Volunteers, Healthy | ADA | Retrospective | 65.8 | |
| Saxena, M. | 2014 | India | Mixed | T2DM | YES | Healthy, Stuff Members | Doctor's diagnosis | Retrospective | 49.2 | 47.8 |
| Karadeniz, M. (T2DM w/t DKD) | 2014 | Turkey | Middle Eastern | T2DM without DKD | NA | Healthy | Doctor's diagnosis | Retrospective | 52.2 | 54.2 |
| Karadeniz, M. (T2DM w/o DKD) | 2014 | Turkey | Middle Eastern | T2DM with DKD | NA | Healthy | Doctor's diagnosis | Retrospective | 58.3 | 54.2 |
| Zhang, X. | 2011 | China | Chinese | T2DM | YES | Healthy | WHO 1999 criteria | Retrospective | 57.4 | 56.8 |
| Bouhaha, R. | 2010 | Tunisia | Middle Eastern | T2DM | NO | Healthy | ADA | Retrospective | 60.6 | 43.8 |
| Xiao, L. M. | 2009 | China | Chinese | T2DM | YES | Healthy, Community | WHO 1999 criteria | Retrospective | 59.7 | 51.6 |
| Danielsson, P. | 2005 | Sweden | Caucasian | T2DM | YES | Healthy | NA | Retrospective | 74.0 | 75.0 |
| Tsiavou, A. | 2004 | Greece | Caucasian | T2DM | NO | Healthy | Doctor's diagnosis | Retrospective | 51.0 | 44.0 |
| Vozarova, B. | 2003 | Spain | Caucasian | T2DM | NA | NA | NA | Retrospective | 58.6 | 56.7 |
| Vozarova, B. | 2003 | USA | Caucasian | T2DM | NA | NA | NA | Retrospective | 29.2 | 63.9 |
| Males (%) | BMI (kg/m2) | HbA1c (%) | FPG (mg/dL) | PGG (mg/dL) | TG (mmol/L) | TC (mmol/L) | HDL (mmol/L) | LDL (mmol/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls | Cases | Controls |
| 0.640 | 0.571 | 34.1 | 29.4 | 1.4 | 1.1 | 4.2 | 4.9 | 1.3 | 1.5 | 2.3 | 3.0 | ||||||
| 0.343 | 0.571 | 34.5 | 29.4 | 1.8 | 1.1 | 3.9 | 4.9 | 1.1 | 1.5 | 2.0 | 3.0 | ||||||
| 0.300 | 0.530 | ||||||||||||||||
| 0.368 | 0.456 | 7.8 | |||||||||||||||
| 26.88 | 7.59 | 143.06 | 199.71 | 2.39 | 4.88 | 1.12 | 2.69 | ||||||||||
| 26.55 | 8.52 | 154.31 | 215.16 | 2.22 | 4.46 | 0.97 | 2.40 | ||||||||||
| 0.186 | 0.194 | 8.9 | 126.5 | 85.3 | 203 | ||||||||||||
| 0.600 | 0.670 | 20.8 | 24.9 | ||||||||||||||
| 0.600 | 0.600 | 25.88 | 23.16 | 7.04 | 5.89 | 183.64 | 122.94 | ||||||||||
| 0.600 | 0.600 | 24.3 | 23.16 | 9.8 | 5.89 | 212.76 | 122.94 | ||||||||||
| 0.425 | 0.442 | 28.5 | 27.3 | ||||||||||||||
| 0.460 | 28.1 | 7.7 | 2.10 | 4.70 | 1.5 | ||||||||||||
| 0.509 | 26.8 | 7.9 | 1.70 | 4.90 | 1.2 | ||||||||||||
| 0.600 | 0.662 | 24.05 | 23.33 | 173.81 | 83.87 | 272.75 | 139.65 | 1.27 | 1.42 | 5.74 | 4.77 | 1.17 | 1.25 | 3.96 | 1.66 | ||
| 6.68 | 5.13 | 140.53 | 85.77 | 193.9 | 121.3 | 1.89 | 1.53 | 5.36 | 4.45 | 1.24 | 1.37 | 3.51 | 2.81 | ||||
| 8.02 | 5.13 | 154.77 | 85.77 | 218.5 | 121.3 | 2.27 | 1.53 | 5.35 | 4.45 | 1.22 | 1.37 | 3.39 | 2.81 | ||||
| 0.576 | 0.571 | 24.5 | 22.4 | 8.74.7 | 153 | 82.8 | 244.8 | 102.6 | 2.69 | 1.59 | 4.69 | 4.86 | 1.04 | 1.16 | 2.51 | 2.48 | |
| 0.367 | 0.715 | 29.29 | 27.43 | 174.42 | 96.66 | 167.76 | 1.73 | 4.81 | 1.25 | ||||||||
| 0.273 | 0.311 | 24.22 | 22.76 | ||||||||||||||
| 1.000 | 0.600 | 6.75 | 4.65 | 1.22 | 0.95 | 1.2 | 1.46 | 2.9 | 3.35 | ||||||||
| 0.250 | 0.615 | ||||||||||||||||
| 0.412 | 0.449 | ||||||||||||||||
| 0.315 | 0.476 | ||||||||||||||||
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; DKD, diabetic kidney disease; FPG, fasting plasma glucose; HbA1c, haemoglobin A1c; HDL, high‐density lipoprotein cholesterol; LDL, low‐density lipoprotein cholesterol. Vacant panes denote the unavailability of data; PPG, postprandial glucose; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglyceride; w/o, without; w/t, with.
