Abstract
Frailty is a common comorbidity associated with adverse events in patients with heart failure, and early recognition is key to improving its management. We hypothesized that the AST to ALT ratio (AAR) could be a marker of frailty in patients with heart failure. Data from the FRAGILE-HF study were analyzed. A total of 1327 patients aged ≥ 65 years hospitalized with heart failure were categorized into three groups based on their AAR at discharge: low AAR (AAR < 1.16, n = 434); middle AAR (1.16 ≤ AAR < 1.70, n = 487); high AAR (AAR ≥ 1.70, n = 406). The primary endpoint was one-year mortality. The association between AAR and physical function was also assessed. High AAR was associated with lower short physical performance battery and shorter 6-min walk distance, and these associations were independent of age and sex. Logistic regression analysis revealed that high AAR was an independent marker of physical frailty after adjustment for age, sex and body mass index. During follow-up, all-cause death occurred in 161 patients. After adjusting for confounding factors, high AAR was associated with all-cause death (low AAR vs. high AAR, hazard ratio: 1.57, 95% confidence interval, 1.02–2.42; P = 0.040). In conclusion, AAR is a marker of frailty and prognostic for all-cause mortality in older patients with heart failure.
Subject terms: Heart failure, Prognostic markers, Geriatrics
Introduction
Frailty is a geriatric syndrome characterized by a decline in functional reserve and increased vulnerability to stressors1. Frailty is common and associated with worse clinical outcomes in patients with acute2 and chronic heart failure3,4. Since frailty is reversible and patients can possibly return to a healthy state, early identification is essential. However, simple and readily available markers for frailty are yet to be found.
We previously demonstrated a significant association between high aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio (AAR) and low body mass index, malnutrition, and worse outcomes in patients with acute heart failure5, suggesting high AAR as a marker of frailty status in patients with heart failure. However, the direct association between AAR and physical frailty or exercise capacity has not been evaluated in older patients with heart failure. Therefore, we aimed to evaluate the association between AAR and physical frailty, exercise capacity, and prognosis in older patients with heart failure.
Results
During our study period, 1332 hospitalized heart failure patients aged ≥ 65 years were registered in the FRAGILE-HF study. Patients lacking AST or ALT values (n = 5) were excluded; thus, a total of 1327 patients (age: 80.2 ± 7.8 years, 43% females) were enrolled in the analysis. Patients were assigned into three groups according to the AAR cut-off values: low AAR, AAR < 1.16 (n = 434); middle AAR, 1.16 ≤ AAR < 1.70 (n = 487); and high AAR, AAR ≥ 1.70 (n = 406) (Fig. 1). The baseline characteristics are described in Table 1. Patients with high AAR were older in age, more of females, and had New York Heart Association class III/IV, prior history of heart failure and use of loop diuretics, lower body mass index, and higher left ventricular ejection fraction. Laboratory data showed that patients with high AAR had higher brain natriuretic peptide (BNP) and lower albumin, AST, ALT, estimated glomerular filtration rate, and hemoglobin levels.
Figure 1.
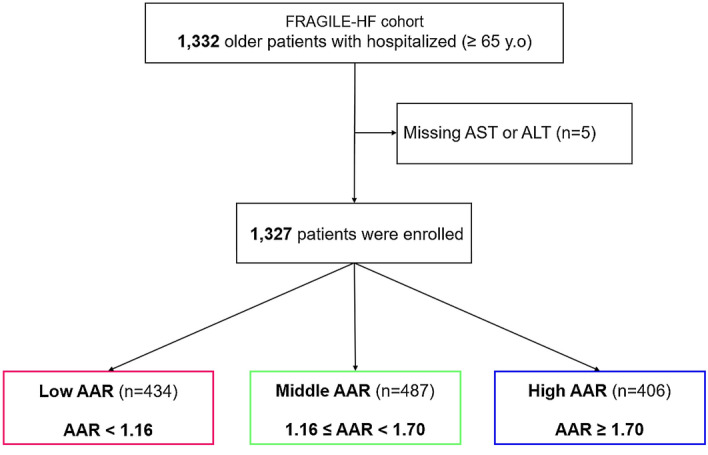
Study flowchart: Participants were divided into three groups based on their aspartate aminotransferase to alanine aminotransferase ratio. AAR aspartate aminotransferase to alanine aminotransferase ratio, ALT alanine aminotransferase, AST aspartate aminotransferase, HF heart failure.
