Abstract
Traditional refrigeration technologies based on compression cycles of greenhouse gases pose serious threats to the environment and cannot be downscaled to electronic device dimensions. Solid-state cooling exploits the thermal response of caloric materials to changes in the applied external fields (i.e., magnetic, electric and/or mechanical stress) and represents a promising alternative to current refrigeration methods. However, most of the caloric materials known to date present relatively small adiabatic temperature changes ( to 10 K) and/or limiting irreversibility issues resulting from significant phase-transition hysteresis. Here, we predict by using molecular dynamics simulations the existence of colossal barocaloric effects induced by pressure (isothermal entropy changes of J K kg) in the energy material LiBH. Specifically, we estimate J K kg and K for a small pressure shift of P = 0.1 GPa at K. The disclosed colossal barocaloric effects are originated by a fairly reversible order–disorder phase transformation involving coexistence of Li diffusion and (BH) reorientational motion at high temperatures.
Subject terms: Condensed-matter physics, Materials science, Materials for energy and catalysis
Solid-state cooling is an environmentally friendly, energy efficient, and highly scalable technology that can solve most of the problems associated with conventional refrigeration methods based on compression cycles of greenhouse gases (i.e., environmental harm and lack of downsize scaling). Upon application of small or moderate magnetic, electric and/or mechanical stress field shifts good caloric materials undergo noticeable temperature changes ( to 10 K) as a result of induced phase transformations that involve large entropy variations ( to 100 J K kg)1–11. For instance, the archetypal elastocaloric compound NiTi exhibits an adiabatic temperature change of K/GPa12,13 and the eminent magnetocaloric crystal GdSiGe a of K/T14. Solid-state cooling capitalizes on such substantial caloric effects to engineer refrigeration cycles. From a performance point of view, that is, largest and (although these are not the only parameters defining cooling efficiency15,16), mechanocaloric effects driven by small uniaxial stresses (i.e., elastocaloric effects) and hydrostatic pressure (i.e., barocaloric effects) appear to be the most promising1–3,7,9–13.
Recently, colossal barocaloric effects (defined here as J K kg) have been measured in two different families of materials that display intriguing order–disorder phase transitions17–19. First, giant barocaloric effects have been theoretically predicted20 and experimentally observed in the archetypal superionic compound AgI17. AgI exhibits a first-order normal (low-entropy) to superionic (high-entropy) phase transition that responds to both temperature and pressure21–23 and which involves the presence of highly mobile silver ions in the high-T superionic state24,25. Likewise, the entropy changes estimated for other superionic phase transitions are also large in general26–29. And second, colossal barocaloric effects have been reported for the molecular solid neopentylglycol18,19, (CH)C(CHOH), and other plastic crystals15. In these solids, molecules reorient almost freely around their centers of mass, which remain localized at well-defined lattice positions. Molecular rotations lead to orientational disorder, which renders high entropy. By using hydrostatic pressure, it is possible to block such molecular reorientational motion and thus induce a fully ordered state characterized by low entropy29. The barocaloric effects resulting from this class of first-order order–disorder phase transitions are huge and comparable in magnitude to those achieved in conventional refrigerators with environmentally harmful gases15,18,19.
Here, we report the prediction of colossal barocaloric effects ( J K kg) in the energy material LiBH (LBH), a complex hydride that is already known from the fields of hydrogen storage30,31 and solid-state batteries32–34. By using molecular dynamics simulations, we identify a pressure-induced isothermal entropy change of J K kg and an adiabatic temperature change of K at K. These colossal entropy and temperature changes are driven by small hydrostatic pressure shifts of GPa, thus yielding gigantic barocaloric strengths of J K kg GPa and K GPa. The colossal barocaloric effects disclosed in bulk LBH are originated by simultaneous frustration of Li diffusion and (BH) icosahedra reorientational motion driven by hydrostatic pressure. Thus, alkali-metal complex borohydrides (BH, Li, Na, K, Cs35,36) emerge as a promising new family of caloric materials in which the salient phase-transition features of fast-ion conductors and plastic crystals coexist and cooperate to render colossal barocaloric effects.
Results
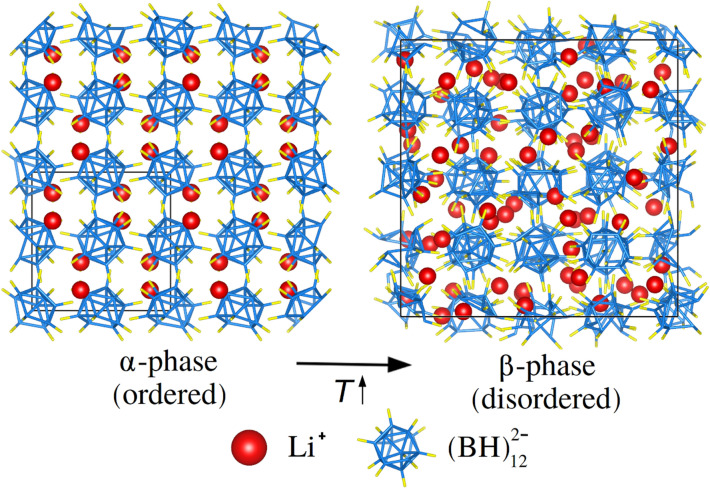
At ambient conditions, lithium dodecahydrododecaborate (LiBH), LBH, presents an ordered cubic phase (), referred to as hereafter, which is characterized by Li cations residing on near-trigonal-planar sites surrounded by three (BH) icosahedron anions. In turn, each (BH) anion resides in an octahedral cage surrounded by six Li cations (Fig. 1)30. A symmetry preserving order–disorder phase transition occurs at high temperatures ( K) that stabilises a disordered state, referred to as hereafter, in which the Li cations are mobile and the (BH) anions present reorientational motion (Fig. 1; Supplementary Fig. 1)32,38. The relative volume expansion that has been experimentally measured for such a first-order order–disorder phase transition is %32 and the corresponding phase transition enthalpy kJ kg37. The huge volume and enthalpy variations accompanying the transformation could be promising in the context of barocaloric effects if the involved phase transition was responsive to small hydrostatic pressure shifts of GPa. To the best of our knowledge, this possibility has never been hitherto explored. We performed classical molecular dynamics (MD) simulations based on a recently proposed LBH force field38 to fill up such a knowledge gap (“Methods” and Supplementary Methods) and thus assess the potential of complex hydrides as barocaloric materials.
