Abstract
Countless studies in animals have shown how housing environments and behaviors can significantly affect anxiety and brain health, giving valuable insight as to whether this is applicable in the human context. The relationship between housing, behavior, brain health, and mental wellbeing in humans remains poorly understood. We therefore explored the interaction of housing quality, weekend/holiday sedentary behavior, brain structure, and anxiety in healthy Japanese adults. Whole-brain structural magnetic resonance imaging (MRI) methods based on gray matter volume and fractional anisotropy were used as markers for brain health. Correlation tests were conducted, and then adjusted for multiple comparisons using the False Discovery Rate method. Housing quality and weekend/holiday sedentary behavior were associated with fractional anisotropy, but not with gray matter volume. Fractional anisotropy showed significant associations with anxiety. Lastly, both weekend/holiday sedentary behavior and housing quality were indirectly associated with anxiety through fractional anisotropy. These results add to the limited evidence surrounding the relationship among housing, behavior, and the brain. Furthermore, these results show that behavior and housing qualities can have an indirect impact on anxiety through neurobiological markers such as fractional anisotropy.
Subject terms: Diagnostic markers, Predictive markers, Development of the nervous system, Stress and resilience, Synaptic plasticity, Human behaviour
Introduction
The ill effects of the coronavirus disease 2019 (COVID 19) have spilled over into various mental health problems1,2, which may have been caused by strict lockdown and social distancing measures3. In response to the advent of new lifestyles, staying physically and mentally fit whilst staying at home have become of great interest. Living environments evoke various experiences and behavioral activity, which overtime considerably affect psychological dispositions4. This is highly evident in Enriched Environment (EE) experiments in animals. EEs are housing environments designed to increase physical, social, and cognitive activity. A substantial amount of literature has shown that exposure to EEs reduces anxiety5–7 and stress8. Such environments are also shown to have profound effects on brain plasticity, a key component in healthy brain aging. Histological analyses of animal brains post experiment showed an increase in neurons9,10, synaptic activity11,12, and overall brain volume13,14. These findings provide valuable insight as to whether EEs in the human context affect anxiety and brain health.
Multiple systematic reviews have shown the positive effects of physical activity on human structural neuroplasticity. In general, higher cardiorespiratory fitness or doses of physical activity seem to have protective effects on gray matter15,16 and white matter volumes17–19. Intriguingly, in light of the recent shift to home-based lifestyles, sedentariness is increasingly being shown as a separate risk factor. Multiple studies have shown that sedentary behavior is associated with brain atrophy, independent of physical activity20–22. Although the detrimental health effects of sedentariness can be reduced through physical activity, but this approach may be impractical for the general population. A study showed thatan individual needs to perform around 60-75 minutes of moderately intense physical activity throughout the day in order to reduce mortality risks associated to sedentary lifestyles, which is well beyond the guidelines set by the World Health Organization (150 minutes of moderately intense physical activity throughout the week)23. In terms of psychological outcomes, it was only until recently that researchers started to investigate the effects of sedentary behavior on anxiety. A prospective study showed that sitting for more than 42 hours per week is associated with a 31% increased risk of developing a mental disorder compared with sitting for less than 10.5 hours per week24. In a recent systematic review, sedentary behavior was shown to have positive associations with various mental health disorders, such as an increased risk for developing anxiety25.
In addition to physical activity and sedentariness, the efficacy of an EE also rests on its ability to stimulate extended social and cognitive behaviors26. Housing environments, however, comprise of several other “trivial” elements such as indoor thermal conditions, lighting, and humidity. These housing qualities are oftentimes overlooked but are shown to influence experimental outcomes27. For example, the standard housing temperature of 22 °C, compared to 30 °C, already puts mice under a certain degree of stress at baseline28,29.
In humans, the quality of housing varies across different socioeconomic stratums (SES). Census data from the American Housing Survey dated 1989–2001 shows that individuals who lived in substandard housing conditions belonged to lower income brackets30. In another longitudinal study (1995–2013), lead exposure was shown to be more concentrated in lower SES individuals and neighborhoods31.
Relatedly, SES is consistently shown to affect brain health32. Cross-sectional MRI analyses have shown that higher years of education and household income have positive associations with gray matter volume33 and white matter integrity34,35 in both children and adults. In a prospective study, a significant association was found between childhood SES and white matter integrity, measured during adulthood36. Given that SES affects brain structure across multiple age groups, it is possible that markers related to SES, such as housing qualities, may also have associations with brain structures.
Evidence on the effects of human housing qualities, such as indoor thermal conditions, on structural brain markers appears to be scant. A previous study by Dufford and Kim (2017), although not directly related to indoor thermal conditions, is the only study that investigated the impact of poor housing qualities on fractional anisotropy. Their study assessed housing quality in terms of structural defects, maintenance, childhood resources, safety hazards, and cleanliness. They found that individuals exposed to poor housing qualities had lower fractional anisotropy scores37.