3.3. Overall analyses: −174G/C polymorphism and T2DM
The overall association of IL‐6 gene −174G/C polymorphism with T2DM was assessed under three different genetic models, as illustrated in Figure 2. The mutation of this polymorphism was related to a reduced risk of T2DM, albeit no detectable statistical significance. For example, carriers of −174CC genotype had an 18% lower risk than those with −174GG genotype (OR: 0.82, 95% CI: 0.56 to 1.21). There was statistically significant between‐study heterogeneity across three genetic models, with the I 2 around 80% (P <.001).
FIGURE 2.
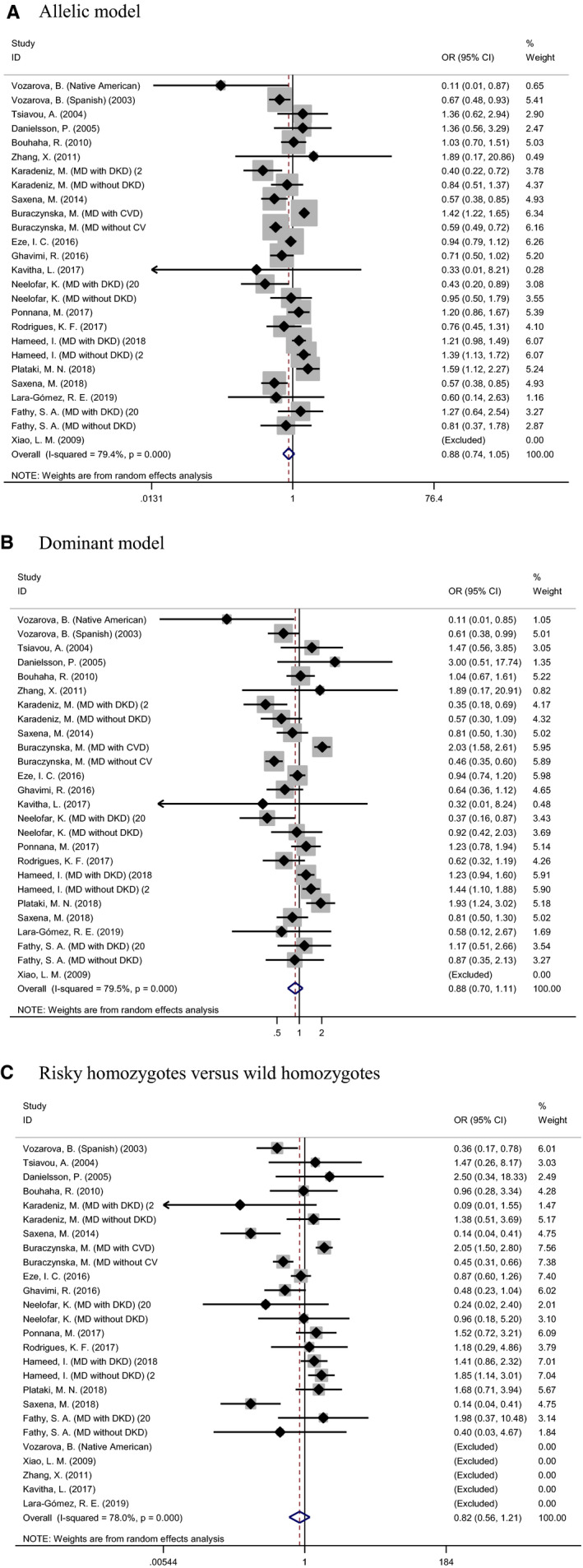
Forest plots of interleukin 6 gene −174G/C polymorphism associated with type 2 diabetes mellitus under three genetic models
3.4. Subsidiary analyses: −174G/C polymorphism and T2DM
Due to significant heterogeneity in overall analyses, a wide panel of subsidiary analyses was carried out separately according to sample sizes, races, countries, diagnostic criteria of T2DM, disease status of patients with T2DM, matched statue and study designs (Table 2). Only subgroups involving at least 2 studies are listed in this meta‐analysis. Heterogeneity was improved in some subgroups, such as in the subgroups with total samples <324 (I 2: 19.4%), and studies with matched patients and controls (I 2: 42.0%). In populations with mixed races, the mutation of IL‐6 gene −174G/C polymorphism was associated with a 37% reduced risk of T2DM (OR: 0.63, 95% CI: 0.46 to 0.86, P: 0.004). No significance was noted for the other subgroups (P >.05).
