Abstract
Background and aims:
Fecal microbiota transplantation (FMT) is a commonly used therapy for multiply recurrent Clostridioides difficile (mrCDI). By altering the gut microbiota, there is the potential for FMT to impact the risk for cardiometabolic, intestinal or immune-mediated conditions. Likewise, the microbiota disturbance associated with mrCDI could potentially lead to these conditions. We aimed to assess the associations of mrCDI and FMT with cardiometabolic, immune-mediated diseases, and irritable bowel syndrome.
Methods:
This retrospective cohort study using a United States commercial claims database included persons diagnosed with CDI or undergoing FMT. We created two pairwise comparisons: mrCDI versus non-mrCDI, and non-mrCDI or mrCDI treated with FMT vs. mrCDI without FMT.
Results:
We found no significant association between mrCDI (vs. non-mrCDI) and inflammatory bowel disease (adjusted hazard ratio (aHR)=1.65; 95% confidence interval, 0.67-4.04), rheumatoid arthritis (HR=0.86; 0.47-1.56), psoriasis (HR=0.72; 0.23-2.27), diabetes (aHR=0.97; 0.67-1.40), hypertension (aHR=1.05; 0.76-1.44), myocardial infarction (aHR=0.82; 0.63-1.06), stroke (aHR=0.83; 0.62-1.12), or irritable bowel syndrome (HR=0.94; 0.61-1.45). Similarly, we found no association of CDI with FMT (vs. mrCDI without FMT) and diabetes (aHR=0.92; 0.27-3.11), hypertension (aHR=1.41; 0.64-3.15), stroke (aHR=1.27; 0.69-2.34) or inflammatory bowel syndrome (aHR=0.80; 0.26-2.46). However, the incidence of myocardial infarction was increased following FMT (aHR=1.68; 1.01-2.81).
Conclusion:
Relative to those with CDI, persons with mrCDI do not appear to be intrinsically at higher risk of cardiometabolic, immune-mediated diseases, or irritable bowel syndrome.
However, those who underwent FMT for CDI had a higher incidence of myocardial infarction. Future studies should assess this association to assess reproducibility.
Keywords: Clostridioides difficile, fecal microbiota transplantation, irritable bowel syndrome, diabetes, hypertension, myocardial infarction, stroke, inflammatory bowel disease, rheumatoid arthritis, psoriasis
INTRODUCTION
Clostridioides difficile infection (CDI) is an increasingly common nosocomial and community-acquired infection.1 The incidence of multiply recurrent CDI (mrCDI) has increased disproportionately to that of CDI that resolves with one or two courses of antibiotics.1 Fecal microbiota transplantation is increasingly used to treat of mrCDI.2 While extremely efficacious, the long-term safety of FMT is unknown.2
Alterations of the composition of the human gut microbiota, either as a consequence of disease or secondary to interventions such as FMT, may have significant effects on cardiometabolic processes, digestive diseases and immune function. Variation in the composition of the gut microbiota associated with a disease state, “dysbiosis”, has been reported among patients with obesity,3, 4 diabetes mellitus,4 cardiovascular diseases,5 inflammatory arthritis,6, 7 inflammatory bowel diseases8, 9 and irritable bowel syndrome.10 FMT has shown potential therapeutic benefit in the treatment of some of these conditions.11-13
To the extent that FMT can improve conditions associated with dysbiosis, there exists the corollary hypothesis that FMT could induce these same conditions. Moreover, to the extent that such changes are associated with FMT, it is important to establish whether the underlying indication for FMT (i.e. CDI) or the FMT procedure is more likely to be associated with the development of these chronic conditions. We conducted this cohort study to assess whether mrCDI and FMT are associated with metabolic disorders, cardiovascular disease, immune-mediated conditions, and irritable bowel syndrome.
METHODS
Data source and overall design
We performed two retrospective cohort studies using Optum’s de-identified Clinformatics® Data Mart Database. The data provide information on inpatient and outpatient diagnoses and procedure claims, prescription pharmacy claims, demographics, and dates of health plans enrollment. The first cohort study compared outcomes following mrCDI versus non-mrCDI. The second cohort study compared outcomes following mrCDI without FMT treatment versus CDI treated with FMT. These studies used data from May 2000 to June 2019. At the University of Pennsylvania, studies using Optum’s Clinformatics Database are categorized as exempt from requiring Institutional Review Board approval.
Inclusion and exclusion criteria
We included persons who had a diagnosis of CDI or procedure for CDI testing and had at least 12 months of continuous enrollment in medical and pharmacy benefits (i.e., lookback period) prior to their first CDI diagnosis. International Classification of Diseases (ICD) diagnosis codes of CDI have been validated previously and were found to have a sensitivity= 78%-81% and specificity= 99%.14 We excluded persons who: 1) had a prescription of metronidazole, oral vancomycin, or oral fidaxomicin prior to first CDI diagnosis or 2) had missing values for location of residence, sex, or birth year. For the cohort study comparing mrCDI versus non-mrCDI, persons with FMT prior to the first CDI diagnosis were excluded. We excluded those with the outcome of interest or a prescription for a drug that is used to treat each outcome during the lookback period. For example, the outcome model for diabetes excluded patients with a prior diabetes diagnosis but included those with prior diagnoses of the other outcomes of interest. Figure 1 provides additional details of the creation of the cohorts for studies 1 and 2.
Figure 1.