The improvements in certain housing qualities, such as the installation of central heating, has been shown to reduce symptoms of anxiety38,39. In a randomized study, retrofit insulation improved mental health and was associated with fewer hospital visits40. In another study, air-conditioning had significant associations with anxiety41. In terms of brain function, an interventional study showed that the manipulation of ambient temperatures during fMRI scans activates brain regions related to behavioral adaptations to external stimuli42,43. Similarly, thermal stimulations were shown to activate specific brain regions related to positive and negative emotion44. In a randomized trial, hyperthermia affected executive function and reduced neural efficiency in healthy participants45. More notably, a study by Kim et al. investigated the effects of different thermal sensations on emotional responses by measuring brain activity. Kim and colleagues were able to show that different emotional responses can be attained by changing the ambient temperature. Pleasant emotions were seen when the ambient temperature was slightly warm, and unpleasant in all other cases46. The results from these studies already show, to a certain extent, that indoor environments affect the brain at the state level. The current evidence can be further enriched by investigating the effects on structural brain markers, as these markers are usually not affected by transient psychological conditions47.
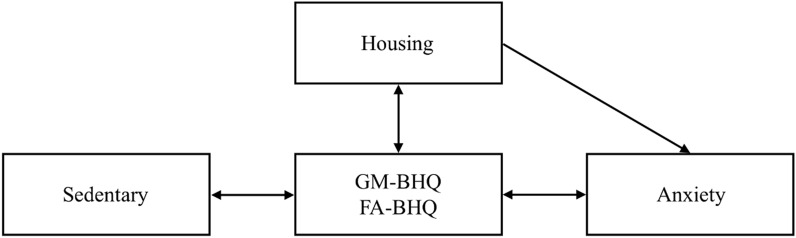
We also speculate that differences in behavioral choices during weekdays and weekend-days may have differential effects on anxiety, as one study showed that anxiety is more pronounced during the weekend48. Therefore, this study aimed to investigate the impact of housing quality and weekend/holiday sedentary behavior on the central nervous system, specifically on brain structure and anxiety (see Fig. 1).
Figure 1.
Hypothesized associations among housing quality, weekend/holiday sedentary behavior, brain health, and anxiety. The relationships shown below are assumptions based on the current literature, and are not in any way definitive or causal.
Materials and methods
Subjects
One hundred and nine healthy individuals (47 females and 62 males), aged 25–69 [mean (M) ± standard deviation (SD): 48.239 ± 8.773 years] participated in the experiment, which was conducted in Kyoto University (Kyoto City, Japan) and the University of Tokyo (Tokyo City, Japan). The experiment required each participant to undergo an MRI brain scan and answer a set of questionnaires to assess their housing quality, their weekend/holiday sedentary behavior at home, and their current anxiety level.
This study was approved by the ethics committees of Kyoto University (approval number 27-P-13) and the University of Tokyo (Tokyo, Japan; approval number 402-2), and performed in accordance with the guidelines and regulations of the institute, respecting the Declaration of Helsinki. All participants gave written informed consent prior to participation, and participant anonymity was preserved.
Housing quality and weekend/holiday sedentary behavior
This study focused on indoor insulation and space heating/cooling, which was measured using a housing quality assessment tool called the Comprehensive Assessment System for Built Environment Efficiency (CASBEE)49–51. This tool evaluates various housing qualities such as quietness, dampness or presence of mold, safety, peace of mind, indoor temperature, etc. Since this study focuses on indoor thermal environment, the following questions were included in the analysis: (1) “During summer, do you feel hot without using space cooling?” (2) “During winter, do you feel cold without using space heating?” Participants were asked to rate how hot/cold they felt during the summer/winter without using space cooling/heating facilities, using a four-point Likert scale (0—Extremely uncomfortable to 3—Very Comfortable). Higher scores indicate lower levels of discomfort.
Weekend/holiday sedentary behavior was measured using the International Physical Activity Questionnaire (IPAQ)52. Participants indicated the amount of time they spend sitting/lying down during weekend/holidays (in minutes).
Anxiety
Anxiety was measured using the Brief Job Stress Questionnaire (BJSQ). Developed by the Japanese Ministry of Labor and health, this instrument has been widely used in research and practice in the field of workplace mental health in Japan53. The BJSQ asks the participant how he/she has felt during the past month, in terms of Liveliness, Irritability, Tiredness, Anxiety, Depression, and Physical complaints. The questions were answered through a three-point Likert scale (0—Strongly Agree to 3—Strongly Disagree). Three questions were related to Anxiety: “I have been full of energy”, “I have felt worried or insecure”, and “I have felt restless”. The sum of the scores of these 3 questions was used for analysis.
MRI data acquisition
Similar to the previous MRI experiment by Nemoto and colleagues54, all magnetic resonance imaging (MRI) data were collected using a 3-T Siemens scanner (Verio, Siemens Medical Solutions, Erlangen, Germany or MAGNETOM Prisma, Siemens, Munich, Germany) equipped with a 32- or 64-channel head array coil at Kyoto University and the University of Tokyo. A high-resolution structural image was acquired using a three-dimensional (3D) T1-weighted magnetization-prepared rapid-acquisition gradient echo (MP-RAGE) pulse sequence. The parameters were as follows: repetition time (TR), 1900 ms; echo time (TE), 2.52 ms; inversion time (TI), 900 ms; flip angle, 9°; matrix size, 256 × 256; field of view (FOV), 256 mm; and slice thickness, 1 mm54.
