Abstract
Introduction:
The authors tested the hypothesis that the EEG feature generalized polyspike train (GPT) is associated with drug-resistant idiopathic generalized epilepsy (IGE).
Methods:
The authors conducted a single-center case–control study of patients with IGE who had outpatient EEGs performed between 2016 and 2020. The authors classified patients as drug-resistant or drug-responsive based on clinical review and in a masked manner reviewed EEG data for the presence and timing of GPT (a burst of generalized rhythmic spikes lasting less than 1 second) and other EEG features. A relationship between GPT and drug resistance was tested before and after controlling for EEG duration. The EEG duration needed to observe GPT was also calculated.
Results:
One hundred three patients were included (70% drug-responsive and 30% drug-resistant patients). Generalized polyspike train was more prevalent in drug-resistant IGE (odds ratio, 3.8; 95% confidence interval, 1.3–11.4; P = 0.02). This finding persisted when controlling for EEG duration both with stratification and with survival analysis. A median of 6.5 hours (interquartile range, 0.5–12.7 hours) of EEG recording was required to capture the first occurrence of GPT.
Conclusions:
The findings support the hypothesis that GPT is associated with drug-resistant IGE. Prolonged EEG recording is required to identify this feature. Thus, >24-hour EEG recording early in the evaluation of patients with IGE may facilitate prognostication.
Keywords: Idiopathic generalized epilepsy, EEG, Prognostication
Although most patients with idiopathic generalized epilepsy (IGE) are well-controlled on anti-seizure medications (ASMs), one third of them continue to have refractory seizures.1-5 Accurately predicting drug resistance would facilitate early aggressive management. The newly defined EEG feature generalized polyspike train (GPT) was reported to predict drug resistance, although this finding was not confirmed in a subsequent study.6,7 Several limitations prevent us from using GPT in clinical practice. First, the association between GPT and drug resistance remains unclear given these conflicting results. Second, EEG duration may be an unmeasured source of bias if we are more likely to capture GPT in patients with longer EEG durations and if clinicians tend to order more frequent or long-duration EEGs in drug-resistant patients. Finally, the probability of identifying GPT on a routine EEG is low,7 and we do not know the EEG duration required to capture this feature.
Given that these unanswered questions limit our understanding of the relationship between GPT and drug-resistant IGE and the utility of this biomarker in clinical practice, we performed a retrospective study of patients with IGE. We compared the presence and timing of several EEG features between drug-resistant and drug-responsive patients. Our goals were to (1) determine the relationship between GPT and drug resistance before and after controlling for EEG duration—testing the hypothesis that EEG duration confounds the apparent relationship between GPT and drug resistance—and (2) identify how long we must record EEG to capture GPT.
METHODS
Patients
This study was approved by the institutional review board at the University of Pennsylvania. Written informed consent was waived by the institutional review board. We performed a single-center retrospective case–control study, enrolling patients who were evaluated at University of Pennsylvania for IGE and had outpatient EEGs between January 1, 2016 and February 28, 2020. We queried our EEG database to include EEGs with either generalized spike–wave discharges (GSW) or polyspike-and-wave discharges (PSW). We excluded patients with (1) a clinical history inconsistent with IGE (as interpreted by the treating epileptologist), (2) cortically based epileptogenic lesions on neuroimaging, (3) focal neurologic deficits, (4) intellectual disability, (5) shorter than 1 year of follow-up or anti-seizure drug treatment, (6) abnormal EEG background, or (7) no available outpatient EEGs.
Study Procedure and Clinical Chart Review
EEG and clinical charts were reviewed by five clinical epilepsy fellows with at least 6 months experience in epilepsy and EEG review (E.C.C., N.C., T.G., J.J.G., and E.T.) and one board-certified epileptologist (C.A.E.). No reviewer was assigned the same patient’s EEG and clinical chart to ensure blinding. Clinical reviewers assessed charts for exclusion criteria and determined whether patients were drug resistant at their most recent clinical encounter, using the International League Against Epilepsy definition of continued unprovoked seizures in the previous 12 months despite adequate trials of two tolerated, appropriately chosen ASMs (noting that the longest pretreatment interseizure interval was often unknown).8,9 Appropriately chosen ASMs included valproic acid, clobazam, clonazepam, ethosuximide, felbamate, lamotrigine, levetiracetam, topiramate, zonisamide, and perampanel. Next, reviewers determined the IGE syndrome,10-12 age of epilepsy onset, presence or absence of family history of epilepsy, number of seizure types, and number of ASMs tried.
