Abstract
Genes with cross-cancer aberrations are most likely to be functional genes or potential therapeutic targets. Here, we found a total of 137 genes were ectopically expressed in eight cancer types, of which Holliday junction recognition protein (HJURP) was significantly upregulated in prostate cancer (PCa). Moreover, patients with higher HJURP mRNA and protein levels had poorer outcomes, and the protein levels served as an independent prognosis factor for the overall survival of PCa patients. Functionally, ectopic HJURP expression promoted PCa cells proliferation in vitro and in vivo. Mechanistically, HJURP increased the ubiquitination of cyclin-dependent kinase inhibitor 1 (CDKN1A) via the GSK3β/JNK signaling pathway and decreased its stability. This study investigated the role of HJURP in PCa proliferation and may provide a novel prognostic and therapeutic target for PCa.
Subject terms: Tumour biomarkers, Prostate cancer, Cell growth, Diseases, Molecular biology
Introduction
The incidence of prostate cancer (PCa) has continued to rise in recent years and is currently ranked first among all male malignancies in the USA.1 Therefore, it is imperative to identify new diagnostic and therapeutic targets for PCa. Many studies have reported that genes with cross-cancer aberrations are most likely to be functional genes and potential therapeutic targets2,3. Thus, we analyzed the RNA-seq data of eight cancer types, including PCa, from the FireBrowse database (http://firebrowse.org/) to determine the cross-oncogenes. The results showed that 137 genes are differentially expressed across all tumors assessed, and HJURP was most significantly upregulated in data from PCa.
In mammals, HJURP is a molecular chaperone of centromere protein-A (CENP-A) and mediates the deposition of CENP-A in centromeres to promote chromosome separation and mitosis4. Furthermore, HJURP inhibits senescence through the p53-dependent pathway and plays a role in cell viability regulation5. Kato et al.6 revealed that, in cancer cells, HJURP promotes homologous recombination and rDNA stability through interaction with human mutS homolog 5 (hMSH5) and MRE11-RAD50-NBS1 protein complex, which contributes to immortality and genomic stability in osteosarcoma, lung, and testicular cancer. Moreover, Cao et al.7 reported that HJURP-silencing could activate PPARγ and inhibit the p-SIRT1/t-SIRT1 signaling pathways in bladder cancer cells, which can lead to an increase in ROS and cause apoptosis. In addition, HJURP is elevated in ovarian8 and breast cancer9 and is correlated with poor outcomes.
Similarly, HJURP is an unfavorable factor in PCa10, but the specific functions and mechanisms underlying its involvement remain unclear. In this study, we demonstrated that HJURP was upregulated in PCa and its expression was positively correlated with unfavorable outcomes. Moreover, HJURP increased the ubiquitination of CDKN1A through the GSK3β/JNK signaling pathway, which promoted the proliferation of PCa cells in vitro and in vivo.
Results
A total of 137 genes were ectopically expressed in eight cancer types, of which HJURP was most significantly upregulated in PCa
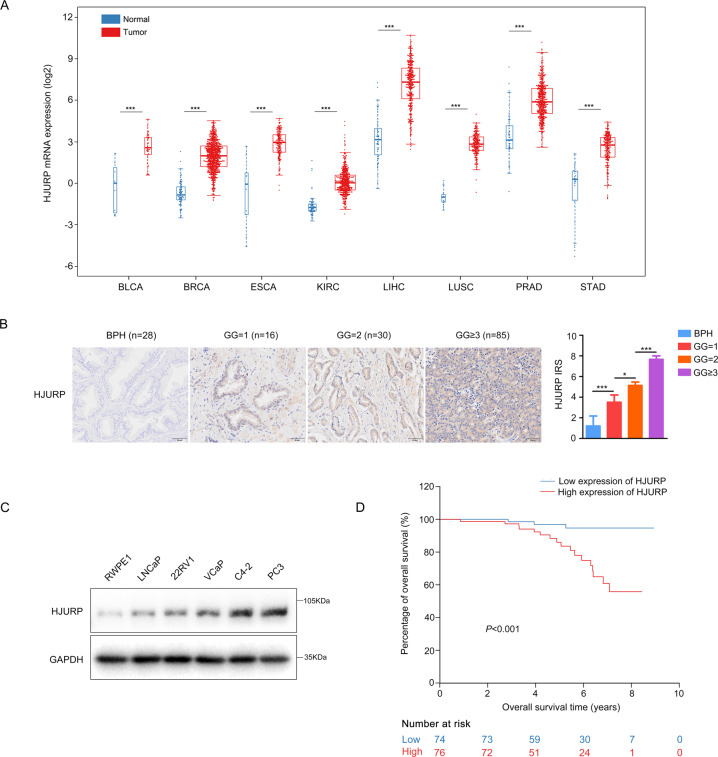
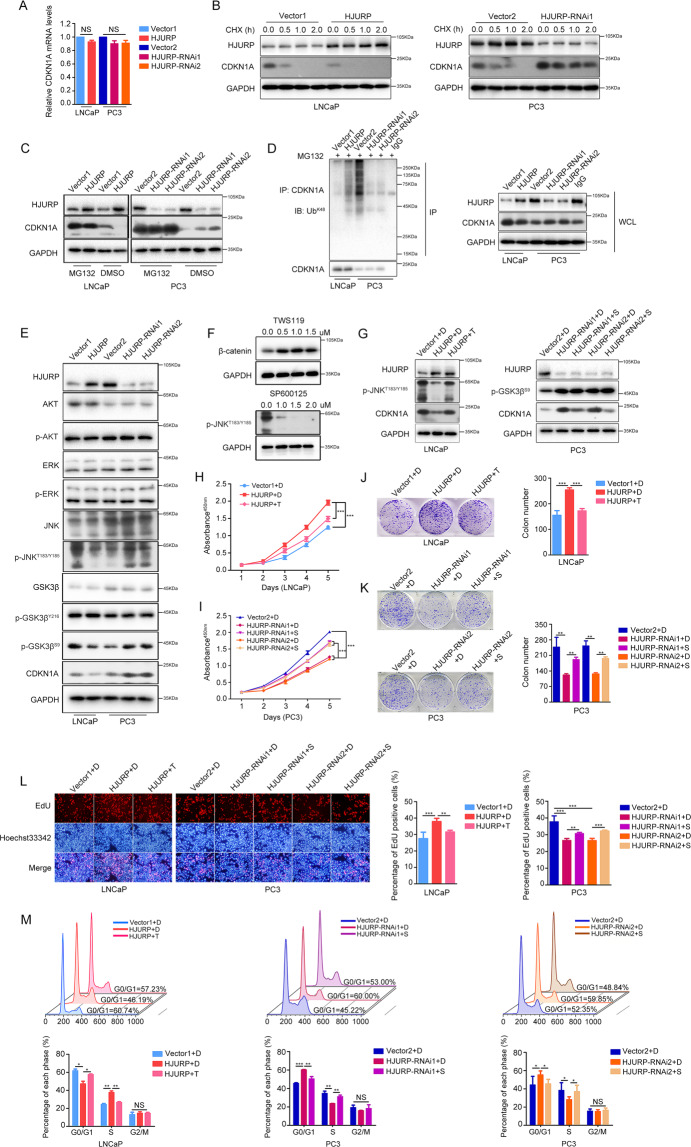
The analysis of RNA-seq data from FireBrowse showed that a total of 137 genes were differentially expressed across all tumors (Fig. 1A, Supplementary Fig. S1A, and Table S3). In PCa, 137 genes were differentially expressed between tumor and normal tissues (Supplementary Fig. S1B), which HJURP was most significantly upregulated in PCa (fold change = 6.2, P < 0.05, Supplementary Fig. S1C), and its mRNA levels were substantially elevated among the eight tumors (Fig. 2A). Moreover, IHC staining confirmed that compared with BPH, HJURP was notably increased in PCa tissues and positively correlated with Gleason grade (GG) (Fig. 2B and Supplementary Fig. S1D). Western blotting also showed that compared with RWPE1 cells, HJURP was significantly upregulated in LNCaP, 22Rv1, VCaP, C4-2, and PC3 cells (Fig. 2C).