DTI data were collected with spin-echo echo-planar imaging (SE-EPI) with GRAPPA (generalized autocalibrating partially parallel acquisitions). The image slices were parallel to the orbitomeatal (OM) line. The parameters were as follows: TR, 14,100 ms; TE, 81 ms, flip angle, 90°; matrix size, 114 × 114; FOV, 224 mm; slice thickness, 2 mm. A baseline image (b = 0 s/mm2) and 30 different diffusion orientations were acquired with a b value of 1000 s/mm2, similar to the study by Nemoto et al.54.
MRI data analysis
The calculation of the gray matter volume in this study is identical to the calculation methods used in our previous neuroimaging study54. In summary, gray matter images were segmented from T1-weighted images using Statistical Parametric Mapping 12 (SPM12; Wellcome Trust Centre for Neuroimaging, London, UK) running on MATLAB R2015b (Mathworks Inc., Sherborn, MA, USA), followed by spatial normalization using diffeomorphic anatomical registration through an exponentiated lie algebra (DARTEL) algorithm55 and modulation to preserve the GM volume. All normalized, segmented, and modulated images were smoothed with an 8-mm full width at half-maximum (FWHM) Gaussian kernel. Additionally, intracranial volume (ICV) was calculated by summing the GM, white matter, and cerebrospinal fluid images for each subject. Proportional GM images were generated by dividing smoothed GM images by ICV to control for differences in whole-brain volume across participants. Using these proportional GM images, images for the mean and standard deviation (SD) across participants were generated. Then, we calculated the gray matter volume using the following formula: 100 + 15 × (individual proportional GM − mean)/SD. Regional GM quotients were then extracted using an automated anatomical labeling (AAL) atlas56 and averaged across regions to produce participant-specific gray matter volumes.
DTI data were preprocessed using FMRIB Software Library (FSL) 5.0.957. First, all diffusion images were aligned with the initial b0 image, and motion correction and eddy current distortion correction was performed using eddy_correct. Following these corrections, FA images were calculated using dtifit. FA images were then spatially normalized into the standard Montreal Neurological Institute (MNI) space using FLIRT and FNIRT. FLIRT, a linear registration tool, was used to roughly align a set of brains to MNI space. Then FNIRT, a non-linear registration tool, was used to achieve better registration. After spatial normalization we smoothed the data with an 8-mm FWHM. Mean and SD images were generated from all the FA images, and both individual FA quotient images and GM-BHQ images were calculated. Individual FA quotient images were calculated using the following formula: 100 + 15 × (individual FA − mean)/SD. Regional FA quotients were extracted using Johns Hopkins University (JHU) DTI-based white-matter atlases and averaged across regions to produce participant-specific FA-BHQs53.
Statistical analysis
Preliminary statistical analysis was performed using SPSS version 26 (IBM Corporation, Armonk, NY, USA) and AMOS. The preliminary analysis included four tests: (1) two Student’s t-test (two-tailed) to determine if the mean values of the predictor variables were strongly influenced by sex and the experiment location (Kyoto City or Tokyo City), (2) a chi-square test to determine if the experiment location and sex had significant distributional differences, (3) zero-order correlation analysis to find out if the predictor variables had associations with anxiety, and (4) partial correlation analysis to determine if the combination of housing and weekend/holiday sedentary behavior as a whole affected anxiety. The main analysis was a path analysis to explore the direct and indirect relationships among housing quality, weekend/holiday sedentary behavior, overall white matter integrity, overall gray matter volume, and anxiety. The path analysis was calculated using AMOS Version 26 (IBM Corp., Armonk, NY, USA). For brevity purposes, “Housing quality” was renamed to “Housing” and “Weekend/holiday sedentary behavior” was renamed to “Sedentary”.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committees of Kyoto University (approval number 27-P-13) and the University of Tokyo (approval number 402). The patients/participants provided their written informed consent to participate in this study.
Results
Results from the first t-test in Table 1 showed statistical mean difference between men and women when it comes to FA-BHQ (t = − 2.487, p = 0.014) and GM-BHQ scores (t = − 5.807, p < 0.001). The results from the chi-square test, also in Table 1, showed a p-value less than the 0.001 significance level, suggesting that there is a relationship between sex and experiment location.
Table 1.
Student’s t test (two-tailed) and chi square test for the statistical difference between male and female participants.
| Male | Female | t | p | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age | 49.610 | 8.912 | 46.430 | 8.335 | 1.901 | 0.060 |
| FA-BHQ | 99.180 | 4.291 | 101.090 | 3.488 | − 2.487 | 0.014* |
| GM-BHQ | 96.540 | 7.124 | 104.640 | 7.329 | − 5.807 | 0.000*** |
| Housing | 3.190 | 1.587 | 3.490 | 1.502 | − 0.986 | 0.326 |
| Sedentary | 366.450 | 220.707 | 379.790 | 227.037 | − 0.309 | 0.758 |
| Anxiety | 4.650 | 1.700 | 4.020 | 2.142 | 1.696 | 0.093 |
| n | % | n | % | χ2 | p | |
|---|---|---|---|---|---|---|
| Kyoto | 22 | 20% | 36 | 33% | 18.149 | 0.000*** |
| Tokyo | 40 | 37% | 11 | 10% |
Results from the second t-test in Table 2 also showed significant mean differences between all continuous predictor variables and the location of the MRI system used for the experiment (except for Sedentary). Therefore, the succeeding correlation and path analyses controlled for age, sex, and experiment location.