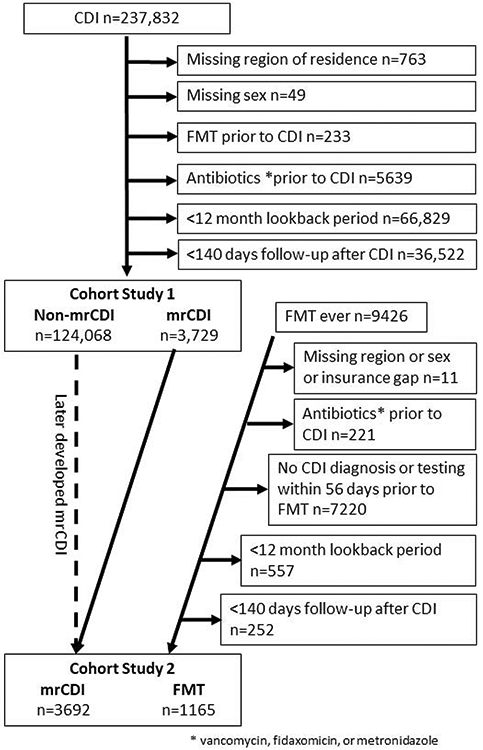
Cohorts included in study 1 and study 2
Exposure Ascertainment
A). CDI definition
Persons were considered to have CDI when any of the following criteria were met: 1) ICD-9-CM and ICD-10-CM code indicative of CDI presenting on inpatient claims with at least one prescription of CDI antibiotics (i.e., oral or intravenous metronidazole, oral vancomycin, or oral fidaxomicin) dispensed within 3 days following hospital discharge; 2) an inpatient claim indicative of CDI with a length of hospital stay of ≥ 10 days; 3) current procedural terminology (CPT) codes indicative of testing for C. difficile and at least one prescription of CDI antibiotics dispensed within 10 days following testing; and 4) ICD-9-CM or ICD-10-CM code for CDI presenting on an outpatient claims (any position) with at least one prescription of CDI antibiotics dispensed within 10 days following the date of CDI diagnosis.1
B). mrCDI definition
Persons were considered to have mrCDI if they: 1) met any of the CDI definitions listed above; 2) received a total of 3 courses of CDI antibiotics with each subsequent course starting at least 14 days and no more than 56 days after the start of the prior antibiotic course; and 3) received at least one prescription for vancomycin or fidaxomicin.1 When there were multiple antibiotics prescriptions within 14 days of each other, to be considered a new course of antibiotics required at least 14 days separating the end of one prescription and the start of the next. The date of the initial CDI episode was considered the date of diagnosis of mrCDI.
C). FMT definition
Persons were considered to have received FMT based on the presence of CPT codes indicative of FMT (Supplemental Table S1). Persons were also required to have diagnosis or testing for CDI during the 56 days prior to FMT, but the CDI diagnosis did not need to meet the administrative claims definition of mrCDI prior to FMT.
Follow-up
For the study comparing mrCDI versus non-mrCDI, follow-up started 140 days (index-date) after the first diagnosis of CDI that led to the diagnosis of mrCDI for those in the mrCDI group or the first CDI diagnosis for those with non-mrCDI, respectively. The 140-day window allows for up to 14 days each for two courses of antibiotics and two 56 day intervals between courses of antibiotics. Follow-up ended at the earliest occurrence of: 1) an outcome of interest; 2) FMT; 3) disenrollment; or 4) end of study period.
For the study comparing CDI treated with FMT versus mrCDI without FMT, the index date was 140 days after the first diagnosis or testing for CDI prior to FMT or 140 days after the first diagnosis or testing of CDI that led to the diagnosis of mrCDI. FMT was treated as a unidirectional time-varying exposure such that a person could contribute follow-up time to the mrCDI group until they underwent FMT. From that point forward, their follow-up time was contributed to the FMT group. Persons were followed from the index-date until the occurrence of the first of the following: 1) an outcome of interest; 2) disenrollment; or 3) end of study period.
Outcomes of Interest
The primary study outcomes were a new diagnosis of inflammatory bowel diseases, rheumatoid arthritis, psoriasis, diabetes, hypertension, myocardial infarction, stroke and irritable bowel syndrome. A new diagnosis entailed no diagnosis or treatment for the outcome prior to the index date. Outcomes were based on previously validated algorithms using ICD-9-CM and ICD-10-CM codes. See supplemental methods for additional details. List of ICD-9-CM and ICD-10-CM codes and additional lookback period requirements are listed in the Supplemental Table S2.
Covariates
We ascertained baseline covariates during the lookback period preceding the first CDI diagnosis. We included data on demographics (age, sex, census region), diagnosis location (inpatient, outpatient or nursing home), antibiotics use within 90 days preceding CDI diagnosis, antibiotics use within 90 days after CDI diagnosis, proton pump inhibitors (PPIs) use within 90 days preceding CDI diagnosis, PPIs use within 90 days after CDI diagnosis, corticosteroids use within 90 days preceding CDI diagnosis, corticosteroids use within 90 days after CDI diagnosis, calendar year, Charlson comorbidity score, and measures of healthcare utilization (e.g., total number of inpatient visits, total number of outpatient visits). For the myocardial infarction outcome model only, we adjusted for prior history of coronary artery bypass graft surgery (CABG), percutaneous coronary intervention (PCI) or prescription of an antiplatelet drug other than aspirin (e.g. clopidogrel).