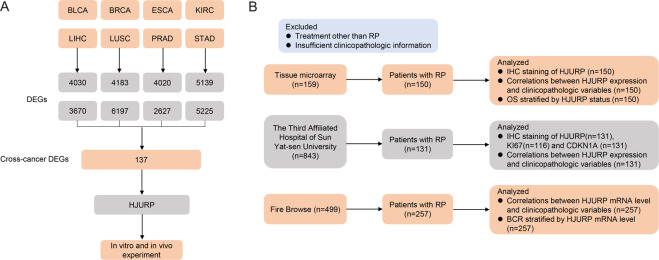
Fig. 1. Flowchart depicting study enrollment.
A Screening of cross-oncogene in eight tumors. B The patients with prostate cancer enrolled in this study. BLCA bladder urothelial carcinoma, BRCA breast invasive carcinoma, ESCA esophageal carcinoma, KIRC kidney renal clear cell carcinoma, LIHC liver hepatocellular carcinoma, LUSC lung squamous cell carcinoma, PRAD prostate adenocarcinoma, STAD stomach adenocarcinoma, DEGs differentially expressed genes, RP radical prostatectomy, IHC Immunohistochemistry, OS overall survival, BCR biochemical recurrence.
Fig. 2. HJURP was upregulated in PCa and positively correlated with poor overall survival (OS).
A HJURP mRNA levels were significantly elevated in all eight types of tumors. B Representative IHC staining of HJURP in PCa and BPH tissues from our hospital (scale bar: 50 µm; magnification: ×200). C Western blotting was used to measure HJURP expression in different PCa cell lines. D Kaplan–Meier plots demonstrating OS time stratified by HJURP protein levels. BPH benign prostate hyperplasia, IRS immunoreactivity score, GG Gleason grade.
These results indicate that HJURP is a cross-oncogene, notably upregulated in PCa.
High HJURP mRNA and protein levels are positively correlated with poor outcomes
The IHC staining of PCa specimens from our hospital (n = 131) and tissue array (n = 150) both showed that HJURP protein level was strongly associated with the pathologic GG group (Supplementary Table S4). Moreover, the HJURP mRNA levels (n = 257) were also positively correlated with the pathologic GG group (Supplementary Table S4). Kaplan–Meier survival analyses from the tissue array indicated that patients with higher HJURP protein levels had shorter overall survival (OS) time (P < 0.001, Fig. 2D). Furthermore, univariate and multivariate Cox regression analyses showed that high HJURP protein expression was an independent prognostic factor for the poor OS of PCa patients (HR = 5.62, 95% CI = 1.53–20.60, P = 0.009, Table 1). Moreover, the high HJURP mRNA levels were also positively associated with increased biochemical recurrence rate (BCR) after RP (P = 0.024) in PCa patients from the FireBrowse database (Supplementary Fig. S1E). However, multivariate Cox regression analyses failed to confirm the HJURP mRNA level as an independent prognostic factor for BCR of PCa patients (data not shown).
Table 1.
Univariate and multivariate Cox regression analyses for overall survival in PCa patients enrolled in tissue microarray.
| Univariable models | Multivariable models | Multivariable models | ||||
|---|---|---|---|---|---|---|
| Variables | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value |
| Age (years) | ||||||
| ≤65 | Ref. | Ref. | Ref. | |||
| >65 | 11.60 (1.55, 86.75) | 0.017 | 14.11 (1.67, 119.22) | 0.015 | 13.31 (1.63, 108.51) | <0.001 |
| Gleason grade group at RP | – | – | ||||
| 1 | Ref. | |||||
| 2 | 0.49 (0.04, 5.44) | 0.563 | ||||
| ≥3 | 2.35 (0.31, 17.67) | 0.408 | ||||
| Pathological T stage | – | |||||
| T2 | Ref. | Ref. | ||||
| T3a | 4.36 (1.47, 12.95) | 0.008 | 0.79 (0.20, 3.10) | 0.730 | ||
| T3b | 9.17 (2.49, 33.86) | 0.001 | 0.78 (0.16, 3.83) | 0.758 | ||
| Pathological N stage | ||||||
| N0 | Ref. | Ref. | Ref. | |||
| N1 | 14.27 (4.74, 42.95) | <0.001 | 34.48 (6.67, 178.34) | <0.001 | 28.32 (7.89, 101.60) | <0.001 |
| IRS of HJURP | ||||||
| ≤6 | Ref. | Ref. | Ref. | |||
| >6 | 6.63 (1.94, 22.64) | 0.003 | 5.84 (1.56, 21.85) | 0.009 | 5.62 (1.53, 20.60) | 0.009 |
| Surgical margins | ||||||
| Negative | Ref. | Ref. | Ref. | |||
| Positive | 6.01 (2.44, 14.84) | <0.001 | 11.12 (3.60, 34.31) | <0.001 | 10.38 (3.55, 30.42) | <0.001 |
IRS immunoreactivity score, RP radical prostatectomy.
In addition, the T stage of patients from the Third Affiliated Hospital denotes statistically significant differences between the high- and low-expression groups of HJURP, but there was no significant difference in patients from the tissue array (Supplementary Table S4). We think it is caused by differences in health care and insurance systems. The Third Affiliated Hospital mainly treats patients from undeveloped areas with limited medical resources and a lack of early screening for PCa, resulting in a higher T stage. However, most of the patients enrolled in the tissue array come from developed regions with better health care, for the timely treatment prevented the PCa from progression.