Table 2.
Student’s t test for the statistical difference between two experiment locations (Kyoto City, Tokyo City).
| Kyoto | Tokyo | t | p | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age | 44.520 | 8.168 | 52.470 | 7.474 | − 5.277 | 0.000*** |
| FA-BHQ | 101.710 | 3.356 | 98.060 | 3.942 | 5.219 | 0.000*** |
| GM-BHQ | 103.497 | 8.044 | 96.094 | 6.549 | 5.223 | 0.000*** |
| Housing | 3.620 | 1.497 | 2.980 | 1.556 | 2.188 | 0.031** |
| Sedentary | 392.760 | 238.525 | 348.820 | 202.58 | 1.029 | 0.306 |
| Anxiety | 3.970 | 1.901 | 4.840 | 1.848 | − 2.437 | 0.016* |
Table 3 shows the descriptive statistics and results from the full and partial correlation tests. Subjects comprised a total of one hundred and nine participants (n = 109, 47 females and 62 males), aged 25–69 [mean (M) ± standard deviation (SD): 48.239 ± 8.773 years]. Due to the multiple comparisons (5 variables), p-values were corrected through FDR post-test. The adjusted p-value (q) was calculated using the standard threshold of 0.05. The following full correlations survived the FDR correction: Anxiety and Sedentary (r = 0.211, p = 0.028, q = 0.030), Housing and FA-BHQ (r = 0.237, p = 0.013, q = 0.035), Anxiety and Housing (r = − 0.243, p = 0.011, q = 0.040), and Anxiety and FA-BHQ (r = − 0.281, p = 0.003, q = 0.045).
Table 3.
Descriptive statistics and correlations. n = 109. Correlations appear below the diagonal and partial correlations (controlled for age and sex, and experiment location) above diagonal. R values with an asterisk (*) denote significance after FDR correction.
| Variable | Mean | SD | Anxiety | Housing | GM-BHQ | FA-BHQ | Sedentary |
|---|---|---|---|---|---|---|---|
| Anxiety | 4.376 | 1.919 | 1.000 | − 0.209* | 0.013 | − 0.206* | 0.242 |
| Housing | 3.321 | 1.551 | − 0.243 | 1.000 | 0.070 | 0.223* | − 0.063 |
| GM-BHQ | 100.033 | 8.233 | − 0.134 | 0.081 | 1.000 | 0.153 | − 0.05 |
| FA-BHQ | 100.000 | 4.060 | − 0.281 | 0.237 | 0.483* | 1.000 | − 0.253* |
| Sedentary | 372.202 | 222.512 | 0.211 | − 0.044 | 0.026 | − 0.153 | 1.000 |
Partial correlation coefficients are placed above the diagonal. After FDR correction, Anxiety was still associated with FA-BHQ (r = − 0.206, p = 0.034, q = 0.030), although marginal. Housing (r = 0.209, p = 0.031, q = 0.035), and Sedentary (r = 0.242, p = 0.013, q = 0.045) still remained significantly associated with Anxiety. There was a moderate, negative association between FA-BHQ (100.000 ± 4.060) and Sedentary (372.202 ± 222.512), which was statistically significant (r = − 0.253, p = 0.009, q = 0.050). This association was not seen in the prior zero-order correlation test (r = − 0.153, p = 0.112), indicating that age, sex, and experiment location played a significant role in controlling for the relationship between FA-BHQ and Sedentary. GM-BHQ did not have a significant association with anxiety in the full and partial correlation tests. Therefore, GM-BHQ was not included in the succeeding path analysis.
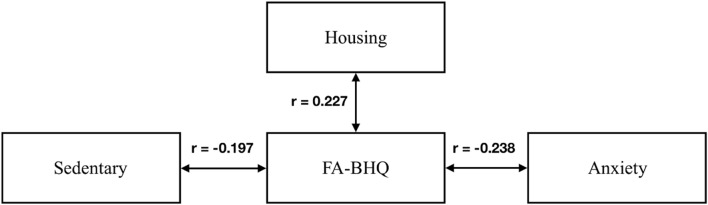
To explore the interactions among housing, weekend/holiday sedentary behavior, brain health, and anxiety, we conducted a path analysis, as shown in Fig. 2. The figures of the standardized path coefficient, calculated using AMOS Version 26 (IBM Corp., Armonk, NY, USA), are also shown. The figures are the standardized path coefficient. FDR correction was also applied post-test. The adjusted p-value (q) was calculated using the standard threshold of 0.05. This test controlled for age and experiment location (sex was not used in the path model as it has a strong correlation with experiment location as shown in Table 1). All paths still showed significant associations after FDR correction.