TABLE 2.
Subsidiary analysis of IL‐6 gene −174G/C polymorphism in association with T2DM under the allelic model
| Subgroups | Number of Studies | OR | 95% CI | P | I 2 (P) |
|---|---|---|---|---|---|
| Sample size | |||||
| Total sample size <324 | 12 | 0.81 | 0.63 to 1.04 | .104 | 19.4% (.259) |
| Total sample size ≥324 | 14 | 0.91 | 0.74 to 1.13 | .830 | 87.0% (<.001) |
| Race | |||||
| Caucasian | 10 | 0.98 | 0.73 to 1.32 | .896 | 86.7% (<.001) |
| Chinese | 2 | 1.89 | 0.17 to 20.86 | .604 | NA |
| Indian | 6 | 0.96 | 0.71 to 1.30 | .797 | 77.7% (<.001) |
| Middle Eastern | 4 | 0.73 | 0.52 to 1.04 | .080 | 58.0% (.067) |
| Mixed | 4 | 0.63 | 0.46 to 0.86 | .004 | 0.0% (.823) |
| Country | |||||
| Asia | 15 | 0.83 | 0.65 to 1.06 | .129 | 73.0% (<.001) |
| Europe | 7 | 1.02 | 0.73 to 1.43 | .905 | 90.4% (<.001) |
| North America | 2 | 0.30 | 0.06 to 1.61 | .160 | 44.9% (.178) |
| Diagnosis of T2DM | |||||
| ADA | 8 | 0.93 | 0.68 to 1.26 | .692 | 88.7% (<.001) |
| Doctor diagnosis | 12 | 0.91 | 0.72 to 1.16 | .460 | 69.0% (<.001) |
| WHO 1999 criteria | 2 | 1.89 | 0.17 to 20.86 | .604 | NA |
| NA | 4 | 0.66 | 0.43 to 1.00 | .052 | 49.7% (.113) |
| Disease status in cases | |||||
| T2DM | 15 | 0.86 | 0.67 to 1.09 | .217 | 62.8% (.001) |
| T2DM with DKD | 4 | 0.74 | 0.39 to 1.40 | .350 | 83.2% (<.001) |
| T2DM without DKD | 4 | 1.08 | 0.78 to 1.48 | .650 | 43.8% (.148) |
| Matched status | |||||
| YES | 11 | 0.83 | 0.68 to 1.02 | .079 | 42.0% (.078) |
| NO | 2 | 1.09 | 0.77 to 1.54 | .642 | 0.0% (.530) |
| NA | 13 | 0.88 | 0.67 to 1.15 | .342 | 87.4% (<.001) |
| Study design | |||||
| Prospective | 6 | 0.96 | 0.71 to 1.30 | .797 | 77.7% (<.001) |
| Retrospective | 20 | 0.85 | 0.69 to 1.06 | .154 | 79.4% (<.001) |
Abbreviations: 95% CI, 95% confidence interval; ADA, American Diabetes Association; DKD, diabetic kidney disease; I 2, inconsistence index; NA, not available; OR, odds ratio; T2DM, type 2 diabetes mellitus; WHO, World Health Organization.
3.5. Cumulative and influential analyses: −174G/C polymorphism and T2DM
In cumulative analyses, there was no suggestion of significant influence from the first publication on subsequent publications for IL‐6 gene −174G/C polymorphism associated with T2DM under three genetic models (Figure S1). The influential analyses indicated no significant influence of any one studies on overall estimates under three genetic models (Figure S2).