Statistical analyses
Separate Cox proportional hazard regression models were developed for each outcome for non-mrCDI (referent) vs. mrCDI and mrCDI (referent) vs CDI followed by FMT. For each model, we examined potential confounders one at a time and included in the final models only those that resulted in a 10% or greater change in the unadjusted hazard ratio (HR). For the FMT vs. without FMT comparison, we were not able to run the adjusted models for inflammatory bowel disease, rheumatoid arthritis and psoriasis due the small number of events (n< 20). Given that FMT was relatively uncommon prior to 2010, we conducted a sensitivity analysis that limited the cohort to those with an index date of January 1, 2010 or later.
Results
Cohort characteristics
a). mrCDI versus non-mrCDI
A total of 127,797 persons met the inclusion criteria for the first cohort study (n= 3,729 for mrCDI and n= 124,068 for non-mrCDI). Depending on the outcome under study, the range of average follow-up time was 1.99-2.39 years for the mrCDI group and 2.13-2.47 years for the non-mrCDI group. Compared to persons in the non-mrCDI groups, those with mrCDI were older (27% vs. 24% were 70- 79 years old), more likely to be female (65% vs. 61%), and more likely in the 90 days prior to CDI diagnosis to use antibiotics (64% vs. 54%) or corticosteroids (17% vs. 15%), and to have a Charlson comorbidity score ≥ 2 (76% vs. 72%) (Table 1).
Table 1.
Demographics and clinical characteristics in the non-multiply recurrent Clostridioides difficile (non-mrCDI) vs. mrCDI group
| Characteristic, % | Non-mrCDI (N=124,068) |
mrCDI (N=3,729) |
P-value |
|---|---|---|---|
| Age group | |||
| < 10 | 1.5 | 0.5 | <.0001 |
| 10-19 | 2.4 | 1.6 | <.0001 |
| 20-29 | 4.0 | 2.9 | <.0001 |
| 30-39 | 7.0 | 5.0 | <.0001 |
| 40-49 | 10.3 | 7.2 | <.0001 |
| 50-59 | 15.2 | 13.4 | <.0001 |
| 60-69 | 18.2 | 18.3 | <.0001 |
| 70-79 | 23.6 | 27.3 | 0.0007 |
| 80+ | 17.6 | 23.9 | |
| Female | 61.2 | 65.0 | <.0001 |
| Census level Division based on US State | |||
| EAST NORTH CENTRAL | 15.2 | 18.2 | 0.8686 |
| EAST SOUTH CENTRAL | 3.9 | 2.7 | <.0001 |
| MIDDLE ATLANTIC | 6.5 | 6.6 | 0.042 |
| MOUNTAIN | 9.7 | 11.3 | 0.7914 |
| NEW ENGLAND | 4.2 | 5.8 | 0.0737 |
| PACIFIC | 10.7 | 12.7 | |
| SOUTH ATLANTIC | 25.7 | 20.2 | <.0001 |
| WEST NORTH CENTRAL | 12.2 | 15.6 | 0.2474 |
| WEST SOUTH CENTRAL | 11.9 | 7.1 | <.0001 |
| Diagnosis location | |||
| Inpatient | 40.7 | 36.7 | |
| Outpatient | 56.4 | 58.1 | 0.0001 |
| Nursing home | 2.9 | 5.2 | <.0001 |
| First antibiotic used to treat CDI | |||
| Fidaxomicin | 0.3 | 1.0 | <.0001 |
| Metronidazole | 92.1 | 73.0 | |
| Vancomycin | 7.6 | 26.0 | <.0001 |
| Antibiotic use within 90 days prior to CDI diagnosis | 54.1 | 63.6 | <.0001 |
| Antibiotic use within 90 days after to CDI diagnosis | 41.7 | 39.9 | 0.029 |
| PPI use within 90 days prior to CDI diagnosis | 21.0 | 21.4 | 0.5423 |
| PPI use within 90 days after to CDI diagnosis | 23.3 | 24.2 | 0.2096 |
| Corticosteroid use within 90 days prior to CDI diagnosis | 14.6 | 16.9 | 0.0001 |
| Corticosteroid use within 90 days after to CDI diagnosis | 13.7 | 14.3 | 0.2409 |
| Antiplatelet Rx during baseline | 8.3 | 7.1 | 0.0079 |
| CABG/PCI during baseline | 4.1 | 3.7 | 0.1332 |
| Charlson score | |||
| 0 | 23.1 | 19.1 | |
| 1 | 4.8 | 5.0 | 0.007 |
| 2+ | 72.1 | 75.9 | <.0001 |
| Calendar Year (per 1-year increase) | |||
| 2001 | 1.1 | 1.0 | <.0001 |
| 2002 | 2.2 | 1.3 | |
| 2003 | 2.4 | 1.5 | |
| 2004 | 2.6 | 2.0 | |
| 2005 | 3.2 | 2.3 | |
| 2006 | 3.9 | 4.2 | |
| 2007 | 4.3 | 4.6 | |
| 2008 | 5.1 | 5.7 | |
| 2009 | 5.3 | 5.3 | |
| 2010 | 5.7 | 5.3 | |
| 2011 | 6.3 | 5.9 | |
| 2012 | 6.9 | 7.2 | |
| 2013 | 7.1 | 6.6 | |
| 2014 | 6.7 | 5.9 | |
| 2015 | 7.6 | 7.6 | |
| 2016 | 8.3 | 8.2 | |
| 2017 | 9.7 | 11.4 | |
| 2018 | 10.4 | 12.3 | |
| 2019 | 1.1 | 1.8 | |