Figure 2.
Path diagram for the resulting interactions among Sedentary, Housing, FA-BHQ, and anxiety, after controlling for Age and experiment location. FDR correction was applied using a threshold of 0.05. All pathways shown are significant.
The path analysis showed that Housing had an indirect association with Anxiety through FA-BHQ. Sedentary also had an indirect association with Anxiety through FA-BHQ. Sedentary did not have direct associations with Anxiety. Sedentary was marginally associated with FA-BHQ (r = − 0.197, p = 0.013, q = 0.01). Housing had significant associations with FA-BHQ (r = 0.227, p = 0.006, q = 0.020). FA-BHQ showed a negative direct association with Anxiety (r = − 0.238, p = 0.010, q = 0.015). Between Sedentary and Housing, Housing showed a stronger association with FA-BHQ.
Discussion
Countless studies in animals have shown that exposure to EEs improves brain health through structural brain plasticity5,6,9,10 and promotes anxiolytic behaviors5–7. The convincing evidence begs the question as to whether the same applies in the human context. Human housing qualities are markedly influenced by SES, and recent evidence shows that SES affects brain structures across multiple age groups. It is therefore possible that markers related to SES may also affect brain structures. This exploratory study evaluated the effects of human housing quality and weekend sedentary behaviors on whole brain structure and anxiety. Whole brain structure was examined using two MRI measures based on gray matter volume and fractional anisotropy (called the GM-BHQ and FA-BHQ, respectively).
Our results reveal that housing quality and behavior are associated with white matter integrity, but not with whole brain gray matter volume. The null finding on gray matter volume seems to be in congruence with the previous study by Arnardottir et al.21. In their 5-year longitudinal cohort trial, only the decrease in white matter volume was associated with sedentary behavior. This shows a possibility that structural changes in white matter integrity in healthy adults is a sensitive biomarker that precedes gray matter volume loss. Interestingly, previous temporal studies have also posited that changes in white matter precedes volumetric changes in gray matter during normal aging58,59. Future neuroimaging studies may further investigate this speculation in order to shed light on the extent to which the changes in fractional anisotropy and gray matter volume are related.
Indices of white matter, such as fractional anisotropy, are said to be representations of brain integrity/resiliency60,61. Indeed, numerous observational and intervention studies have shown fractional anisotropy as a possible biomarker for psychological disorders such as anxiety. Kim et al.62 and Lu et al.63 demonstrated that trait anxiety in healthy individuals is inversely correlated with FA scores62,63. Tromp et al. showed that FA scores in Generalized Anxiety Disorder (GAD) patients was significantly lower compared to healthy controls64. Our findings, although cross-sectional, add to the extant literature on the relationship between white matter integrity and symptoms of anxiety in healthy individuals. This kind of study is particularly important, as the recent pandemic has greatly reduced and disrupted social connections and relationships, which has led to the surge in mental health issues1-3. It is possible that the effects could be more pronounced on vulnerable groups, such as communities or individuals on the lower end of the SES spectrum.
This study also presents a significant contribution to the identification of environmental risk factors of anxiety through neurobiological markers. More specifically, this study showed housing quality measures as possible surrogate markers for SES characteristics that affect behavior and brain structure. We believe these factors should be further explored as points of intervention in prospective and/or randomized trials. For example, investing in proper centralized heating or other insulation facilities in homes may be beneficial for brain development and aging. Investigating the housing qualities identified by the World Health Organization as physical/mental health risks (wall thickness, shading from direct sunlight, natural and artificial ventilation)65 may also have neurobiological effects.
It is also worth noting that the use of multiple MRI facilities could also increase the generalizability of the results from this study. However, at the same time, we also recognize that this can also be a limitation. We addressed this by conducting multiple t-tests (Tables 2, 3) prior to the main analysis. The t-tests results showed significant mean differences in terms of FA-BHQ values. Therefore, the succeeding correlation and path analyses controlled for age, gender and experiment location. We believe this is still a reasonable procedure because whole brain measurements are not significantly altered. However, if specific brain regions are to be evaluated, more steps are needed in order to account for the difference in MRI facility66,67.
Limitations and future work
The data used is cross-sectional in nature, therefore causal relationships and directionalities cannot be formed. Second, our sample only consists of Japanese individuals which could be prone to nationality bias. Changes in whole brain structures (gray matter volume and white matter integrity) may be different in other nationalities. Third, a larger number of samples may have increased the generalizability of the findings. Nevertheless, prospective and randomized investigations exploring the link between white matter integrity and actual behaviors using larger sample sizes and diverse nationalities are warranted in order to further elucidate the mechanisms connecting housing quality, sedentary behavior and anxiety. Fourth, the assessment of weekend/holiday sedentary behavior and anxiety were rather simplified. Future research utilizing more objective clinical evaluations of anxiety and sedentary are needed. Fifth, variables related to geographic location and income, which were not collected in this study, are also valid potential confounding variables. Including these variables in future experiments are also needed.
