Abstract
Purpose
A derangement of the coagulation process and thromboinflammatory events has emerged as pathologic characteristics of severe COVID-19, characterized by severe respiratory failure. C—C motive chemokine ligand 2 (CCL2), a chemokine originally described as a chemotactic agent for monocytes, is involved in inflammation, coagulation activation and neoangiogenesis. We investigated the association of CCL2 levels with coagulation derangement and respiratory impairment in patients with COVID-19.
Methods
We retrospectively evaluated 281 patients admitted to two hospitals in Italy with COVID-19. Among them, CCL2 values were compared in different groups (identified according to D-dimer levels and the lowest PaO2/FiO2 recorded during hospital stay, P/Fnadir) by Jonckheere-Terpstra tests; linear regression analysis was used to analyse the relationship between CCL2 and P/Fnadir. We performed Mann-Whitney test and Kaplan-Meier curves to investigate the role of CCL2 according to different clinical outcomes (survival and endotracheal intubation [ETI]).
Results
CCL2 levels were progressively higher in patients with increasing D-dimer levels and with worse gas exchange impairment; there was a statistically significant linear correlation between log CCL2 and log P/Fnadir. CCL2 levels were significantly higher in patients with unfavourable clinical outcomes; Kaplan-Meier curves for the composite outcome death and/or need for ETI showed a significantly worse prognosis for patients with higher (> median) CCL2 levels.
Conclusions
CCL2 correlates with both indices of activation of the coagulation cascade and respiratory impairment severity, which are likely closely related in COVID-19 pathology, thus suggesting that CCL2 could be involved in the thromboinflammatory events characterizing this disease.
Keywords: CCL2, Thrombosis, Inflammation, COVID-19, Acute lung injury
1. Introduction
Since its outbreak in late 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected more than 122 million people, causing the so-called coronavirus disease 2019 (COVID-19), with more than 2.7 million deaths worldwide, as of March 20, 2021 [1]. While the disease severity is variable, one in five patients requires hospitalization, mainly for severe pneumonia with respiratory failure, which can lead to intensive care unit (ICU) admission, with a relevant mortality rate [2]. The most severe cases generally fit the Berlin criteria for the acute respiratory distress syndrome (ARDS) [3]; however, in ARDS caused by SARS-CoV-2 lung compliance is relatively preserved, and an intrapulmonary vascular disease process seems to play a major role [4]. Indeed, in a small series of autopsy studies on COVID-19 patients, histological signs of severe lung endothelial injury, with a marked, widespread thrombosis of the small vessels and alveolar capillaries, along with evidence of intussusceptive neoangiogenesis, were found [5]. Moreover, circulating markers of deranged coagulation have been demonstrated in these patients, correlating with excess thromboembolic events [6] and prognosis [7]. The role of blood coagulation in early, innate immune response to external pathogens is well known, since many coagulation factors are involved in inflammatory and anti-inflammatory reactions through their interactions with the protease activated receptors located on immune cells [8]; these interactions induce several cytokines and chemokines synthesis. This complex crosstalk between inflammation and coagulation is now referred to as thromboinflammation (or immunothrombosis [9]); while its importance in ARDS is already known [10], its fundamental role in COVID-19 pathology has been recently highlighted [11].
C—C motive chemokine ligand 2 (CCL2, also known as monocyte chemoattractant protein-1, MCP-1) is a member of the C—C chemokine family; while monocytes/macrophages represent its main source, CCL2 is also synthesized by many different cell types, including endothelial, epithelial, smooth muscle and fibroblast cells. CCL2 is a potent chemotactic factor for monocytes, and it is involved in many biological processes, including inflammation, angiogenesis and coagulation [12]. Its synthesis is induced during inflammatory processes at sites of injury, where CCL2 is involved in the recruitment of innate immune cells, including monocytes/macrophages which can in turn activate the coagulation cascade, by expressing tissue factor (TF, the principal activator of the extrinsic coagulation pathway) not only on their surface, but also through the release of extracellular vesicles (EVs), which also carry TF on their outer membrane [12,13].
Higher levels of CCL2 have already been suggested as potential prognostic markers in COVID-19 [14,15]; however, their relationships with markers of activation of the coagulation cascade and respiratory impairment have not been specifically investigated.
Thus, since diffuse lung microangiopathy (characterized by alveolar capillary thrombosis and intussusceptive angiogenesis) and coagulation derangement seem to play a key role in severe COVID-19, and CCL2 has a known potential pro-thrombotic and pro-angiogenic activity, we conducted the present study to test the hypothesis that CCL2 levels are associated with markers of deranged coagulation in COVID-19 patients, reflecting a more severe respiratory impairment and a worse outcome in terms of survival and endotracheal intubation (ETI) requirement.