EEG Acquisition and Classification
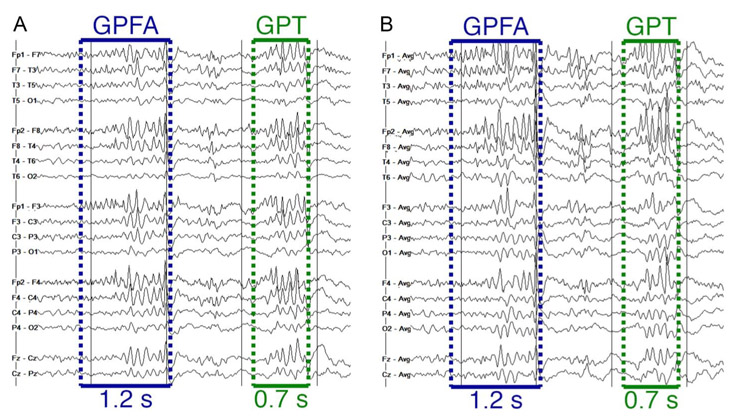
We acquired EEG by 21-channel electrodes according to the international 10 to 20 system of placement with a sampling rate of 256 Hz. We reviewed EEG data using Natus Database Version 8.5. We manually reviewed the entire duration of all available outpatient EEGs for each patient. Following the protocol from the original report on GPT,6 we recorded the presence of GSW, PSW, GPT, generalized paroxysmal fast activity (GPFA), generalized low-voltage fast activity, focal slowing, and focal discharges. We defined PSW as two or more generalized discharges in a row followed by a surface-negative slow wave. Generalized polyspike train was defined as a high-amplitude burst of at least five generalized rhythmic discharges with frontal predominance lasting less than 1 second (Fig. 1). Generalized paroxysmal fast activity was defined as a high-amplitude burst of generalized rhythmic discharges with frontal predominance lasting more than 1 second. We defined generalized low-voltage fast activity as a noticeable generalized decrease of voltage with a marked increase of signal frequency. We marked whether each feature was present during sleep and/or wakefulness. Next, a second reviewer (E.C.C. or C.A.E.) reviewed EEGs again to confirm EEG markings, to identify the time to the first occurrence of each feature, and to determine whether instances of GPT occurred during periods of hyperventilation or photic stimulation. Cases of disagreement between the first and second EEG reviewer or ambiguous EEG features were adjudicated by a board-certified epileptologist (C.A.E.).
FIG. 1.
Example of a single patient showing an occurrence of both GPFA (>1 second) and GPT (<1 second) in close temporal proximity on a bipolar montage (A) and average referential montage (B). In this example, the two electrographic features have similar morphology. GPFA, generalized paroxysmal fast activity; GPT, generalized polyspike train.
Statistical Analysis
We compared clinical factors between drug-responsive and drug-resistant patients. We used a Fisher exact test to compare categorical variables with two levels, a χ2 test of independence to compare categorical variables with more than two levels, and a Wilcoxon rank sum test to compare continuous variables.
We next examined the association between GPT and drug resistance, ignoring EEG duration. We performed univariate analyses comparing the prevalence of EEG features in drug-resistant and drug-responsive patients using Fisher exact tests. We then performed the same analyses stratifying EEG features by whether they occurred during sleep or wakefulness. We next tested for an association between GPT and drug resistance after redefining drug resistance to require a trial of valproic acid.6 We also compared the prevalence of GPT across different IGE syndromes using a χ2 test of independence. Finally, we used logistic regression to test for an independent relationship between GPT and drug resistance, controlling for clinical variables (sex, age of epilepsy onset, age at EEG, number of seizure types, and whether valproic acid was tried). We excluded family history and IGE syndrome so as not to exclude patients with missing data for these variables. We also excluded the number of ASMs tried as this variable was thought to closely reflect the definition of drug resistance.
Next, we used two methods—stratification and survival analysis—to test for a relationship between GPT and drug responsiveness controlling for EEG duration. We first performed separate Fisher exact tests to compare the presence of GPT amongst drug-responsive and drug-resistant patients in those with EEG <1 hour (N = 57) and those with EEG >1 hour (N = 46). We used a Cochran–Mantel–Haenszel test to combine odds ratios (ORs) across strata.13 Separately, we performed a survival analysis comparing the time to the first occurrence of GPT between drug-responsive and drug-resistant patients. We used a right-censored log-rank test with the end of EEG recording taken as censoring time.