Finally, future RCTs and Longitudinal studies are also warranted in order to investigate the causal directionality and dose–response relationships among housing qualities, behavior, brain structure, and psychological outcomes. We believe this research direction may find its way to personalized healthcare and promote non-hospital/medicinal treatments of psychological and neurological disorders.
Author contributions
P.J.C. and K.K. prepared the tables. P.J.C. wrote the main manuscript text. P.J.C. drew Figs. 1 and 2. K.K. amended the work of P.J.C. Y.Y. and T.I. were responsible for the conceptualization, data curation, funding acquisition, and project administration. All authors reviewed and edited the manuscript.
Funding
This work was supported by the ImPACT Program of Council for Science, Technology and Innovation (Cabinet Office, Government of Japan), and the Japan Society for the Promotion of Science (JSPS), Kakenhi Grant Number 17H06151.
Data availability
All datasets generated for this study are available upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang Y, Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 epidemic in China: A web-based cross-sectional survey. Psychiatry Res. 2020;288:112954. doi: 10.1016/j.psychres.2020.112954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazza MG, et al. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benke C, Autenrieth LK, Asselmann E, Pané-Farré CA. Lockdown, quarantine measures, and social distancing: Associations with depression, anxiety and distress at the beginning of the COVID-19 pandemic among adults from Germany. Psychiatry Res. 2020;293:113462. doi: 10.1016/j.psychres.2020.113462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kempermann G. Environmental enrichment, new neurons and the neurobiology of individuality. Nat. Rev. Neurosci. 2019;20:235–245. doi: 10.1038/s41583-019-0120-x. [DOI] [PubMed] [Google Scholar]
- 5.Gong X, Chen Y, Chang J, Huang Y, Cai M, Zhang M. Environmental enrichment reduces adolescent anxiety- and depression-like behaviors of rats subjected to infant nerve injury. J. Neuroinflamm. 2018;15:262. doi: 10.1186/s12974-018-1301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravenelle R, Santolucito H, Byrnes E, Byrnes J, Donaldson S. Housing environment modulates physiological and behavioral responses to anxiogenic stimuli in trait anxiety male rats. Neuroscience. 2014;270:76–87. doi: 10.1016/j.neuroscience.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mora-Gallegos A, Fornaguera J. The effects of environmental enrichment and social isolation and their reversion on anxiety and fear conditioning. Behav. Processes. 2019;158:59–69. doi: 10.1016/j.beproc.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Bailey KR, Rustay NR, Crawley JN. Behavioral phenotyping of transgenic and knockout mice: Practical concerns and potential pitfalls. ILAR J. 2006;47:124–131. doi: 10.1093/ilar.47.2.124. [DOI] [PubMed] [Google Scholar]
- 9.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 10.Van Praag H, Kempermann G, Gage FH. Neural consequences of enviromental enrichment. Nat. Rev. Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 11.Greenough W, West R, DeVoogd T. Subsynaptic plate perforations: Changes with age and experience in the rat. Science. 1978;202:1096–1098. doi: 10.1126/science.715459. [DOI] [PubMed] [Google Scholar]
- 12.Gogolla N, Galimberti I, Deguchi Y, Caroni P. Wnt signaling mediates experience-related regulation of synapse numbers and mossy fiber Connectivities in the adult hippocampus. Neuron. 2009;62:510–525. doi: 10.1016/j.neuron.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Darmopil S, Petanjek Z, Mohammed AH, Bogdanović N. Environmental enrichment alters dentate granule cell morphology in oldest-old rat. J. Cell Mol. Med. 2008;13:1845–1856. doi: 10.1111/j.1582-4934.2008.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monteiro BM, Moreira FA, Massensini AR, Moraes MF, Pereira GS. Enriched environment increases neurogenesis and improves social memory persistence in socially isolated adult mice. Hippocampus. 2013;24:239–248. doi: 10.1002/hipo.22218. [DOI] [PubMed] [Google Scholar]
- 15.Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiol. Aging. 2014;35:S20–S28. doi: 10.1016/j.neurobiolaging.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen F, et al. The effect of exercise training on brain structure and function in older adults: A systematic review based on evidence from randomized control trials. J. Clin. Med. 2020;9:914. doi: 10.3390/jcm9040914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sexton CE, Betts JF, Demnitz N, Dawes H, Ebmeier KP, Johansen-Berg H. A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. Neuroimage. 2016;131:81–90. doi: 10.1016/j.neuroimage.2015.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wassenaar TM, Yaffe K, Van der Werf YD, Sexton CE. Associations between modifiable risk factors and white matter of the aging brain: Insights from diffusion tensor imaging studies. Neurobiol. Aging. 2019;80:56–70. doi: 10.1016/j.neurobiolaging.