Abstract
Obstructive sleep apnoea (OSA) is associated with repetitive breathing obstructions during sleep. These episodes of hypoxia and associated arousals from sleep induce physiological stress and nocturnal over-activation of the sympathetic nervous system (SNS). One consequence of OSA is impairment in a range of cognitive domains. Previous research into cognitive impairment in OSA have focussed on intermittent hypoxia and disrupted sleep, but not nocturnal over-activation of the SNS. Therefore, we investigated whether nocturnal over-activity of the SNS was associated with cognitive impairments in OSA. The extent of nocturnal SNS activation was estimated from heart rate variability (HRV), pulse wave amplitude (PWA) and stress response biomarkers (cortisol and glucose levels). OSA severity was significantly associated with PWA indices and the HRV low frequency/ high frequency ratio (p < 0.05). Morning blood glucose levels were significantly associated with the duration of a blood oxygen saturation (SaO2) < 90% (p < 0.01). PWA and HRV were significantly associated with the time taken to perform a task involving visuospatial functioning (p < 0.05), but not with impairments in sustained attention, reaction time or autobiographical memory. These results suggest that the visuospatial dysfunction observed in people with OSA is associated with increased nocturnal activity of the SNS.
Subject terms: Neuroscience, Physiology, Biomarkers, Medical research
Introduction
Obstructive sleep apnoea (OSA) is a sleep-related breathing disorder that involves repetitive obstruction of breathing during sleep, which is caused by the narrowing and/or complete collapse of the upper airway. One consequence of OSA is the impairment of a wide range of cognitive domains including memory, attention, psychomotor speed and visuospatial skills1, and studies have shown that hypoxia and sleep fragmentation contribute to these impairments2–5. However, as these two factors only account for 30% to 40% of the variance6, additional factors must be involved. Studies of healthy, middle-aged subjects have shown that SNS over-activity during wakefulness is associated with cognitive impairment7. The episodes of hypoxia and/or arousals that accompany breathing cessation provoke a stress response, which is indicated by increased activation of the sympathetic nervous system (SNS)8. In spite of that, severe hypoxia has been documented to be associated with higher SNS activity9. Therefore, one potential mechanism behind the cognitive impairments seen in OSA may be nocturnal overactivation of the SNS.
When healthy individuals fall asleep, the level of SNS activation decreases and heart rate and blood pressure are reduced accordingly10. In OSA patients, the activation of the SNS is increased as a function of the duration and severity of apnoeic events rather than by the stages of sleep11. Heart rate variability (HRV), a non-invasive method that is based on the differences in the time interval between individual heartbeats, is used to evaluate autonomic nervous system modulation12. Sequeira, et al. 13 reviewed HRV in adult OSA patients and concluded that people with OSA exhibit reduced vagal tone and higher sympathetic sensitivity. Decreases in pulse wave amplitude (PWA) is another indicator of increased SNS activation14. The PWA signal is measured via plethysmography, which is linked to blood flow in the fingers15. PWA drops are used to detect vasoconstriction, which reflect autonomic activation16,17. Randerath et al.18 found that untreated OSA patients displayed more PWA drops during sleep than OSA patients who received treatment with continuous positive airway pressure (CPAP) therapy. Serum cortisol and glucose levels also increase as a result of the stress response during sleep in OSA patients19,20; however, adherence to CPAP treatment has been shown to decrease the levels of these stress biomarkers21,22.
In this study, we examined whether nocturnal over-activation of the SNS was associated with impairments in the domains of sustained attention, reaction time, autobiographical memory and visuospatial skills, and whether SNS over-activation was correlated with OSA severity. We hypothesised that SNS over-activation would be associated with cognitive impairments in OSA.
Results
Seventy-two participants met the study inclusion criteria (48 males and 26 females) with a mean age of 40.1 years (standard deviation (SD) of 12.8 years) and a mean body-mass index (BMI) of 33.3 (SD = 9.1 kg/m2). Polysomnography results revealed that 12 of the 74 participants did not have OSA, while 26, 16 and 20 participants had mild, moderate and severe OSA, respectively.
A comparison of the demographic characteristics, daytime sleepiness, nocturnal SNS indices and polysomnography data between the four OSA severity groups is shown in Table 1. The severe and moderate groups were significantly older than those in the non-OSA group. There was no variation in BMI, depression or Epworth Sleepiness Scores (ESS) among the groups. From the polysomnography, we found that the duration of SaO2 below 90% and the respiratory arousal index were significantly increased with OSA severity [based on the Apnoea-hypopnoea index (AHI)]. No significant differences were found between the OSA severity groups in terms of sleep efficiency, sleep onset latency time, or the durations of non-rapid eye movement (NREM) sleep stages 2 and 3 (i.e. the percentage of the sleep-period time). However, the duration of NREM sleep stage 1 sleep was significantly longer in the severe OSA group, but the rapid eye movement (REM) was significantly shorter in the severe OSA group. For the nocturnal SNS indices, both the PWA drop index and PWA drop duration index increased significantly with OSA severity. In addition, the HRV low frequency/high frequency (LF/HF) ratio was significantly higher in the severe OSA group than in the non-OSA group. However, there were no significant differences in the morning urine and blood cortisol levels and blood glucose levels between the OSA severity groups.
Table 1.
Comparison of demographic variables, depression, daytime sleepiness, polysomnography parameters and nocturnal sympathetic nervous system indices by OSA severity group.