| Prevalent diseases ǁ | |||
| Inflammatory bowel disease | 12.6 | 14.6 | 0.0002 |
| Rheumatoid arthritis | 11.4 | 11.4 | 0.8994 |
| Psoriasis | 43.6 | 46.4 | 0.0009 |
| Diabetes mellitus | 36.8 | 35.6 | 0.1214 |
| Hypertension | 75.1 | 79.8 | <.0001 |
| Myocardial infarction | 10.7 | 12.0 | 0.0132 |
| Stroke | 18.0 | 19.9 | 0.0038 |
| Inflammatory bowel syndrome | 30.0 | 30.6 | 0.4241 |
| CVD during baseline | 30.8 | 27.1 | <.0001 |
| Baseline healthcare utilization, mean | |||
| # Inpatient visits during baseline | 2.0 | 1.6 | <.0001 |
| # Inpatient visits during baseline collapsed | 1.3 | 1.1 | <.0001 |
| # Outpatient visits during baseline | 28.6 | 25.8 | <.0001 |
| # Ambulatory care visits during baseline | 24.2 | 21.8 | <.0001 |
Coronary artery bypass grafting, CABG, Clostridium difficile, CDI, confidence interval, CI, multiply recurrent Clostridium difficile, cardiovascular disease, CVD, mrCDI, non-multiply recurrent Clostridium difficile, non-mrCDI, odds ratio, OR, percutaneous coronary intervention, PCI, proton pump inhibitors, PPI
those persons were excluded from each corresponding outcome model
b). mrCDI without FMT versus CDI treated with FMT
A total of 4,857 persons met the inclusion criteria for the second cohort study (n= 3,692 for mrCDI without FMT and n= 1,165 for CDI treated with FMT). In the FMT cohort, 92% of patients had an insurance claim for CDI diagnosis in the 56 days prior to FMT and the remainder had CDI testing but did not have an ICD-9-CM or ICD-10-CM code for CDI diagnosis. Depending on the outcome under study, the range of average follow-up time was 1.96-2.34 years for the mrCDI group and 1.59-1.80 years for the CDI treated with FMT group. Compared to the mrCDI group, those with CDI treated with FMT were slightly younger (11% vs. 13% were 50- 59 years), and less likely within the 90 days prior to CDI diagnosis to use antibiotics (29% vs. 64%), PPIs (16% vs. 22%), and corticosteroids (14% vs. 17%). However, those with CDI treated with FMT were more likely to have a Charlson comorbidity score ≥ 2 (83% vs. 76%) (Table 2).
Table 2.
Demographics and clinical characteristics in the multiply recurrent Clostridioides difficile (mrCDI) group without fecal microbiota transplantation (FMT) vs. CDI treated with FMT
| Characteristic, % | Without FMT (N=3,692) |
With FMT (N=1,165) |
P-value |
|---|---|---|---|
| Age group | |||
| < 10 | 0.5 | 1.0 | 0.0836 |
| 10-19 | 1.6 | 1.6 | 0.648 |
| 20-29 | 2.9 | 3.1 | 0.7278 |
| 30-39 | 4.9 | 4.7 | 0.2762 |
| 40-49 | 7.3 | 6.8 | 0.1258 |
| 50-59 | 13.4 | 11.0 | 0.0036 |
| 60-69 | 18.3 | 17.1 | 0.0408 |
| 70-79 | 27.4 | 27.3 | 0.1028 |
| 80+ | 23.7 | 27.4 | |
| Female | 64.7 | 64.0 | 0.6747 |
| Census level Division based on US State | |||
| East North Central | 18.1 | 23.0 | <.0001 |
| East South Central | 2.7 | 3.4 | 0.0154 |
| Middle Atlantic | 6.6 | 6.7 | 0.103 |
| Mountain | 11.0 | 13.9 | 0.0004 |
| New England | 5.9 | 4.8 | 0.7157 |
| Pacific | 12.7 | 9.8 | |
| South Atlantic | 20.6 | 16.3 | 0.8193 |
| West North Central | 15.4 | 14.8 | 0.1027 |
| West South Central | 7.1 | 7.3 | 0.074 |
| Diagnosis location | |||
| Inpatient | 36.8 | 15.5 | |
| Outpatient | 57.9 | 76.5 | <.0001 |
| Nursing home | 5.3 | 0.2 | 0.0003 |
| First antibiotic used to treat CDI | |||
| Fidaxomicin | 1.1 | 2.5 | 0.0019 |
| Metronidazole | 72.8 | 75.0 | |
| Vancomycin | 26.1 | 22.5 | 0.0428 |
| Antibiotic use within 90 days prior to CDI diagnosis | 63.5 | 29.0 | <.0001 |
| Antibiotic use within 90 days after to CDI diagnosis | 40.2 | 19.2 | <.0001 |
| PPI use within 90 days prior to CDI diagnosis | 21.7 | 15.8 | <.0001 |
| PPI use within 90 days after to CDI diagnosis | 24.5 | 15.7 | <.0001 |
| Corticosteroid use within 90 days prior to CDI diagnosis | 16.8 | 14.3 | 0.0474 |
| Corticosteroid use within 90 days after to CDI diagnosis | 14.4 | 13.9 | 0.6871 |