Figure 1.
Low-T (ordered) and high-T (disordered) phases of bulk LiBH. The low-T phase () presents cubic symmetry and space group 30. In the high-T phase (), cubic symmetry is preserved but the Li ions are highly mobile and the (BH) icosahedra present reorientational disorder32. The T-induced phase transition is an order–disorder isosymmetric transformation that is regarded as of first order32. Li, B, and H ions are represented with red, blue, and yellow colours, respectively.
Figure 2 shows the P–T phase diagram of bulk LBH theoretically estimated with MD techniques (“Methods”). We determined the coexistence line of the and phases by conducting numerous MD simulations at small P–T shifts of 0.05 GPa and 12.5 K, and by monitoring the structural, Li diffusion, and (BH) reorientational properties of the system (“Methods”). In consistency with the experiments, a point in the – coexistence line of Fig. 2 was identified by sharp and simultaneous changes in the volume, Li diffusion coefficient (), and (BH) reorientational frequency () of bulk LBH (errorbars in Fig. 2 correspond to statistical uncertainties obtained through time averages). It was found that the critical temperature of the transformation can be certainly modified by hydrostatic pressure. Specifically, increases under increasing pressure at an approximate rate of K GPa; a simple second-order polynomial fit to our set of predicted – coexistence points renders the analytical expression , in which the temperature and pressure are respectively expressed in units of K and GPa.
Figure 2.
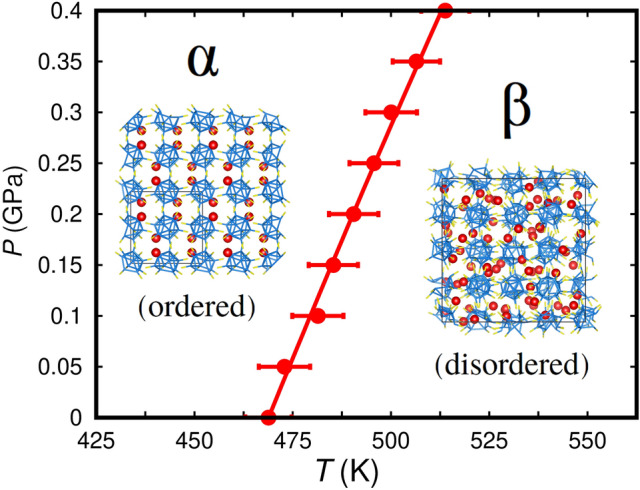
Estimated LiBH phase diagram expressed as a function of pressure (P) and temperature (T). Results were obtained from classical molecular dynamics simulations performed with the LBH force field reported in work38. The coexistence line separating the stability region of the and phases, represented with a solid red line, was determined through a polynomial fit to the theoretical phase-transition data (solid red dots and error bars) and corresponds to the analytical expression , where the temperature and pressure are respectively expressed in units of K and GPa. Error bars represent statistical uncertainties estimated with time-average techniques (“Methods”).
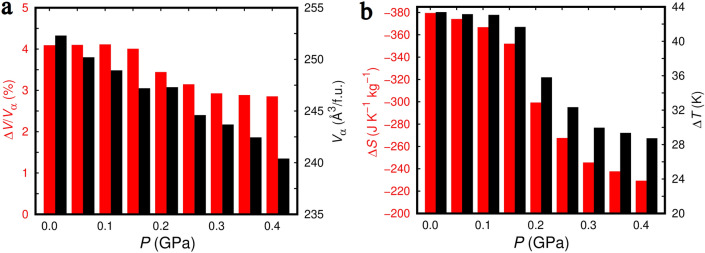
The predicted volume change associated with the phase transition expressed as a function of pressure are shown in Fig. 3a. At zero pressure, our MD simulations render a transition volume change, , relative to the volume of the phase at (namely, Å per formula unit—f.u.) of %. This result significantly underestimates the corresponding experimental value of %32. The main reason for this discrepancy is that the employed force field is not perfect and overestimates the zero-pressure volume of the and phases at by % and 5% (e.g., Å/f.u.32), respectively. Also, it should be noted that in our simulations we considered perfect and stoichiometric bulk LBH configurations whereas in real samples crystal defects like vacancies, grain boundaries and dislocations are always present; such crystal defects typically have little effect on standard first-order phase transitions (i.e., the agreement between experimental results obtained for single crystals and polycrystalline samples is consistent most of the times) but they are known to play a critical role on the physical properties of fast-ion conductors like LBH39–41.