| Variable | Non-OSA (n = 12) | Mild OSA (n = 26) | Moderate OSA (n = 16) | Severe OSA (n = 20) | p-value |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (years) | 33.7 (14.8)3,4 | 37.4 (11.5) | 44.1 (12.4)1 | 46.5 (10.4)1 | < 0.01 |
| Body Mass Index (kg/m2) | 30.2 (9.7) | 31.2 (8.1) | 33.3 (9.9) | 37.3 (9.9) | 0.09 |
| Current smoker (%)+ | 1 (8) | 5 (22) | 4 (14) | 7 (42) | 0.13 |
| DASS-21 depression subscale | 8.3 (8.3) | 13.0 (9.3) | 11.5 (10.7) | 12.2 (8.8) | 0.51 |
| ESS | 9.1 (5.3) | 9.0 (3.9) | 11.0 (6.7) | 13.3 (6.4) | 0.06 |
| Apnoea-hypopnea Index (AHI) | 2.3 (1.3)3,4 | 9.2 (2.9)3,4 | 21.1 (4.9)1,2,4 | 49.2 (20.6)1,2,3 | < 0.01 |
| Oxygen Desaturation Index (ODI) | 2.6 (1.8)4 | 9.5 (6.9)4 | 16.8 (13.7)4 | 47.9 (25.9)1,2,3 | < 0.01 |
| SaO2 time < 90% (mins) | 0 (1)4 | 2 (7)4 | 11 (28) 4 | 32 (48)1,2,3 | < 0.01 |
| Minimum SaO2 | 90.0 (3.9)4 | 88.6 (3.32)4 | 85.1 (7.2)4 | 78.2 (10.0)1,2,3 | < 0.01 |
| Total sleep time (mins) | 299 (64)2,4 | 248 (65)1 | 270 (46) | 230 (60)1 | 0.01 |
| Sleep efficiency (%) | 78.1(13.4)2,4 | 63.7 (14.8)1 | 73.3 (10.8) | 63.2 (15.5)1 | < 0.01 |
| Sleep latency time (mins) | 19 (14) | 50 (48) | 30 (21) | 35 (28) | 0.07 |
| Waking after sleep onset time (mins) | 63 (51) | 73 (48) | 68 (38) | 99 (47) | 0.08 |
| Respiratory Arousal Index | 1.4 (1.1)3,4 | 5.5 (4.9)4 | 11.2 (4.9)1,4 | 26.9 (16.9)1,2,3 | < 0.01 |
| N1 time duration (%) | 08 (04)4 | 11 (8)4 | 12 (7) 4 | 22 (13)1,2,3 | < 0.01 |
| N2 time duration (%) | 49 (10) | 45 (12) | 55 (16) | 40 (13) | 0.23 |
| N3 time duration (%) | 22 (09) | 26 (13) | 21 (16) | 119 (20) | 0.53 |
| REM time duration (%) | 14 (08)3,4 | 09 (07) | 07 (06)4 | 06 (07)4 | 0.01 |
| PWA drop index | 2.7 (3.0)4 | 3.4 (5.5)4 | 6.0 (4.6)4 | 12.3 (7.0)1,2,3 | < 0.01 |
| PWA drop time duration index | 2.1 (2.6) 4 | 2.9 (2.6)4 | 4.0 (3.2)4 | 9.6 (6.0)1,2,3 | < 0.01 |
| HRV LF/HF ratio (log) | 0.1 (0.6)4 | 0.04 (0.8) | 0.04 (0.8) | 0.6 (0.7)1 | 0.04 |
| Morning urine cortisol (mmol/L) | 75.1 (67.5) | 90.2 (143.0) | 102.5 (177.3) | 152.0 (163.4) | 0.20 |
| Morning blood cortisol (mmol/L) | 232.0 (115.2) | 240.3 (138.0) | 292.0 (135.8) | 287.0 (122.0) | 0.13 |
| Morning blood glucose (mmol/L) | 5.2 (0.9) | 6.0 (1.9) | 5.4 (1.0) | 6.0 (2.3) | 0.39 |
OSA severity cut-off values were: 5–14 AHI (mild); 15–29 AHI (moderate); ≥ 30 AHI (severe); OSA, obstructive sleep apnoea; Significant differences between groups were defined by: 1p < 0.05 vs. non-OSA; 2p < 0.05 vs. mild OSA; 3p < 0.05 vs. moderate OSA; 4p < 0.05 vs. severe OSA; + : Pearson’s chi-square; Log logarithmic transformation, DASS-21 depression, anxiety and stress scale-21, ESS Epworth sleepiness scale, SaO2 arterial blood oxygen saturation, PWA pulse wave amplitude, HRV heart rate variability, LF low frequency, HF high frequency, N1 sleep stage 1, N2 sleep stage 2, N3 sleep stage 3, REM rapid eye movement sleep stage.
Bolded p-values indicate statistically significant effects
In addition, there were no significant differences in the cognitive test scores between the OSA groups, except for total episodic memory and episodic childhood memory, which were positively correlated with OSA severity (Table 2).
Table 2.
Comparison of cognitive tests by OSA severity group.
| Variable | Non-OSA (n = 12) |
Mild OSA (n = 26) |
Moderate OSA (n = 16) |
Severe OSA (n = 20) |
p-value |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| PVT RT | 317.1 (73.5) | 296.0 (43.3) | 323.8 (72.4) | 323.8 (72.5) | 0.53 |
| PVT RT slowest 10% | 569.5 (204.9) | 478.5 (104.7) | 591.9 (310.6) | 549.9 (223.2) | 0.34 |
| PVT RT-10-lapses > 500 ms | 5.2 (9.8) | 3.0 (3.4) | 4.3 (3.4) | 5.1 (9.5) | 0.78 |
| AM-time (mins) | 35.9 (20.0) | 38.5 (12.4) | 45.5 (11.9) | 42.7 (18.3) | 0.433 |
| AM-errors | 10.0 (6.6) | 14.7 (8.8) | 12.2 (5.6) | 10.7 (6.5) | 0.16 |
| Total semantic memory | 58.6 (3.7) | 56.8 (4.9) | 57.8 (4.3) | 58.1 (3.8) | 0.26 |
| Childhood semantic memory | 19.5 (1.7) | 18.0 (3.1) | 19.4 (1.6) | 19.5 (1.9) | 0.11 |
| Early adult life semantic memory | 19.0 (2.1) | 19.0 (2.7) | 19.2 (2.0) | 19.2 (2.4) | 0.98 |
| Recent life semantic memory | 20.0 (1.6) | 18.4 (2.6) | 19.6 (1.7) | 19.5 (1.7) | 0.09 |
| Total episodic memory | 22.1 (4.5)2,4 | 16.8 (5.9)1,4 | 17.2 (5.1) | 15.8 (5.4)1,2 | 0.01 |
| Episodic childhood memory | 7.8 (1.6)2,4 | 5.6 (2.6)1,4 | 5.8 (2.1) | 5.1 (2.3)1,2 | 0.01 |
| Episodic early adult life memory | 6.8 (2.2) | 5.3 (2.4) | 5.9 (1.9) | 5.1 (2.5) | 0.17 |
| Episodic recent life memory | 7.5 (1.5) | 5.8 (2.7) | 5.6 (2.1) | 5.5 (2.1) | 0.05 |
OSA severity cut-off values were: 5–14 AHI (mild); 15–29 AHI (moderate); ≥ 30 AHI (severe); Significant differences between groups were defined by: 1p < 0.05 vs. non-OSA; 2p < 0.05 vs. mild OSA; 3p < 0.05 vs. moderate OSA; 4p < 0.05 vs. severe OSA; PVT, psychomotor vigilance test; RT, reaction time; AM, Austin Maze; ms, milliseconds.
Bolded p-values indicate statistically significant effects
Multiple linear regression analyses (Table 3) demonstrated that nocturnal PWA was significantly associated with the respiratory arousal index. In addition, the HRV LF/HF ratio was associated with age and the respiratory arousal index. Only the morning blood glucose level was significantly associated with an SaO2 time < 90%. Urine and blood cortisol levels were not significantly associated with any of the OSA predictors.
Table 3.
Multiple regression analysis for age, SaO2 time < 90%, respiratory arousal index and body mass index to predict nocturnal pulse wave amplitude, heart rate variability and morning blood glucose levels.
| Variable | R2 | pmodel | Predictor | B | SE | β | sr | p-value |
|---|---|---|---|---|---|---|---|---|
| PWA drop index | 0.40 | < 0.01* | Body Mass Index | 0.10 | 0.08 | 0.14 | 0.13 | 0.19 |
| Age (years) | 0.08 | 0.06 | 0.14 | 0.13 | 0.19 | |||
| Respiratory Arousal Index | 0.28 | 0.06 | 0.56 | 0.46 | < 0.01 | |||
| SaO2 time < 90% | 0.00 | 0.00 | 0.09 | 0.07 | 0.47 | |||
| PWA drop time index | 0.36 | < 0.01* | Body Mass Index | 0.06 | 0.06 | 0.09 | 0.09 | 0.34 |
| Age (years) | 0.05 | 0.05 | 0.10 | 0.09 | 0.33 | |||
| Respiratory Arousal Index | 0.23 | 0.05 | 0.57 | 0.46 | < 0.01 | |||
| SaO2 time < 90% | 0.00 | 0.00 | 0.05 | 0.43 | 0.74 | |||
| HRV LF/HF ratio (log) | 0.25 | < 0.01* | Body Mass Index | 0.00 | 0.00 | 0.10 | 0.09 | 0.40 |
| Age (years) | 0.03 | 0.00 | 0.44 | 0.40 | < 0.01 | |||
| Respiratory Arousal Index | 0.01 | 0.00 | 0.36 | 0.31 | < 0.01 | |||
| SaO2 time < 90% | 0.00 | 0.00 | 0.21 | 0.17 | 0.17 | |||
| Morning blood glucose (mmol/L) | 0.26 | < 0.01* | Body Mass Index | 0.02 | 0.03 | 0.07 | 0.07 | 0.53 |
| Age (years) | 0.01 | 0.02 | 0.08 | 0.07 | 0.48 | |||
| Respiratory Arousal Index | 0.02 | 0.02 | 0.16 | 0.13 | 0.21 | |||
| SaO2 time < 90% | 0.00 | 0.00 | 0.34 | 0.26 | 0.01 |
*Significant model (p < 0.05) after false discovery rate adjustment; log logarithmic transformation, R2 the models' multiple correlations, B unstandardised regression coefficient, β standardised regression coefficient, PWA pulse wave amplitude, HRV heart rate variability, LF low frequency, HF high frequency, SaO2 arterial blood oxygen saturation.