| Antiplatelet Rx during baseline | 6.1 | 8.4 | 0.0121 |
| CABG/PCI during baseline | 5.2 | 4.3 | 0.1581 |
| Charlson score | |||
| 0 | 19.0 | 13.2 | |
| 1 | 4.8 | 3.4 | 0.9028 |
| 2+ | 76.2 | 83.3 | <.0001 |
| Calendar Year (per 1-year increase) | |||
| 2001 | 1.0 | 0.3 | <.0001 |
| 2002 | 1.4 | 0.7 | |
| 2003 | 1.6 | 0.5 | |
| 2004 | 2.1 | 0.2 | |
| 2005 | 2.4 | 0.3 | |
| 2006 | 4.3 | 0.3 | |
| 2007 | 4.7 | 0.6 | |
| 2008 | 6.0 | 0.4 | |
| 2009 | 5.4 | 0.5 | |
| 2010 | 5.5 | 0.4 | |
| 2011 | 5.9 | 0.7 | |
| 2012 | 7.3 | 2.4 | |
| 2013 | 6.5 | 6.2 | |
| 2014 | 5.9 | 7.6 | |
| 2015 | 7.6 | 14.2 | |
| 2016 | 7.8 | 18.5 | |
| 2017 | 11.1 | 20.3 | |
| 2018 | 12.0 | 22.6 | |
| 2019 | 1.8 | 3.3 | |
| Prevalent diseases ǁ | |||
| Inflammatory bowel disease | 14.7 | 24.4 | <.0001 |
| Rheumatoid arthritis | 11.2 | 15.4 | 0.0002 |
| Psoriasis | 46.3 | 46.0 | 0.8797 |
| Diabetes mellitus | 35.9 | 33.8 | 0.198 |
| Hypertension | 80.0 | 80.9 | 0.4855 |
| Myocardial infarction | 12.3 | 11.5 | 0.4835 |
| Stroke | 20.1 | 19.8 | 0.8262 |
| Inflammatory bowel syndrome | 30.6 | 47.0 | <.0001 |
| CVD during baseline | 35.5 | 31.0 | 0.0050 |
| Baseline healthcare utilization, mean | |||
| # Inpatient visits during baseline | 3.5 | 2.0 | <.0001 |
| # Inpatient visits during baseline collapsed | 2.2 | 1.3 | <.0001 |
| # Outpatient visits during baseline | 40.6 | 29.0 | <.0001 |
| # Ambulatory care visits during baseline | 34.2 | 24.4 | <.0001 |
Coronary artery bypass grafting, CABG, Clostridium difficile, CDI, confidence interval, CI, cardiovascular disease, CVD, fecal microbiota transplantation, FMT, odds ratio, OR, percutaneous coronary intervention, PCI, proton pump inhibitors, PPI
those persons were excluded from each corresponding outcome model
Relative risk of inflammatory bowel disease, rheumatoid arthritis and psoriasis with mrCDI and FMT
Among patients with mrCDI and non-mrCDI, respectively, the incidence rate per 1000py (IR) and 95% confidence interval (CI) for inflammatory bowel disease was 0.7 (0.2-1.6) vs. 0.5 (0.4-0.6), for rheumatoid arthritis 1.4 (0.7-2.6) vs. 1.7 (1.5-1.8) and for psoriasis 0.8 (0.2- 2.2) vs. 1.0 (0.9-1.2) (Supplemental Table 3). After adjustment, we did not identify an association between mrCDI (vs. non-mrCDI) and inflammatory bowel disease (adjusted hazard ratio (aHR), 1.65 (0.67-4.04), rheumatoid arthritis (HR, 0.86; 0.47-1.56), or psoriasis (HR, 0.72; 0.23- 2.27) (Table 3).
Table 3.
Unadjusted and adjusted estimates of each outcome in the mrCDI vs. non-mrCDI groups
| Outcome | Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
Model adjusted for the listed variables |
|---|---|---|---|
| Inflammatory bowel disease | 1.41 (0.58-3.45) | 1.65 (0.67-4.04) | age |
| Rheumatoid arthritis | 0.86 (0.47-1.56) | Π | no covariates changed HR by 10% or more |
| Psoriasis | 0.72 (0.23-2.27) | Π | no covariates changed HR by 10% or more |
| Diabetes mellitus | 0.88 (0.61-1.27) | 0.97 (0.67-1.40) | calendar year |
| Hypertension | 1.26 (0.91-1.74) | 1.05 (0.76-1.44) | age |
| Myocardial infarction | 0.95 (0.74-1.23) | 0.82 (0.63-1.06) | age |
| Stroke | 0.98 (0.73-1.32) | 0.83 (0.62-1.12) | age |
| Irritable bowel syndrome | 0.94 (0.61-1.45) | Π | no covariates changed HR by 10% or more |
Confidence interval, CI, hazard ratio, HR
no further adjusted was made as none of the covariates changed the hazard ratio by ≥ 10%
Among patients with CDI treated with FMT and mrCDI without FMT, respectively, the IR (per 1000py) for inflammatory bowel disease was 1.3 (0.2- 4.7) vs. 0.7 (0.2-1.6), rheumatoid arthritis 2.8 (0.9- 6.6) vs. 1.4 (0.7-2.6), and psoriasis 2.0 (0.2-7.2) vs. 0.8 (0.2- 2.2) (Supplemental Table 4). Unadjusted and adjusted HRs are summarized in Table 4. We did not identify associations between FMT and these outcomes.
Table 4.