Table 1.
Baseline clinical characteristics of the patients.
| AAR tertile | P value | |||
|---|---|---|---|---|
| Low AAR | Middle AAR | High AAR | ||
| n = 434 | n = 487 | n = 406 | ||
| AAR at discharge | 0.92 [0.79–1.05] | 1.40 [1.29–1.53] | 2.00 [1.86–2.33] | <0.001 |
| Clinical demographics | ||||
| Age, years | 78 [71–84] | 81 [74–86] | 84 [78–88] | <0.001 |
| Male, n (%) | 320 (73.7%) | 278 (57.1%) | 159 (39.2%) | <0.001 |
| Body mass index, kg/m² | 21.4 [19.6–23.7] | 21.2 [19.1–23.7] | 20.0 [17.8–22.1] | <0.001 |
| Current smoker, n (%) | 58 (13.4%) | 46 (9.4%) | 25 (6.2%) | 0.002 |
| NHYA class III/IV, n (%) | 48 (11.1%) | 71 (14.6%) | 78 (19.2%) | 0.004 |
| Systolic blood pressure, mmHg | 112 [102–125] | 112 [102–126] | 112 [102–124] | 0.529 |
| Diastolic blood pressure, mmHg | 62 [56–68] | 61 [56–70] | 60 [54–68] | 0.031 |
| Heart rate, bpm | 70 [60–79] | 70 [60–79] | 70 [61–80] | 0.235 |
| Left ventricular ejection fraction, % | 40 [31–58] | 45 [32–60] | 50 [34–62] | <0.001 |
| Prior history of heart failure, n (%) | 145 (33.4%) | 204 (41.9%) | 200 (49.3%) | <0.001 |
| Hypertension, n (%) | 312 (71.9%) | 352 (72.3%) | 281 (69.2%) | 0.560 |
| Dyslipidemia, n (%) | 162 (37.3%) | 189 (38.8%) | 123 (30.3%) | 0.021 |
| Diabetes mellitus, n (%) | 156 (35.9%) | 199 (40.9%) | 119 (29.3%) | 0.002 |
| Coronary artery disease, n (%) | 153 (35.3%) | 173 (35.5%) | 142 (35.0%) | 0.986 |
| COPD, n (%) | 53 (12.2%) | 49 (10.1%) | 41 (10.1%) | 0.501 |
| Laboratory data | ||||
| Albumin, g/dL | 3.6 [3.2–3.8] | 3.5 [3.2–3.8] | 3.3 [3.0–3.6] | <0.001 |
| Blood urea nitrogen, mg/dL | 25 [19–34] | 25 [20–35] | 28 [20–38] | 0.026 |
| Creatinine, mg/dL | 1.12 [0.90–1.44] | 1.16 [0.93–1.60] | 1.23 [0.92–1.69] | 0.008 |
| eGFR, mL/min/1.73m² | 60 [44–74] | 52 [36–69] | 46 [31–62] | <0.001 |
| Aspartate aminotransferase, U/L | 25 [19–32] | 22 [18–28] | 21 [17–27] | <0.001 |
| Alanine aminotransferase, U/L | 28 [20–40] | 16 [12–20] | 10 [8–13] | <0.001 |
| Sodium, mEq/L | 139 [137–141] | 139 [137–141] | 140 [137–142] | 0.099 |
| Hemoglobin, g/dL | 12.5 [11.2–14.0] | 11.7 [10.4–13.0] | 10.7 [9.6–12.0] | <0.001 |
| Brain natriuretic peptide, pg/mL | 231.6 [118.7–451.2] | 271.8 [130.1–477.8] | 331.8 [162.0–601.5] | <0.001 |
| Medication | ||||
| ACE-I/ARB, n (%) | 304 (70.0%) | 338 (69.4%) | 249 (61.3%) | 0.011 |
| β-blockers, n (%) | 337 (77.6%) | 376 (77.2%) | 253 (62.3%) | <0.001 |
| Loop diuretics, n (%) | 359 (82.7%) | 435 (89.3%) | 364 (89.7%) | 0.002 |
| MRA, n (%) | 242 (55.8%) | 240 (49.3%) | 178 (43.8%) | 0.003 |
Values are presented as number (%) or median [interquartile range].
AAR, aspartate aminotransferase to alanine aminotransferase ratio; NYHA, New York Heart Associations; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; ACE-I, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; MRA, mineralocorticoid receptor antagonists.