Bolded p-values indicate statistically significant effects
Factors (in Table 3) that were not significantly related to OSA severity or parameters of interest were excluded from further analysis. To identify confounders correlated with the cognitive test results, Pearson’s correlations were calculated (Table 4). These indicated that most of the psychomotor vigilance test (PVT) indices and both of the Austin Maze indices were associated with age. However, for autobiographical memory, age was only associated with episodic memory (total episodic memory, episodic early adult life memory and episodic recent life memory). Smoking was positively correlated with Austin Maze errors and recent life semantic memory. ESS scores were not correlated with performances on any of the cognitive tests.
Table 4.
Pearson’s correlation analysis between age, smoking, ESS and all cognitive tests.
| Variables | Age | Smoking | ESS |
|---|---|---|---|
| PVT RT-mean | R = 0.28 (p = 0.04) | R = 0.10 (p = 0.38) | R = 0.15 (p = 0.19) |
| PVT RT slowest 10% | R = 0.19 (p = 0.10) | R = 0.00 (p = 0.94) | R = 0.17 (p = 0.14) |
| PVT RT-10-lapses > 500 ms | R = 0.26 (p = 0.03) | R = 0.06 (p = 0.61) | R = 0.19 (p = 0.09) |
| AM-time (mins) | R = 0.60 (p = < 0.01) | R = 0.14 (p = 0.25) | R = 0.10 (p = 0.39) |
| AM-errors | R = 0.30 (p = < 0.01) | R = 0.25 (p = 0.02) | R = 0.10 (p = 0.36) |
| Total semantic memory | R = − 0.01 (p = 0.91) | R = − 0.17 (p = 0.10) | R = − 0.14 (p = 0.24) |
| Childhood semantic memory | R = − 0.05 (p = 0.65) | R = − 0.06 (p = 0.15) | R = − 0.00 (p = 0.97) |
| Early adult life semantic memory | R = 0.05 (p = 0.70) | R = 0.15 (p = 0.20) | R = 0.09 (p = 0.39) |
| Recent life semantic memory | R = 0.02 (p = 0.86) | R = 0.21 (p = 0.04) | R = 0.07 (p = 0.50) |
| Total episodic memory | R = − 0.33 (p = < 0.01) | R = 0.04 (p = 0.70) | R = − 0.02 (p = 0.86) |
| Episodic childhood memory | R = − 0.19 (p = 0.09) | R = 0.005 (p = 0.96) | R = 0.06 (p = 0.55) |
| Episodic early adult life memory | R = − 0.25 (p = < 0.01) | R = − 0.04 (p = 0.78) | R = − 0.03 (p = 0.76) |
| Episodic recent life memory | R = − 0.39 (p = < 0.01) | R = − 0.08 (p = 0.49) | R = − 0.02 (p = 0.81) |
R correlation, PVT psychomotor vigilance test, RT reaction time, AM Austin Maze, ms milliseconds, ESS Epworth sleepiness scale.
Bolded p-values indicate statistically significant effects
Multiple linear regression analyses were used to determine the effects of the SNS indices (PWA indices, the HRV LF/HF ratio and morning blood glucose levels) on the PVT, the Austin Maze and autobiographical memory indices after controlling for hypoxia, sleep fragmentation, depression, age and smoking status (Fig. 1; Tables 5 and 6). None of the regression models displayed multicollinearity (variance inflation factors (VIF) < 2.0). After conducting a false discovery rate (FDR) adjustment for multiple comparisons, models that included the PVT, Austin Maze, total semantic memory and recent life episodic memory remained significant (p < 0.05). However, performance on the PVT, autobiographical memory and Austin Maze errors were not associated with any of the nocturnal SNS indices. The Austin Maze time was associated with the PWA index, PWA drop time duration index and the HRV LF/HF (Table 5).
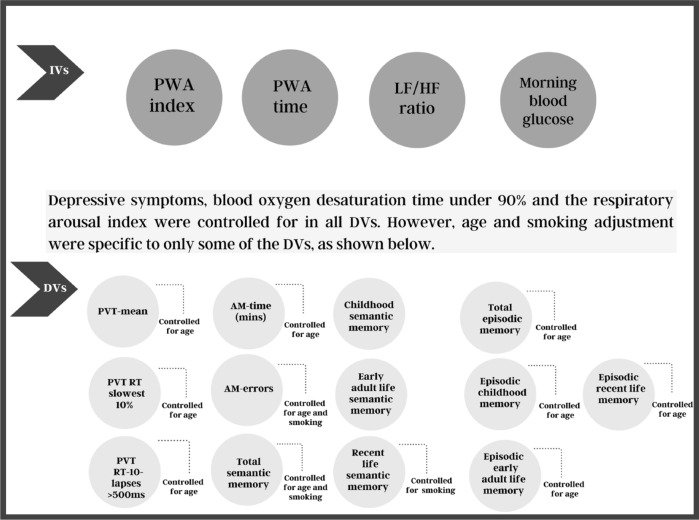
Figure 1.
This graph shows the processes of the main analysis for the independent variables, dependent variables and covariates used in the study. PWA pulse wave amplitude, LF low frequency, HF high frequency, PVT psychomotor vigilance test, RT reaction time, AM Austin Maze, ms milliseconds, IVs independent variables, DVs dependent variables.
Table 5.
Multiple linear regression analyses showing the association between the psychomotor vigilance test and Austin Maze and the sympathetic nervous system activity indices.