Unadjusted and adjusted estimates of each outcome in CDI treated with FMT vs. mrCDI without FMT
| Outcome | Unadjusted HR (95% CI) |
Adjusted HR (95% CI) |
Model adjusted for the listed variables |
|---|---|---|---|
| Inflammatory bowel disease | 1.60 (0.31-8.27) | ǁ | |
| Rheumatoid arthritis | 2.20 (0.75-6.50) | ǁ | |
| Psoriasis | 2.49 (0.41-15.03) | ǁ | |
| Diabetes mellitus | 0.39 (0.12-1.30) | 0.78 (0.23-2.71) | inpatient CDI diagnosis, calendar year, number of inpatient admissions |
| Hypertension | 1.08 (0.52-2.24) | 1.41 (0.64-3.15) | antibiotic use 90 days before , antibiotic use 90 days after, Charlson score, calendar year |
| Myocardial infarction | 1.78 (1.10-2.88) | 1.68 (1.01-2.81) | inpatient CDI diagnosis, Charlson score, number of inpatient admissions |
| Stroke | 1.27 (0.70-2.29) | 1.05 (0.60-1.96) | age, inpatient CDI diagnosis, number of inpatient admissions |
| Irritable bowel syndrome | 0.96 (0.33-2.83) | 0.85 (0.27-2.68) | age, antibiotic use 90 days before , antibiotic use 90 days after, Charlson score, number of inpatient admissions, number of ambulatory care visits |
Confidence interval, CI, hazard ratio, HR
Not calculated due to the small number of event
CDI C difficile infection
Relative risk of diabetes and cardiovascular diseases with mrCDI and FMT
Among patients with mrCDI and non-mrCDI, respectively, the IR (per 1000py) of diabetes mellitus was 5.2 (3.5-7.5) vs. 5.9 (5.6-6.2), hypertension 22.1 (15.6-30.3) vs. 17.4 (16.5-18.4), myocardial infarction 7.5 (5.7-9.7) vs. 7.9 (7.6- 8.2), and stroke 6.3 (4.5-8.4) vs. 6.4 (6.1-6.7) (Supplemental Table 3). After adjustment, we found no association between mrCDI (vs. non-mrCDI) and diabetes (aHR, 0.97; 0.67-1.40), hypertension (aHR, 1.05; 0.76-1.44), myocardial infarction (aHR, 0.82; 0.63-1.06) or stroke (aHR, 0.83; 0.62- 1.12) (Table 3).
Among patients with CDI treated with FMT and mrCDI without FMT, respectively, the IR (per 1000py) of diabetes was 2.3 (0.5-6.6) vs. 5.5 (3.7-7.8), hypertension 23.3 (10.6-44.2) vs. 21.9 (15.4-30.2), myocardial infarction 13.3 (8.5-19.7) vs. 7.6 (5.8-9.8) and stroke 9.0 (5.0-14.9) vs. 6.3 (4.6-8.4) (Supplemental Table 4). After adjustment, there was no association between FMT (vs. without FMT) and diabetes (aHR, 0.92; 0.27- 3.11), hypertension (aHR, 1.41; 0.64-3.15), or stroke (aHR, 1.27; 0.69-2.34). A possible increased incidence of myocardial infarction was seen among persons receiving FMT when compared with those with mrCDI who did not undergo FMT (aHR, 1.68; 1.01-2.81) (Table 4). The median time (interquartile range) from FMT to myocardial infarction was 585.5 (276.5 – 1127) days. The shortest time from FMT to myocardial infarction was 146 days, thus none occurred in close proximity to the FMT procedure.
Relative risk of irritable bowel syndrome with mrCDI and FMT
The IRs (per 1000py) for irritable bowel syndrome were 3.9 (2.4-5.9) vs. 4.1 (3.8-4.4) for mrCDI and non-mrCDI, respectively (Supplemental Table 3). The unadjusted HR was 0.94 (0.61-1.45). No covariates were identified as confounders of the association between mrCDI and irritable bowel syndrome (Table 3).
The IRs (per 1000py) for irritable bowel syndrome were 4.0 (1.1-10.2) vs. 3.8 (2.3-5.7) for mrCDI with FMT and without FMT, respectively (Supplemental Table 4). After adjustment, we found no association between FMT (vs. without FMT) and irritable bowel syndrome (aHR, 0.80; 0.26-2.46) (Table 4).
Sensitivity analysis limited to the period beginning on January 1, 2010
The sensitivity analyses included 2,690 with mrCDI vs. 86,733 with non-mrCDI and 1,119 with FMT vs. 2,633 without FMT. The results were similar to the primary analyses. See Supplemental Tables 5 for all results of the sensitivity analyses.
Discussion
While antibiotics are effective in many patients with CDI, the rising incidence of mrCDI has led to the need for novel therapeutic strategies. FMT has become a standard therapeutic approach for patients with mrCDI and has been demonstrated to be highly efficacious in randomized controlled trials.15 Given the link between the gut microbiota and multiple health conditions, there is the theoretical potential for alteration of the gut microbiota via FMT to influence the risk for cardiometabolic, immune-mediated or other adverse events. In this large cohort study, we demonstrated no increased risk of a variety of immune-mediated diseases, irritable bowel syndrome, diabetes, hypertension, stroke or myocardial infarction among patients with mrCDI compared to those with simple CDI that required only one or two courses of antibiotics. Similarly, no increased incidence of rheumatoid arthritis, inflammatory bowel disease, irritable bowel syndrome, hypertension, diabetes mellitus, or stroke was observed among patients receiving FMT. However, there was a possible small increased incidence of acute myocardial infarction following FMT (aHR, 1.68; 1.01-2.81).