Regarding frailty and nutritional status, univariable and multivariable linear regression analysis revealed that handgrip strength, 6-min walk distance, short physical performance battery (SPPB), and geriatric nutritional risk index (GNRI) were significantly lower in patients with AAR ≥ 1.70 compared to those with AAR < 1.16 after adjustment for age and sex (Table 2). Univariate and multivariable logistic regression showed that AAR ≥ 1.70 was significantly associated with physical frailty even after adjustment for age, sex and body mass index (Table 3). We also checked the association between all these factors and AAR as a continuous variable on univariate/multivariable linear regression analysis and found that these findings were not significantly changed (handgrip strength, t value − 4.69, P < 0.001; 6-min walk distance, t value − 2.04, P = 0.041; SPPB, t value − 1.70, P = 0.089; GNRI, t value − 4.36, P < 0.001).
Table 2.
Association between AAR and physical status.
| Unadjusted model | Adjusted model* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | Standard error | t value | P value | Coefficient | 95% CI | Standard error | t value | R² | P value | |
| Hand grip strength | 0.503 | ||||||||||
| Low vs. middle AAR | − 2.97 | − 3.94–− 2.00 | 0.50 | − 5.99 | <0.001 | − 0.90 | − 1.64–− 0.16 | 0.38 | − 2.38 | 0.017 | |
| Low vs. high AAR | − 6.55 | − 7.57–− 5.53 | 0.52 | − 12.61 | <0.001 | − 2.10 | − 2.91–− 1.28 | 0.41 | − 5.05 | <0.001 | |
| 6− minute walk distance | 0.243 | ||||||||||
| Low vs. middle AAR | − 25.01 | − 41.63–− 8.38 | 8.47 | − 2.95 | 0.003 | − 3.18 | − 18.23–11.87 | 7.67 | − 0.41 | 0.678 | |
| Low vs. high AAR | − 70.37 | − 87.99–52.74 | 8.98 | − 7.83 | <0.001 | − 22.04 | − 38.75–− 5.33 | 8.52 | − 2.59 | 0.010 | |
| SPPB | 0.252 | ||||||||||
| Low vs. middle AAR | − 0.65 | − 1.07–− 0.24 | 0.21 | − 3.12 | 0.002 | − 0.10 | − 0.47–0.27 | 0.19 | − 0.52 | 0.600 | |
| Low vs. high AAR | − 1.77 | − 2.20–− 1.34 | 0.22 | − 8.06 | <0.001 | − 0.51 | − 0.92–− 0.10 | 0.21 | − 2.46 | 0.014 | |
| GNRI | 0.093 | ||||||||||
| Low vs. middle AAR | − 0.93 | − 2.34–0.48 | 0.72 | − 1.29 | 0.197 | − 0.35 | − 1.74–1.05 | 0.71 | − 0.49 | 0.624 | |
| Low vs. high AAR | − 5.33 | − 6.80–− 3.82 | 0.76 | − 7.05 | <0.001 | − 3.94 | − 5.47–− 2.40 | 0.78 | − 5.02 | <0.001 | |
*Adjusted for age and gender.
AAR, aspartate aminotransferase to alanine aminotransferase ratio; CI, confidence interval; GNRI, geriatric nutritional risk index; SPPB, short physical performance battery.
Table 3.
Logistic analysis for physical frailty according to the Fried phenotype model.
| Unadjusted model | Adjusted model* | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P value | Odds ratio | 95% CI | P value | |
| AAR as categorical variable | ||||||
| Low AAR | 1 (reference) | 1 (reference) | ||||
| Middle AAR | 1.37 | 1.05–1.80 | 0.022 | 1.24 | 0.94–1.64 | 0.127 |
| High AAR | 1.91 | 1.43–2.56 | <0.001 | 1.45 | 1.06–1.99 | 0.021 |
| AAR as continuous variable | 1.66 | 1.35–2.05 | <0.001 | 1.39 | 1.11–1.73 | 0.004 |
* Adjusted for age, gender and body mass index.
AAR, aspartate aminotransferase to alanine aminotransferase ratio; CI, confidence interval.