3.6. Publication bias: −174G/C polymorphism and T2DM
Begg's plots and filled funnel plots are presented in Figure 3 for the association between IL‐6 gene −174G/C polymorphism and T2DM under three genetic models. The Begg's funnel plots seemed symmetrical, and there was no statistical evidence of publication bias. In addition, there were no theoretically missing studies in filled funnel plots.
FIGURE 3.

Begg's (the left) and filled (the right) funnel plots of interleukin 6 gene −174G/C polymorphism associated with type 2 diabetes mellitus under three genetic models
3.7. Circulating IL‐6 concentrations across −174G/C genotypes
Figure 4 illustrates the changes of circulating IL‐6 concentrations across the genotypes of IL‐6 gene −174G/C polymorphism. Taking the carriers of −174GG genotype as a reference group, carriers of −174CC and −174CG genotypes had 0.10 and 0.03 lower concentrations of circulating IL‐6 in pg/mL, albeit no detectable significance.
FIGURE 4.

Changes of circulating interleukin 6 concentrations across of the genotypes of −174G/C polymorphism. Abbreviations: SMD, standard mean difference; 95% CI, 95% confidence interval
3.8. Other circulating biomarkers across −174G/C genotypes
The changes in other circulating biomarkers, including LDL, HDL, TC, TG, HbA1c, and FPG, across the genotypes of IL‐6 gene −174G/C polymorphism are separately summarized in Figure S3.
4. DISCUSSION
This study was designed to meta‐analytically examine the association of IL‐6 gene −174G/C polymorphism with T2DM, and circulating IL‐6 changes across −174G/C genotypes. Albeit no detectable significance between this polymorphism and T2DM, our genotype‐phenotype analyses provided suggestive evidence on a dose‐dependent relation between the number of −174G/C mutant alleles and circulating IL‐6 concentrations, indicating possible implication of IL‐6 gene in the pathogenesis of T2DM. Additionally, our subsidiary analyses revealed that ethnicity and matched status were underlying sources for the obvious between‐study heterogeneity.
In 2006, Qi and colleagues meta‐analysed the association of IL‐6 gene −174G/C polymorphism with T2DM by pooling the results of 10 articles, and they found that the −174GG homozygotes were not significantly associated with the risk of T2DM compared with −174CC genotype or −174GG plus −174GC genotypes, 45 in line with the overall findings of the current study. With the accumulating data afterwards, on the basis of the meta‐analysis by Qi and colleagues, 45 we synthesized the results from 20 eligible articles to examine the association between this polymorphism and T2DM under three genetic models in the current meta‐analysis. Importantly, such a relative large number of eligible studies permitted us to seek underlying sources of heterogeneity. In spite of no detectable significance in both overall and subsidiary analyses, we observed that the association between IL‐6 gene −174G/C polymorphism and T2DM was more obvious under the dominant model and the relation between circulating IL‐6 concentrations across −174G/C genotypes followed a dose‐dependent manner. We cannot preclude the possibility that IL‐6 gene −174G/C polymorphism may not, by itself, exhibit significant predisposition to T2DM, mainly because its effect is small and may be dependent on the presence of other mutations. We agree that further large, well‐designed, prospective investigations are warranted to confirm the susceptible role of IL‐6 gene in the pathogenesis of T2DM.
Extending the findings of previous meta‐analysis by Qi and colleagues, 45 we noticed that race and matched status were underlying causes of previously conflicting reports. Indeed, there is a wide recognition that the development of T2DM is complex, and divergent genetic determinants or linkage profiles might account for these differences. 46 , 47 A variant may be a candidate locus for T2DM in one ethnic group, but not in another, which was further reinforced in the current meta‐analysis, when analysing the association of IL‐6 gene −174G/C polymorphism with the risk for T2DM upon stratification by races. Another important aspect is the confounding that results from unmatched cases and controls. In fact, our effect‐size estimates in the current meta‐analysis were derived from allele or genotype counts, overlooking the consideration of other confounding factors, such as age, gender and lifestyle factors. 48 The disparities in the findings of previous studies may be attributable to unaccounted residual confounding. 49 A potentially power approach to avoiding residual confounding is through Mendelian randomization. 50 Due to the non‐significant observations in genotype‐disease and genotype‐phenotype analyses, Mendelian randomization cannot be further conducted, as this approach requires genotypes that influence the variable of interest are directly related to the outcome.