Although FMT has appeared generally safe in clinical trials,15 larger observational studies are required to assess the relative risk of serious adverse events that are less common and/or require a long duration after FMT to occur. This cohort study included more than 1000 FMT recipients and more than 3000 patients with mrCDI with average follow-up time of approximately 2 years, which is substantially larger than any prior clinical trial or even pooled analyses of clinical trials of FMT for mrCDI.15 Despite this, we may still have been underpowered to exclude some of the associations explored in this study. Perhaps by chance or perhaps reflecting insufficient statistical power, in the analysis of FMT vs. mrCDI, the hazard ratio for most of the outcomes assessed was greater than 1, but with confidence intervals crossing unity. The exceptions were diabetes mellitus and irritable bowel syndrome, both being conditions where some randomized controlled trials of FMT have shown a benefit.12, 16
A theoretical increased risk of myocardial infarction among patients who received FMT could be due to alteration of the gut microbiota. Trimethylamine (TMA) is derived from food by the gut microbiota. After absorption, it is converted to trimethylamine-N-oxide (TMAO).17 Higher plasma levels of TMAO are associated with an increased risk of cardiovascular disease.18 Alternatively, most FMT in the U.S. is administered via colonoscopy, a procedure with known risks of inducing cardiac ischemia or arrhythmias.19 Notably, the minimum time from FMT to myocardial infarction was 146 days, suggesting that colonoscopy was unlikely to contribute to the observed HR in this study.
The primary indication for FMT is mrCDI. If patients with mrCDI were intrinsically at increased risk for the outcomes of interest (i.e., confounding by indication), the results of the comparisons between those undergoing FMT and those with mrCDI without FMT could be biased. We minimized this risk by assuring that all FMT patients had a diagnosis of CDI (92%) or testing for CDI (8%) prior the procedure. However, we did not require that the full mrCDI administrative definition be met prior to FMT since nearly all FMT in the United States is for CDI. To assess whether confounding by indication was likely, we also compared the incidence of the outcomes of interest among patients with non-mrCDI and mrCDI. Notably, no associations were observed suggesting that confounding by indication is unlikely to explain the observed results for FMT. The major strengths of this study include the largest sample size to date to study the safety of FMT, the heterogeneous population included in the study and the analyses to assess for confounding by indication. The cohort had relatively long follow-up time after CDI and FMT. However, it is possible that for some of the outcomes even longer follow-up time may be needed to see an association with mrCDI or CDI treated with FMT. One limitation is the potential for residual confounding by unmeasured variables, including features of the donors. We lacked data on smoking and obesity that are associated with several of our outcomes under study. The incidence of CDI appears to be independent of obesity,20-22 but potentially linked to smoking.23, 24 In one cohort study, current and former smokers were less likely to be cured within 14 days, but did not have a higher rate of 30-day readmission, CDI recurrence, death before treatment completion, or CDI severity 48-hours after diagnosis.25 Thus, it seems relatively unlikely that obesity or smoking are strong confounders of the associations examined in this study. We were also limited in our ability to adjust for confounders due to small numbers of events so we individually tested for variables that could be confounders based on change in the hazard ratio by 10% or more in adjusted models. Fortunately, few variables met this criterion. Another potential limitation is the reliability of the diagnosis and procedure codes to identify mrCDI, FMT and the outcomes of interest. Medication exposure misclassification is possible as data in the current study reflect dispensed medications but it is unknown if the patient took the medication. We added additional restrictions (e.g. requirement of initiation of CDI antibiotics) to minimize the potential of misclassifying CDI events. Given the lack of a validated algorithm of mrCDI, we developed our own algorithm. This case definition was derived from a prior study in Veterans which suggested that the mean relapse time between an incident and mrCDI is 14 and not more than 43-60 days.26 Where possible, we used validated algorithms to identify the outcomes of interest. Any misclassification of the outcomes was likely non-differential and as such would result in bias toward the null. As such, it is possible that we have under-estimated the association of FMT with some of the outcomes of interest. We are not aware of a validated algorithm for FMT. We relied on billing codes to identify FMT but it is possible that our algorithm has less than 100% sensitivity. To the extent that patients in the mrCDI group received FMT that was not captured by our algorithm, this would likely bias the results toward the null, again suggesting that we could have underestimated true associations. Finally, the risk of complications following FMT could be related to characteristics of the donor, such as age, gender, or comorbidities, but we have no data on donors.
In conclusion, we have demonstrated that patients with mrCDI or CDI treated with FMT do not appear to be intrinsically at higher risk of immune-mediated diseases, hypertension, diabetes mellitus, myocardial infarction, stroke or irritable bowel syndrome. Future studies should assess the potential association of FMT with myocardial infarction in other large data sets, particularly those with access to data not routinely available in insurance claims data. If confirmed, subsequent studies examining changes in the gut microbiota and the host metabolome could be informative to understand the mechanisms behind the association.
Supplementary Material
Background
Fecal microbiota transplantation is a commonly used therapy for multiply recurrent Clostridioides difficile. By altering the gut microbiome, there is the potential for fecal microbiota transplantation to impact the risk for cardiometabolic, intestinal or immune-mediated conditions.