Finally, we determined the recording duration needed to capture different EEG features. We compared the time to the first occurrence of the four most common EEG features (GSW, PSW, GPT, and GPFA) in subjects who experienced these events. We used a Kruskal–Wallis test with post-hoc Dunn–Sidak tests to test for individual significant intergroup differences. We confirmed significant pairwise differences from this analysis with an additional test for partially overlapping samples.14-16 For each pair of EEG features with a significant difference in time to capture the feature with the above analysis, we separated patients into those with both features present and those with just one of the features present. For patients with both features, we performed a Wilcoxon signed rank test to compare the time to first occurrence. For patients with only one feature, we performed a Wilcoxon rank sum test. We combined the paired and unpaired P-values from the two tests using Fisher17 combined probability test.
All analyses were performed in Matlab R2019a (The Mathworks, Natick, MA) except for the log-rank test, which was performed using the Survival package in R.18,19 All de-identified data, along with Matlab and R scripts to perform the analysis, are available on GitHub (https://github.com/erinconrad/ige_project).
RESULTS
One hundred ninety-five EEGs from 152 patients were reviewed, representing 2,532 hours of EEG data. Forty-nine patients were excluded: 38 met clinical exclusion criteria (10 for less than 1 year of follow-up or ASM treatment, 27 for clinical phenotype inconsistent with IGE, and 1 for abnormal neurologic examination) and an additional 11 met EEG exclusion criteria (two for abnormal EEG backgrounds and nine for inaccessible outpatient EEGs). This resulted in 103 included patients. Table 1 shows summary clinical characteristics.
TABLE 1.
Summary of Patient Characteristics
| Parameter | Drug-Responsive Patients, n = 72 |
Drug-Resistant Patients, n = 31 |
Statistic | P |
|---|---|---|---|---|
| Total number | 72 (69.9%) | 31 (30.1%) | — | |
| Sex | ||||
| Men | 24 (33.3%) | 11 (35.5%) | 1.1 (0.5–2.7) | 0.83 |
| Women | 48 (66.7%) | 20 (64.5%) | — | |
| Syndrome | ||||
| CAE | 8 (11.4%) | 6 (19.4%) | 4.5 (df = 4) | 0.34 |
| JAE | 9 (12.9%) | 7 (22.6%) | — | |
| JME | 29 (41.4%) | 12 (38.7%) | — | |
| GTCA | 20 (28.6%) | 4 (12.9%) | — | |
| Unclassified | 4 (5.7%) | 2 (6.5%) | — | |
| Age at epilepsy onset mean (SD) | 14.7 (8.5) | 13.4 (8.3) | 756.5 | 0.30 |
| Age at first EEG in study mean (SD) | 32.4 (13.5) | 30.0 (13.1) | 1,013 | 0.46 |
| Number of seizure types mean (SD) | 2.0 (0.8) | 2.1 (0.8) | 1,051.5 | 0.62 |
| Family history of epilepsy | ||||
| Yes | 39 (54.2%) | 17 (54.8%) | 1.9 (0.7–5.1) | 0.24 |
| No | 30 (41.7%) | 7 (22.6%) | — | |
| Number of ASMs tried mean (SD) | 3.1 (1.9) | 5.1 (2.7) | 584 | <0.001 |
| Tried VPA | ||||
| Yes | 35 (48.6%) | 22 (71.0%) | 2.6 (1.0–6.4) | 0.05 |
| No | 37 (51.4%) | 9 (29.0%) | — | |
| Total EEG duration (minutes) median (IQR) | 43.0 (42.0–2764.5) | 1,440.0 (42.0–2984.5) | 951.5 | 0.23 |
| EEG captured sleep | ||||
| Yes | 61 (84.7%) | 30 (96.8%) | 5.4 (0.7–43.9) | 0.10 |
| No | 11 (15.3%) | 1 (3.2%) | — |
For categorical variables with two levels (sex, family history of epilepsy, trial of valproic acid, and sleep captured), test statistics are ORs and 95% CIs, and P-values are obtained from a Fisher exact test. For categorical variables with more than two levels (IGE syndrome), test statistics are χ2 statistics from a χ2 test of independence. For continuous variables (age at first EEG, duration), test statistics are U statistics, and P-values are obtained from a Wilcoxon rank sum test. Ten patients were excluded from the family history analysis and two patients from the IGE syndrome analysis because of incomplete documentation precluding assignment of family history or IGE syndrome (and so percentages do not add up to 100% for these analyses). Of the six patients with “unclassified” IGE, two had Jeavons syndrome, one had CAE evolving to JAE with tonic seizures, two had adult onset of absences and convulsions, and one had convulsions and poorly characterized confusional episodes.