The contribution of IL‐6, as a pro‐inflammatory cytokine to the pathogenesis of T2DM, is biologically plausible. 51 Actually, IL‐6 acts via two distinct signalling pathways in the development of diabetes, that is, classic signalling and trans‐signalling. The final biological effects of these two signalling modes that lead to activation of the same receptor subunit are completely different. 23 Knockout experiments showed that the expression of IL‐6 was significantly elevated in insulin‐resistant individuals. 52 Although IL‐6 is an indicator of inflammation, the study by Mauer and colleagues demonstrated that it can limit inflammation by promoting the alternative activation of macrophages to curb inflammation. 53 In addition, IL‐6 is considered to be involved in the development of inflammation, insulin resistance, as well as β‐cell dysfunction. 23 The interaction between IL‐6 and TNF‐α can exacerbate oxidative stress and reduce phosphorylation of endothelial nitric oxide synthase (eNOS), which may cause various complications. 54 On the basis of above evidence, it is reasonable to speculate that IL‐6 gene is a possible candidate in susceptibility to the development of diabetes.
Several limitations should be acknowledged for the current meta‐analysis. The first limitation lied in the analysis of only one polymorphism in IL‐6 gene. The second limitation was that only retrieved articles in English were analysed in this study, and the ‘grey’ literature was not included. The exclusion of ‘grey’ literature from meta‐analysis may result in an overestimate of an association impact by an average of 12%. 55 The third limitation was about publication bias. Although there was a low probability, the possibility of missing small or negative studies that had not yet been published was still existed. The fourth limitation was about heterogeneity. Although a set of auxiliary analyses had been conducted, the heterogeneity was still significant in some subgroups, which limited the interpretation of combined risk estimates.
Taken together, albeit no detectable significance between IL‐6 gene −174G/C polymorphism and T2DM, our genotype‐phenotype analyses provided suggestive evidence on a dose‐dependent relation between the number of −174G/C mutant alleles and circulating IL‐6 concentrations, indicating possible implication of IL‐6 gene in the pathogenesis of T2DM. For practical reasons, our hope is that this meta‐analysis will not represent just another endpoint of investigations, instead of a start to clarify the association of other genetic defects in IL‐6 gene with the risk for T2DM, as well as to elucidate the underlying molecular mechanisms of circulating IL‐6 concentrations in the onset and progression of T2DM.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTION
Hao Cheng: Data curation (equal); Formal analysis (equal); Writing‐original draft (lead). Wenbin Zhu: Data curation (lead); Writing‐original draft (equal). Mou Zhu: Methodology (lead). Yan Sun: Methodology (equal); Project administration (equal). Xiaojie Sun: Methodology (equal); Project administration (equal). Di Jia: Project administration (lead). Chao Yang: Data curation (equal); Project administration (equal). Haitao Yu: Supervision (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Chunjing Zhang: Conceptualization (lead); Supervision (lead); Writing‐original draft (equal); Writing‐review & editing (equal).
Supporting information
Supporting information
ACKNOWLEDGEMENT
The work was supported by the Natural Science Foundation of Heilongjiang Province (Grant No: LH2020H129) and the Research Projects of Basic Scientific Research of Provincial Universities in Heilongjiang Province (Grant No: 2017‐QYKYYWF‐0747).
Cheng H, Zhu W, Zhu M, et al. Meta‐analysis: Interleukin 6 gene ‐174G/C polymorphism associated with type 2 diabetes mellitus and interleukin 6 changes. J Cell Mol Med. 2021;25:5628–5639. 10.1111/jcmm.16575
Hao Cheng and Wenbin Zhu contributed equally to this work.
Contributor Information
Haitao Yu, Email: yht422@126.com.
Chunjing Zhang, Email: cjzhang2005@163.com.
DATA AVAILABILITY STATEMENT
Data involved in this study are available upon reasonable request.
REFERENCES
- 1. Diabetes. https://www.who.int/health‐topics/diabetes (accessed on March 21, 2021).