Overall, the results indicate that high HJURP mRNA and protein levels are both significantly correlated with a poor outcome, and HJURP protein levels serve as an independent prognosis factor for OS of PCa patients.
HJURP promotes the proliferation of PCa cells in vitro and in vivo
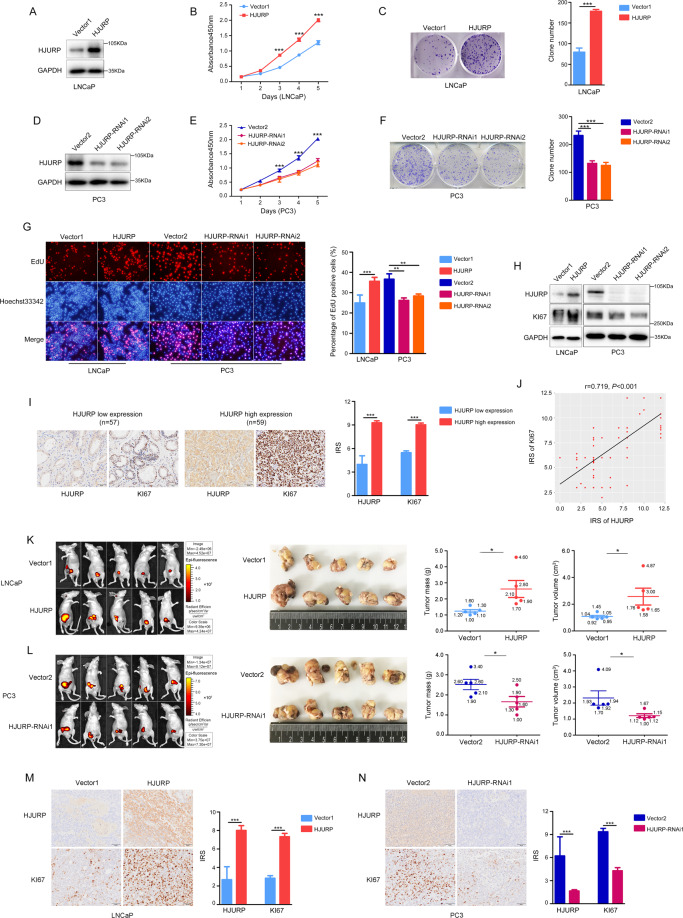
To investigate the specific function of HJURP in PCa cells, we overexpressed HJURP in LNCaP cells (Fig. 3A). A CCK8 assay showed that the viability of LNCaP cells significantly increased (Fig. 3B). Conversely, HJURP was silenced in PC3 cells (Fig. 3D), and cell viability was significantly decreased (Fig. 3E). Similar results were also obtained from the colony-formation assay (Fig. 3C, F). In addition, HJURP overexpression can significantly increase the proliferative capacity of LNCaP cells, and HJURP knockdown inhibited the proliferative capacity of PC3 cells (Fig. 3G). Similarly, HJURP knockdown in LNCaP cells and overexpression in PC3 cells have the same results (Supplementary Fig. 2A–G). Western blotting established that KI67 was positively correlated with HJURP (Fig. 3H). Notably, the IHC staining of PCa tissues (n = 116) from our hospital also showed a high correlation between HJURP and KI67 (r = 0.719, P < 0.001, Fig. 3I, J), which provided strong evidence for HJURP association with PCa proliferation. However, HJURP was weakly affected the apoptosis of LNCaP and PC3 cells (Supplementary Fig. S2H). Consistent with the results described, the in vivo growth of LNCaP cells with stable HJURP expression was notably enhanced (Fig. 3K), whereas that of PC3 cells with HJURP silencing was significantly impaired (Fig. 3L). IHC staining of the xenograft demonstrated that KI67 expression was positively correlated with HJURP levels (Fig. 3M, N).
Fig. 3. HJURP promotes the proliferation of PCa cells in vitro and in vivo.
A, D Western blotting of HJURP-overexpression (A) and knockdown (D) efficiency. B, E CCK8 assay to measure PCa cells viability after HJURP-overexpression (B) and knockdown (E). C, F Colony-formation assay to measure the cell viability of PCa cells after HJURP overexpression (C) and silencing (F). G EdU assay to measure the effects of HJURP on the proliferative capacity of PCa cells. H Western blotting quantification of KI67 expression after HJURP overexpression or knockdown in PCa cells. I Representative IHC staining of HJURP and KI67 in different Gleason grade PCa tissues from our hospital (n = 131, scale bar: 50 µm; magnification: ×200). J HJURP and KI67 protein expression are highly correlated in PCa (n = 131). K, L HJURP can affect the in vivo growth of PCa cells. M, N Representative IHC staining of HJURP and KI67 in PCa-cell xenograft tissues (scale bar: 50 µm; magnification: ×200).
In summary, we found that HJURP can promote PCa cell proliferation, but not inhibit apoptosis in vitro and in vivo.
HJURP promotes G1/S phase transition through CDKN1A inhibition in PCa cells
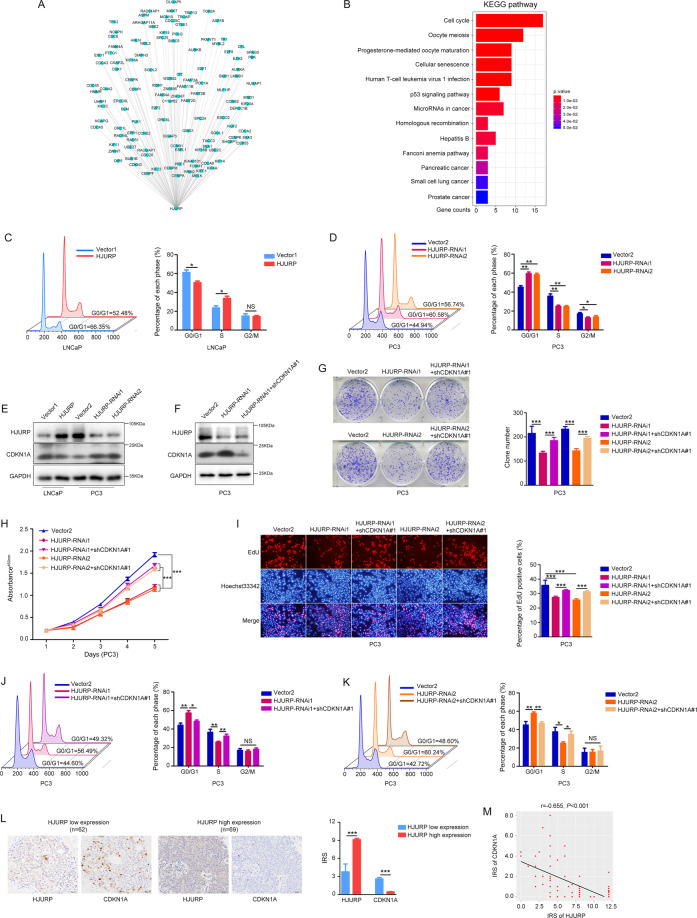
Genes with similar co-expression patterns may play similar roles in cancer cells11. Therefore, co-expression and KEGG enrichment analysis of HJURP were performed. The results indicated that a total of 119 genes were highly correlated with HJURP (r ≥ 0.6, P < 0.05, Fig. 4A and Supplementary Table S5) and the cell-cycle pathway had the highest number of enriched genes (Fig. 4B), indicating that HJURP may primarily regulate the cell cycle in PCa cells. Consistent with this result, we detected that HJURP overexpression promoted G1/S phase transition, whereas HJURP-silencing generally induced G0/G1 arrest in PCa cells (Fig. 4C, D and Supplementary Fig. S3A, B).