Data regarding 1-year mortality were collected for 97.5% of the 1,327 study patients, and all-cause death occurred in 161 (12.4%) patients. Kaplan–Meier curves showed that event-free rates were lower among patients with high AAR than in the other two groups (Fig. 2, log-rank P < 0.001). Cox proportional hazards analysis after excluding 214 patients (16.1%) with missing data on prognosis or adjustment variables showed that patients with high AAR had a significant higher risk of 1-year mortality than those with low AAR even after adjusting for the Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) risk score and log-transformed BNP (Table 4). We also performed multiple imputations by creating 20 imputed datasets as sensitivity analysis and obtained the same results in the adjusted model (hazard ratio: 0.92, 95% CI: 0.60–1.42 for middle AAR, and hazard ratio: 1.51, 95% CI: 1.01–2.26, P = 0.043 for high AAR). Moreover, the interaction between AAR and sex was investigated because sex may have significant interaction with frailty. Consequently, we found that there was no significant interaction between AAR and sex (interaction P = 0.430).
Figure 2.
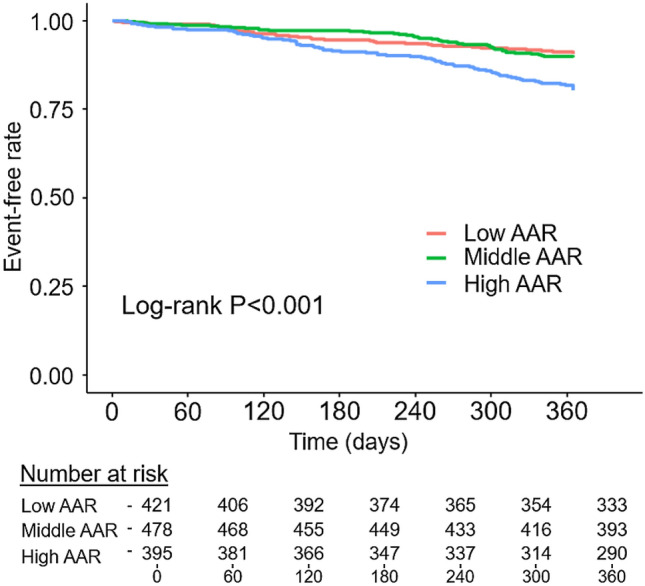
Kaplan–Meier curves of all-cause death stratified by aspartate aminotransferase to alanine aminotransferase ratio. AAR aspartate aminotransferase to alanine aminotransferase ratio.
Table 4.
Cox proportional hazard analysis for all-cause death.
| Unadjusted model | Adjusted model* | |||||||
|---|---|---|---|---|---|---|---|---|
| C-index | Hazard ratio | 95% CI | P value | C-index | Hazard ratio | 95% CI | P value | |
| Groups | 0.59 | 0.71 | ||||||
| Low AAR | 1 (reference | 1 (reference) | ||||||
| Middle AAR | 1.08 | 0.70–1.67 | 0.715 | 0.98 | 0.62–1.56 | 0.938 | ||
| High AAR | 2.19 | 1.48–3.25 | <0.001 | 1.57 | 1.02–2.42 | 0.040 | ||
* Adjusted for Meta-Analysis Global Group in Chronic Heart Failure risk score and log brain natriuretic peptide.
AAR, aspartate aminotransferase to alanine aminotransferase ratio; CI, confidence interval.
To test if this association was mainly driven by AST alone or ALT alone, we performed the same analysis by segmenting the entire cohort into three groups stratified by the tertile of AST and ALT. For ALT, a higher tertile group was associated with lower handgrip strength and higher GNRI, but not with SPPB and 6-min walk distance after adjustment for age and sex (Supplementary Table 1). Likewise, higher tertile groups of AST were not associated with handgrip strength, 6-min walk distance, SPPB, and GNRI (Supplementary Table 2). We compared the prognostic predictability of AST, ALT, and AAR using the area under the receiver operating characteristic curve (AUC) (AAR: AUC 0.60, 95% confidence interval [CI] 0.56–0.65; AST: AUC 0.51, 95% CI 0.46–0.56; ALT: AUC 0.56, 95% CI 0.51–0.61) and found that AAR exceeded AST and ALT in AUC (AAR vs. AST, P = 0.014; AAR vs. ALT, P = 0.017). Moreover, neither AST nor ALT was associated with 1-year mortality after adjusting for the MAGGIC risk score and log-transformed BNP (Supplementary Table 3).
Discussion
In the current study evaluating patients hospitalized for heart failure, we demonstrated that: 1) high AAR was associated with physical frailty, malnutrition, and lower exercise capacity independent of age and sex, 2) high AAR was independently associated with greater 1-year mortality. Our study results imply that AAR can be a potential surrogate marker of frailty in patients with heart failure.