- 2. Temneanu OR, Trandafir LM, Purcarea MR. Type 2 diabetes mellitus in children and adolescents: a relatively new clinical problem within pediatric practice. J Med Life. 2016;9:235‐239. [PMC free article] [PubMed] [Google Scholar]
- 3. The L. Diabetes: a dynamic disease. Lancet (London, England). 2017;389:2163. [DOI] [PubMed] [Google Scholar]
- 4. Cui ZH, Lu XT, Xiao KL, Chen Y, Li HQ. Association of interleukin‐6 ‐174G/C polymorphism with the risk of diabetic nephropathy in type 2 Diabetes: a meta‐analysis. Curr Med Sci. 2019;39:250‐258. [DOI] [PubMed] [Google Scholar]
- 5. Karamitri A, Jockers R. Melatonin in type 2 diabetes mellitus and obesity. Nat Rev Endocrinol. 2019;15:105‐125. [DOI] [PubMed] [Google Scholar]
- 6. Durda P, Sabourin J, Lange EM, et al. Plasma levels of soluble interleukin‐2 receptor α: associations with clinical cardiovascular events and genome‐wide association scan. Arterioscler Thromb Vasc Biol. 2015;35:2246‐2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lowe WL Jr, Scholtens DM, Sandler V, Hayes MG. Genetics of gestational diabetes mellitus and maternal metabolism. Curr Diab Rep. 2016;16:15. [DOI] [PubMed] [Google Scholar]
- 8. Prasad RB, Groop L. Genetics of type 2 diabetes‐pitfalls and possibilities. Genes (Basel). 2015;6:87‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davegardh C, Garcia‐Calzon S, Bacos K, Ling C. DNA methylation in the pathogenesis of type 2 diabetes in humans. Mol Metab. 2018;14:12‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang X, Ma L, Peng F, et al. The endothelial dysfunction in patients with type 2 diabetes mellitus is associated with IL‐6 gene promoter polymorphism in Chinese population. Endocrine. 2011;40:124‐129. [DOI] [PubMed] [Google Scholar]
- 11. Vozarova B, Fernández‐Real JM, Knowler WC, et al. The interleukin‐6 (‐174) G/C promoter polymorphism is associated with type‐2 diabetes mellitus in Native Americans and Caucasians. Hum Genet. 2003;112:409‐413. [DOI] [PubMed] [Google Scholar]
- 12. Rehman K, Akash MSH, Liaqat A, Kamal S, Qadir MI, Rasul A. Role of interleukin‐6 in development of insulin resistance and type 2 diabetes mellitus. Crit Rev Eukaryot Gene Expr. 2017;27:229‐236. [DOI] [PubMed] [Google Scholar]
- 13. Panagi L, Poole L, Hackett RA, Steptoe A. Sex differences in interleukin‐6 stress responses in people with Type 2 diabetes. Psychophysiology. 2019;56:e13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. da Silva CB, Vieira DA, de Melo LF, et al. Interleukin‐6‐174G/C polymorphism is associated with a decreased risk of type 2 diabetes in patients with chronic hepatitis C virus. World J Hepatol. 2020;12:137‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cirelli T, Nepomuceno R, Rios ACS, et al. Genetic polymorphisms in the Interleukins IL1B, IL4, and IL6 are associated with concomitant periodontitis and type 2 diabetes mellitus in Brazilian patients. J Periodontal Res. 2020. [DOI] [PubMed] [Google Scholar]
- 16. Plataki MN, Zervou MI, Samonis G, Daraki V, Goulielmos GN, Kofteridis DP. Association of the Interleukin‐6 rs1800795 Polymorphism with Type 2 Diabetes Mellitus in the Population of the Island of Crete, Greece. Genet Test Mol Biomarkers. 2018;22:448‐452. [DOI] [PubMed] [Google Scholar]
- 17. Feng Y, Jiang CD, Chang AM, et al. Interactions among insulin resistance, inflammation factors, obesity‐related gene polymorphisms, environmental risk factors, and diet in the development of gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2019;32:339‐347. [DOI] [PubMed] [Google Scholar]
- 18. Lu QK, Zhang JT, Zhao N, Wang HY, Tong QH, Wang SL. Association of IL‐6 Gene (‐174 and ‐572 G/C) polymorphisms with proliferative diabetic retinopathy of type 2 diabetes in a Chinese population. Ophthalmic Res. 2017;58:162‐167. [DOI] [PubMed] [Google Scholar]
- 19. Ghavimi R, Sharifi M, Mohaghegh MA, Mohammadian H, Khadempar S, Rezaei H. Lack of association between rs1800795 (‐174 G/C) polymorphism in the promoter region of interleukin‐6 gene and susceptibility to type 2 diabetes in Isfahan population. Adv Biomed Res. 2016;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ururahy MA, de Souza KS, Oliveira YM, et al. Association of polymorphisms in IL6 gene promoter region with type 1 diabetes and increased albumin‐to‐creatinine ratio. Diabetes Metab Res Rev. 2015;31:500‐506. [DOI] [PubMed] [Google Scholar]