CAE, childhood absence epilepsy; 95% CI, 95% confidence interval; IGE, idiopathic generalized epilepsy; JAE, juvenile absence epilepsy; IQR, interquartile range; OR, odds ratio; VPA, valproic acid.
Relationship Between GPT and Drug Responsiveness
Figure 1 shows an example of a single time point of EEG from one patient with both GPT and GPFA on a single page, demonstrating that these features sometimes had similar morphology. Table 2 shows the prevalence of EEG features. Generalized polyspike train was associated with drug resistance (P = 0.02), although this finding was nonsignificant when correcting for testing multiple EEG features (α = 0.006). The association between GPT and drug resistance persisted when we required a trial of valproic acid to meet criteria for drug resistance, as in the original report on GPT.6 Seven of 22 (31.8%) drug-resistant patients who had tried valproic acid had GPT, and 9 of 81 (11.1%) remaining patients had GPT (Fisher exact test: OR, 3.7; 95% confidence interval [CI], 1.2–11.6; P = 0.04). The association between GPT and drug resistance remained when controlling for clinical variables using a logistic regression model (adjusted OR, 3.6; 95% CI, 1.0–13.0; P = 0.04).
TABLE 2.
Relationship Between EEG Features and Drug Resistance
| Feature | Responsive Patients With Feature, N (%) |
Resistant Patients With Feature, N (%) |
OR of Resistant-To-Responsive (95% CI) |
P |
|---|---|---|---|---|
| GSW | 66 (91.7) | 29 (93.5) | 1.3 (0.3–6.9) | 1.0 |
| PSW | 40 (55.6) | 21 (67.7) | 1.7 (0.7–4.1) | 0.28 |
| GPT | 7 (9.7) | 9 (29.0) | 3.8 (1.3–11.4) | 0.02 |
| GPFA | 4 (5.6) | 6 (19.4) | 4.1 (1.1–15.7) | 0.06 |
| GPT or GPFA | 8 (11.1) | 10 (32.3) | 3.8 (1.3–10.9) | 0.02 |
| GLVFA | 0 (0.0) | 2 (6.5) | — | 0.09 |
| Focal discharges | 1 (1.4) | 3 (9.7) | 7.6 (0.8–76.3) | 0.08 |
| Focal slowing | 3 (4.2) | 2 (6.5) | 1.6 (0.3–10.0) | 0.64 |
A higher OR implies greater prevalence of the feature in drug-resistant patients.
95% CI, 95% confidence interval; GLVFA, generalized low-voltage fast activity; GPFA, generalized paroxysmal fast activity; GPT, generalized polyspike train; GSW, generalized spike–wave; OR, odds ratio; PSW, polyspike-and-wave; —, not estimable.
We also compared the prevalence of GPT across IGE syndromes. Two patients were excluded because of incomplete documentation precluding determination of IGE syndrome. Generalized polyspike train was present in 1 of 14 (7.1%) patient with childhood absence epilepsy, 3 of 16 (18.8%) patients with juvenile absence epilepsy, 5 of 41 (12.2%) patients with juvenile myoclonic epilepsy, 3 of 24 (12.5%) patients with epilepsy with generalized tonic–clonic seizures alone, and 3 of 6 (50%) patients with unclassified IGE. There was no significant association between the presence of GPT and IGE syndrome ( = 7.0, P = 0.13).
Table 3 shows the relationship between EEG features and drug responsiveness in sleep versus wakefulness. There was no significant relationship between any EEG feature and drug resistance when stratifying by sleep and wakefulness. There were nonsignificant associations (P < 0.05, α = 0.003) of more frequent GPT during sleep, GPFA during sleep, GPT or GPFA during sleep, and focal discharges during wakefulness among drug-resistant patients. The ORs for the relationship between GPT and drug resistance were similar during sleep (3.8) and wakefulness (4.4). Of the eight patients who had GPT while awake, five (62.5%) had EEGs including hyperventilation and six (75%) had EEGs including photic stimulation. None of these patients had GPT during hyperventilation or photic stimulation.