Physical frailty is known as a clinical syndrome composed of five physical conditions, including weakness, slow gait speed, exhaustion, weight loss, and low physical activities6. It progresses through a vicious circle of decrease in muscle strength, metabolic rate, energy expenditure, and worsening nutritional status7. Thus, decreased physical activity and malnutrition are the important components of physical frailty. In the current study, we showed that high AAR was associated with low SPPB, short 6-min walk distance, poor hand grip strength, and worse nutritional status. Although we demonstrated the association between high AAR and poor prognosis, the mechanism behind the association of AAR and physical function has not been well demonstrated. However, several possible mechanisms have been proposed for the association between high AAR and frailty. For instance, ALT plays an essential role in gluconeogenesis, the process of breaking down muscle protein into amino acids, converting alanine to α-ketoglutarate to produce energy8. Moreover, a previous study on a diabetes mellitus cohort reported that low ALT was correlated with low handgrip strength9. Given these study results, it could be that ALT is the main driver of the association between higher AAR and frailty; however, as we clearly showed in our study results, AAR was more strongly associated with frailty, exercise capacity, and 1-year mortality independently of other covariates, than ALT alone. This may because the proportion of AST and ALT offsets the impact of liver dysfunction and makes AAR more specific to muscle capacity, which cannot be evaluated otherwise. AAR is thus a potential prognostic predictor independent of other covariates in patients with heart failure.
Frailty may be associated with sex, and a previous study including 10% of patients with heart failure suggested that frailty had a significant interaction with sex on survival10. However, we cannot find any interaction between sex and frailty. The discrepancy may be explained by the differences in baseline characteristics. Our cohort was all heart failure patients, and was older and had lower body mass index than a previous cohort. However, we should keep “the sex difference” in mind.
We also reconfirmed the association between AAR and malnutrition, which we have previously described in patients with acute heart failure5. Malnutrition often leads to a deficiency of pyridoxal-5′-phosphatase11, which is the biologically active form of vitamin B6. ALT is more vulnerable to pyridoxal-5′-phosphatase deprivation than AST12, which may explain the association between malnutrition and higher AAR. Indeed, in healthy populations aged ≥ 65 years, lower ALT was associated with lower vitamin B6 levels and physical frailty13. Reconfirmation of this association in the FRAGILE-HF cohort with predominantly older patients strengthens our hypothesis; however, it should be further investigated in future studies as GNRI is not a gold standard of nutritional status in patients with heart failure, and pyridoxal-5-phosphatase was not directly evaluated in this study.
In summary, our study results suggest that high AAR may reflect poor physical function and malnutrition, which are important components of the frailty cycle. This implies the possibility of AAR to serve as a marker for screening/monitoring of frailty and subsequent poor prognostic outcomes in older patients with heart failure. As both AST and ALT are readily available and are easily measurable biomarkers in daily clinical practice, early identification of those at a high risk of frailty among older patients with heart failure may lead to early intervention, including nutritional intervention or exercise rehabilitation, and subsequently, a better prognosis. Muscle training and nutritional supplements may be effective for frail patients with heart failure. Aerobic endurance training and resistance training were associated with an improvement in exercise capacity and quality of life in patients with heart failure14,15. However, this hypothesis should be evaluated in large-scale randomized controlled trials in the future.
There are several limitations in the current study. First, we could not exclude those with a history of liver disease or heavy consumption of alcohol that can impact liver function tests and subsequently AAR and its association with prognosis. Second, because laboratory data were obtained only at discharge, serial changes in AAR were not available, and we could not analyze their clinical characteristics and prognostic implications. Third, as our current study did not include patients under hemodialysis and those aged < 65 years; whether our study results apply to such patients should be tested in future studies. Fourth, although we performed multivariable analyses adjusting for confounding variables, the results may yet be confounded by other factors. Finally, our study included only Japanese patients, and our cohort (mean body mass index, 21.4 ± 3.8 kg/m2) was much smaller physically than a general Western cohort. Therefore, whether the results of our study can apply to Western populations is unknown.
In conclusion, AAR, which is readily available at a low cost, is strongly associated with frailty and poor prognosis in older patients with hospitalized heart failure.