Fig. 4. HJURP inhibits CDKN1A to promote the G1/S phase transition.
A Correlation analysis showed that 119 genes were highly correlated with HJURP (r ≥ 0.06, P < 0.05). B KEGG enrichment analysis of HJURP and its highly correlated genes. C, D Flow cytometry used to measure the proportion of cells in various phases of the cell cycle in HJURP-overexpression (C) and -knockdown (D). E Western blotting quantified CDKN1A expression after HJURP-overexpression or -knockdown in PCa cells. F Western blotting results of CDKN1A knockdown efficiency. G–K CDKN1A knockdown significantly reversed changes in clonogenic potential (G), cell viability (H), proliferative capacity (I), and cell cycle (J, K) in PC3 cells caused by HJURP inhibition. L Representative IHC staining of HJURP and CDKN1A in different Gleason grade PCa tissues from our hospital (n = 131, scale bar: 50 µm; magnification: ×200). M HJURP and CDKN1A protein expression are highly negatively correlated in PCa (n = 131).
Chen et al.12 reported that HJURP could promote hepatocellular carcinoma cell proliferation through the CDKN1A protein, a critical factor for G1/S phase transition13. Therefore, we hypothesized that HJURP also regulates the cell cycle via the CDKN1A protein in PCa cells. Western blotting confirmed the hypothesis that HJURP overexpression in LNCaP cells decreased CDKN1A protein levels, whereas HJURP silencing in PC3 cells increased CDKN1A protein levels (Fig. 4E). Moreover, CCK8, colony formation, EdU, and flow cytometry assays showed that the impaired proliferative capacity of PC3 cells with HJURP-silencing was significantly reversed after CDKN1A knockdown (Fig. 4F–K and Supplementary Fig. S3C–3H). More convincingly, HJURP expression was negatively correlated with CDKN1A levels in PCa tissues (n = 131) from our hospital (r = −0.655, P < 0.001, Fig. 4L, M).
The results show that HJURP promotes G1/S phase transition via inhibiting CDKN1A protein expression in PCa cells.
HJURP promotes CDKN1A ubiquitin-dependent proteasome degradation
To investigate the specific mechanism by which HJURP regulates CDKN1A, we first measured the mRNA levels of CDKN1A after HJURP overexpression and knockdown. The results showed that HJURP did not affect CDKN1A mRNA transcription (Fig. 5A), indicating that HJURP may affect CDKN1A expression through post-transcriptional regulation. To validate this hypothesis, we treated PCa cells with a translation inhibitor, cycloheximide (CHX). The results demonstrated that HJURP overexpression in LNCaP cells accelerated the degradation of CDKN1A protein, whereas HJURP-silencing in PC3 cells decreased its degradation rate (Fig. 5B). In addition, the proteasome inhibitor, MG132, significantly reversed CDKN1A protein regulation by HJURP (Fig. 5C). Together, these results suggest that HJURP promotes CDKN1A protein degradation through the proteasomal pathway. However, the CDKN1A protein can be degraded by ubiquitin-dependent or -independent proteasomal pathways;14 therefore, we further tested whether HJURP affected CDKN1A ubiquitination levels. Protein co-immunoprecipitation showed that the ectopic expression of HJURP increased CDKN1A ubiquitination levels, whereas HJURP-silencing significantly decreased CDKN1A ubiquitination levels (Fig. 5D).
Fig. 5. HJURP promotes CDKN1A ubiquitin-dependent proteasomal degradation through the GSK3β/JNK pathway.
A qPCR quantification of CDKN1A mRNA levels in PCa cells after HJURP-overexpression or -silencing. B CHX (50 µg/mL) was used to treat LNCaP and PC3 cells for 0, 0.5, 1.0, and 2.0 h, and western blotting was applied to quantify CDKN1A stability. C MG132 (10 µM) was employed to incubate cells for 6 h, and western blotting was utilized to quantify CDKN1A levels after HJURP -overexpression or -knockdown in PCa cells. D CO-IP quantification of the effects of HJURP on CDKN1A ubiquitination. E Western blotting of AKT, p-AKT, ERK1/2, p-ERK1/2, JNK, p-JNKT183/Y185, GSK3β, p-GSK3βY216, p-GSK3βS9, and CDKN1A levels in PCa cells after HJURP-overexpression or -silencing. F Western blotting quantified optimal TWS119 and SP600125 concentrations (0.5 µM TWS119 and 1.0 µM SP600125 were selected). G TWS119 could reverse HJURP-induced reduction in p-JNKT183/Y185 and CDKN1A, whereas SP600125 could reverse HJURP-induced CDKN1A elevation, but did not affect p-GSK3βS9 levels. H–M TWS119 and SP600125 could reverse HJURP-induced changes in cell viability (H), clonogenic potential (J), proliferative capacity (L), and cell cycle (M) in PCa cells. CHX cycloheximide, DMSO dimethyl sulfoxide, Ub ubiquitination.
In summary, HJURP can activate ubiquitin-dependent proteasomal degradation of the CDKN1A protein.