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson NF, Bahrani AA, Powell DK, Jicha GA, Gold BT. Cardiorespiratory fitness diminishes the effects of age on white matter hyperintensity volume. PLoS ONE. 2020;15:e0236986. doi: 10.1371/journal.pone.0236986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burzynska AZ, et al. Physical activity and cardiorespiratory fitness are beneficial for white matter in low-fit older adults. PLoS ONE. 2014;9:e107413. doi: 10.1371/journal.pone.0107413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnardottir NY, et al. Association of change in brain structure to objectively measured physical activity and sedentary behavior in older adults: Age, gene/Environment Susceptibility-Reykjavik study. Behav. Brain Res. 2016;296:118–124. doi: 10.1016/j.bbr.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siddarth P, Burggren AC, Eyre HA, Small GW, Merrill DA. Sedentary behavior associated with reduced medial temporal lobe thickness in middle-aged and older adults. PLoS ONE. 2018;13:e0195549. doi: 10.1371/journal.pone.0195549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekelund U, et al. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet. 2016;388:1302–1310. doi: 10.1016/s0140-6736(16)30370-1. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Villegas A, Ara I, Guillen-Grima F, Bes-Rastrollo M, Varo-Cenarruzabeitia JJ, Martinez-Gonzalez MA. Physical activity, sedentary index, and mental disorders in the SUN cohort study. Med. Sci. Sports Exerc. 2008;40:827–834. doi: 10.1249/mss.0b013e31816348b9. [DOI] [PubMed] [Google Scholar]
- 25.Teychenne M, Costigan SA, Parker K. The association between sedentary behaviour and risk of anxiety: A systematic review. BMC Public Health. 2015;15:513. doi: 10.1186/s12889-015-1843-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clemenson GD, Gage FH, Stark CE. Environmental enrichment and neuronal plasticity. Oxford Handb. Dev. Neural Plast. 2018 doi: 10.1093/oxfordhb/9780190635374.013.13. [DOI] [Google Scholar]
- 27.Hylander BL, Gordon CJ, Repasky EA. Manipulation of ambient housing temperature to study the impact of chronic stress on immunity and cancer in mice. J. Immunol. 2019;202:631–636. doi: 10.4049/jimmunol.1800621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Eng JW, et al. Housing temperature-induced stress drives therapeutic resistance in murine tumour models through β2-adrenergic receptor activation. Nat. Commun. 2015;6:6426. doi: 10.1038/ncomms7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs DE. Environmental health disparities in housing. Am. J. Public Health. 2011;101(S1):S115–S122. doi: 10.2105/ajph.2010.300058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sampson, R. J., & Winter, A. The racial ecology of lead poisoning, toxic inequality in Chicago neighborhoods, 1995–2013. DuBois Review: Social Science Research on Race, 13(2). https://scholar.harvard.edu/sampson/publications/racial-ecology-lead-poisoning-toxic-inequality-chicago-neighborhoods-1995-2013. (2016). Accessed 1 Aug 2019.
- 32.Chan MY, Na J, Agres PF, Savalia NK, Park DC, Wig GS. Socioeconomic status moderates age-related differences in the brain’s functional network organization and anatomy across the adult lifespan. Proc. Natl. Acad. Sci. 2018;115(22):E5144–E5153. doi: 10.1073/pnas.1714021115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lotze M, Domin M, Schmidt CO, Hosten N, Grabe HJ, Neumann N. Income is associated with hippocampal/amygdala and education with cingulate cortex grey matter volume. Sci. Rep. 2020 doi: 10.1038/s41598-020-75809-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gianaros PJ, Marsland AL, Sheu LK, Erickson KI, Verstynen TD. Inflammatory pathways link socioeconomic inequalities to white matter architecture. Cereb. Cortex. 2012;23(9):2058–2071. doi: 10.1093/cercor/bhs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ursache A, Noble KG. Socioeconomic status, white matter, and executive function in children. Brain Behav. 2016;6(10):e00531. doi: 10.1002/brb3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dufford AJ, Evans GW, Dmitrieva J, Swain JE, Liberzon I, Kim P. Prospective associations, longitudinal patterns of childhood socioeconomic status, and white matter organization in adulthood. Hum. Brain Mapp. 2020;41(13):3580–3593. doi: 10.1002/hbm.25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dufford AJ, Kim P. Family income, cumulative risk exposure, and white matter structure in middle childhood. Front. Hum. Neurosci. 2017;11:547. doi: 10.3389/fnhum.2017.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunt SM, McKenna SP. The impact of housing quality on mental and physical health. Hous. Rev. 1992;41:47–49. [Google Scholar]
- 39.Halpern D. Mental Health and the Built Environment: More than Bricks and Mortar? Routledge; 2014. [Google Scholar]
- 40.Howden-Chapman P, et al. Effect of insulating existing houses on health inequality: Cluster randomised study in the community. BMJ. 2007;334:1–9. doi: 10.1136/bmj.39070.573032.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandberg JC, et al. Association between housing quality and individual health characteristics on sleep quality among Latino farmworkers. J. Immigr. Minor Health. 2012;16:265–272. doi: 10.1007/s10903-012-9746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becerra LR, et al. Human brain activation under controlled thermal stimulation and habituation to noxious heat: An fMRI study. Magn. Reson. Med. 1999;41:1044–1057. doi: 10.1002/(sici)1522-2594(199905)41:5<1044::aid-mrm25>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 43.Oi H, et al. Neural correlates of ambient thermal sensation: An fMRI study. Sci. Rep. 2017;7:11279. doi: 10.1038/s41598-017-11802-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aizawa Y, et al. Assessment of brain mechanisms involved in the processes of thermal sensation, pleasantness/unpleasantness, and evaluation. IBRO Rep. 2019;6:54–63. doi: 10.1016/j.ibror.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu K, et al. The impact of passive hyperthermia on human attention networks: An fMRI study. Behav. Brain Res. 2013;243:220–230. doi: 10.1016/j.bbr.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 46.Kim M, Chong SC, Chun C, Choi Y. Effect of thermal sensation on emotional responses as measured through brain waves. Build. Environ. 2017;118:32–39. doi: 10.1016/j.buildenv.2017.03.023. [DOI] [Google Scholar]