2. Methods
2.1. Study design and subjects
This was an observational, retrospective, bi-centric study enrolling consecutive patients admitted to the Pisa University Hospital and to the Lucca Community Hospital, Italy, from March 4 to April 30, 2020. We included patients ≥18 years, admitted because of pneumonia and laboratory confirmed COVID-19, diagnosed by a positive SARS-CoV-2 real time-PCR test on a nasopharyngeal swab. The only exclusion criterion was the lack of CCL2 data. We recorded information about demographics, comorbidities, ongoing treatments, need for advanced respiratory support through ETI, arterial gas exchange values and laboratory findings (including a cytokine panel encompassing CCL2) on peripheral venous blood samples.
In order to analyse the relationships between CCL2 and coagulation abnormalities, we decided to use D-dimer as a marker of coagulation cascade activation, and we therefore divided the whole sample in four groups according to the D-dimer quartiles, thus comparing CCL2 levels among these groups. Since D-dimer was repeatedly measured per standard clinical practice, we selected the value closest to the date of CCL2 determination; only D-dimer levels obtained within 72 h of CCL2 determination were included in the analysis.
Furthermore, we explored the relationships between CCL2 and respiratory impairment, evaluated by using arterial partial pressure of oxygen (PaO2) to fraction of inspired oxygen (FiO2) ratio (P/F), recorded both at admission (P/Fadm) and at the lowest value during the hospital stay (P/Fnadir), the latter representing the most severe respiratory impairment recorded in the patient. Moreover, we divided the whole sample in four groups, according to the following predefined, clinically significant P/Fnadir values intervals [3]: P/Fnadir > 300 mmHg, 200 < P/Fnadir ≤ 300 mmHg; 100 < P/Fnadir ≤ 200 mmHg; P/Fnadir ≤ 100 mmHg, and compared CCL2 levels among them.
Last, we performed predefined analyses of the possible prognostic value for CCL2, by dividing patients according to their vital status (survivors vs. non-survivors), need for mechanical ventilation via ETI during hospital stay, and a composite outcome of death and/or need for ETI.
Since all the procedures described in this study were part of routine care for COVID-19 patients in our two hospitals (including cytokines dosage, performed in order to evaluate patients' eligibility and response to specific biological treatments, such as tocilizumab), the study was conducted in compliance with the Declaration of Helsinki and with the approval of the local Ethic Committee, but patients' informed consent was waived.
2.2. Procedures
Gas exchange parameters were evaluated in arterial blood samples, collected according to standard clinical practice. Routine laboratory parameters (total and differential white blood cells count and platelets; hepatic and renal function, coagulation parameters, inflammatory markers, including C-reactive protein and procalcitonin) were evaluated in venous blood samples collected within 72 h from hospital admission and were processed according to standard laboratory techniques by the Pisa University Hospital and the Lucca Community Hospital clinical laboratories. Venous blood samples for cytokines (including CCL2, as well as interleukin [IL]1-β, IL6, IL10, tumour necrosis factor-α [TNF-α]), were taken at approximately 7:00 am, in fasting condition, within 96 h of hospital admission. CCL2 analysis was performed in the Laboratory of Clinical Pathology in the Pisa hospital for both Pisa and Lucca patients. The plasma concentration of CCL2 was determined by a fully automated ELISA processing system, DSX, DINEX Technologies, using a commercial ELISA kit (Human CCL2/MCP-1 Quantikine® ELISA Kit; R&D System, Minneapolis, MN, USA). The assay was performed according to the manufacturer's instructions.
2.3. Statistical analysis
Data are presented as medians and interquartile ranges (IQR) except when otherwise specified. To test for orderly distributions of multiple samples we used the Jonckheere-Terpstra test for independent samples. Since both P/Fnadir and CCL2 values were non-normally distributed, we applied a logarithmic transformation in order to perform a linear Pearson correlation between these two variables. We used the Mann-Whitney U test and the Kruskal-Wallis rank test for binary and multiple comparisons, respectively. Simple logistic regression was used to evaluate the performance of CCL2 in predicting binary outcomes (namely, probability of survival and ETI).