HJURP regulates CDKN1A ubiquitination through the GSK3β/JNK signaling pathway
The ubiquitination of CDKN1A protein is affected by its phosphorylation status. For example, extracellular signal-regulated kinase 1/2 (ERK1/2)15, glycogen synthase kinase (GSK) 3β16, c-Jun N-terminal kinase (JNK)17, and AKT18 can regulate CDKN1A ubiquitination through different phosphorylation sites. Therefore, we hypothesized that HJURP might regulate CDKN1A ubiquitination through these kinases. Western blotting demonstrated that HJURP overexpression decreased GSK3β S9, and JNK T183/Y185 phosphorylation levels, whereas HJURP-silencing increased phosphorylation levels at these sites (Fig. 5E). Moreover, many studies have reported that GSK3β can act upstream of JNK to exert positive or negative regulation19–21. Thus, to determine the specific relationship between GSK3β and JNK, we used TWS119 to inhibit GSK3β activity and SP600125 to inhibit JNK activity (Fig. 5F). The results showed that TWS119 significantly reversed JNK T183/Y185 phosphorylation and CDKN1A protein levels in HJURP-overexpression LNCaP cells and enhanced that in HJURP-knockdown PC3 cells, however, SP600125 failed to affect the phosphorylation level of GSK3β S9 in both cells (Fig. 5G and Supplementary Fig. S4A). These results suggest that HJURP regulates CDKN1A ubiquitination through the GSK3β/JNK signaling pathway. Based on the results of CCK8, colony formation, Edu, and flow cytometry assay, the growth affected by HJURP were significantly reversed by treatment with TWS119 and SP600125 in HJURP ectopic expression-LNCaP cells and HJURP-knockdown PC3 cells, respectively (Fig. 5H–M). Moreover, the proliferation ability of HJURP-overexpression LNCaP cells was enhanced by SP600125, and that of HJURP-knockdown PC3 cells were impaired by TWS119 (Supplementary Fig. S4B–4G).
Taken together, HJURP promotes ubiquitin-dependent proteasome degradation of CDKN1A via the GSK3β/JNK signaling pathway (Fig. 6).
Fig. 6. Mechanisms of HJURP in PCa cells.
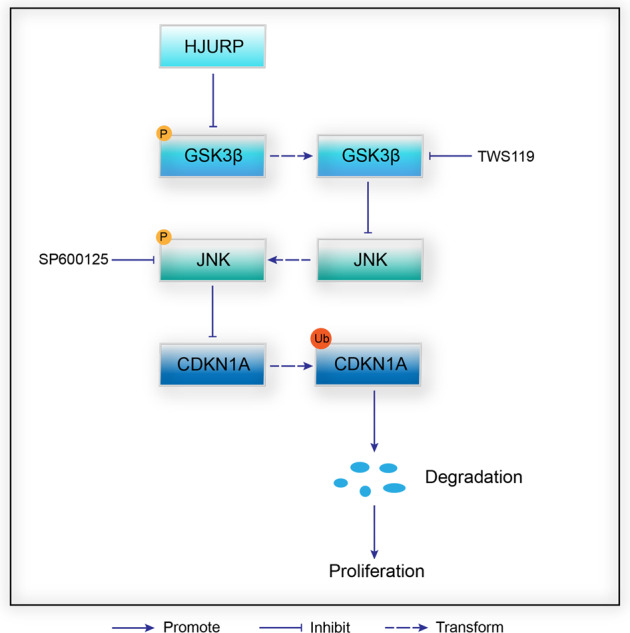
HJURP inhibits the phosphorylation of GSK3β S9 and JNK T183/Y185, which activates the ubiquitin-dependent proteasomal degradation pathway of CDKN1A and ultimately promotes the proliferation of PCa cells. P phosphorylation, Ub ubiquitination.
Discussion
In this study, we discovered 137 cross-oncogenes from eight types of tumors, of which HJURP was the most significantly upregulated gene for PCa. Moreover, HJURP protein levels were an independent prognostic factor for short OS time. Furthermore, HJURP can promote G1/S phase transition through increasing degradation of CDKN1A protein via the GSK3β/JNK signaling pathway, which leads to proliferation of PCa cells in vitro and in vivo.
It is well-known that the stability of CDKN1A, a crucial factor for transition from G1 to S phase22, depends on its phosphorylation status23. GSK3β is a CDKN1A kinase that phosphorylates different sites to promote ubiquitin-dependent or -independent proteasomal degradation of CDKN1A14,24. Conversely, JNK can decrease CDKN1A ubiquitination levels to increase its stability13,25. Moreover, GSK3β can also regulate JNK as an upstream factor: Liu et al.19 found that GSK3β is a negative regulator of growth factor-induced activation of JNK in mouse embryonic fibroblasts. Abell et al.20 reported that GSK3β binds to the C-terminal kinase domain of MEKK4 in COS-7 cells, which blocks MEKK4 dimerization and ultimately inhibits JNK activity. Furthermore, Yuri et al.21 reported that in glioblastoma cells, JNK phosphorylation levels decreased after GSK3β was inhibited. These studies demonstrated that GSK3β could positively or negatively regulate JNK as an upstream factor. In this study, HJURP could activate GSK3β while inhibiting JNK activity in PCa cells. Moreover, treating HJURP-overexpression PCa cells with TWS119 could significantly reverse p-JNKT183/Y185 levels, however, the expression of p-GSK3βS9 did not change after incubation with SP600125 in HJURP-knockdown PCa cells. Thus, we conclude that HJURP inhibits CDKN1A expression via the GSK3β/JNK signaling pathway, which promotes the proliferation of PCa cells in vitro and in vivo ultimately.
Interestingly, the subcellular location of HJURP was initially reported to be nucleus4, however, we found that it was mainly stained in the cytoplasm of PCa cells according to the IHC. This is consistent with prior studies in PCa10. In addition, in hepatocellular carcinoma12,26, HJURP is also stained in the cytoplasm. However, in pancreatic cancer27 and colorectal cancer28, its localization is mainly in the nucleus. In glioma29, HJURP is stained in both cytoplasm and nucleus. These results suggested that the subcellular localization of HJURP varies in different kinds of cancer, and different localization of HJURP may lead to different functions, but the specific mechanism needs further investigation.
In conclusion, our findings revealed that HJURP, a cross-oncogene, negatively correlated with outcomes of PCa patients, promoted proliferation in PCa cells via inhibition of CDKN1A expression through the GSK3β/JNK signaling pathway. These findings may provide a novel prognostic and therapeutic target for PCa.
Materials and methods
Analysis of RNA-seq and clinical data from public database
The RNA-seq data of eight types of cancer with the most comprehensive information were downloaded from the FireBrowse database (http://firebrowse.org/) (Fig. 1A and Supplementary Table S1). The R 3.5.1 and Perl 5.24.1 were employed to analyze the data.
Clinicopathological information from 499 PCa patients was obtained from the FireBrowse database; 257 patients who underwent radical prostatectomy (RP) provided sufficient information to be included in the present analysis (Fig. 1B and Supplementary Tables S1 and S2), the detailed inclusion and exclusion criteria are exhibited in Supplementary Methods. Patients were divided into high-expression and low-expression groups according to the median RNA-Seq by Expectation-Maximization (RSEM) of HJURP mRNA.