TABLE 3.
Relationship Between EEG Features and Drug Resistance Stratified by Sleep and Wakefulness
| Feature | Responsive Patients With Feature, N (%) |
Resistant Patients With Feature, N (%) |
OR of Resistant-To-Responsive (95% CI) |
P |
|---|---|---|---|---|
| GSW awake | 51 (70.8) | 22 (71.0) | 1.0 (0.4–2.5) | 1.0 |
| GSW asleep | 47 (65.3) | 26 (83.9) | 2.8 (0.9–8.1) | 0.06 |
| PSW awake | 19 (26.4) | 14 (45.2) | 2.3 (1.0–5.5) | 0.07 |
| PSW asleep | 35 (48.6) | 18 (58.1) | 1.5 (0.6–3.4) | 0.40 |
| GPT awake | 3 (4.2) | 5 (16.1) | 4.4 (1.0–19.8) | 0.05 |
| GPT asleep | 6 (8.3) | 8 (25.8) | 3.8 (1.2–12.2) | 0.03 |
| GPFA awake | 4 (5.6) | 4 (12.9) | 2.5 (0.6–10.8) | 0.24 |
| GPFA asleep | 1 (1.4) | 4 (12.9) | 10.5 (1.1–98.4) | 0.03 |
| GPT or GPFA awake | 4 (5.6) | 6 (19.4) | 4.1 (1.1–15.7) | 0.06 |
| GPT or GPFA asleep | 6 (8.3) | 9 (29.0) | 4.5 (1.4–14.1) | 0.01 |
| GLVFA awake | 0 (0.0) | 0 (0.0) | — | — |
| GLVFA asleep | 0 (0.0) | 2 (6.5) | — | 0.09 |
| Focal discharges awake | 0 (0.0) | 3 (9.7) | — | 0.03 |
| Focal discharges asleep | 1 (1.4) | 0 (0.0) | — | 1.0 |
| Focal slowing awake | 3 (4.2) | 2 (6.5) | 1.6 (0.3–10.0) | 0.64 |
| Focal slowing asleep | 0 (0.0) | 0 (0.0) | — | — |
A higher OR implies greater prevalence of the feature in drug-resistant patients.
95% CI, 95% confidence interval; GLVFA, generalized low-voltage fast activity; GPFA, generalized paroxysmal fast activity; GPT, generalized polyspike train; GSW, generalized spike–wave; OR, odds ratio; PSW, polyspike-and-wave; —, not estimable.
Relationship Between GPT and Drug Responsiveness, Controlling for Duration
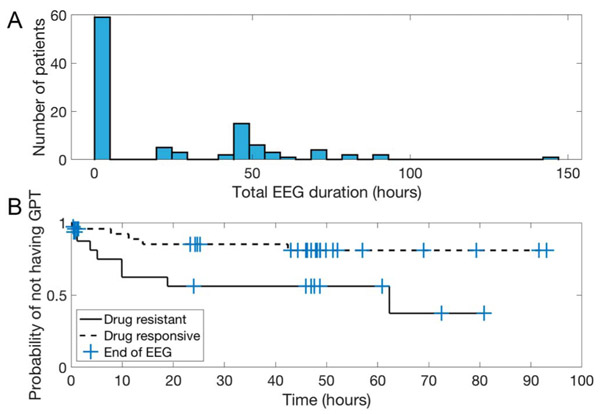
Figure 2A shows a histogram of EEG durations across patients. This highlights that most patients (N = 57) had less than 1 hour of recording and the remainder (N = 46) had a broad distribution of longer studies.
FIG. 2.
A, Histogram showing the distribution of total EEG durations across patients. Most patients had less than 1 hour of EEG recording. B, Kaplan–Meier curve showing the probability of a patient remaining without GPT as a function of recording duration. Check marks indicate the end times of EEG recording for each patient, taken as censoring times. GPT, generalized polyspike train.
Restricting analysis to patients with less than 1 hour of EEG recording, there was no difference in the prevalence of GPT among drug-responsive (N = 2/42, 4.8%) and drug-resistant patients (N = 1/15, 6.7%; Fisher exact test: OR, 1.4; 95% CI, 0.1–17.0; P = 1.00). Restricting analysis to patients with more than 1 hour of EEG recording, 5 of 30 drug-responsive patients (16.7%) and 8 of 16 drug-resistant patients (50.0%) had GPT (OR, 5.0; 95% CI, 1.3–19.7; P = 0.04). The combined OR across strata was 3.7 (Cochran–Mantel–Haenszel test: 95% CI, 1.2–11.8; = 5.0; P = 0.03). This suggests a persistent association of higher rates of GPT when controlling for duration.