| Cognitive tests | Predictors | R2 | SE | β | sr | p-value |
|---|---|---|---|---|---|---|
| PVT RT-mean | PWA drops (index)* | 0.28 | 1.11 | 0.02 | 0.01 | 0.87 |
| PWA drops time durations (index)* | 0.28 | 1.37 | 0.08 | 0.06 | 0.56 | |
| HRV LF/HF ratio (log)* | 0.30 | 8.20 | 0.08 | 0.07 | 0.50 | |
| Morning blood glucose (mmol/L)* | 0.27 | 3.38 | 0.05 | 0.04 | 0.65 | |
| PVT slowest 10% | PWA drops (index)* | 0.20 | 4.12 | 0.11 | 0.08 | 0.43 |
| PWA drops time durations (index)* | 0.20 | 5.07 | 0.01 | 0.11 | 0.92 | |
| HRV LF/HF ratio (log)* | 0.22 | 3.55 | 0.09 | 0.08 | 0.47 | |
| Morning blood glucose (mmol/L)* | 0.22 | 12.30 | 0.15 | 0.13 | 0.22 | |
| PVT RT-10-lapses > 500 ms | PWA drops (index)* | 0.36 | 0.12 | 0.09 | 0.07 | 0.47 |
| PWA drops time durations (index)* | 0.36 | 0.14 | 0.00 | 0.00 | 0.98 | |
| HRV LF/HF ratio (log)* | 0.38 | 0.88 | 0.14 | 0.12 | 0.22 | |
| Morning blood glucose (mmol/L)* | 0.36 | 0.35 | 0.05 | 0.04 | 0.68 | |
| AM-10 T-time | PWA drops (index)* | 0.45 | 0.24 | 0.31 | 0.29 | < 0.01 |
| PWA drops time durations (index)* | 0.45 | 0.39 | 0.27 | 0.25 | 0.02 | |
| HRV LF/HF ratio (log)* | 0.43 | 2.04 | 0.22 | 0.19 | 0.04 | |
| Morning blood glucose (mmol/L)* | 0.45 | 0.82 | 0.09 | 0.07 | 0.46 | |
| AM-10 T-errors | PWA drops (index)* | 0.30 | 0.14 | 0.07 | 0.05 | 0.61 |
| PWA drops time durations (index)* | 0.23 | 0.18 | 0.05 | 0.04 | 0.72 | |
| HRV LF/HF ratio (log)* | 0.25 | 1.10 | 0.13 | 0.12 | 0.28 | |
| Morning blood glucose (mmol/L)* | 0.25 | 0.44 | 0.15 | 0.12 | 0.25 |
*Significant model (p < 0.05) after false discovery rate adjustment; The model was adjusted for demographic cofounders, hypoxia, sleep fragmentation and depressive symptoms; R2 the models' multiple correlations, SE standard error, β standardized regression coefficient, sr semi-partial correlation, log logarithmic transformation, PVT psychomotor vigilance test, RT reaction time, AM Austin Maze, ms milliseconds, PWA pulse wave amplitude, HRV heart rate variability, LF low frequency, HF high frequency, mins minutes.
Bolded p-values indicate statistically significant effects
Table 6.
Multiple linear regression analyses of the association between autobiographical memory interview indices and sympathetic nervous system activity indices.
| Cognitive tests | Predictors | R2 | SE | β | sr | p-value |
|---|---|---|---|---|---|---|
| Total semantic memory | PWA drops (index) | 0.13 | 0.09 | 0.07 | 0.06 | 0.62 |
| PWA drops time durations (index) | 0.15 | 0.11 | 0.10 | 0.08 | 0.47 | |
| HRV LF/HF ratio (log) | 0.14 | 0.67 | 0.02 | 0.02 | 0.86 | |
| Morning blood glucose (mmol/L) | 0.14 | 0.27 | 0.01 | 0.01 | 0.96 | |
| Childhood semantic memory | PWA drops (index) | 0.15 | 0.05 | 0.03 | 0.02 | 0.84 |
| PWA drops time durations (index) | 0.16 | 0.06 | 0.01 | 0.00 | 0.94 | |
| HRV LF/HF ratio (log) | 0.20 | − 0.35 | − 0.22 | − 0.21 | 0.06 | |
| Morning blood glucose (mmol/L) | 0.16 | 0.15 | 0.02 | 0.02 | 0.89 | |
| Early adult life semantic memory | PWA drops (index) | 0.07 | 0.05 | 0.05 | 0.04 | 0.75 |
| PWA drops time durations (index) | 0.08 | 0.06 | 0.02 | 0.02 | 0.88 | |
| HRV LF/HF ratio (log) | 0.08 | 0.37 | 0.11 | 0.10 | 0.38 | |
| Morning blood glucose (mmol/L) | 0.08 | 0.15 | 0.09 | 0.09 | 0.46 | |
| Recent life semantic memory | PWA drops (index) | 0.12 | 0.05 | 0.05 | 0.04 | 0.75 |
| PWA drops time durations (index) | 0.12 | 0.05 | 0.02 | 0.01 | 0.90 | |
| HRV LF/HF ratio (log) | 0.12 | 0.33 | 0.09 | 0.08 | 0.49 | |
| Morning blood glucose (mmol/L) | 0.12 | 0.14 | 0.06 | 0.05 | 0.67 | |
| Total episodic memory | PWA drops (index) | 0.17 | 0.12 | 0.09 | 0.07 | 0.53 |
| PWA drops time durations (index) | 0.15 | 0.15 | 0.07 | 0.06 | 0.60 | |
| HRV LF/HF ratio (log) | 0.23 | 0.87 | 0.13 | 0.10 | 0.09 | |
| Morning blood glucose (mmol/L) | 0.15 | 0.37 | 0.09 | 0.08 | 0.48 | |
| Episodic childhood memory | PWA drops (index) | 0.02 | 0.07 | 0.00 | 0.00 | 0.96 |
| PWA drops time durations (index) | 0.01 | 0.06 | 0.03 | 0.02 | 0.86 | |
| HRV LF/HF ratio (log) | 0.05 | 0.39 | 0.20 | 0.19 | 0.13 | |
| Morning blood glucose (mmol/L) | 0.06 | 3.80 | 0.12 | 0.10 | 0.38 | |
| Episodic early adult life memory | PWA drops (index) | 0.17 | 0.05 | 0.13 | 0.10 | 0.36 |
| PWA drops time durations (index) | 0.10 | 0.06 | 0.09 | 0.08 | 0.50 | |
| HRV LF/HF ratio (log) | 0.18 | 0.36 | 0.16 | 0.14 | 0.14 | |
| Morning blood glucose (mmol/L) | 0.11 | 0.15 | 0.03 | 0.02 | 0.84 | |
| Episodic recent life memory | PWA drops (index)* | 0.22 | 0.05 | 0.11 | 0.09 | 0.42 |
| PWA drops time durations (index)* | 0.20 | 0.06 | 0.10 | 0.08 | 0.46 | |
| HRV LF/HF ratio (log)* | 0.21 | 0.36 | 0.17 | 0.15 | 0.17 | |
| Morning blood glucose (mmol/L)* | 0.20 | 0.14 | 0.12 | 0.10 | 0.34 |
*Significant model (p < 0.05) after false discovery rate adjustment; The model was adjusted for demographic cofounders, hypoxia, sleep fragmentation and depressive symptoms; R2 the models' multiple correlations, SE standard error, β standardized regression coefficient, sr semi-partial correlation, log logarithmic transformation.
Discussion
The present study estimated nocturnal over-activity of the SNS through a variety of measures including PWA, HRV and stress response biomarkers. Nocturnal over-activity of the SNS, as measured by PWA and HRV, was linked to visuospatial dysfunction but not to sustained attention, response time or autobiographical memory. Morning blood glucose level was not associated with cognitive impairment.
The current results indicate that the stress associated with an SaO2 < 90% is sufficient to elevate blood glucose levels for sustained periods of time and remain elevated upon waking. However, despite confirming the relationship between morning blood glucose levels and time spent with an SaO2 < 90%, glucose levels were not associated with any cognitive impairments. However, previous studies of patients without OSA have reported a strong relationship between blood glucose levels and memory impairments23 and visuospatial dysfunction24.
In this study, HRV and PWA were associated with impairments on a test of visuospatial function, which supports the link between nocturnal over-activity of the SNS and visuospatial dysfunction. A systematic review of HRV and cognitive dysfunction in non-OSA patients by Forte et al.25 concluded that lower HRV was associated with poor visuospatial implementation. In contrast, Idiaquez et al.26 reported that performance on the Trail Making Test-B, which measures visuospatial skills27, and nocturnal over-activation of the SNS were not related to one another in OSA patients26. The inconsistency between our findings and those of Idiaquez and colleagues may be attributed to their recruitment of a male-only sample and/or the different measures of the visuospatial function used between their study and ours. Regardless of the reason, our results revealed a robust relationship between SNS over-activation during sleep in OSA patients and performance on the Austin Maze test. This finding suggests that nocturnal over-activity of the SNS should be taken into consideration in future studies of cognitive impairment in OSA patients.