Figure 3.
Effect of pressure on the structural and barocaloric properties of LiBH estimated with classical molecular dynamics techniques and the force field reported in work38. (a) Relative volume change associated with the T-induced phase transition expressed as a function of pressure (red); volume of the phase per formula unit (f.u.), , at the phase-transition conditions (black). (b) Isothermal entropy (red) and adiabatic temperature (black) changes associated with the barocaloric response of bulk LiBH expressed as a function of pressure.
Nevertheless, in spite of the differences between our MD results and experiments, which to some extent were expected and are insurmontable, our theoretical estimations for bulk LBH can be regarded overall as physically meaningful and reasonably accurate. For example, by using simple thermodynamic relations, the analytical expression reported above and the results enclosed in Fig. 3a, we estimated a zero-pressure transition enthalpy of kJ kg, which is fairly consistent with the reported experimental value of kJ kg37. Moreover, as it is shown in work38 and the Supplementary Discussion, the employed interatomic potential satisfactorily reproduces the general structural and dynamical properties (i.e., Li ion diffusion and (BH) icosahedra reorientational motion) of bulk LBH as determined from experiments and first-principles molecular dynamics simulations based on density functional theory (which are virtually free of the limitations affecting interatomic potentials42,43).
Therefore, we proceeded to estimate the barocaloric isothermal entropy, , and adiabatic temperature, , changes induced by pressure shifts of GPa in bulk LBH. For this end, and in view of the first-order nature of the phase transformation32, we employed the Clausius–Clapeyron method2,3, which involves the knowledge of the – coexistence line slope (Fig. 2), and the (Fig. 3a) and heat capacity (Supplementary Fig. 2) data estimated for bulk LBH as a function of pressure and temperature (“Methods”). The resulting and values enclosed in Fig. 3b in fact render colossal barocaloric effects. For example, at K and GPa we calculated an isothermal entropy change of J K kg and an adiabatic temperature change of K. The size of and were found to gradually decrease under pressure (e.g., at K and GPa we estimated J K kg and K, respectively). Meanwhile, the predicted LBH barocaloric effects are conventional, that is, , because the low-entropy ordered phase () is stabilized over the high-entropy disordered phase () as a result of applying pressure (). In “Discussion” section, we will compare the barocaloric performance of LBH with those of other well-known barocaloric materials. In what follows, the atomistic origins of the extraordinary and values reported in Fig. 3b are discussed.
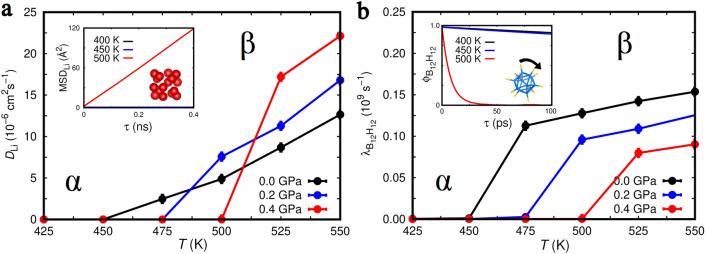
There are two possible sources of large entropy variation in LBH, one stemming from Li ionic diffusion and the other from (BH) icosahedra reorientational motion. When hydrostatic pressure is applied on the disordered phase and the ordered phase is stabilized, both ionic diffusion and molecular orientational disorder are drastically reduced, thus the entropy of the crystal significantly decreases. This conclusion is straightforwardly deduced from the P-induced variation of the Li diffusion coefficient, , and reorientational (BH) frequency, , shown in Fig. 4a,b (“Methods”). For instance, at K and zero pressure and amount to cms and s, respectively, whereas at GPa and same temperature both quantities are practically zero. (It is noted in passing that the theoretical value computed at zero pressure and is in very good agreement with the corresponding experimental value of s37.) The two resulting entropy change contributions are large and of the same sign, thus they sum up giving rise to colossal values (Fig. 3b).
Figure 4.
Order parameter changes associated with the T-induced phase transition occurring in bulk LiBH at different pressures (estimated with classical molecular dynamics techniques and the force field reported in work38). (a) Computed lithium ion diffusion coefficient, , expressed as a function of temperature and pressure. The inset shows the Li mean-squared displacement (MSD) data employed for the calculation of at GPa (“Methods”). (b) Estimated (BH) icosahedra reorientational rate, , expressed as a function of temperature and pressure. The inset shows the (BH) icosahedra angular auto-correlation function () data employed for the calculation of at GPa (“Methods”).