We divided the whole sample in two groups, using the CCL2 median as a cut-off value, to explore the predefined composite outcome (death and/or ETI) with log-rank test of Kaplan-Meier curves; moreover, a chi square test was performed to compare comorbidities distribution among the same two groups. We arbitrarily used the median for these analyses because CCL2 values distribution in COVID-19 can be variable, as shown by other studies [[14], [15], [16]], and a predefined cut-off for CCL2 has not been clearly identified in this disease.
Multivariate Cox proportional regression analysis was performed to investigate the performance of the different cytokines that were part of the original panel in predicting the same predefined outcomes. SPSS® 26 for MacOS® (IBM, Armonk, NY, USA) was used for statistical analysis and 2-tails p values <0.05 were considered statistically significant. Graphs were prepared with Prism® 8.0 (GraphPad, San Diego, CA, USA).
3. Results
Of the 382 patients admitted to the two participating hospitals during the study period, CCL2 levels were not available in 101. All the following analyses are therefore related to 281 patients (221 from the Pisa hospital and 60 from the Lucca hospital). Age ranged from 18 to 98 years (mean ± standard deviation: 66 ± 15) and males represented 70% of the sample. At the end of the study period, we recorded 51 deaths, corresponding to 18% in-hospital mortality rate. Among the whole population, 87% of all patients received supplemental oxygen, 59 patients (21% of total) required ETI. The most common comorbidities were systemic arterial hypertension (46%), chronic heart disease (30%) and diabetes (18%). Table 1 reports the most relevant clinical characteristics of the study population, including the main pharmacological treatments administered for COVID-19 during hospital stay.
Table 1.
Clinical characteristics of the study population (P/Fadm: arterial partial pressure of oxygen [PaO2] to fraction of inspired oxygen [FiO2] ratio [P/F] at admission; P/Fnadir: the lowest P/F value during the hospital stay, CCL2: C—C motive chemokine ligand 2; IL: interleukin; TNF- α: tumour necrosis factor-α; ETI: endotracheal intubation; COPD: chronic obstructive pulmonary disease; SD: standard deviation; IQR: interquartile range).
| Patients' characteristics: | |
|---|---|
| Sex (male/female) | 196/85 |
| Age, years (mean ± SD) | 66 ± 15 |
| P/Fadm mmHg (median [IQR]; missing values) | 276 [123–336]; 7 |
| P/Fnadir mmHg (median [IQR]; missing values) | 144 [90–276]; 4 |
| CCL2 ng/mL (median [IQR]; missing values) | 0.61 [0.42–0.93]; 0 |
| IL6 pg/mL (median [IQR]; missing values) | 21 [11–43]; 2 |
| TNF-α pg/mL (median [IQR]; missing values) | 9 [4–15]; 10 |
| IL1-β pg/mL (median [IQR]; missing values) | 1.4 [0.9–2.0]; 10 |
| D-dimer mg/L (median [IQR]; missing values)a | 0.53 [0.24–1.2]; 25 |
| C-reactive protein mg/dL (median [IQR]; missing values) | 6.3 [2.8–13.6]; 10 |
| Procalcitonin ng/mL (median [IQR]; missing values) | 0.12 [0.06–0.25]; 39 |
| White blood cells/μL (median [IQR]; missing values) | 6495 [4960–9133]; 3 |
| Neutrophils/μL (median [IQR]; missing values) | 4685 [3313–6955]; 5 |
| Lymphocytes/μL (median [IQR]; missing values) | 950 [628–1273]; 3 |
| Monocytes/μL (median [IQR]; missing values) | 500 [340–798]; 5 |
| Creatinine mg/dL (median [IQR]; missing values) | 0.96 [0.79–1.17]; 4 |
| Outcomes: | |
| Vital status (survived/deceased; missing values) | 230/51; 0 |
| ETI (no/yes; missing values) | 217/59; 5 |
| Comorbidities: | |
| Heart diseases, n (%) | 85 (30) |
| Hypertension, n (%) | 128 (46) |
| Diabetes, n (%) | 50 (18) |
| COPD, n (%) | 27 (10) |
| Treatments: | |
| Low-molecular weight heparin, n (%); missing values | 242 (86); 0 |
| Prophylactic dose, n (%) | 147 (61) |
| Intermediate dose, n (%) | 57 (24) |
| Anticoagulation dose, n (%) | 15 (6) |
| Unknown dose, n (%) | 23 (9) |
| Systemic glucocorticosteroids, n (%); missing values | 121 (43); 0 |
| Lopinavir/ritonavir, n (%); missing values | 206 (73); 0 |
| Remdesevir, n (%); missing values | 16 (6); 0 |
| Hydroxychloroquine, n (%); missing values | 242 (86); 0 |
| Tocilizumab, n (%); missing values | 26 (9); 0 |
| Baricitinib, n (%); missing values | 34 (12); 0 |
D-dimer closest to the date of CCL2 determination.