We next performed a survival analysis comparing the time to first occurrence of GPT amongst drug-responsive versus drug-resistant patients, censoring by the end of EEG. There was again an association of earlier time-to-first occurrence of GPT among drug-resistant patients (log-rank test: = 4.6, P = 0.03). Figure 2B shows a Kaplan–Meier curve of the probability of remaining without occurrence of GPT over time.
Association of EEG Features with EEG Duration
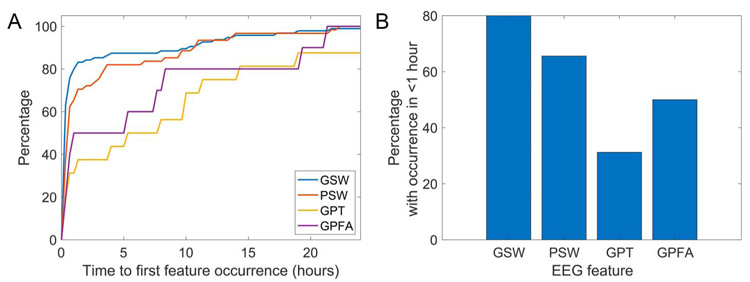
Figure 3 shows the recording duration needed to capture various EEG features. The median time to the first occurrence of EEG features among patients with the feature was 12.0 minutes (interquartile range, 5.0–39.5 minutes) for GSW, 29.0 minutes (12.5–174.2) for PSW, 387.0 minutes (27.0–759.0) for GPT, and 175.0 minutes (21.0–489.0) for GPFA. Among patients with GPT present, 31.2% of them had GPT in the first hour, and 90% had GPT within the first 42.4 hours. EEG features differed in the time to first occurrence (Kruskal–Wallis: = 22.8, P < 0.001). The time to first occurrence was higher for GPT than for GSW (post-hoc Dunn–Sidak test, P < 0.001) and for PSW than for GSW (P = 0.04). These differences remained using our additional test for overlapping samples (GSW-PSW comparison: P < 0.001, GSW-GPT comparison: P = 0.005, α = 0.008). Other comparisons were nonsignificant (GSW vs. GPFA: P = 0.08, PSW vs. GPT: P = 0.12, PSW vs. GPFA: P = 0.85, GPT vs. GPFA: P = 0.99).
FIG. 3.
A, The percentage of patients with each EEG feature whose feature occurred by a given time. Only patients who ever had the listed EEG feature are included. B, The percentage of patients with each EEG feature whose feature occurred within the first hour of recording. This is the proportion of patients in whom a routine outpatient EEG is expected to capture a feature, given that it is present in the patient. GPFA, generalized paroxysmal fast activity; GPT, generalized polyspike train; GSW, generalized spike-wave; PSW, polyspike-and-wave.
DISCUSSION
Our study supports the hypothesis that GPT is a biomarker of drug-resistant IGE and offers two additional findings: (1) the relationship between GPT and drug resistance is not explained by longer EEG recordings in drug-resistant patients and (2) prolonged EEG recordings are needed to capture GPT.
We found a consistent association between GPT and drug resistance (OR, 3.8; 95% CI, 1.3–11.4; P = 0.02) across several analyses. This finding was significant when taken alone as a test of our primary hypothesis, although nonsignificant when correcting for testing multiple EEG features. Given the significant association in the original GPT study and the nonsignificant association in the 2020 follow-up study, our findings support a true association between GPT and drug resistance.
We hypothesized a source of bias whereby GPT may merely signify longer EEG recordings rather than independently mark drug resistance. We based this hypothesis on the expectation that (1) GPT may be captured more frequently in longer EEG recording and (2) clinicians may be more likely to order more or longer EEGs in drug-resistant patients.8 The persistent association between GPT and drug resistance controlling for EEG duration oppose this hypothesis.