Which of these two P-induced order-restoring effects is most relevant for the barocaloric performance of bulk LBH? To qualitatively answer this question, we performed constrained MD simulations in which we forced the Li and (BH) ions to remain localized around their equilibrium positions at any temperature during separate MD runs (Supplementary Fig. 3). This type of artificial conditions in principle cannot be imposed in the experiments but can be easily enforced in the atomistic simulations (e.g., by disproportionately increasing the mass of the targeted ions). Specifically, we assumed that the phase transition entropy change could be decomposed in the sum of terms: , where the first term corresponds to pure Li contributions (obtained by freezing the (BH) anions), the second to pure (BH) contributions (obtained by freezing the Li cations), and the third to correlated Li(BH) contributions (obtained by substracting the sum of the pure terms and to ). The partial entropy change values were obtained by considering the Clausius-Clapeyron relation, the volume changes obtained from constrained MD simulations, and the – coexistence line obtained from standard MD simulations. The entropy contributions estimated by following this approximate partition ansatz are shown in Supplementary Fig. 3. It was found that mixed ionic-molecular Li(BH) correlations contribute the most to the phase transition entropy change. In particular, pure Li entropy change contributions amount to %, pure (BH) to to 5% (depending on pressure), and mixed Li(BH) to to 85%. This result is not surprising since the interplay between ionic diffusion and molecular reorientational motion in LBH38 and other akin complex materials44 typically is very strong.
Figure 4a shows that at K the diffusivity of Li ions increases under increasing pressure. For example, at K and zero pressure we estimate cm s whereas at GPa and same temperature we obtain cm s. This ionic diffusion behaviour is highly anomalous since hydrostatic compression typically hinders ionic transport23,25,26. On the other hand, the reorientational motion of the (BH) icosahedra behaves quite normally, that is, decreases under pressure3,15. For instance, at K and zero pressure we estimate s whereas at GPa and same temperature we obtain s (Fig. 4b). In view of the physical insight previously gathered on , we hypothesized that such anomalous P-induced lithium diffusion enhancement could be related to the strong interplay between Li and (BH) ions. In particular, high anionic reorientational motion could make the (BH) centers of mass (CMs) to fluctuate wildly thus blocking partially the ionic current channels44. Consistent to this hypothesis, and for small and moderate pressures, large (BH) CMs fluctuations should be accompanied by reduced Li diffusivity while small (BH) CMs fluctuations should be accompanied by enhanced Li diffusivity. The P-dependent MD results obtained for the fluctuations of anionic CMs shown in Supplementary Fig. 4 appear to confirm this hypothesis. Specifically, at high temperatures and GPa the fluctuations of the (BH) CMs are about 5% smaller than estimated at zero pressure and same temperature, which may explain the origin of the larger ’s found in the former case. Nevertheless, the identified anomalous lithium diffusivity ceases at GPa since under higher pressures gradually decreases (Supplementary Fig. 5). Analysis of such anomalous P-induced Li diffusion enhancement effect predicted by MD simulations deserves further theoretical exploration using highly accurate but computationally expensive first-principles methods (which is out of the scope of the present study).
Discussion
To date, large barocaloric effects have been experimentally measured for a number of shape-memory alloys45,46, antiferromagnetic metallic alloys47,48, polar compounds49, organic–inorganic hybrid perovskites50,51, fluoride-based materials52, polymers53, the fast-ion conductor AgI17 and molecular crystals18,19,54. In Table 1, we compare the predicted barocaloric performance of bulk LBH with the experimentally measured barocaloric performance of some representative materials1–3. It is noted that the and values reported here for LBH do not take into account the likely existence of adverse hysteresis and reversibility effects (see below), which tend to reduce significantly their size and have been included in some experimental studies17,19,45,46,49. Therefore, comparison between our theoretical predictions and experimental results may be regarded as indicative in some cases.
Table 1.
Materials presenting giant ( J K kg) and colossal ( J K kg) barocaloric effects.
| 330 | 0.25 | 41.0 | 4.0 | 164 | 16.0 | 45 (Expt.) | |
| 310 | 0.11 | 12.5 | 8.1 | 114 | 73.6 | 46 (Expt.) | |
| 262–290 | 47,48 (Expt.) | ||||||
| 220 | 0.10 | 130.0 | 8.0 | 1300 | 80.0 | 49 (Expt.) | |
| 330 | 0.01 | 30.5 | 4.1 | 3050 | 410.0 | 50 (Expt.) | |
| 262 | 0.03 | 80.0 | 3.0 | 2667 | 100.0 | 54 (Expt.) | |
| 320 | 0.25 | 445.0 | 30–45 | 1780 | 120–180 | 18,19 (Expt.) | |
| 400 | 0.25 | 62.0 | 36.0 | 248 | 144.0 | 17 (Expt.) | |
| 480 | 0.10 | 367.0 | 43.0 | 3670 | 430.0 |
T working temperature, P applied pressure, isothermal entropy change, adiabatic temperature change, barocaloric strength, SMA shape-memory alloy, AMA antiferromagnetic metallic alloy (X = Ga,Ni), FE ferroelectric, OIH organic–inorganic hybrid perovskite, MC molecular crystal, FIC fast-ion conductor. All references correspond to experimental works (Expt.) except for the present computational study (Theory).