Immunohistochemical (IHC) staining
The tissue array was purchased from Wuhan Servicebio Biotech Co. (Shanghai, China). Moreover, PCa tissue specimens of 131 patients and benign prostate hyperplasia (BPH) tissue specimens of 28 patients were obtained from the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China (Fig. 1B). The inclusion and exclusion criteria of tissue array and specimens were presented in Supplementary Methods. Patient characteristics from our study cohort are displayed in Supplementary Table S2. This study was approved by the Ethics Review Committee of the Third Affiliated Hospital of Sun Yat-sen University and registered in the Chinese Clinical Trial Registry (ChiCTR2000033835). Written informed consent was received from patients according to the guidelines of the Declaration of Helsinki. IHC staining was based on the methods described previously30. The primary antibodies included HJURP (Abcam, ab175577, USA), KI67 (Abcam, ab16667, USA), and CDKN1A (CST, 2947, USA). The tissue samples were evaluated according to immunoreactive score (IRS). IRS > 6 was defined as a high expression of HJURP while IRS ≤ 6 was defined as a low expression of HJURP.
IRS = staining intensity × percentage of stained cells. Staining intensity: negative, 0; weak, 1; moderate, 2; strong, 3; percentage of stained cells: 0%, 0; <10%, 1; 11–50%, 2; 51–80%, 3; >80%, 4.
Cell culture
LNCaP and PC3 cells were purchased from the American Type Culture Collection (Manassas, USA). LNCaP and PC3 cells were cultured in RPMI-1640 (HyClone, USA) with 10% fetal bovine serum (FBS; Bovogen, Australia). All cells were incubated in a humidified incubator at 37 °C with 5% CO2. All cells used in this study were identified by short tandem repeat authentication and excluded the mycoplasma contamination.
Construction and transfection of plasmids and lentivirus
Lentivirus: HJURP-overexpressing and knockdown lentiviruses and control viruses were purchased from OBiO Technology Corporation (Shanghai, China). HJURP-overexpressing and control lentiviruses were transfected into LNCaP cells, HJURP knockdown and control lentiviruses were transfected into PC3 cells according to the manufacturer’s instructions. Puromycin at 3 µg/mL (LNCaP) or 5 µg/mL (PC3) was employed to screen stably transfected cells; HJURP-RNAi1, 5’-GTATGGAAGTTCGATATCA-3’; HJURP-RNAi2, 5’-GTGACACCCTCGAAGTATT-3’.
Plasmids: CDKN1A-shRNA was constructed using pSUPER.retro.neo vector, and the empty vector was regarded as the control. Lipofectamine 3000 (Invitrogen, USA) was used for transfection according to the manufacturer’s instructions; shCDKN1A#1: 5’- GATCCCCGATGGAACTTCGACTTTGTTTCAAGAGACTACCTTGAAGCTGAAACATTTTTA-3’; shCDKN1A#2: 5’-GATCCCCGAGACTCTCAGGGTCGAAATTCAAGAGACTCTGAGAGTCCCAGCTTTTTTTTA-3’.
Western blotting
Western blotting was performed as previously described30. Primary antibodies included those for HJURP (Abcam, ab100800, USA), KI67 (Abcam, ab16667, USA), CDKN1A (CST, 2947, USA), AKT (CST, 4691, USA), p-AKTT308 (CST, 13038, USA), ERK1/2 (CST, 4695, USA), p-ERK1/2 (CST, 4370, USA), JNK (CST, 9252, USA), p-JNKT183/Y185 (CST, 4668, USA), GSK3β (Abcam, ab93926, USA), p-GSK3βY216 (Abcam, ab75745, USA), p-GSK3βS9 (CST, 9322, USA), β-catenin (CST, 8480, USA), and GAPDH (ABclonal, A19056, China). Other reagents included cycloheximide, MG132, TWS119, and SP600125 (Sigma).
Cell viability assay
The Cell Counting Kit-8 Assay (Dojindo, Japan) was used to measure cell viability.
To investigate whether HJURP affected the viability of PCa cells, LNCaP (3 × 103 cells/well) and PC3 cells (2 × 103 cells/well) were seeded into 96-well plates and cultured in a humidified incubator at 37 °C with 5% CO2 for 1–5 days. After the indicated number of days, the supernatants were replaced by fresh culture medium containing 10% CCK8 and then incubated for an hour at 37 °C. A microplate reader (Bio Rad, USA) was used to measure absorbance at 450 nm.
Colony-formation assay
Stably infected LNCaP and PC3 cells were seeded into six-well plates at a density of 1 × 103 cells/well. After incubation for 14 days, the plates were washed with phosphate-buffered saline (PBS) three times, and 4% paraformaldehyde was applied for 15 min to fix the cells. Subsequently, 0.5% crystal violet solution (KeyGEN, China) was used to stain the cells. After 10 min, the plates were washed with pure water for further counting and analysis.
EdU assay
The cells were seeded in 12-well plates and incubated with EdU (Ribobio, C10310, China) for 4 h at 37 °C with 5% CO2. Subsequently, the supernatants were removed and 500 µl of 1 × Apollo® reaction cocktail was employed to treat cells for 30 min. Following this, the DNA contents of the cells were stained with 500 µl of Hoechst33342 (5 µg/ml) for 30 min and detected under a fluorescence microscope (Olympus Optical, Japan).
Mouse xenograft assay
Four- to six-week-old BALB/c nude male mice were purchased from the Laboratory Animal Center of Sun Yat-sen University (Guangzhou, China), were used in this study. Mice were housed in a specific pathogen-free environment with sterile food and water. All animal experiments underwent the necessary ethical review (SYSU-IACUC-2020-000202) and were performed with strict adherence to the regulations of the Sun Yat-Sen University Animal Care and Use Committee.
The sample size of mice was decided from previous reports30 and practical considerations, blinding and randomization method were employed to the group.
After anesthetizing the mice with sodium pentobarbital (40 mg/kg) (Sigma), LNCaP cells stably expressing HJURP (5 × 106 cells/ mice) or PC3 cells with stable HJURP-silencing (1 × 106 cells/ mice) and their control cells, resuspended in 100 µl Hanks’ balanced salt solution (HBSS, HyClone), were injected into the prostate with a 29-gauge needle (These mice were divided into four groups: LNCaP-Vector1, LNCaP-HJURP, PC3-Vector2, and PC3-HJURP-RNAi1, with five mice/group), of which the bilateral testes of mice in the PC3 group were simultaneously resected. After 4 weeks, mice were anesthetized and imaged using an IVIS system (PerkinElmer, USA). Subsequently, mice were euthanized and tumors were extracted for further histomorphological analysis. The carcasses were disposed of by the Laboratory Animal Center.