Since COVID-19 has been associated with the derangement of blood coagulation, we first investigated the potential association between CCL2 levels and D-dimer, a well-established marker of the activation of the coagulation and fibrinolytic cascades. Fig. 1 shows that patients with progressively increasing D-dimer have significantly higher circulating levels of CCL2; Jonckheere-Terpstra test confirmed the statistically significant trend among the quartiles. In order to identify a possible effect of thromboembolic events (TEV) excess on D-dimer levels, we checked for total TEV during the hospital stay. We found that only 8 on 281 patients (less than 3%) had a clinical demonstration of TEV (by computed tomography pulmonary angiography and/or venous Doppler ultrasound), thus reasonably excluding this possibility.
Fig. 1.
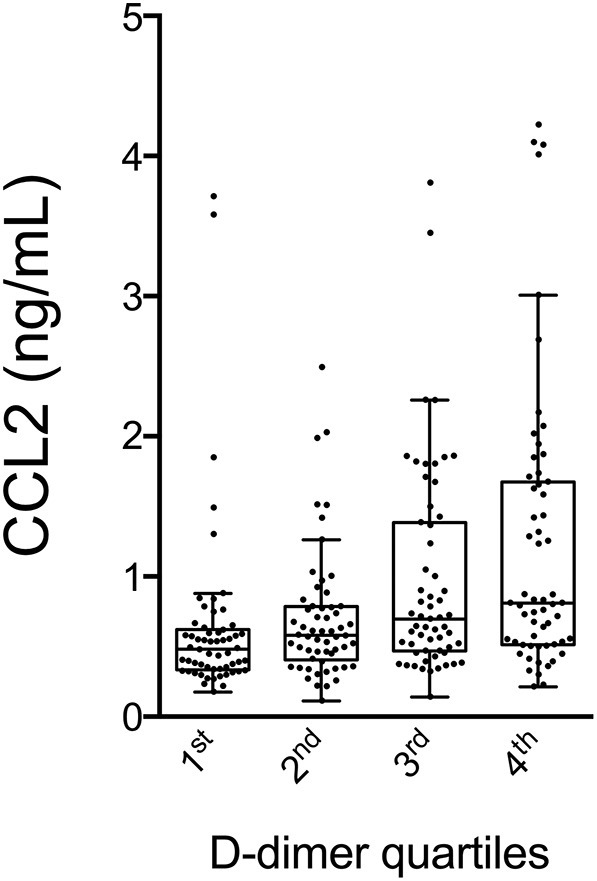
CCL2 values according to different quartiles of D-dimer. p for trend <0.001 by Jonckheere-Terpstra test.
CCL2: C—C motive chemokine ligand 2.
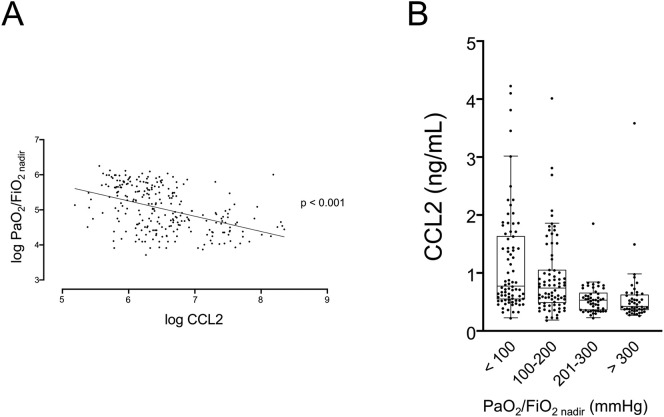
Next, in order to evaluate whether CCL2 levels were associated with severity of gas exchange impairment, we performed a linear regression between CCL2 levels and the lowest level of P/F recorded in a single patient (P/Fnadir). We log-transformed the data since neither value was normally distributed. As shown in Fig. 2A, there is a statistically significant, negative linear relationship between log-CCL2 and log-P/Fnadir (r = −0.439; p < 0.001). When patients were divided according to predefined levels of disease severity, according to the classification of ARDS severity based on the Berlin definition [3], a significant difference among the four groups in terms of CCL2 levels was confirmed by Jonckheere-Terpstra test (Fig. 2B).
Fig. 2.