The isothermal entropy change induced in LBH by a small hydrostatic pressure of 0.1 GPa, 367 J K kg, is comparable in magnitude to the record that has been recently reported for the plastic crystal neopentylglycol by considering a similar pressure shift, namely, J K kg18,19. The rest of materials in Table 1 present isothermal entropy changes that are appreciably smaller, made the exception of the polar crystal (NH)SO which registers 130 J K kg. As regards , the clear contestants of LBH are the fast-ion conductor AgI (36 K)17 and again the plastic crystal (CH)C(CHOH) (30–45 K)18,19. In terms of the barocaloric strengths and , defined as and , LBH is placed amongst the best performers. For instance, the organic–inorganic hybrid perovskite [TPrA] [Mn(dca)] displays the largest experimental BSS and BST coefficients of all crystals, J K kg GPa and K GPa, which are comparable in magnitude to those predicted for bulk LBH. Likewise, the experimental barocaloric strengths reported for the plastic crystal neopentylglycol are close to those predicted for LBH, which hints at the common order–disorder origin of their relevant phase transformations.
As it was mentioned in the Introduction, the size of the and shifts are not the only parameters defining the barocaloric performance of a material. The degree of reversibility of the involved P-induced phase transition, for instance, is another important barocaloric descriptor that provides information on the materials efficiency during successive pressure application and removal cycles. Specifically, the hysteresis of the transition makes the materials behaviour to depend on its cycling history and to increase the value of the external field that is required to bring the phase transition to completion15,16. As a consequence, the barocaloric performance of a hysteretic material can be significantly worse than that of its ideal non-hysteretic counterpart. In order to quantify the degree of reversibility associated with the phase transition in LBH, we performed a series of long MD simulations ( ns) in which the pressure was kept fixed while the temperature was varied steadily first from 425 up to 625 K and subsequently from 625 back to 425 K. The results of these simulations indicate that the degree of reversibility of the order–disorder phase transition is fairly good (Supplementary Fig. 6). In particular, by monitoring the variation of the system volume we found that at zero pressure the difference between the transition temperatures observed during the heating and cooling switches was K. This predicted temperature hysteresis value is in reasonably good agreement with the corresponding experimental value of K37. According to our simulations, however, the hysteresis of the transformation tends to increase under increasing pressure (e.g., K at GPa). It is worth noting that, due to obvious computational limitations, our estimation should be regarded as tentative since the temperature change rate adopted in our simulations ( K/s) is orders of magnitude faster than in the real experiments ( K/s), and in practice phase-transition hysteresis may depend strongly on this parameter.
Arguably the only weakness of bulk LBH in terms of barocaloric potential is that the critical temperature of the order–disorder phase transition is significantly higher than room temperature. However, this practical problem can be solved effectively by means of doping and alloying strategies. In fact, recently it has been experimentally shown that carbon-substituted LBH, LiCBH, presents a much lower transition temperature of K55, and that the disordered phase is already stabilized at room temperature in structurally similar Li(CBH)–Li(CBH) solid solutions56. Moreover, the type of isosymmetric order–disorder phase transition underlying the exceptional barocaloric behaviour of LBH also occurs in analogous alkali–metal complex hydrides (ABH, A = Na, K, Cs)57 and other earth-abundant and non-toxic materials like KHPO, NaAlSiO and KNO58,59. Thus the present theoretical study should motivate experimental searches of colossal barocaloric effects in LBH and related materials exhibiting lower transition temperatures, so that they can be employed in technological solid-state refrigeration applications.
In conclusion, we have predicted the existence of colossal barocaloric effects rendering isothermal entropy changes of J K kg and adiabatic temperature changes of K in the complex hydride LiBH, which are driven by moderate pressure shifts of GPa. The phase transition underlying such colossal barocaloric effects is remarkable as it combines key ingredients of fast-ion conductors (i.e., ionic diffusion) and molecular crystals (i.e., reorientational motion), materials that individually have been proven to be excellent barocaloric materials. This same type of isosymmetric order–disorder phase transition is likely to occur in other economically affordable and innocuous compounds (e.g., NaBH and KNO), thus broadening significantly the spectrum of caloric materials with commercial potential for solid-state cooling applications. We believe that our simulation study will stimulate experimental research on this new family of barocaloric materials, namely, alkali-metal complex hydrides, which are already known from other technological disciplines (e.g., hydrogen storage and electrochemical devices) and are routinely synthesized in the laboratory.
Methods
Classical molecular dynamics simulations
Molecular dynamics (MD) (N, P, T) simulations60 were performed with the LAMMPS code61. The pressure and temperature in the system were kept fluctuating around a set-point value by using thermostatting and barostatting techniques in which some dynamic variables are coupled to the particle velocities and simulation box dimensions. The interactions between atoms were modeled with the harmonic Coulomb–Buckingham interatomic potential reported in work38, the details of which are provided in the Supplementary Methods. The employed interatomic potential reproduces satisfactorily structural properties and lithium diffusion coefficients of bulk LBH as obtained with first-principles methods and experiments (Ref.38 and Supplementary Discussion). A particle–particle particle-mesh k-space solver was used to compute long-range van der Waals and Coulomb interactions beyond a cut-off distance of 12 Å at each time step. We employed simulation boxes containing 6656 atoms and applied periodic boundary conditions along the three Cartesian directions. Newton’s equations of motion were integrated by using the customary Verlet’s algorithm with a time-step length of 0.5 fs. In most cases, the simulations were initialized from the ordered phase. The standard duration of a MD (N, P, T) run was of 2 ns and statistical time averages typically were calculated over the last 1 ns interval. Numerical convergence tests were carried out that demonstrate the adequacy of these technical parameters and statistical procedure (Supplementary Fig. 7).