Gene co-expression and KEGG enrichment analysis
To obtain genes highly correlated with HJURP (r ≥ 0.6, P < 0.05), RNA-seq data from PCa cases emanating from FireBrowse were analyzed using Hmisc package in R 3.5.1. Then, the clusterProfiler package in R 3.5.1 was applied for KEGG enrichment analysis of the genes.
Flow cytometry
Cell-cycle analysis: Pre-cooled 75% ethanol was used to fix PCa cells (1 × 106 cells) at 4 °C overnight. Subsequently, the cells were washed with PBS three times and resuspended with 500 µl propidium iodide (PI) (Becton, Dickinson and Company, USA) at room temperature. After 15 min, the cells were loaded into the flow cytometer (Calibur, BD Bioscience, USA) for analysis. FlowJo software (Tree Star, San Carlos, USA) was used to evaluate the results.
Cell apoptosis analysis: The PCa cells (1 × 106 cells) were washed with pre-cooled PBS twice and resuspended in 1 ml of 1× binding buffer. Then, 5 µl FITC Annexin V and 5 µl PI (Becton, Dickinson and Company) were added to 100 µl of cell suspension (1 × 105 cells), and the cells were incubated for 15 min at room temperature. Finally, 200 µl of 1× binding buffer was added to each tube, and cell apoptosis analysis was detected via a flow cytometer (Calibur, BD Bioscience). FlowJo software (Tree Star) was used to analyze the results.
RT-qPCR
Trizol (Invitrogen) was employed to extract total RNA from PCa cells, and superscript III reverse transcriptase (Invitrogen) was used for the reverse transcription of 1 µg of the total RNA. SYBR Green qPCR reagent (GenStar, China) was used for fluorescence real-time PCR, and the mRNA levels of GAPDH were utilized as the internal reference genes.
qPCR primers: CDKN1A, upstream primer: 5’-CGATGGAACTTCGACTTTGTCA-3’, downstream primer: 5’-GCACAAGGGTACAAGACAGTG-3’; GAPDH, upstream primer: 5’-GACTCATGACCACAGTCCATGC-3’, downstream primer: 5’-AGAGGCAGGGATGATGTTCTG-3’.
Co-immunoprecipitation and ubiquitination assays
The PCa cells were seeded into six-well plates, and the supernatants were replaced with fresh culture medium containing 10 µM/L MG132 when the cell density was 70–80%. After 6 h, the cells were washed with PBS three times and lysed in 120 µl lysis buffers (containing 0.1% protease inhibitor, 1% phosphatase inhibitor, and 1% phenylmethanesulfonyl fluoride). The homogenates were incubated on ice for 30 min and the samples were centrifuged at 16,000 × g for 10 min at 4 °C. Then, 25 µl of whole-cell lysates were used for western blotting, and the remaining 95 µl of lysates were incubated at 95 °C for 10 min with 5 µl of 20% SDS. Subsequently, the cell lysates were used for immunoprecipitation with CDKN1A (CST) or IgG primary antibodies (Abcam, ab6715, USA), and the samples were incubated at 4 °C in a shaking incubator. After 5 h, protein-A/G mix beads (Thermo Scientific) were added and the samples were incubated at 4 °C overnight. Following this, the immunoprecipitates were collected and washed with lysis buffer three times and prepared for western blotting. Ubiquitination antibodies (Millipore, 05-1307, USA) were used for the detection of CDKN1A ubiquitination levels.
Statistical analysis
All experiments were performed in triplicate. The R 3.5.1, Perl 5.24.1, and the Statistical Package for the Social Sciences (SPSS) v.22.0 software packages (SPSS Inc., Chicago, USA) were used to perform statistical analyses, and the data were expressed as mean ± standard deviation (SD). The relationship between HJURP and clinicopathological parameters was analyzed using χ2 test or Fisher’s exact test, whenever appropriate. The correlations between HJURP and OS were assessed using the log-rank test for Kaplan–Meier methods, and the median IRS of HJURP was used as the cutoff point to separate the samples into high- and low-expression groups. The median RNA-Seq by RSEM of HJURP mRNA was the cutoff point dividing the patients from FireBrowse into high- and low-level groups when assessed the correlation between HJURP and BCR. OS data were analyzed using univariate analysis initially, and covariates with a P value ≤0.05 were enrolled in multivariate Cox regression analysis subsequently. The results of the CCK8 assay, colony-formation assay, EdU assay, cell-cycle and apoptosis flow cytometry, and animal experiments were evaluated with independent-sample t test. A P < 0.05 was considered to be statistically significant. * represents P < 0.05, ** represents P < 0.01, and *** represents P < 0.001.
Supplementary information
Acknowledgements
The authors acknowledge Qilong Wang and Chensi Zhao for technical assistance, and they are all experienced technicians from Key Laboratory of Gene Engineering of the Ministry of Education, School of Life Sciences, Sun Yat-sen University, Guangzhou, China.
Author contributions
W.X. and L.M. performed study concept and design; L.W., Z.W., and X.C. performed the experiments; L.W. and Z.W. wrote the manuscript; L.W., L.X., and H.Y. analyzed the data; W.Y. and Z.J. participated in the clinical specimen detection; L.Y. provided technical and material support. All authors have read and approved the final manuscript.
Funding information
This work was supported by the National Natural Science Foundation of China (82072820, 81874095), and the Guangdong Basic and Applied Basic Research Project Major Program of China (2019B1515120007).
Data availability
All data and materials generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
The related computerized programs for the analysis using R or Perl are exhibited in Supplementary Methods.
Ethics approval and consent to participate
The study of human tumor tissues was approved by the Ethics Review Committee of the Third Affiliated Hospital of Sun Yat-sen University (No. 2020-02-105). Written informed consents were obtained from all patients who participated. All animal experiments underwent the necessary ethical review (SYSU-IACUC-2020-000202) and were performed with strict adherence to the regulations of the Sun Yat-Sen University Animal Care and Use Committee. This study was performed in accordance with the Declaration of Helsinki.
Conflict of interest
The authors declare no competing interests.
Footnotes
Edited by S. Tait
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mingqiang Li, Email: limq567@mail.sysu.edu.cn.
Xingqiao Wen, Email: wenxq@mail.sysu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41419-021-03870-x.