Linear regression of log CCL2 and log PaO2/FiO2nadir. p < 0.001 (A). CCL2 values according to different predefined levels of severity of gas exchange impairment. p for trend <0.001 by Jonckheere-Terpstra test (B).
CCL2: C—C motive chemokine ligand 2; PaO2/FiO2nadir: the lowest arterial partial pressure of oxygen [PaO2] to fraction of inspired oxygen [FiO2] ratio [P/F] value during the hospital stay.
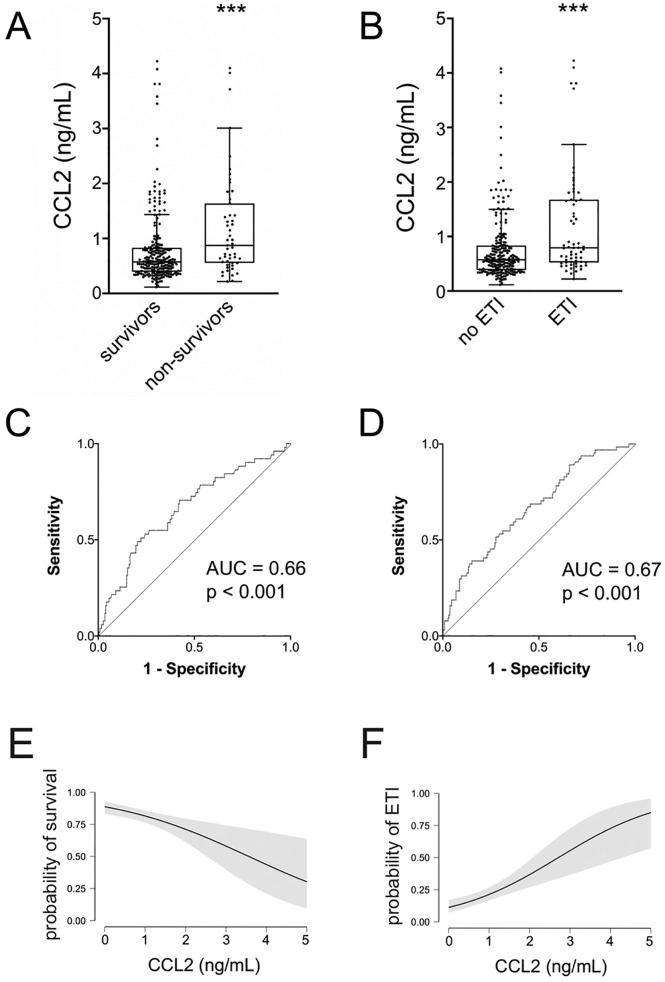
We then examined whether CCL2 levels at admission were different in patients with different outcomes. First, CCL2 levels were significantly higher in non-survivors compared to survivors (Fig. 3A) and in patients that required ETI (Fig. 3B) compared to patients that did not; Fig. 3C and D show the receiving-operating curves (ROC) for CCL2, with respect to these two outcomes (death and need for ETI). For death, the positive and negative predictive values corresponding to a CCL2 value of 625 ng/mL were 27% and 90%, respectively; the corresponding figures for the need for ETI were 31% and 86%. Moreover, a simple binary logistic regression confirmed a significant correlation between CCL2 levels and the previously described outcomes (p < 0.001 for all analyses): Fig. 3E and F respectively show the probability of survival and of the need for ETI associated with increasing levels of CCL2, based on the odd ratios calculated by the logistic regression model.
Fig. 3.
Comparison of CCL2 values according to prespecified outcomes: survival (A) and need for endotracheal intubation (B). *** p < 0.001 by Mann-Whitney test.
C-D: corresponding ROC curves.
Conditional probability of survival (E) and need for endotracheal intubation (F) for increasing values of CCL2. The shaded areas represent the 95% confidence intervals.
CCL2: C—C motive chemokine ligand 2; ETI: endotracheal intubation; AUC: area under curve.