Density functional theory calculations
First-principles calculations based on density functional theory (DFT)42 were performed to analyse the structural and ionic transport properties of LiBH. We performed these calculations with the VASP software62 by following the generalized gradient approximation to the exchange-correlation energy due to Perdew et al.63. The projector augmented-wave method was used to represent the ionic cores64, and the electronic states 1s-2s Li, 1s-2s-2p B and 1s H were considered as valence. Wave functions were represented in a plane-wave basis set truncated at 650 eV. By using these parameters and dense -point grids for Brillouin zone integration, the resulting energies were converged to within 1 meV per formula unit. In the geometry relaxations, a tolerance of 0.01 eV Å was imposed on the atomic forces.
Ab initio molecular dynamics (AIMD) simulations based on DFT were carried out to reassess the reliability of the interatomic potential model employed in the classical molecular dynamics simulations38 (Supplementary Fig. 8 and Supplementary Discussion). The AIMD simulations were performed in the canonical (N, V, T) ensemble, considering constant number of particles, volume and temperature, and were initialized from the ordered phase. The constrained volumes were equal to the equilibrium volumes determined at zero temperature, thus we neglected possible thermal expansion effects. Nevertheless, in view of previous first-principles work43, it is reasonable to expect that thermal expansion effects do not affect significantly the estimation of lithium diffusion coefficients at the considered temperatures. The temperature in the AIMD simulations was kept fluctuating around a set-point value by using Nose–Hoover thermostats. A large simulation box containing 832 atoms was employed in all the simulations, and periodic boundary conditions were applied along the three Cartesian directions. Newton’s equations of motion were integrated by using the customary Verlet’s algorithm and a time-step length of ps. -point sampling for integration within the first Brillouin zone was employed in all the AIMD simulations. The AIMD simulations comprised long simulation times of ps.
Estimation of key quantities
The mean square displacement of lithium ions was estimated with the formula43:
| 1 |
where is the position of the migrating ion i at time (), represents a lag time, , is the total number of mobile ions, and the total number of time steps. The maximum was chosen equal to , hence we could accumulate enough statistics to reduce significantly the fluctuations in at large ’s. The diffusion coefficient of lithium ions then was obtained with the Einstein relation:
| 2 |
by performing linear fits to the averaged values calculated at long .
The angular autocorrelation function of the closoborane (BH) icosahedra was estimated according to the expression38:
| 3 |
where is a unitary vector connecting the center of mass of each closoborane unit with one of its edges and denotes thermal average considering all the closoborane icosahedra. This autocorrelation function typically decays as , where the parameter represents a characteristic reorientational frequency. When the (BH) reorientational motion is significant, that is, is large, the function decreases rapidly to zero with time. In this case, was chosen equal to 100 ps although statistics were gathered through the whole duration of the MD simulations.
In view of the reported first-order nature of the transformation32,58, isothermal entropy changes associated with barocaloric effects were estimated with the Clausius–Clapeyron relation2,3:
| 4 |
where dP/dT represents the slope of the coexistence line separating the stability regions of the and phases, and the volume change associated with the phase transition. The dependence of the involved physical quantities on both pressure and temperature are explicitly noted as “(P, T)”.
Likewise, the accompanying adiabatic temperature shifts were calculated as:
| 5 |
where is the heat capacity of the crystal calculated at constant pressure and temperature and H represents the enthalpy of the system. The heat capacity was computed by numerically estimating the temperature derivative of at fixed (P, T) conditions, where indicates thermal average. The heat capacity entering Eq. (5) was corrected for latent heat contributions17,65 thus for the estimation of we adopted values obtained at temperatures close to the corresponding transition points (Supplementary Fig. 2; recall that strictly speaking the heat capacity of a first-order transformation diverges at the transition point65). Specifically, we found that the corrected was quite insensitive to compression and equal to 4100 J K kg within the analysed pressure and temperature intervals (Supplementary Fig. 2). In order to accurately compute the barocaloric shifts and as well as the volume, , and of bulk LBH, our molecular dynamics calculations were performed for dense grids of (P, T) points spaced by small increments of GPa and K, respectively.
Supplementary Information
Acknowledgements
C.C. acknowledges support from the Spanish Ministry of Science, Innovation and Universities under the “Ramón y Cajal” fellowship RYC2018-024947-I. D. E. acknowledges support from the Spanish Ministry of Science, Innovation and Universities under the Grant PID2019-106383GB-C41 and the Generalitat Valenciana under the Grant Prometeo/2018/123 (EFIMAT). Computational resources and technical assistance were provided by the Australian Government and the Government of Western Australia through the National Computational Infrastructure (NCI) and Magnus under the National Computational Merit Allocation Scheme and the Pawsey Supercomputing Centre, the Informatics Service of the University of Valencia through the Tirant III cluster, and the the Center for Computational Materials Science of the Institute for Materials Research, Tohoku University (Material science Supercomputing system for Advanced Multiscale simulations towards Next-generation-Institute of Material Research) (Project No-20S0021).
Author contributions
K.S. and C.C. conceived the study and planned the research. K.S. performed the molecular dynamics simulations. C.C. performed the first-principles calculations and barocaloric analysis. Results were discussed by all the authors. K.S. and C.C. wrote the manuscript.