Methods
Study design and patient population
This is a post hoc analysis of the FRAGILE-HF cohort study, in which 1,332 hospitalized patients aged ≥ 65 years with decompensation of heart failure, who could ambulate at discharge, were included. The study design and main results have already been published elsewhere16. Briefly, the main objective of FRAGILE-HF was to evaluate the prevalence and prognostic impact of multi-frailty domains in older patients with heart failure who require hospitalization. The exclusion criteria were: (1) previous heart transplantation or left ventricular assist device implantation, (2) chronic peritoneal dialysis or hemodialysis, and (3) acute myocarditis. Patients with missing BNP or N-terminal-proBNP data and patients with a BNP level < 100 pg/mL or N-terminal-proBNP level < 300 pg/mL at admission were also excluded as the diagnosis may be unclear in these cases. We enrolled patients with both heart failure with reduced and preserved ejection fraction. Fifteen hospitals in Japan enrolled patients from September 2016 to March 2018. Before discharge, physical examination, echocardiography, blood samples, and drug history were obtained when patients were stable. From the AST and ALT values obtained before discharge, patients were assigned into three groups based on predetermined AAR cut-off values as follows: low AAR, AAR < 1.16; middle AAR, 1.16 ≤ AAR < 1.70; and high AAR, AAR ≥ 1.705.
All participants were notified regarding their participation in the present study, and it was explained that they were free to opt out of participation at any time. Written, informed consent was obtained from each patient before enrolment. Our study complies with the Declaration of Helsinki and the Japanese Ethical Guideline for Medical and Health Research involving Human Subjects. The study protocol was approved by the Sakakibara Heart Institution of Okayama Research Ethics Committee. Study information, including objectives, inclusion and exclusion criteria, primary outcome, and the names of participating hospitals, were published in the publicly available University Hospital Information Network (UMIN-CTR, unique identifier: UMIN000023929) before the first patient was enrolled.
Assessment of physical frailty and physical function
Physical frailty was defined by the Fried phenotype model, which is the most widely applied model, and generally considered as the standard model for physical frailty6. According to Fried's model, the frailty phenotype consists of the following five elements: slowness (gait speed), weakness (handgrip strength), weight loss, exhaustion, and low physical activity17. Patients who met ≥ 3 out of 5 criteria were considered physically frail according to previous studies and the predefined definition in FRAGILE-HF16,18,19.
We also evaluated SPPB and 6-min walk distance, performed by experienced physical therapists and/or heart failure specialists. The SPPB consists of 3 physical performance tests to assess each frailty domain, including balance (static standing balance), gait speed test (4-m walk time), and weakness (time to complete 5 repeated chair stands)20. Each test is scored from 0–4, for a total score of 0–12. For balance, the participants were asked to maintain their feet in side-by-side, semi-tandem, and tandem positions for 10 s each. For the gait speed assessment test, the participants’ usual speed was timed during a 4-m walk. For the chair stand test, participants were asked to stand up and sit down five times as quickly as possible. The 6-min walk distance was assessed in an unobstructed hallway according to the guideline as follows21: patients were instructed to walk as fast as possible between two points positioned 30-m apart, and the distance walked in 6 min was recorded. Patients were allowed to use an assistive device if needed.
Assessment of nutritional status
GNRI22 was used to evaluate the nutritional status of patients. The index was calculated as follows: 14.89 × serum albumin concentration (g/L) + 41.7 × (weight [kg]/ideal weight [kg]). Ideal weight was defined as: 22 × height (m2).
Outcomes
The prognoses of registered patients within 1 year of discharge were prospectively collected up to March 2019. Our primary outcome was all-cause death. After discharge, most patients were followed up in outpatient clinics at least every 3 months, and additionally on a need basis. For those without in-person follow-up scheduled in clinics, prognostic data were obtained from telephone interviews and medical records of other medical departments that cared for the patient or from the family.
Statistical analysis
Normally distributed data are expressed as mean and standard deviation, and non-normally distributed data are reported as median with interquartile range. Categorical data are shown as numbers and percentages. Data were compared between groups using Student’s t-tests or Mann–Whitney U tests for continuous variables and chi-squared or Fisher exact tests for categorical variables as appropriate. The associations between AAR and physical function and nutritional status were investigated using linear regression analysis. In the multivariable linear regression analyses, age and gender were used as adjustment variables23–29. Association between physical frailty and AAR was evaluated with logistic regression analysis. Age, sex and body mass index were used as adjustment variable in the multivariable logistic regression analyses30. Regarding time-dependent survival analysis, event-free survival curves were constructed using the Kaplan–Meier survival method and compared with log-rank statistics. The AUC for 1-year mortality was used to evaluate the predictive value of AAR for 1-year mortality. As for the prognostic outcome of all-cause death, the MAGGIC risk score was calculated for each patient as previously described31. The discrimination and calibration of this risk score have been well validated in Japanese patients with heart failure32. As adding BNP level at discharge has been shown to be associated with improvement of discrimination with adequate calibration32, we used the MAGGIC risk score and log-transformed BNP as an adjustment variable in a multivariable prognostic model for the outcome of all-cause death.