Previous studies of healthy subjects have reported a relationship between sympathetic over-activation and poor performance on memory tests7,28 and response times29. Our results did not find such associations. This may be because, while other studies examined the effects of acute stress on cognitive performance, we investigated the effect of chronic nocturnal stress on cognitive performance during wakefulness, when stress levels were not elevated. Furthermore, cortisol levels in the blood and urine of our subjects were not associated with any measure of OSA severity or cognitive performance. This indicates that the subjects were not physiologically stressed at the time of waking. Therefore, the relationship between nocturnal over-activity of the SNS and visuospatial dysfunction was likely not the result of acute stress but other factors, such as elevations in nocturnal blood pressure that accompany apnoeic episodes30 and are involved in brain injury31.
This study has some limitations. First, the non-OSA group was smaller than the OSA group. Although this difference was negated using correlational analyses that treated OSA severity as a continuous variable rather than a categorical one, it is possible that the smaller number of participants without OSA decreased the statistical power. Second, the current study recruited participants who had been referred for a sleep study because they were suspected of having a sleep disorder. This selection bias suggests that our results may not be representative of those from a randomly chosen sample. Third, although the non-OSA group were used in the comparison of polysomnography parameters, they cannot be classified as a healthy control group since they were clinically referred for sleep study. Fourth blood glucose and cortisol measurements were based on samples taken upon waking. It is possible that a more dynamic picture of SNS over-activation could have been obtained by continuously sampling blood throughout the night. Fifth, cognitive tests were performed in the evening (at 5:00 pm) prior to bedtime and may have coincided with the wake maintenance zone for participants, which could have masked cognitive impairment. Sixth, severe sleep deprivation has the potential to affect cognitive function and HRV32,33. Since actigraphy or sleep diary data were not collected prior to the PSG study night, some of the variance in cognitive performance and HRV may have been attributable to severe sleep deprivation in some participants. However, we consider this possibility to be unlikely, as the mean ESS score did not differ significantly between the OSA severity groups. Seventh, although the OSA severity was associated with SNS over-activity, medication use was not controlled for. Since only 3 patients were taking medications for asthma or diabetes, this small number is unlikely to have influenced the main outcomes of the study. Finally, the current study did not include brain imaging, so it was not possible to correlate the observed cognitive deficits with structural changes.
Conclusion
This study examined whether there are associations between nocturnal over-activity of the SNS and cognitive impairments in OSA. Our findings revealed that SNS over-activity during sleep, as measured by PWA and HRV, was linked to visuospatial dysfunction among OSA patients. Morning blood glucose level was not related to impairments on any of the cognitive tests we used. These results suggest that there is an association between SNS activity during sleep and visuospatial dysfunction in OSA. Accordingly, interventions that reduce the nocturnal activation of the SNS may contribute to the improvement of visuospatial functioning in these individuals.
Methods
Study participants
A retrospective study was conducted (July to December 2018) of patients suspected to have OSA who had been referred by sleep clinics to the Sleep Medicine and Research Centre, King Abdulaziz University Hospital, Jeddah, Saudi Arabia for overnight polysomnography studies. During this period, consecutive patients were given the opportunity to participate in the study. Potential study participants were excluded if they: (1) used CPAP therapy; (2) had been diagnosed with a neurodegenerative disease; (3) typically slept less than 2 h per night (as the recommended minimum sleep duration for a valid polysomnography is 2 h per night)34; (4) were prescribed cardiovascular medications.
Ethical approval for the study was obtained from the Human Research Ethics Committee of the Royal Melbourne Institute of Technology University (ethics reference number: HREC 21,459) and from the King Abdulaziz University Hospital Human Research Ethics Committee (ethics reference number: 395-18). The study was conducted according to the guidelines and regulations stated in the ethics approvals. All participants provided written, informed consent to participate in the study.
A sample size calculation was conducted using G*POWER software 335. A total sample size of 56 subjects was required to test the null hypothesis for a moderate effect size (r = 0.30) and 0.80 power36. This minimum sample size was increased to provide extra power for multiple comparisons, and a total of 100 participants were recruited into the study. However, 14 participants did not meet the inclusion criteria, and among the remainder, complete data were obtained for all of the study variables in 74 participants. Medication use was documented for all participants included in the study (Table 7).
Table 7.
Documented medications that were taken by the patients during the study period and patients’ OSA severity categories.
| Patients number (n = 3) | Medications used | Patients’ OSA severity categories |
|---|---|---|
| 1 | Insulin and Diamicron (for diabetes) | Mild |
| 2 | Insulin (for diabetes) | Mild |
| 3 | Montelukast and Theophylline (for asthma) | Moderate |
OSA obstructive sleep apnoea.
Procedures and measurements
Once participants were admitted to the sleep laboratory, height and weight were measured, and subjects completed a series of questionnaires designed to collect demographic information and assess daytime sleepiness and mood levels. Cognitive tests were conducted at 5:00 pm. The time to completion all of the tests ranged from 40 to 45 min for all participants. The cognitive tests were administered in the following order: the PVT, Austin Maze and the autobiographical memory interview. Subjects were prepared for the polysomnography at 10:00 pm. After subjects awoke at 6:00 am, blood and urine samples were taken to measure urinary and blood cortisol levels and fasting blood glucose levels. The same procedures and time of all tests and evaluations in the sleep laboratory were applied to the home studies. For home studies, a certified nurse went to the patient’s home at 6:00 am to take blood and urine samples. The samples were transported to the laboratory in less than 2 h and stored at 4–8 °C during transit.
Questionnaires
Epworth sleepiness scale (Arabic version)37,38
The ESS was used to assess the general level of daytime sleepiness. The questionnaire contained eight items, each rated from 0 to 3. Higher numbers represented higher levels of sleepiness, and the eight items were summed to give an overall score. Scores between 0–10 indicated no sleepiness; 11–14 indicated mild sleepiness; 15–17 indicated moderate sleepiness; and scores above 17 suggested severe daytime sleepiness.
Depression, anxiety, stress scale-21 (Arabic version)39,40
The Depression, Anxiety, Stress Scale-21 (DASS) was used to assess the level of three emotional states: depression, anxiety and stress. There is convergent validity between the DASS depression and anxiety subscales and the Beck depression and anxiety inventories41.
Polysomnography evaluation
Overnight polysomnography (SOMNO Medics Plus, SOMNOmedics, Randersacker, Germany) was used to evaluate sleep duration and quality, breathing and other sleep-related parameters. Most participants underwent studies in the sleep laboratory, but five subjects underwent home sleep studies. All participants (including home studies) used the same devices and procedures as subjects in the sleep laboratory and were allowed to conduct the study at home for patient convenience and mobility. For all of the sleep parameters, a sleep technician applied sensors 30 min before sleep time. The polysomnography consisted of continuous recordings from surface leads for electroencephalography (EEG), electrooculography, electromyography (from muscles in the sub-mental space and the tibialis anterior muscles bilaterally) and electrocardiography (ECG). The polysomnography consisted of a 10-channel recording montage (Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1 and O2), which was used to measure EEG activity and left/right electrooculography. The nasal pressure was recorded, and nasal and oral airflow were measured by thermocouple device, chest and abdominal impedance belts measured respiratory muscle effort, a pulse oximeter assessed SaO2 and pulse waves, a tracheal microphone measured snoring, and body position sensors measured the sleep position. Polysomnographic procedures were repeated for three participants (using the procedures outlined above) due to technical issues and loss of data during the initial studies.
Autonomic nervous system measurements
Autonomic nervous system activity was estimated using HRV, PWA, and cortisol and glucose levels, not directly measured at the neuronal level.