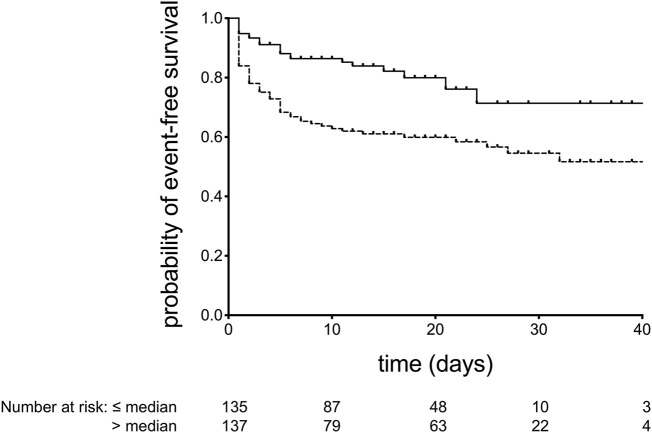
Finally, as shown in Fig. 4 , Kaplan-Meier curves were obtained to describe the event-free survival (where event was defined as a combination of death and/or need for ETI) in patients with CCL2 above and below the median value. The curves differ significantly by the log-rank test. No statistically significant difference was observed between comorbidities distribution in the same two groups, except for arterial hypertension (not shown).
Fig. 4.
Kaplan-Meier survival curves for the probability of event (death and/or need for endotracheal intubation)-free survival for patients with CCL2 values ≤ (solid line) or > (dashed line) median. p < 0.001 by log-rank test.
To evaluate the role of other cytokines available in our database in predicting the outcome death and/or need for ETI, we performed a multivariate analysis using Cox Regression Model. As shown in Table 2 , from the original panel including IL1-β, IL10, IL6, TNF-α and CCL2, only CCL2 and IL6 retained statistical significance.
Table 2.
Multivariate Cox regression analysis for the association of different cytokines with the composite outcome death and/or need for endotracheal intubation (CCL2: C—C motive chemokine ligand 2; IL: interleukin; TNF-α: tumour necrosis factor-α).
| Cytokine | HR (95% CI) | p Value |
|---|---|---|
| CCL2 (ng/mL) | 1.413 (1.073–1.861) | 0.014 |
| IL1-β (pg/mL) | 0.999 (0.993–1.005) | 0.720 |
| IL6 (pg/mL) | 1.003 (0.999–1.006) | 0.041 |
| IL10 (pg/mL) | 0.988 (0.968–1.008) | 0.245 |
| TNF-α (pg/mL) | 1.002 (0.996–1.007) | 0.180 |
4. Discussion
We have hypothesized that circulating CCL2 could be related to coagulation derangement and severity of respiratory impairment, expressing a potential role for CCL2 in thromboinflammatory processes in COVID-19. Indeed, we found that CCL2 levels were associated to both higher D-dimer levels and worse P/Fnadir; moreover, CCL2 levels were related to prognostic indices (survival and need for ETI).
First, we investigated the possible correlation between CCL2 and coagulation abnormalities in COVID-19, finding a statistically significant, direct association between circulating CCL2 and D-dimer levels (Fig. 1). D-dimer is a fibrin degradation product, and its circulating levels are therefore altered in all conditions characterized by the activation of the coagulation and fibrinolytic cascades, including thrombosis and acute infections [17]. In COVID-19 patients, D-dimer has been demonstrated to represent a prognostic marker [18], with higher levels associated with in-hospital death, critical illness with ICU admission, thrombotic events and the risk for adverse events during hospitalization [19]. The relationship we have observed between CCL2 and D-dimer highlights the interplay between inflammation and coagulation in COVID-19. Indeed, along with an altered inflammatory response, patients affected by COVID-19 showed a significant and clinically relevant deranged coagulation, with widespread thrombosis of the small pulmonary vessels [5] and a higher rate of clinically detectable thromboembolic events [6]. Moreover, both a close interaction between platelets and monocytes in promoting thromboinflammation [20] and elevated levels of circulating, TF-bearing EVs (probably derived from monocytes and associated to disease severity) [21] have been demonstrated in COVID-19. Last, it is well known that cytokines recruit inflammatory cells, raising a biological circle including, among others, the release of the so-called neutrophil extracellular traps (NETs) by neutrophils in the injured lung. NETs also exert procoagulant activities by promoting recruitment, adhesion and aggregation of platelets to the sites of inflammations, thus producing microvascular thrombosis, a pathological finding in ARDS [22], which seems to be predominant in COVID-19 most severe cases [5]. Indeed, NETs-containing microthrombi have been found in alveolar capillaries in COVID-19 patients [23]. In this light, our data are coherent with the abovementioned observation, and lend further support to the hypothesized relevant role for thromboinflammation in COVID-19 patients [11].