- 47.Farah MJ. The neuroscience of socioeconomic status: Correlates, causes, and consequences. Neuron. 2017;96(1):56–71. doi: 10.1016/j.neuron.2017.08.034. [DOI] [PubMed] [Google Scholar]
- 48.Kao L, et al. Weekly and holiday-related patterns of panic attacks in panic disorder: A population-based study. PLoS ONE. 2014;9:e100913. doi: 10.1371/journal.pone.0100913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takayanagi E, Ikaga T, Murakami S, Seike T, Nakano J. Validation of the effectiveness of residential environment assessment tool for health promotion. J. Environ. Eng. 2011;76:1101–1108. doi: 10.3130/aije.76.1101. [DOI] [Google Scholar]
- 50.Kawakubo S, Ikaga T, Murakami S, Hoshi T, Ando S. Influence of residential performance on residents’ health promotion, Nationwide survey of environmental performance of detached houses and residents' health status. J. Environ. Eng. 2014;79:555–561. doi: 10.3130/aije.79.555. [DOI] [Google Scholar]
- 51.Murakami, S., Iwamura, K., & Cole, R. J. CASBEE, a decade of development and application of an environmental assessment system for the built environment. Institute for Building Environment and Energy Conservation, Tokyo. https://www.ibec.or.jp/CASBEE/english/downloadE.html(2019). Accessed 1 Jul 2019.
- 52.Lee PH, Macfarlane DJ, Lam T, Stewart SM. Validity of the international physical activity questionnaire short form (IPAQ-SF): A systematic review. Int. J. Behav. Nutr. Phys. Act. 2011;8:115. doi: 10.1186/1479-5868-8-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inoue A, et al. Development of a short questionnaire to measure an extended set of job demands, job resources, and positive health outcomes: The new brief job stress questionnaire. Ind. Health. 2014;52:175–189. doi: 10.2486/indhealth.2013-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nemoto K, Oka H, Fukuda H, Yamakawa Y. MRI-based brain healthcare quotients: A bridge between neural and behavioral analyses for keeping the brain healthy. PLoS One. 2017;12:e0187137. doi: 10.1371/journal.pone.0187137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 56.Tzourio-Mazoyer N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical Parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 57.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2011;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 58.Bartzokis G. Age-related myelin breakdown: A developmental model of cognitive decline and Alzheimer’s disease. Neurobiol. Aging. 2004;25:5–18. doi: 10.1016/j.neurobiolaging.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Bartzokis G, Sultzer D, Lu PH, Nuechterlein KH, Mintz J, Cummings JL. Heterogeneous age-related breakdown of white matter structural integrity: Implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiol. Aging. 2004;25:843–851. doi: 10.1016/j.neurobiolaging.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 60.Rathee R, Rallabandi VS, Roy PK. Age-related differences in white matter integrity in healthy human brain: Evidence from structural Mri and diffusion tensor imaging. Magn. Reson. Insights. 2016;9:9–20. doi: 10.4137/mri.s39666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jenkins LM, et al. Shared white matter alterations across emotional disorders: A voxel-based meta-analysis of fractional anisotropy. Neuroimage Clin. 2016;12:1022–1034. doi: 10.1016/j.nicl.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J. Neurosci. 2009;29:11614–11618. doi: 10.1523/jneurosci.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu M, Yang C, Chu T, Wu S. Cerebral white matter changes in young healthy individuals with high trait anxiety: A tract-based spatial statistics study. Front. Neurol. 2018;9:704. doi: 10.3389/fneur.2018.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tromp DP, et al. Reduced structural connectivity of a major Frontolimbic pathway in generalized anxiety disorder. Arch. Gen. Psychiatry. 2012;69:925. doi: 10.1001/archgenpsychiatry.2011.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.WHO housing and health guidelines. (2018). https://www.who.int/sustainable-development/publications/housing-health-guidelines/en/. Accessed 1 Apr 2020.
- 66.Mechelli A, et al. Neuroanatomical abnormalities that predate the onset of psychosis. Arch. Gen. Psychiatry. 2011;68:489. doi: 10.1001/archgenpsychiatry.2011.42. [DOI] [PubMed] [Google Scholar]
- 67.Nemoto K, et al. Differentiation of schizophrenia using structural MRI with consideration of scanner differences: A real-world multisite study. Psychiatry Clin. Neurosci. 2019;74:56–63. doi: 10.1111/pcn.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are available upon request.