Findings
The subsequent incidence of cardiometabolic, intestinal or immune-mediated conditions did not differ between patients with multiply recurrent Clostridioides difficile and those with Clostridioides difficile treated with one or two courses of antibiotics or between patients with multiply recurrent Clostridioides difficile and those with Clostridioides difficile treated with fecal microbiota transplantation. The one exception was an increased incidence of myocardial infarction in patients with Clostridioides difficile treated with fecal microbiota transplantation as compared to those with multiply recurrent Clostridioides difficile treated with antibiotics alone.
Implications for patient care
These results support the long term safety of fecal microbiota transplantation for multiply recurrent Clostridioides difficile with the possible exception of a small increased incidence of myocardial infarction. This finding requires validation in additional studies.
Acknowledgments
Funding: This work was supported by NIH grant R24 AI118629
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
Dr. Lewis reports consulting for Merck and Pfizer, outside of submitted work. Dr. Lewis and Dr. Wu reports having a patent, “Compositions and methods comprising a defined microbiome and methods of use thereof,”
Dr. Kelly reports research support from Finch Therapeutics for a clinical trial. Unpaid clinical advisor to OpenBiome
References
- 1.Ma GK, Brensinger CM, Wu Q, et al. Increasing Incidence of Multiply Recurrent Clostridium difficile Infection in the United States: A Cohort Study. Ann Intern Med 2017;167:152–158. [DOI] [PubMed] [Google Scholar]
- 2.Kelly CR, Kahn S, Kashyap P, et al. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology 2015;149:223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maruvada P, Leone V, Kaplan LM, et al. The Human Microbiome and Obesity: Moving beyond Associations. Cell Host Microbe 2017;22:589–599. [DOI] [PubMed] [Google Scholar]
- 4.Tilg H, Moschen AR. Microbiota and diabetes: an evolving relationship. Gut 2014;63:1513–21. [DOI] [PubMed] [Google Scholar]
- 5.Tang WHW, Backhed F, Landmesser U, et al. Intestinal Microbiota in Cardiovascular Health and Disease: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;73:2089–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horta-Baas G, Romero-Figueroa MDS, Montiel-Jarquín AJ, et al. Intestinal Dysbiosis and Rheumatoid Arthritis: A Link between Gut Microbiota and the Pathogenesis of Rheumatoid Arthritis. J Immunol Res 2017;2017:4835189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horton DB. Juvenile Idiopathic Arthritis and the Gut Microbiome: More Clues, More Questions. Arthritis Rheumatol 2019;71:842–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni J, Wu GD, Albenberg L, et al. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol 2017;14:573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishida A, Inoue R, Inatomi O, et al. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol 2018;11:1–10. [DOI] [PubMed] [Google Scholar]
- 10.Tap J, Derrien M, Tornblom H, et al. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology 2017; 152:111–123 e8. [DOI] [PubMed] [Google Scholar]
- 11.Narula N, Kassam Z, Yuan Y, et al. Systematic Review and Meta-analysis: Fecal Microbiota Transplantation for Treatment of Active Ulcerative Colitis. Inflamm Bowel Dis 2017;23:1702–1709. [DOI] [PubMed] [Google Scholar]
- 12.Xu D, Chen VL, Steiner CA, et al. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome. The American Journal of Gastroenterology 2019;114:1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Mocanu V, Cai C, et al. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome-A Systematic Review. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubberke ER, Reske KA, McDonald LC, et al. ICD-9 codes and surveillance for Clostridium difficile-associated disease. Emerg Infect Dis 2006;12:1576–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hui W, Li T, Liu W, et al. Fecal microbiota transplantation for treatment of recurrent C. difficile infection: An updated randomized controlled trial meta-analysis. PLOS ONE 2019;14:e0210016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Zhang, Mocanu, Cai, et al. Impact of Fecal Microbiota Transplantation on Obesity and Metabolic Syndrome—A Systematic Review. Nutrients 2019;11:2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koeth RA, Wang ZE, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nature Medicine 2013;19:576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013;368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George AT, Davis C, Rangaraj A, et al. Cardiac ischaemia and rhythm disturbances during elective colonoscopy. Frontline Gastroenterol 2010;1:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madan R, Petri WA Jr. Role of obesity and adipose tissue-derived cytokine leptin during Clostridium difficile infection. Anaerobe 2015;34:182–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meier K, Nordestgaard AT, Eid AI, et al. Obesity as protective against, rather than a risk factor for, postoperative Clostridium difficile infection: A nationwide retrospective analysis of 1,426,807 surgical patients. J Trauma Acute Care Surg 2019;86:1001–1009. [DOI] [PubMed] [Google Scholar]
- 22.Chandradas S, Khalili H, Ananthakrishnan A, et al. Does Obesity Influence the Risk of Clostridium difficile Infection Among Patients with Ulcerative Colitis? Dig Dis Sci 2018;63:2445–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bovonratwet P, Bohl DD, Russo GS, et al. How Common-and How Serious- Is Clostridium difficile Colitis After Geriatric Hip Fracture? Findings from the NSQIP Dataset. Clin Orthop Relat Res 2018;476:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers MA, Greene MT, Saint S, et al. Higher rates of Clostridium difficile infection among smokers. PLoS One 2012;7:e42091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barker AK, Van Galen A, Sethi AK, et al. Tobacco use as a screener for Clostridium difficile infection outcomes. J Hosp Infect 2018;98:36–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reveles KR, Mortensen EM, Koeller JM, et al. Derivation and Validation of a Clostridium difficile Infection Recurrence Prediction Rule in a National Cohort of Veterans. Pharmacotherapy 2018;38:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