Generalized polyspike train and GPFA demonstrated substantial overlap (about half of patients who had either feature had both). These features also had similar associations (OR 3.8 for GPT, 4.1 for GPFA) with drug-resistant IGE. Furthermore, we found multiple instances (Fig. 1) in which GPFA and GPT occurred in the same patient in close temporal proximity and with similar morphology. These findings, combined with the somewhat arbitrary cutoff of >1 second for GPFA and < 1 second for GPT6,20 and the fact that the relative frequencies of these features have varied widely across studies,6,7 suggest that GPT and GPFA likely represent a continuum of the same electrographic phenomenon and perhaps the same underlying pathophysiology.
Generalized paroxysmal fast activity is frequently described in patients with Lennox–Gastaut syndrome, a generalized epilepsy syndrome characterized by intractable seizures and encephalopathy.20,21 Generalized paroxysmal fast activity has also been increasingly recognized in intractable IGE.22,23 It is hypothesized that the prolonged burst of discharges seen in GPFA may reflect a failure of GABAergic inhibition.24,25 This inhibitory breakdown may link drug-resistant IGE and Lennox–Gastaut syndrome. If this is true, our findings would suggest that both GPT and GPFA represent different points along the continuum of failed inhibition.
The median time to first occurrence of GPT was 6.5 hours, and 42.4 hours were needed to achieve a 90% sensitivity for GPT in our dataset. This implies that most patients require prolonged EEGs—specifically, a >24-hour study—to capture GPT. Generalized polyspike train was about twice as common in sleep as in wakefulness, consistent with previous studies,6,7 suggesting that a routine EEG including sleep may increase the chance of capturing GPT. Because all our prolonged EEG recordings included sleep, we could not separate the effect of prolonged recordings from that of sleep recordings on likelihood of capturing GPT. We did not replicate the finding in the original report on GPT that GPT during sleep in particular was associated with drug resistance.6,7
We determined drug resistance at the time of the most recent clinical encounter; however, we reviewed all EEG data since 2016. It is possible that some patients classified as drug-responsive would have been classified as drug-resistant at the time their EEGs were obtained, and vice versa. This limitation is hard to avoid: attempts to align EEGs to clinical assessments are impeded by the fact that EEGs are often obtained several months away from patients’ nearest clinical encounters. An additional limitation is that our method of enrolling patients according to the reported presence of GSW or PSW on an EEG possibly introduces bias. If GSW and PSW are themselves associated with drug-resistant IGE, then we may have preferentially selected for a drug-resistant cohort. However, the rate of drug resistance in our population (30.1%) is similar to that reported in other studies.3,5-7 Also, other recent studies suggest that GSW and PSW do not predict drug resistance.6,7 We believe that our patient population is representative of the larger cohort of IGE patients in our health system. We also do not know the rate of pseudoresistance in our population, or the rate of seizure underreporting, as these are challenging to determine clinically.8 These phenomena would both lead to misclassification of some drug-resistant patients as responsive, and vice versa. Assuming these phenomena are independent of GPT, we would expect this effect to decrease the sensitivity and specificity of GPT for drug resistance, rendering the study less powerful.
Our study suggests that GPT is more common in drug-resistant IGE and that prolonged recordings are needed to capture GPT. These combined findings argue for a change in practice toward obtaining >24-hour EEGs earlier in IGE management. Earlier recognition of GPT in patients with IGE may facilitate prognostication in IGE and alter future management.