A two-tailed P value < 0.05 was considered statistically significant. Statistical analyses were performed using R version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria; ISBN 3-900051-07-0, URL http://www.R-project.org).
Supplementary Information
Author contributions
D.M. and Y.M. contributed to the conception or design of the work. D.M., N.K., K.J., K.S., K.K., H.S., Y.O., E.M., M.K., T.Ki., K.Iw., H.W., M.H., T.D., T.S., T.Ka., H.N., T.O., K.Iz., S.Y., N.A., R.Y., K.O., S.M. and Y.M. contributed to the acquisition, analysis, or interpretation of data for the work. D.M. and Y.M. drafted the manuscript. K.J. and M.K. critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Funding
FRAGILE-HF was supported by Novartis Pharma Research Grants and a Japan Heart Foundation Research Grant. This work was also partially supported by JSPS KAKENHI Grant Number 18K15862.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
Dr. Yuya Matsue and Takatoshi Kasai are affiliated with a department endowed by Philips Respironics, ResMed, Teijin Home Healthcare, and Fukuda Denshi, and Dr. Yuya Matsue received an honorarium from Otsuka Pharmaceutical Co., Ltd., and Novartis Japan. Dr. Kagiyama reports grants from Philips, Asahi KASEI Corporation, Toho Holdings Co., Ltd, and Inter Reha Co., Ltd. outside the submitted work. Dr. Kamiya has received research funding from Eiken Chemical Co. Ltd. Other authors have nothing to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-91368-z.
References
- 1.Hoogendijk EO, et al. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 2.Vidán MT, et al. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur. J. Heart Fail. 2016;18:869–875. doi: 10.1002/ejhf.518. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhry SI, Wang Y, Gill TM, Krumholz HM. Geriatric conditions and subsequent mortality in older patients with heart failure. J. Am. Coll. Cardiol. 2010;55:309–316. doi: 10.1016/j.jacc.2009.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang, X. et al. Impact of frailty on mortality and hospitalization in chronic heart failure: A systematic review and meta-analysis. J. Am. Heart Assoc.7, (2018). [DOI] [PMC free article] [PubMed]
- 5.Maeda D, et al. Relation of aspartate aminotransferase to alanine aminotransferase ratio to nutritional status and prognosis in patients with acute heart failure. Am. J. Cardiol. 2021;139:64–70. doi: 10.1016/j.amjcard.2020.10.036. [DOI] [PubMed] [Google Scholar]
- 6.Fried LP, et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001;56:146–157. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 7.Xue QL, Bandeen-Roche K, Varadhan R, Zhou J, Fried LP. Initial manifestations of frailty criteria and the development of frailty phenotype in the women’s health and aging study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008;63:984–990. doi: 10.1093/gerona/63.9.984. [DOI] [PubMed] [Google Scholar]
- 8.Le Couteur DG, et al. The association of alanine transaminase with aging, frailty, and mortality. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010;65A:712–717. doi: 10.1093/gerona/glq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung SM, Moon JS, Yoon JS, Won KC, Lee HW. Low alanine aminotransferase levels predict low muscle strength in older patients with diabetes: A nationwide cross-sectional study in Korea. Geriatr. Gerontol. Int. 2020;20:271–276. doi: 10.1111/ggi.13868. [DOI] [PubMed] [Google Scholar]
- 10.Corbi G, et al. Inter-relationships between Gender, Frailty and 10-Year Survival in Older Italian Adults: an observational longitudinal study. Sci. Rep. 2019;9:1–7. doi: 10.1038/s41598-019-54897-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kjeldby IK, Fosnes GS, Ligaarden SC, Farup PG. Vitamin B6 deficiency and diseases in elderly people—a study in nursing homes. BMC Geriatr. 2013;13:1–8. doi: 10.1186/1471-2318-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elinav E, et al. Low alanine aminotransferase activity in older people is associated with greater long-term mortality. J. Am. Geriatr. Soc. 2006;54:1719–1724. doi: 10.1111/j.1532-5415.2006.00921.x. [DOI] [PubMed] [Google Scholar]