Heart rate variability
HRV is derived from an analysis of the intervals between regular heartbeats (i.e. the time spent between R-waves—the RR intervals)42 to measure autonomic nervous system activity43. The spectrum of RR intervals was analysed within two bands: LF at 0.04–0.15 Hz and HF at 0.15–0.4 Hz. Previous studies have indicated that HRV HF power primarily indicates parasympathetic nervous system (PNS) activity, while HRV LF power indicates SNS activity44,45. However, more recent studies have indicated that HRV LF can be influenced by parasympathetic activity46,47. In addition, HRV HF can exhibit unstable results as a measure of PNS activity, which may be attributable to individual differences, the nature of breathing, and sleeping posture48–51. Malliani52 has suggested that the HRV LF/HF ratio be considered an index of sympatho-vagal balance. Thus, the present study used only the HRV LF/HF ratio to measure SNS over-activity.
Pulse wave amplitude
PWA is a signal obtained from finger plethysmography and is directly and positively associated with blood flow15. Decreases in PWA can indicate increased sympathetic activation53,54. A ≥ 30% drop in the PWA has been recommended as the cut-off value for identifying arousals and respiratory events in OSA patients16,55.
Biochemical markers
The stress response during sleep was assessed from a morning urine sample (urinary cortisol) and morning serum cortisol and glucose levels. The urine sample was collected using a sanitised kit, and the serum samples were taken from the median cubital vein between 5:00 a.m. and 6:00 a.m. The blood sampling was performed by a nurse, and urine and blood samples were stored for no longer than 2 h between 2 and 8 °C prior to being assayed.
Neurobehavioural evaluations
Psychomotor vigilance test-10 minute version56
PVT is a computerised visual test that evaluates the ability to sustain attention and rapidly respond with a button press to cues presented on a digital screen. The reliability and validity of the 10-min version of the test has been confirmed56. The test is sensitive to sleep fragmentation and identifies lapses in sustained attention57. Three outcome measures were used: (1) mean response time; (2) mean of the slowest 10% response time; and (3) number of lapses with response times > 500 ms. A response time > 100 ms was considered valid. A false start was recorded when the response time was < 100 ms or when it occurred without a stimulus presentation.
Austin maze-10 trials58
The Austin Maze is a computerised maze that measures visuospatial ability and memory59,60. Subjects plot a course through a chequerboard maze by pushing buttons and identifying the correct order through trial and error. Each time the correct button is pushed, a green light is displayed. When an incorrect button is pushed, a red light is displayed and a buzzer sounds. As previous studies have confirmed that 10 trials are enough to accurately assess visuospatial ability and memory61, 10 trials were used in this study.
The autobiographical memory interview62
An autobiographical memory interview was used to assess both episodic and semantic memory. For this study, memories of three time points in each participant’s lifespan were assessed: childhood (before high school); early adulthood (including career, relationships, marriage and children); and recent life (including present and previous hospital or institution stays over the previous 5 years, as well as recent holidays or travel). Scoring was based on published guidelines62. For episodic memory, subjects received a score of 3 for full recall that included specifics of time and place, 2 for recall that was personal but general, 1 for an unclear personal memory, and 0 for no answer or a semantic memory. The maximum possible score for each time period was 9, and the maximum total score was 27. For semantic memory, responses were weighted for the level of detail retained (e.g. house number, street name and district). The maximum possible score for each time period was 21 points, and the total maximum was 63. The autobiographical memory interview has achieved high levels of accuracy, reliability and validity62 and was translated from English into Arabic. Two Arabic-speaking researchers reviewed the translation and suggested refinements in terms of expression, phrasing and concepts. To confirm the accuracy of the translation from English to Arabic, the Arabic text was translated back into English by an independent bilingual translator with no knowledge of the topic. A comparison of the original and translated interviews revealed no significant differences in content or meaning.
Analyses
BMI was estimated according to international standards63. The polysomnography results were scored manually according to the American Academy of Sleep Medicine (AASM) 2012 scoring protocol64, and the classification of abnormal breathing events was based on AASM’s recommendations64,65. Apnoea was defined as ≥ 90% reduction in airflow from baseline for ≥ 10 s. Hypopnoea was defined as a discernible reduction in airflow of ≥ 30% of the pre-event baseline for ≥ 10 s using nasal pressure, as well as an associated reduction in oxygen saturation of at least 3% and/or subsequent arousal. The severity of OSA was estimated from the AHI. The degree of hypoxia was based on the cumulative time (in seconds) spent with an SaO2 below 90%, while the degree of sleep fragmentation was assessed using the respiratory arousal index, which was calculated by dividing the total number of respiratory arousals by the number of hours of sleep. The duration of NREM sleep stages N1, N2 and N3 and the duration of the REM sleep stage were analysed. Three technicians verified all polysomnography scores to ensure the quality of the scoring process. The technicians also randomly selected and scored cases to confirm inter-observer reliability and accuracy. Bolded p-values in Tables 1, 2, 3, 4, and 5 indicate statistically significant effects.
HRV was analysed using LAB CHART-PRO analysis software (AD INSTRUMENTS, Sydney, Australia). During the polysomnography, the ECG was continuously acquired at 1 kHz. The ECG record for the entire night's sleep was divided into 5-min segments for analysis. Noise was manually removed. The remaining normal-to-normal RR intervals were analysed using the fast fourier transform algorithm.
Plethysmography data were extracted at 128 Hz during the polysomnography, and artefacts were removed using LAB CHART-PRO analysis software (AD INSTRUMENTS, Sydney, Australia). The analysis was limited to PWA drops of ≥ 30% for more than 3 s and less than 60 s66. Nocturnal SNS activity was estimated via two measures: the PWA drop index during sleep, which was calculated by dividing the total number of PWA drops into the number of sleep hours; and the PWA drop time duration index, calculated by dividing the number of sleep minutes by the cumulative minutes spent within PWA drops.
Biochemical markers, including urine cortisol levels, and serum cortisol and glucose levels were analysed in a single laboratory by the same biochemist. The biochemist was blinded to the OSA severity of the study participants. Cortisol samples were analysed using an ATELLICA IM cortisol analyser (SIEMENS HEALTHCARE GMBH, Erlangen, Germany) using a sample volume of 20 µL. Serum glucose was analysed using the DIMENSION VISTA 500 system (SIEMENS HEALTHCARE GMBH). The sample volume was 1.2 µL. The µg/dL units were converted to mmol/L for easy readability.
Statistical analyses were conducted using SPSS version 26 (IBM Corp., Armonk, NY, US). Continuous data were checked for normality. The following variables (found to be non-normally distributed) were log-transformed prior to statistical analysis: the HRV LF/HF ratio, PVT slowest 10%, Austin Maze time, Austin Maze errors, autobiographical childhood semantic memory, autobiographical adult early life memory and autobiographical recent life memory. Data are expressed as means and SDs for continuous variables and as frequencies and percentages for categorical variables. Multivariate analysis of variance using a Bonferroni post-hoc analysis was used to determine significant differences between groups based on OSA levels and demographic variables, depressive symptoms, daytime sleepiness, nocturnal SNS indices, polysomnography parameters and the cognitive tests. Between-group comparisons of categorical data were made using Pearson’s Chi-square tests.
As part of a pre-analysis step, a multiple linear regression analysis was conducted to uncover the predictors of the SNS indices. The predictors were selected on the basis of the main OSA predictors (hypoxia and arousal) and the main demographic variables (age and BMI). Covariates were selected by two methods: (1) they were based on the results of our recent study6, which was conducted on the same sample as the current study. Thus, depressive symptoms, hypoxia and sleep fragmentation factors were controlled; and (2) the sensitivity of the common factors in OSA, such as excessive daytime sleepiness and main demographic data (including age and smoking), to the cognitive tests that were determined using Pearson’s correlational analyses.