References
- 1.Miller KD, et al. Cancer treatment and survivorship statistics. 2019. CA-Cancer J. Clin. 2019;69:363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 2.Kaczkowski B, et al. Transcriptome analysis of recurrently deregulated genes across multiple cancers identifies new pan-cancer biomarkers. Cancer Res. 2016;76:216–226. doi: 10.1158/0008-5472.CAN-15-0484. [DOI] [PubMed] [Google Scholar]
- 3.Weinstein JN, et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013;45:1113–1120. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunleavy EM, et al. HJURP is a cell-cycle-dependent maintenance and deposition factor of CENP-A at centromeres. Cell. 2009;137:485–497. doi: 10.1016/j.cell.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 5.Heo JI, Cho JH, Kim JR. HJURP regulates cellular senescence in human fibroblasts and endothelial cells via a p53-dependent pathway. J. Gerontol. Ser. A-Biol. Sci. Med. Sci. 2013;68:914–925. doi: 10.1093/gerona/gls257. [DOI] [PubMed] [Google Scholar]
- 6.Kato T, et al. Activation of Holliday junction recognizing protein involved in the chromosomal stability and immortality of cancer cells. Cancer Res. 2007;67:8544–8553. doi: 10.1158/0008-5472.CAN-07-1307. [DOI] [PubMed] [Google Scholar]
- 7.Cao R, et al. Silencing of HJURP induces dysregulation of cell cycle and ROS metabolism in bladder cancer cells via PPARγ-SIRT1 feedback loop. J. Cancer. 2017;8:2282–2295. doi: 10.7150/jca.19967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Li X, Meng Q, Khan AQ, Chen X. Increased expression of Holliday junction-recognizing protein (HJURP) as an independent prognostic biomarker in advanced-stage serous ovarian carcinoma. Med. Sci. Monit. 2018;24:3050–3055. doi: 10.12659/MSM.906647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montes de Oca R, et al. The histone chaperone HJURP is a new independent prognostic marker for luminal A breast carcinoma. Mol. Oncol. 2015;9:657–674. doi: 10.1016/j.molonc.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YF, et al. Upregulation of Holliday junction recognition protein predicts poor prognosis and biochemical recurrence in patients with prostate cancer. Oncol. Lett. 2019;18:6697–6703. doi: 10.3892/ol.2019.11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat. Appl. Genet. Mol. Biol. 2005;4:e17. doi: 10.2202/1544-6115.1128. [DOI] [PubMed] [Google Scholar]
- 12.Chen T, et al. HJURP promotes hepatocellular carcinoma proliferation by destabilizing p21 via the MAPK/ERK1/2 and AKT/GSK3β signaling pathways. J. Exp. Clin. Cancer Res. 2018;37:193. doi: 10.1186/s13046-018-0866-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat. Rev. Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Z, Hunter T. Ubiquitylation and proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2) CDK inhibitors. Cell Cycle. 2010;9:2342–2352. doi: 10.4161/cc.9.12.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, et al. Hippocalcin-like 1 suppresses hepatocellular carcinoma progression by promoting p21(Waf/Cip1) stabilization by activating the ERK1/2-MAPK pathway. Hepatology. 2016;63:880–897. doi: 10.1002/hep.28395. [DOI] [PubMed] [Google Scholar]
- 16.Lee JY, Yu SJ, Park YG, Kim J, Sohn J. Glycogen synthase kinase 3beta phosphorylates p21WAF1/CIP1 for proteasomal degradation after UV irradiation. Mol. Cell. Biol. 2007;27:3187–3198. doi: 10.1128/MCB.01461-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milano A, et al. Oxidative DNA damage and activation of c-Jun N-terminal kinase pathway in fibroblasts from patients with hereditary spastic paraplegia. Cell. Mol. Neurobiol. 2005;25:1245–1254. doi: 10.1007/s10571-005-8501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Dowbenko D, Lasky LA. AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J. Biol. Chem. 2002;277:11352–11361. doi: 10.1074/jbc.M109062200. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, et al. Glycogen synthase kinase 3beta is a negative regulator of growth factor-induced activation of the c-Jun N-terminal kinase. J. Biol. Chem. 2004;279:51075–51081. doi: 10.1074/jbc.M408607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abell AN, Granger DA, Johnson GL. MEKK4 stimulation of p38 and JNK activity is negatively regulated by GSK3beta. J. Biol. Chem. 2007;282:30476–30484. doi: 10.1074/jbc.M705783200. [DOI] [PubMed] [Google Scholar]
- 21.Chikano Y, et al. Glycogen synthase kinase 3β sustains invasion of glioblastoma via the focal adhesion kinase, Rac1, and c-Jun N-terminal kinase-mediated pathway. Mol. Cancer Ther. 2015;14:564–574. doi: 10.1158/1535-7163.MCT-14-0479. [DOI] [PubMed] [Google Scholar]
- 22.Karimian A, Ahmadi Y, Yousefi B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair. 2016;42:63–71. doi: 10.1016/j.dnarep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Child ES, Mann DJ. The intricacies of p21 phosphorylation: protein/protein interactions, subcellular localization and stability. Cell Cycle. 2006;5:1313–1319. doi: 10.4161/cc.5.12.2863. [DOI] [PubMed] [Google Scholar]
- 24.Rössig L, Badorff C, Holzmann Y, Zeiher AM, Dimmeler S. Glycogen synthase kinase-3 couples AKT-dependent signaling to the regulation of p21Cip1 degradation. J. Biol. Chem. 2002;277:9684–9689. doi: 10.1074/jbc.M106157200. [DOI] [PubMed] [Google Scholar]
- 25.Park MH, et al. Parkin knockout inhibits neuronal development via regulation of proteasomal degradation of p21. Theranostics. 2017;7:2033–2045. doi: 10.7150/thno.19824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen T, et al. HJURP promotes epithelial-to-mesenchymal transition via upregulating SPHK1 in hepatocellular carcinoma. Int. J. Biol. Sci. 2019;15:1139–1147. doi: 10.7150/ijbs.30904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang CJ, et al. Holliday junction recognition protein promotes pancreatic cancer growth and metastasis via modulation of the MDM2/p53 signaling. Cell Death Dis. 2020;11:386. doi: 10.1038/s41419-020-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang DH, et al. Prognostic relevance of HJURP expression in patients with surgically resected colorectal cancer. Int. J. Mol. Sci. 2020;21:7928. doi: 10.3390/ijms21217928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Tayrac M, et al. Prognostic significance of EDN/RB, HJURP, p60/CAF-1 and PDLI4, four new markers in high-grade gliomas. PLoS ONE. 2013;8:e73332. doi: 10.1371/journal.pone.0073332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, et al. Hyperglycaemia-induced miR-301a promotes cell proliferation by repressing p21 and Smad4 in prostate cancer. Cancer Lett. 2018;418:211–220. doi: 10.1016/j.canlet.2018.01.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
The related computerized programs for the analysis using R or Perl are exhibited in Supplementary Methods.