- 21. Saxena M, Agrawal CG, Srivastava N, Banerjee M. Interleukin‐6 (IL‐6)‐597 A/G (rs1800797) & ‐174 G/C (rs1800795) gene polymorphisms in type 2 diabetes. Indian J Med Res. 2014;140:60‐68. [PMC free article] [PubMed] [Google Scholar]
- 22. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C‐reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327‐334. [DOI] [PubMed] [Google Scholar]
- 23. Akbari M, Hassan‐Zadeh V. IL‐6 signalling pathways and the development of type 2 diabetes. Inflammopharmacology. 2018;26:685‐698. [DOI] [PubMed] [Google Scholar]
- 24. Lainampetch J, Panprathip P, Phosat C, et al. Association of tumor necrosis factor alpha, interleukin 6, and C‐reactive protein with the risk of developing type 2 diabetes: a retrospective cohort study of rural thais. J Diabetes Res. 2019;2019:9051929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ (Clinical research ed). 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buraczynska M, Zukowski P, Drop B, Baranowicz‐Gaszczyk I, Ksiazek A. Effect of G(‐174)C polymorphism in interleukin‐6 gene on cardiovascular disease in type 2 diabetes patients. Cytokine. 2016;79:7‐11. [DOI] [PubMed] [Google Scholar]
- 27. Rodrigues KF, Pietrani NT, Bosco AA, Campos FMF, Sandrim VC, Gomes KB. IL‐6, TNF‐α, and IL‐10 levels/polymorphisms and their association with type 2 diabetes mellitus and obesity in Brazilian individuals. Arch Endocrinol Metab. 2017;61:438‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bouhaha R, Baroudi T, Ennafaa H, et al. Study of TNFalpha ‐308G/A and IL6 ‐174G/C polymorphisms in type 2 diabetes and obesity risk in the Tunisian population. Clin Biochem. 2010;43:549‐552. [DOI] [PubMed] [Google Scholar]
- 29. Danielsson P, Truedsson L, Eriksson KF, Norgren L. Inflammatory markers and IL‐6 polymorphism in peripheral arterial disease with and without diabetes mellitus. Vasc Med. 2005;10:191‐198. [DOI] [PubMed] [Google Scholar]
- 30. Eze IC, Imboden M, Kumar A, et al. A common functional variant on the pro‐inflammatory Interleukin‐6 gene may modify the association between long‐term PM10 exposure and diabetes. Environ Health. 2016;15:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fathy SA, Mohamed MR, Ali MAM, El‐Helaly AE, Alattar AT. Influence of IL‐6, IL‐10, IFN‐γ and TNF‐α genetic variants on susceptibility to diabetic kidney disease in type 2 diabetes mellitus patients. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals. 2019;24:43‐55. [DOI] [PubMed] [Google Scholar]
- 32. Hameed I, Masoodi SR, Malik PA, Mir SA, Ghazanfar K, Ganai BA. Genetic variations in key inflammatory cytokines exacerbates the risk of diabetic nephropathy by influencing the gene expression. Gene. 2018;661:51‐59. [DOI] [PubMed] [Google Scholar]
- 33. Karadeniz M, Erdogan M, Berdeli A, Yilmaz C. Association of interleukin‐6 ‐174 G>C promoter polymorphism with increased risk of type 2 diabetes mellitus patients with diabetic nephropathy in. Turkey. 2014;18:62‐65. [DOI] [PubMed] [Google Scholar]
- 34. Kavitha L, Vijayshree Priyadharshini J, Sivapathasundharam B. Association among interleukin‐6 gene polymorphisms, type 2 diabetes mellitus, and chronic periodontitis: a pilot study. J Investig Clin Dent. 2017;8:e12230. [DOI] [PubMed] [Google Scholar]
- 35. Lara‐Gómez RE, Moreno‐Cortes ML, Muñiz‐Salazar R, Zenteno‐Cuevas R. Association of polymorphisms at ‐174 in IL‐6, and ‐308 and ‐238 in TNF‐α, in the development of tuberculosis and type 2 diabetes mellitus in the Mexican population. Gene. 2019;702:1‐7. [DOI] [PubMed] [Google Scholar]
- 36. Neelofar K, Ahmad J, Ahmad A, Alam K. Study of IL4‐590C/T and IL6‐174G/C gene polymorphisms in type 2 diabetic patients with chronic kidney disease in north Indian population. J Cell Biochem. 2017;118:1803‐1809. [DOI] [PubMed] [Google Scholar]