Multiple linear regression analysis examined the associations between nocturnal SNS indices and cognitive performance while controlling for confounders. All of the models were corrected for multiple comparisons with the FDR67, and multicollinearity was demonstrated using a VIF of > 2.0. Accordingly, statistical significance was reported for models that had p < 0.05 after being adjusted for multiple comparisons and/or for models that showed no multicollinearity, as assumed with a VIF of < 2.0.
Acknowledgements
We wish to thank the administration staff, sleep technicians and nurses at the Sleep Medicine and Research Centre at King Abdulaziz University Hospital for their help and support in conducting this research.
Author contributions
R.M.A., S.R.R. & G.A.K. designed the project and obtained ethical approval. Data collection was performed by R.M.A., S.O.W. & F.A. The data was analysed by R.M.A. with guidance from S.R.R., G.A.K. and R.M.A. The manuscript was drafted by R.M.A., S.R.R. & G.A.K. The final version of the manuscript has been read and approved by all of the authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bilyukov RG, et al. Cognitive impairment and affective disorders in patients with obstructive sleep apnea syndrome. Front. Psychiatry. 2018;9:357–357. doi: 10.3389/fpsyt.2018.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verstraeten E. Neurocognitive effects of obstructive sleep apnea syndrome. Curr. Neurol. Neurosci. Rep. 2007;7:161–166. doi: 10.1007/s11910-007-0012-8. [DOI] [PubMed] [Google Scholar]
- 3.Tamilarasan V, et al. Prevalence of cognitive impairment in patients with obstructive sleep apnea. ERJ Open Res. 2019;5:P115. [Google Scholar]
- 4.Canessa N, et al. Obstructive sleep apnea: Brain structural changes and neurocognitive function before and after treatment. Am. J. Respir. Crit. Care Med. 2011;183:1419–1426. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]
- 5.Bucks RS, Olaithe M, Eastwood P. Neurocognitive function in obstructive sleep apnoea: A meta-review. Respirology. 2013;18:61–70. doi: 10.1111/j.1440-1843.2012.02255.x. [DOI] [PubMed] [Google Scholar]
- 6.Alomri RM, Kennedy GA, Wali SO, Ahejaili F, Robinson SR. Differential associations of hypoxia, sleep fragmentation, and depressive symptoms with cognitive dysfunction in obstructive sleep apnea. Sleep. 2020;44:4. doi: 10.1093/sleep/zsaa213. [DOI] [PubMed] [Google Scholar]
- 7.Shah AJ, et al. Is heart rate variability related to memory performance in middle-aged men? Psychosom. Med. 2011;73:475–482. doi: 10.1097/PSY.0b013e3182227d6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohler M, Stradling JR. Mechanisms of vascular damage in obstructive sleep apnea. Nat. Rev. Cardiol. 2010;7:677–685. doi: 10.1038/nrcardio.2010.145. [DOI] [PubMed] [Google Scholar]
- 9.Taranto-Montemurro L, et al. Cardiac sympathetic hyperactivity in patients with chronic obstructive pulmonary disease and obstructive sleep apnea. COPD. 2016;13:706–711. doi: 10.1080/15412555.2016.1199668. [DOI] [PubMed] [Google Scholar]
- 10.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N. Engl. J. Med. 1993;328:303–307. doi: 10.1056/NEJM199302043280502. [DOI] [PubMed] [Google Scholar]
- 11.Narkiewicz K, Somers VK. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol. Scand. 2003;177:385–390. doi: 10.1046/j.1365-201X.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- 12.Catai AM, et al. Heart rate variability: Are you using it properly? Standardisation checklist of procedures. Braz. J. Phys. Ther. 2020;24:91–102. doi: 10.1016/j.bjpt.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sequeira VCC, Bandeira PM, Azevedo JCM. Heart rate variability in adults with obstructive sleep apnea: A systematic review. Sleep Sci. 2019;12:214–221. doi: 10.5935/1984-0063.20190082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delessert A, et al. Pulse wave amplitude drops during sleep are reliable surrogate markers of changes in cortical activity. Sleep. 2010;33:1687–1692. doi: 10.1093/sleep/33.12.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burch GE. Digital Plethysmography: Introducing a Method for Recording Simultaneously the Time Course of the Rate of Blood Flow Into and Out of the Finger Tip. Grune & Stratton; 1954. [Google Scholar]
- 16.Haba-Rubio J, et al. Obstructive sleep apnea syndrome: Effect of respiratory events and arousal on pulse wave amplitude measured by photoplethysmography in NREM sleep. Sleep and Breathing. 2005;9:73–81. doi: 10.1007/s11325-005-0017-y. [DOI] [PubMed] [Google Scholar]
- 17.Catcheside PG, et al. Noninvasive cardiovascular markers of acoustically induced arousal from non-rapid-eye-movement sleep. Sleep. 2002;25:797–804. doi: 10.1093/sleep/25.7.797. [DOI] [PubMed] [Google Scholar]
- 18.Randerath WJ, et al. Parameters of Overnight Pulse Wave under Treatment in Obstructive Sleep Apnea. Respiration. 2016;92:136–143. doi: 10.1159/000448248. [DOI] [PubMed] [Google Scholar]
- 19.Lee DY, Kim E, Choi MH. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep. 2015;48:209–216. doi: 10.5483/BMBRep.2015.48.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: Normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J. Clin. Endocrinol. Metab. 2005;90:3106–3114. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- 21.Kritikou I, et al. Sleep apnoea and the hypothalamic–pituitary–adrenal axis in men and women: Effects of continuous positive airway pressure. Eur. Respir. J. 2016;47:531–540. doi: 10.1183/13993003.00319-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mokhlesi B, Grimaldi D, Beccuti G, Van Cauter E. Effect of one week of CPAP treatment of obstructive sleep apnoea on 24-hour profiles of glucose, insulin and counter-regulatory hormones in type 2 diabetes. Diabetes Obes. Metab. 2017;19:452–456. doi: 10.1111/dom.12823. [DOI] [PubMed] [Google Scholar]
- 23.Weinstein G, et al. Glucose indices are associated with cognitive and structural brain measures in young adults. Neurology. 2015;84:2329–2337. doi: 10.1212/WNL.0000000000001655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Duinkerken E, Ryan CM. Diabetes mellitus in the young and the old: Effects on cognitive functioning across the life span. Neurobiol. Dis. 2020;134:104608. doi: 10.1016/j.nbd.2019.104608. [DOI] [PubMed] [Google Scholar]
- 25.Forte G, Favieri F, Casagrande M. Heart rate variability and cognitive function: A systematic review. Front. Neurosci. 2019;13:1–10. doi: 10.3389/fnins.2019.00710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Idiaquez J, Santos I, Santin J, Del Rio R, Iturriaga R. Neurobehavioral and autonomic alterations in adults with obstructive sleep apnea. Sleep Med. 2014;15:1319–1323. doi: 10.1016/j.sleep.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 27.Allen MD, Owens TE, Fong AK, Richards DR. A functional neuroimaging analysis of the trail making test-B: Implications for clinical application. Behav. Neurol. 2011;24:159–171. doi: 10.1155/2011/476893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frewen J, et al. Cognitive function is associated with impaired heart rate variability in ageing adults: The Irish longitudinal study on ageing wave one results. Clin. Auton. Res. 2013;23:313–323. doi: 10.1007/s10286-013-0214-x. [DOI] [PubMed] [Google Scholar]