Data availability
The data that support the findings of this study are available from the corresponding author (C.C.) upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-91123-4.
References
- 1.Mañosa L, Plaxnes A. Materials with giant mechanocaloric effects: Cooling by strength. Adv. Mater. 2017;29:1603607. doi: 10.1002/adma.201603607. [DOI] [PubMed] [Google Scholar]
- 2.Moya X, Kar-Narayan S, Mathur ND. Caloric materials near ferroic phase transitions. Nat. Mater. 2014;13:439. doi: 10.1038/nmat3951. [DOI] [PubMed] [Google Scholar]
- 3.Cazorla C. Novel mechanocaloric materials for solid-state cooling applications. Appl. Phys. Rev. 2019;6:041316. doi: 10.1063/1.5113620. [DOI] [Google Scholar]
- 4.Kitanovski A. Energy applications of magnetocaloric materials. Adv. Energy Mater. 2020;10:1903741. doi: 10.1002/aenm.201903741. [DOI] [Google Scholar]
- 5.Barman A, Kar-Narayan S, Mukherjee D. Caloric effects in perovskite oxides. Adv. Mater. Interfaces. 2019;6:1900291. doi: 10.1002/admi.201900291. [DOI] [Google Scholar]
- 6.Zarkevich NA, Johnson DD, Pecharsky VK. High-throughput search for caloric materials: The CaloriCool approach. J. Phys. D Appl. Phys. 2018;51:024002. doi: 10.1088/1361-6463/aa9bd0. [DOI] [Google Scholar]
- 7.Qian S, et al. A review of elastocaloric cooling: Materials, cycles and system integrations. Int. J. Refrig. 2016;64:1. doi: 10.1016/j.ijrefrig.2015.12.001. [DOI] [Google Scholar]
- 8.Scott JF. Electrocaloric materials. Annu. Rev. Mater. Res. 2011;41:229. doi: 10.1146/annurev-matsci-062910-100341. [DOI] [Google Scholar]
- 9.Bonnot E, Romero R, Mañosa L, Vives E, Planes A. Elastocaloric effect associated with the martensitic transition in shape-memory alloys. Phys. Rev. Lett. 2008;100:125901. doi: 10.1103/PhysRevLett.100.125901. [DOI] [PubMed] [Google Scholar]
- 10.Tusek J, et al. A regenerative elastocaloric heat pump. Nat. Energy. 2016;1:16134. doi: 10.1038/nenergy.2016.134. [DOI] [Google Scholar]
- 11.Boldrin D. Fantastic barocalorics and where to find them. Appl. Phys. Lett. 2021;118:170502. doi: 10.1063/5.0046416. [DOI] [Google Scholar]
- 12.Cui J, et al. Demonstration of high efficiency elastocaloric cooling with large using NiTi wires. Appl. Phys. Lett. 2012;101:073904. doi: 10.1063/1.4746257. [DOI] [Google Scholar]
- 13.Tusek J, Engelbrecht K, Mikkelsen LP, Pryds N. Elastocaloric effect of Ni-Ti wire for application in a cooling device. J. Appl. Phys. 2015;117:124901. doi: 10.1063/1.4913878. [DOI] [Google Scholar]
- 14.Pecharsky VK, Gschneidner KA., Jr Giant magnetocaloric effect in GdSiGe. Phys. Rev. Lett. 1997;78:4494. doi: 10.1103/PhysRevLett.78.4494. [DOI] [Google Scholar]
- 15.Aznar A, Lloveras P, Barrio M, Negrier Ph, Planes A, Mañosa L, Mathur ND, Moya X, Tamarit J-L. Reversible and irreversible colossal barocaloric effects in plastic crystals. J. Mater. Chem. A. 2020;8:639. doi: 10.1039/C9TA10947A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hess T, et al. Thermal hysteresis and its impact on the efficiency of first-order caloric materials. J. Appl. Phys. 2020;127:075103. doi: 10.1063/1.5132897. [DOI] [Google Scholar]
- 17.Aznar A, Lloveras P, Romanini M, Barrio M, Tamarit JL, Cazorla C, Errandonea D, Mathur ND, Planes A, Moya X, Mañosa L. Giant barocaloric effects over a wide temperature range in superionic conductor AgI. Nat. Commun. 2017;8:1851. doi: 10.1038/s41467-017-01898-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li B, et al. Colossal barocaloric effects in plastic crystals. Nature. 2019;567:506. doi: 10.1038/s41586-019-1042-5. [DOI] [PubMed] [Google Scholar]
- 19.Lloveras P, Aznar A, Barrio M, Negrier Ph, Popescu C, Planes A, Mañosa L, Stern-Taulats E, Avramenko A, Mathur ND, Moya X, Tamarit J-L. Colossal barocaloric effects near room temperature in plastic crystals of neopentylglycol. Nat. Commun. 2019;10:1803. doi: 10.1038/s41467-019-09730-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sagotra AK, Errandonea D, Cazorla C. Mechanocaloric effects in superionic thin films from atomistic simulations. Nat. Commun. 2017;8:963. doi: 10.1038/s41467-017-01081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keen DA, Hull S, Hayes W, Gardner NJG. Structural evidence for a fast-ion transition in the high-pressure rocksalt phase of silver iodide. Phys. Rev. Lett. 1996;77:4914. doi: 10.1103/PhysRevLett.77.4914. [DOI] [PubMed] [Google Scholar]