Second, our data show a strong correlation between CCL2 levels and the lowest P/F recorded during the hospital stay (P/Fnadir). Since patients have been admitted at different time points of their clinical course, P/Fnadir might standardize this heterogeneity, allowing for a direct comparison of respiratory parameters, since it better describes the severity of the acute lung injury experienced by each individual patient. This was further confirmed by evaluating CCL2 levels among clinically significant categories of lung injury severity, chosen according to the currently accepted definition for ARDS severity [3] (Fig. 2). While other works have focused on P/F at admission (which may be highly variable among a large population with a such rapidly evolving disease), respiratory failure occurrence or ICU admittance as markers of respiratory impairment [14,15,24], to our knowledge, the present study is the first to use the P/Fnadir in order to provide a reliable standard for severity impairment of gas exchange evaluation across the whole sample, independently of the patient's respiratory status at hospital admission. Interestingly, diffuse thrombosis of small vessels and pulmonary capillaries has been implied, along with a failure in the hypoxic pulmonary vasoconstriction mechanism, in the pathogenesis of the ventilation/perfusion mismatching, causing the severe hypoxemia in COVID-19 patients [25]. It is thus conceivable that circulating CCL2 could play a role in the thromboinflammatory process causing severe hypoxemia in COVID-19, and the relationships we found between CCL2, D-dimer and P/Fnadir seem to represent an attractive perspective in this direction. Interestingly, CCL2 levels were elevated in autopsy specimens [26] of patients affected by severe acute respiratory syndrome (SARS), caused by SARS-CoV. Moreover, the relevance of CCL2 in SARS pathogenesis has also been demonstrated in laboratories studies on cellular models [27], and genetic analyses have shown that some polymorphisms of CCL2 gene could increase susceptibility to SARS-CoV infection [28]. Indeed, data on ARDS not caused by coronaviruses have already confirmed the importance of CCL2 in the pathophysiology of acute lung injury, especially through a synergistic action with IL8 in neutrophils recruitment [29]. Last, CCL2 involvement in thrombotic and angiogenic events has already been described, specifically in venous thromboembolism and neoplastic neoangiogenesis [12].
Taken together, all these observations, for both COVID-19 and SARS, confirm a potential important role for CCL2 as an active player in the disease pathogenesis, and explain why we have investigated this particular molecule among a broad cytokines panel.
Last, we found both higher CCL2 levels in non-survivors and a significant relationship between CCL2 levels and probability of survival and ETI requirement. Moreover, the Kaplan-Meier analysis conducted on a prespecified composite outcome (death and/or ETI) confirmed that patients with higher CCL2 values experienced a significantly worse prognosis. Cox regression analysis showed that, among a panel of cytokines, CCL2 had the strongest correlation with death/need for ETI. We analysed survival and ETI since they likely represent highly clinically significant endpoints in COVID-19, a disease characterized by high in-hospital mortality rates [2]. Taken together, these results thus could confirm a possible role for CCL2 as a prognostic biomarker in COVID-19, as already hypothesized by other studies on the topic [[14], [15], [16]].
Our study has some evident limits. First, it has a retrospective design, and it involves only two hospitals, located in the same area (the North-West side of Tuscany, Italy), thus potentially not including the whole heterogeneity of the Italian population. Moreover, we have collected CCL2 only at hospital admission, so we were not able to analyse its trajectory during hospitalization, especially relative to dynamic changes in other significant clinical parameters as P/F or D-dimer levels. Last, we use D-dimer to explore coagulation, but one may reasonably argue that a panel including some tests like von Willebrand factor, P-selectin, plasminogen activator inhibitor-1 or other coagulation factors levels or biological activity measurement, as done in other studies [7,20], might have improved the analysis. We decided to use D-dimer mainly because it has rapidly become a valuable and broadly available marker of both thrombotic events and prognosis in COVID-19 [18,19].
5. Conclusions
In conclusion, our study shows that CCL2 levels correlate with coagulation derangement and respiratory impairment severity, which are likely closely related in COVID-19 pathology: from this point of view, it is conceivable that CCL2 could be involved in the thromboinflammatory events that play a relevant role in the pathogenesis of the respiratory failure that characterises the most severe form of COVID-19. Our data must be considered preliminary, thus prompting further studies to better elucidate the most relevant disease mechanisms in COVID-19.
Funding
No funding was received for this work.