- 29.Mahinrad S, et al. 10-Second heart rate variability and cognitive function in old age. Neurology. 2016;86:1120–1127. doi: 10.1212/WNL.0000000000002499. [DOI] [PubMed] [Google Scholar]
- 30.Gąsecki D, Kwarciany M, Nyka W, Narkiewicz K. Hypertension, brain damage and cognitive decline. Curr. Hypertens. Rep. 2013;15:547–558. doi: 10.1007/s11906-013-0398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swan GE, Carmelli D, Larue A. Systolic blood pressure tracking over 25 to 30 years and cognitive performance in older adults. Stroke. 1998;29:2334–2340. doi: 10.1161/01.STR.29.11.2334. [DOI] [PubMed] [Google Scholar]
- 32.Krause AJ, et al. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017;18:404–418. doi: 10.1038/nrn.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takase B, et al. Effects of chronic sleep deprivation on autonomic activity by examining heart rate variability, plasma catecholamine, and intracellular magnesium levels. Biomed. Pharmacother. 2004;58(Suppl 1):S35–39. doi: 10.1016/S0753-3322(04)80007-6. [DOI] [PubMed] [Google Scholar]
- 34.Epstein LJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009;5:263–276. doi: 10.5664/jcsm.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 36.Cohen J. Statistical power analysis. Curr. Dir. Psychol. Sci. 1992;1:98–101. doi: 10.1111/1467-8721.ep10768783. [DOI] [Google Scholar]
- 37.Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 38.Ahmed AE, et al. Validation of the Arabic version of the Epworth sleepiness scale. J. Epidemiol. Glob. Health. 2014;4:297–302. doi: 10.1016/j.jegh.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henry JD, Crawford JR. The short-form version of the Depression anxiety stress scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 2005;44:227–239. doi: 10.1348/014466505X29657. [DOI] [PubMed] [Google Scholar]
- 40.Ali AM, et al. The Arabic version of the depression anxiety stress scale-21: Cumulative scaling and discriminant-validation testing. Asian J. Psychiatr. 2017;30:56–58. doi: 10.1016/j.ajp.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 41.Lovibond PF, Lovibond SH. The structure of negative emotional states: Comparison of the depression anxiety stress scales (DASS) with the beck depression and anxiety inventories. Behav. Res. Ther. 1995;33:335–343. doi: 10.1016/0005-7967(94)00075-U. [DOI] [PubMed] [Google Scholar]
- 42.McCraty R, Shaffer F. Heart rate variability: New Perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Glob. Adv. Health Med. 2015;4:46–61. doi: 10.7453/gahmj.2014.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sztajzel J. Heart rate variability: A noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med. Wkly. 2004;134:514–522. doi: 10.4414/smw.2004.10321. [DOI] [PubMed] [Google Scholar]
- 44.Camm, A.J., et al. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 93, 1043-1065 (1996). [PubMed]
- 45.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010;141:122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 46.Goldstein DS, Bentho O, Park M-Y, Sharabi Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp. Physiol. 2011;96:1255–1261. doi: 10.1113/expphysiol.2010.056259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sassi R, et al. Advances in heart rate variability signal analysis: joint position statement by the e-Cardiology ESC working group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace. 2015;17:1341–1353. doi: 10.1093/europace/euv015. [DOI] [PubMed] [Google Scholar]
- 48.Malpas SC. Neural influences on cardiovascular variability: Possibilities and pitfalls. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H6–H20. doi: 10.1152/ajpheart.2002.282.1.H6. [DOI] [PubMed] [Google Scholar]
- 49.Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–555. doi: 10.1161/01.CIR.98.6.547. [DOI] [PubMed] [Google Scholar]
- 50.Hirsch JA, Bishop B. Respiratory sinus arrhythmia in humans: How breathing pattern modulates heart rate. Am. J. Physiol. Heart Circ. Physiol. 1981;241:H620–H629. doi: 10.1152/ajpheart.1981.241.4.H620. [DOI] [PubMed] [Google Scholar]
- 51.Akselrod S. Components of heart rate variability: Basic studies. Heart Rate Variability. 1995;1:147–163. [Google Scholar]
- 52.Malliani A. Cardiovascular variability is/is not an index of autonomic control of circulation. J. Appl. Physiol. 2006;101:684–688. doi: 10.1152/japplphysiol.00562.2006. [DOI] [PubMed] [Google Scholar]
- 53.Jaryal AK, Selvaraj N, Santhosh J, Anand S, Deepak KK. Monitoring of cardiovascular reactivity to cold stress using digital volume pulse characteristics in health and diabetes. J. Clin. Monit. Comput. 2009;23:123–130. doi: 10.1007/s10877-009-9174-z. [DOI] [PubMed] [Google Scholar]
- 54.Grote L, Zou D, Kraiczi H, Hedner J. Finger plethysmography–a method for monitoring finger blood flow during sleep disordered breathing. Respir. Physiol. Neurobiol. 2003;136:141–152. doi: 10.1016/S1569-9048(03)00090-9. [DOI] [PubMed] [Google Scholar]
- 55.Zacharia A, et al. Sleep apnea syndrome: Improved detection of respiratory events and cortical arousals using oxymetry pulse wave amplitude during polysomnography. Sleep Breath. 2008;12:33–38. doi: 10.1007/s11325-007-0126-x. [DOI] [PubMed] [Google Scholar]
- 56.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav. Res. Methods Instrum. Comput. 1985;17:652–655. doi: 10.3758/BF03200977. [DOI] [Google Scholar]
- 57.Jung CM, Ronda JM, Czeisler CA, Wright KP., Jr Comparison of sustained attention assessed by auditory and visual psychomotor vigilance tasks prior to and during sleep deprivation. J. Sleep Res. 2011;20:348–355. doi: 10.1111/j.1365-2869.2010.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milner B. Visually-guided maze learning in man: Effects of bilateral hippocampal, bilateral frontal, and unilateral cerebral lesions. Neuropsychologia. 1965;3:317–338. doi: 10.1016/0028-3932(65)90005-9. [DOI] [Google Scholar]
- 59.Stolwyk RJ, Lee S, McKay A, Ponsford JL. Exploring what the austin maze measures: A comparison across conventional and computer versions. Brain Impairment. 2013;14:243–252. doi: 10.1017/BrImp.2013.23. [DOI] [Google Scholar]
- 60.Crowe SF, et al. The cognitive determinants of performance on the Austin Maze. J. Int. Neuropsychol. Soc. 1999;5:1–9. doi: 10.1017/S1355617799511016. [DOI] [PubMed] [Google Scholar]
- 61.Bowden S, et al. Healthy adults' performance on the Austin Maze. Clin. Neuropsychol. 1992;6:43–52. doi: 10.1080/13854049208404116. [DOI] [Google Scholar]
- 62.Kopelman MD, Wilson BA, Baddeley AD. The autobiographical memory interview: A new assessment of autobiographical and personal semantic memory in amnesic patients. J. Clin. Exp. Neuropsychol. 1989;11:724–744. doi: 10.1080/01688638908400928. [DOI] [PubMed] [Google Scholar]
- 63.Nuttall FQ. Body mass index: Obesity, BMI, and health: A critical review. Nutr. Today. 2015;50:117–128. doi: 10.1097/NT.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berry RB, et al. The AASM manual for the scoring of sleep and associated events. Rules Terminol. Tech. Spec. 2012;176:2012. [Google Scholar]
- 65.Berry RB, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events: Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirotsu C, et al. Pulse wave amplitude drops during sleep: Clinical significance and characteristics in a general population sample. Sleep. 2020;43:322. doi: 10.1093/sleep/zsz322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 1995;57:289–300. [Google Scholar]