- 13.Vespasiani-Gentilucci U, et al. Low alanine aminotransferase levels in the elderly population: frailty, disability, sarcopenia, and reduced survival. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018;73:925–930. doi: 10.1093/gerona/glx126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandey A, et al. Exercise training in patients with heart failure and preserved ejection fraction meta-analysis of randomized control trials. Circ. Hear. Fail. 2015;8:33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jewiss D, Ostman C, Smart NA. The effect of resistance training on clinical outcomes in heart failure: a systematic review and meta-analysis. Int. J. Cardiol. 2016;221:674–681. doi: 10.1016/j.ijcard.2016.07.046. [DOI] [PubMed] [Google Scholar]
- 16.Matsue Y, et al. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: the FRAGILE-HF cohort study. Eur. J. Heart Fail. 2020;22:2112–2119. doi: 10.1002/ejhf.1926. [DOI] [PubMed] [Google Scholar]
- 17.Bandeen-Roche K, et al. Phenotype of frailty: characterization in the Women’s Health and aging studies. . J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka S, et al. Prevalence and prognostic value of the coexistence of anaemia and frailty in older patients with heart failure. ESC Hear. Fail. 2021;8:625–633. doi: 10.1002/ehf2.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nozaki K, et al. Validity and utility of the questionnaire-based FRAIL scale in older patients with heart failure: findings from the FRAGILE-HF. J. Am. Med. Dir. Assoc. 2021 doi: 10.1016/j.jamda.2021.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Volpato S, et al. Predictive value of the short physical performance battery following hospitalization in older patients. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011;66:89–96. doi: 10.1093/gerona/glq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Issues S, Test MW, Equipment R, Preparation P. American thoracic society ATS statement : guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 22.Bouillanne O, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am. J. Clin. Nutr. 2005;82:777–783. doi: 10.1093/ajcn/82.4.777. [DOI] [PubMed] [Google Scholar]
- 23.Albala C, et al. Frequency of frailty and its association with cognitive status and survival in older Chileans. Clin. Interv. Aging. 2017;12:995–1001. doi: 10.2147/CIA.S136906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirase T, et al. Impact of frailty on chronic pain, activities of daily living and physical activity in community-dwelling older adults: a cross-sectional study. Geriatr. Gerontol. Int. 2018;18:1079–1084. doi: 10.1111/ggi.13314. [DOI] [PubMed] [Google Scholar]
- 25.Toyoshima K, et al. Use of Dementia Assessment Sheet for Community-based Integrated Care System 8-items (DASC-8) for the screening of frailty and components of comprehensive geriatric assessment. Geriatr. Gerontol. Int. 2020;20:1157–1163. doi: 10.1111/ggi.14057. [DOI] [PubMed] [Google Scholar]
- 26.Kootaka Y, et al. The GLIM criteria for defining malnutrition can predict physical function and prognosis in patients with cardiovascular disease. Clin. Nutr. 2021;40:146–152. doi: 10.1016/j.clnu.2020.04.038. [DOI] [PubMed] [Google Scholar]
- 27.Maddocks M, et al. Physical frailty and pulmonary rehabilitation in COPD: a prospective cohort study. Thorax. 2016;71:988–995. doi: 10.1136/thoraxjnl-2016-208460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song X, Mitnitski A, Rockwood K. Prevalence and 10-Year outcomes of frailty in older adults in relation to deficit accumulation. J. Am. Geriatr. Soc. 2010;58:681–687. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 29.Yuki A, et al. Physical frailty and mortality risk in Japanese older adults. Geriatr. Gerontol. Int. 2018;18:1085–1092. doi: 10.1111/ggi.13316. [DOI] [PubMed] [Google Scholar]
- 30.Houghton JSM, et al. Frailty factors and outcomes in vascular surgery patients: a systematic review and meta-analysis. Ann. Surg. 2020;272:266–276. doi: 10.1097/SLA.0000000000003642. [DOI] [PubMed] [Google Scholar]
- 31.Pocock SJ, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur. Heart J. 2013;34:1404–1413. doi: 10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 32.Sawano M, et al. Performance of the MAGGIC heart failure risk score and its modification with the addition of discharge natriuretic peptides. ESC Heart Fail. 2018;5:610–619. doi: 10.1002/ehf2.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.