Acknowledgments
E. C. Conrad received support from Ceribell and NIH R25 NS-065745. J. J. Gugger received support from Ceribell. M. A. Gelfand received research support from Aquestive, Biogen, Cerevel, Eisai, Engage, Otsuka, SK Life Science, and UCB. A. Omole received support from NIH R25 NS-065745. B. M. Decker received support from NIH T32 NS-061779. K. A. Davis received research support from Eisai. The remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Kharazmi E, Peltola M, Fallah M, Keränen T, Peltola J. Idiopathic generalized epilepsies: a follow-up study in a single-center. Acta Neurol Scand 2010;122:196–201. [DOI] [PubMed] [Google Scholar]
- 2.Seneviratne U, Cook M, D’Souza W. The electroencephalogram of idiopathic generalized epilepsy. Epilepsia 2012;53:234–248. [DOI] [PubMed] [Google Scholar]
- 3.Stevelink R, Koeleman BPC, Sander JW, Jansen FE, Braun KPJ. Refractory juvenile myoclonic epilepsy: a meta-analysis of prevalence and risk factors. Eur J Neurol 2019;26:856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geithner J, Schneider F, Wang Z, et al. Predictors for long-term seizure outcome in juvenile myoclonic epilepsy: 25-63 years of follow-up. Epilepsia 2012;53:1379–1386. [DOI] [PubMed] [Google Scholar]
- 5.Gelisse P, Genton P, Thomas P, Rey M, Samuelian JC, Dravet C. Clinical factors of drug resistance in juvenile myoclonic epilepsy. J Neurol Neurosurg Psychiatry 2001;70:240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Y, Seneviratne U, Perucca P, et al. Generalized polyspike train: an EEG biomarker of drug-resistant idiopathic generalized epilepsy. Neurology 2018;91:e1822–e1830. [DOI] [PubMed] [Google Scholar]
- 7.Jensen CD, Gesche J, Krøigård T, Beier CP. Prognostic value of generalized polyspike trains and prolonged epileptiform EEG runs. J Clin Neurophysiol 2019. doi: 10.1097/WNP.0000000000000679. [DOI] [PubMed] [Google Scholar]
- 8.Kwan P, Schachter SC, Brodie MJ. Drug-resistant epilepsy. N Engl J Med 2011;365:919–926. [DOI] [PubMed] [Google Scholar]
- 9.Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010;51:1069–1077. [DOI] [PubMed] [Google Scholar]
- 10.Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia 2017;58:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koutroumanidis M, Bourvari G, Tan SV. Idiopathic generalized epilepsies: clinical and electroencephalogram diagnosis and treatment. Expert Rev Neurother 2005;5:753–767. [DOI] [PubMed] [Google Scholar]
- 12.International League Against Epilepsy. EpilepsyDiagnosis.org diagnostic manual. Available at: https://www.epilepsydiagnosis.org/. Accessed August 1, 2020.
- 13.Agresti A. Categorical data analysis. Hoboken: John Wiley & Sons; 2013. [Google Scholar]
- 14.Derrick B, White P, Toher D. Parametric and non-parametric tests for the comparison of two samples which both include paired and unpaired observations. J Mod Appl Stat Methods 2020;18:9. [Google Scholar]
- 15.Uddin N, Hasan MS. Testing equality of two normal means using combined samples of paired and unpaired data. Commun Stat Simul Comput 2017;46:2430–2446. [Google Scholar]
- 16.Derrick B, Russ B, Toher D, White P. Test statistics for the comparison of means for two samples that include both paired and independent observations. J Mod Appl Stat Methods 2017;16:9. [Google Scholar]
- 17.Fisher RA. Statistical methods for research workers. 4th ed. Edinburgh, Scotland: Oliver & Boyd, 1934. [Google Scholar]
- 18.Therneau TM, Grambsch PM. The cox model. In: Therneau TM, Grambsch PM, eds. Modeling survival data: extending the cox model. New York: Springer, 2000;39–77. [Google Scholar]
- 19.R Core Team. R: a language and environment for statistical computing. 2013. Available at: http://www.R-project.org/. Accessed April 28, 2020.
- 20.Brenner RP, Atkinson R. Generalized paroxysmal fast activity: electro-encephalographic and clinical features. Ann Neurol 1982;11:386–390. [DOI] [PubMed] [Google Scholar]
- 21.Arzimanoglou A, French J, Blume WT, et al. Lennox-Gastaut syndrome: a consensus approach on diagnosis, assessment, management, and trial methodology. Lancet Neurol 2009;8:82–93. [DOI] [PubMed] [Google Scholar]
- 22.Tatum WO, Ho S, Benbadis SR. Polyspike ictal onset absence seizures. J Clin Neurophysiol 2010;27:93–99. [DOI] [PubMed] [Google Scholar]
- 23.Aydin-Özemir Z, Matur Z, Bebek N, Gürses C, Gökyiğit A, Baykan B. Long-term follow-up of adult patients with genetic generalized epilepsy with typical absence seizures and generalized paroxysmal fast activity in their EEG. Seizure 2014;23:607–615. [DOI] [PubMed] [Google Scholar]
- 24.Halász P. Runs of rapid spikes in sleep: a characteristic EEG expression of generalized malignant epileptic encephalopathies. A conceptual review with new pharmacological data. Seizure 1991;2:49–71. [PubMed] [Google Scholar]
- 25.Mohammadi M, Okanishi T, Okanari K, et al. Asymmetrical generalized paroxysmal fast activities in children with intractable localization-related epilepsy. Brain Dev 2015;37:59–65. [DOI] [PubMed] [Google Scholar]