- 37. Ponnana M, Sivangala R, Joshi L, Valluri V, Gaddam S. IL‐6 and IL‐18 cytokine gene variants of pulmonary tuberculosis patients with co‐morbid diabetes mellitus and their household contacts in Hyderabad. Gene. 2017;627:298‐306. [DOI] [PubMed] [Google Scholar]
- 38. Saxena M, Srivastava N, Banerjee M. Cytokine Gene Variants as Predictors of Type 2 Diabetes Mellitus. Curr Diabetes Rev. 2018;14:307‐319. [DOI] [PubMed] [Google Scholar]
- 39. Tsiavou A, Hatziagelaki E, Chaidaroglou A, et al. TNF‐alpha, TGF‐beta1, IL‐10, IL‐6, gene polymorphisms in latent autoimmune diabetes of adults (LADA) and type 2 diabetes mellitus. J Clin Immunol. 2004;24:591‐599. [DOI] [PubMed] [Google Scholar]
- 40. Xiao LM, Yan YX, Xie CJ, et al. Association among interleukin‐6 gene polymorphism, diabetes and periodontitis in a Chinese population. Oral Dis. 2009;15:547‐553. [DOI] [PubMed] [Google Scholar]
- 41. Gomez‐Garcia EF, Cortes‐Sanabria L, Cueto‐Manzano AM, et al. Interactions between diet quality and interleukin‐6 genotypes are associated with metabolic and renal function parameters in mexican patients with type 2 diabetes mellitus. J Ren Nutr. 2020;30:223‐231. [DOI] [PubMed] [Google Scholar]
- 42. Illig T, Bongardt F, Schöpfer A, et al. Significant association of the interleukin‐6 gene polymorphisms C‐174G and A‐598G with type 2 diabetes. J Clin Endocrinol Metab. 2004;89:5053‐5058. [DOI] [PubMed] [Google Scholar]
- 43. Möhlig M, Boeing H, Spranger J, et al. Body mass index and C‐174G interleukin‐6 promoter polymorphism interact in predicting type 2 diabetes. J Clin Endocrinol Metab. 2004;89:1885‐1890. [DOI] [PubMed] [Google Scholar]
- 44. Papaoikonomou S, Tousoulis D, Tentolouris N, et al. Assessment of the effects of 174G/C polymorphism on interleukin 6 gene on macrovascular complications in patients with type 2 diabetes mellitus. Int J Cardiol. 2014;172:e190‐e191. [DOI] [PubMed] [Google Scholar]
- 45. Qi L, van Dam RM, Meigs JB, Manson JE, Hunter D, Hu FB. Genetic variation in IL6 gene and type 2 diabetes: tagging‐SNP haplotype analysis in large‐scale case‐control study and meta‐analysis. Hum Mol Genet. 2006;15:1914‐1920. [DOI] [PubMed] [Google Scholar]
- 46. Cole JB, Florez JC. Genetics of diabetes mellitus and diabetes complications. Nat Rev Nephrol. 2020;16:377‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stancakova A, Laakso M. Genetics of Type 2 Diabetes. Endocr Dev. 2016;31:203‐220. [DOI] [PubMed] [Google Scholar]
- 48. Madias JE. Prevalence of diabetes mellitus in patients with takotsubo syndrome according to age and sex. Am J Cardiol. 2019;123:1190‐1191. [DOI] [PubMed] [Google Scholar]
- 49. Becher H. The concept of residual confounding in regression models and some applications. Stat Med. 1992;11:1747‐1758. [DOI] [PubMed] [Google Scholar]
- 50. Pigeyre M, Sjaarda J, Chong M, et al. ACE and type 2 diabetes risk: a mendelian randomization study. Diabetes Care. 2020;43:835‐842. [DOI] [PubMed] [Google Scholar]
- 51. Magnusson Hanson LL, Virtanen M, Rod NH, et al. Does inflammation provide a link between psychosocial work characteristics and diabetes? Analysis of the role of interleukin‐6 and C‐reactive protein in the Whitehall II cohort study. Brain Behav Immun. 2019;78:153‐160. [DOI] [PubMed] [Google Scholar]
- 52. Liu LF, Kodama K, Wei K, et al. The receptor CD44 is associated with systemic insulin resistance and proinflammatory macrophages in human adipose tissue. Diabetologia. 2015;58:1579‐1586. [DOI] [PubMed] [Google Scholar]
- 53. Mauer J, Chaurasia B, Goldau J, et al. Signaling by IL‐6 promotes alternative activation of macrophages to limit endotoxemia and obesity‐associated resistance to insulin. Nat Immunol. 2014;15:423‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee J, Lee S, Zhang H, Hill MA, Zhang C, Park Y. Interaction of IL‐6 and TNF‐α contributes to endothelial dysfunction in type 2 diabetic mouse hearts. PLoS One. 2017;12:e0187189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta‐analyses? Lancet. 2000;356:1228‐1231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
Data involved in this study are available upon reasonable request.