CRediT authorship contribution statement
DN, TN, AP and AC designed the study; DN, GB, SM, GM, SS, LG, MFal GT, FM, RP, FP, LCar and AC collected data; DM and AC performed statistical analysis; MFra, LCap and AP performed key laboratory test; DN, TN and AC draft and edited the paper; all authors approved the final version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.https://coronavirus.jhu.edu/
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Ranieri V.M., Rubenfeld G.D., Thompson B.T., et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 4.Leisman D.E., Deutschman C.S., Legrand M. Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. 2020;46:1105–1108. doi: 10.1007/s00134-020-06059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scudiero F., Silverio A., Di Maio M., et al. Pulmonary embolism in COVID-19 patients: prevalence, predictors and clinical outcome.[letter] Thromb. Res. 2021;198:34–39. doi: 10.1016/j.thromres.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goshua G., Pine A.B., Meizlish M.L., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Poll T., Herwald H. The coagulation system and its function in early immune defense. Thromb. Haemost. 2014;112:640–648. doi: 10.1160/TH14-01-0053. [DOI] [PubMed] [Google Scholar]
- 9.Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- 10.Frantzeskaki F., Armaganidis A., Orfanos S.E. Immunothrombosis in acute respiratory distress syndrome: cross talks between inflammation and coagulation. Respiration. 2017;93:212–225. doi: 10.1159/000453002. [DOI] [PubMed] [Google Scholar]
- 11.Gu S.X, Tyagi T., Jain K, et al. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol. 2021;18:194–209. doi: 10.1038/s41569-020-00469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deshmane S.L., Kremlev S., Amini S., Sawaya B.E. Monocyte chemoattractant protein-1 (MCP-1): an overview. J. Interf. Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Celi A., Lorenzet R., Furie B.C., Furie B. Microparticles and a P-selectin-mediated pathway of blood coagulation. Dis. Markers. 2004;20:347–352. doi: 10.1155/2004/876031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S., Jiang L., Li X., et al. Clinical and pathological investigation of patients with severe COVID-19. JCI Insight. 2020;5:e138070. doi: 10.1172/jci.insight.138070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chi Y., Ge Y., Wu B., et al. Serum cytokine and chemokine profile in relation to the severity of coronavirus disease 2019 in China. J. Infect. Dis. 2020;222:746–754. doi: 10.1093/infdis/jiaa363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abers M.S., Delmonte O.M., Ricotta E.E., et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight. 2021;6:e144455. doi: 10.1172/jci.insight.144455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weitz J.I., Fredenburgh J.C., Eikelboom J.W. A test in context: D-dimer. J. Am. Coll. Cardiol. 2017;70:2411–2420. doi: 10.1016/j.jacc.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berger J.S., Kunichoff D., Adhikari S., et al. Prevalence and outcomes of D-dimer elevation in hospitalized patients with COVID-19. Arterioscler. Thromb. Vasc. Biol. 2020;40:2539–2547. doi: 10.1161/ATVBAHA.120.314872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taus F., Salvagno G., Canè S., et al. Platelets promote thromboinflammation in SARS-CoV-2 pneumonia. Arterioscler. Thromb. Vasc. Biol. 2020;40:2975–2989. doi: 10.1161/ATVBAHA.120.315175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosell A., Havervall S., von Meijenfeldt F., et al. Patients with COVID-19 have elevated levels of circulating extracellular vesicle tissue factor activity that is associated with severity and mortality—brief report. Arterioscler. Thromb. Vasc. Biol. 2021;41:878–882. doi: 10.1161/ATVBAHA.120.315547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthay M.A., Zemans R.L., Zimmerman G.A., et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Middleton E.A., He X.Y., Denorme F., et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jøntvedt Jørgensen M., Holter J.C., Christensen E.E., et al. Increased interleukin-6 and macrophage chemoattractant protein-1 are associated with respiratory failure in COVID-19. Sci. Rep. 2020;10:21697. doi: 10.1038/s41598-020-78710-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhont S., Derom E., Van Braeckel E., Depuydt P., Lambrecht B.N. The pathophysiology of ‘happy’ hypoxemia in COVID-19. Respir. Res. 2020;21:198. doi: 10.1186/s12931-020-01462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He L., Ding Y., Zhang Q., et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung C.Y., Poon L.L., Ng I.H., et al. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J. Virol. 2005;79:7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tu X., Chong W.P., Zhai Y., et al. Functional polymorphisms of the CCL2 and MBL genes cumulatively increase susceptibility to severe acute respiratory syndrome coronavirus infection. J. Infect. 2015;71:101–109. doi: 10.1016/j.jinf.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams A.E., José R.J., Mercer P.F., et al. Evidence for chemokine synergy during neutrophil migration in ARDS. Thorax. 2017;72:66–73. doi: 10.1136/thoraxjnl-2016-208597. [DOI] [PMC free article] [PubMed] [Google Scholar]