- 22.Hull S, Keen DA. Pressure-induced phase transitions in AgCl, AgBr, and AgI. Phys. Rev. B. 1999;59:750. doi: 10.1103/PhysRevB.59.750. [DOI] [Google Scholar]
- 23.Sagotra AK, Cazorla C. Stress-mediated enhancement of ionic conductivity in fast-ion conductors. ACS Appl. Mater. Interfaces. 2017;9:38773. doi: 10.1021/acsami.7b11687. [DOI] [PubMed] [Google Scholar]
- 24.Aniya M, Okazaki H, Kobayashi M. Static dielectric function of superionic conductor -AgI. Phys. Rev. Lett. 1990;65:1474. doi: 10.1103/PhysRevLett.65.1474. [DOI] [PubMed] [Google Scholar]
- 25.Hull S. Superionics: Crystal structures and conduction processes. Rep. Prog. Phys. 2004;67:1233. doi: 10.1088/0034-4885/67/7/R05. [DOI] [Google Scholar]
- 26.Cazorla C, Errandonea D. Giant mechanocaloric effects in fluorite-structured superionic materials. Nano Lett. 2016;16:3124. doi: 10.1021/acs.nanolett.6b00422. [DOI] [PubMed] [Google Scholar]
- 27.Sagotra AK, Chu D, Cazorla C. Room-temperature mechanocaloric effects in lithium-based superionic materials. Nat. Commun. 2018;9:3337. doi: 10.1038/s41467-018-05835-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Min J, Sagotra AK, Cazorla C. Large barocaloric effects in thermoelectric superionic materials. Phys. Rev. Mater. 2020;4:015403. doi: 10.1103/PhysRevMaterials.4.015403. [DOI] [Google Scholar]
- 29.Cazorla C. Refrigeration based on plastic crystals. Nature. 2019;567:470. doi: 10.1038/d41586-019-00974-5. [DOI] [PubMed] [Google Scholar]
- 30.Her J-H, et al. Crystal structure of LiBH: A possible intermediate species in the decomposition of LiBH. Inorg. Chem. 2008;47:9757. doi: 10.1021/ic801345h. [DOI] [PubMed] [Google Scholar]
- 31.Lai Q, et al. How to design hydrogen storage materials? Fundamentals, synthesis, and storage tanks. Adv. Sustain. Syst. 2019;3:1900043. doi: 10.1002/adsu.201900043. [DOI] [Google Scholar]
- 32.Paskevicius M, et al. First-order phase transition in the LiBH system. Phys. Chem. Chem. Phys. 2013;15:15825. doi: 10.1039/c3cp53090f. [DOI] [PubMed] [Google Scholar]
- 33.Luo X, Rawal A, Cazorla C, Aguey-Zinsou KF. Facile self-forming superionic conductors based on complex borohydrides surface oxidation. Adv. Sustain. Syst. 2020;4:1900113. doi: 10.1002/adsu.201900113. [DOI] [Google Scholar]
- 34.Mohtadi R, Orimo SI. The renaissance of hydrides as energy materials. Nat. Rev. Mater. 2016;2:16091. doi: 10.1038/natrevmats.2016.91. [DOI] [Google Scholar]
- 35.Udovic TJ, et al. Sodium superionic conduction in NaBH. Chem. Commun. 2014;50:3750. doi: 10.1039/C3CC49805K. [DOI] [PubMed] [Google Scholar]
- 36.Jorgensen M, et al. Understanding superionic conductivity in lithium and sodium salts of weakly coordinating closo-hexahalocarbaborate anions. Chem. Mater. 2020;32:1475. doi: 10.1021/acs.chemmater.9b04383. [DOI] [Google Scholar]
- 37.Verdal N, et al. Complex high-temperature phase transitions in LiBH and NaBH. J. Sol. Stat. Chem. 2014;212:81. doi: 10.1016/j.jssc.2014.01.006. [DOI] [Google Scholar]
- 38.Sau K, et al. Reorientational motion and Li ion transport in LiBH system: Molecular dynamics study. Phys. Rev. Mater. 2019;3:075402. doi: 10.1103/PhysRevMaterials.3.075402. [DOI] [Google Scholar]
- 39.Zheng J, Xiao J, Zhang J-G. The roles of oxygen non-stoichiometry on the electrochemical properties of oxide-based cathode materials. Nano Today. 2016;11:678. doi: 10.1016/j.nantod.2016.08.011. [DOI] [Google Scholar]
- 40.Marrocchelli D, Sun L, Yildiz B. Dislocations in SrTiO: Easy to reduce but not so fast for oxygen transport. J. Am. Chem. Soc. 2015;137:4735. doi: 10.1021/ja513176u. [DOI] [PubMed] [Google Scholar]
- 41.Toyoura K, Koyama Y, Kuwabara A, Tanaka I. Effects of off-stoichiometry of LiC on the lithium diffusion mechanism and diffusivity by first-principles calculations. J. Phys. Chem. C. 2010;114:2375. doi: 10.1021/jp910134u. [DOI] [Google Scholar]
- 42.Cazorla C, Boronat J. Simulation and understanding of atomic and molecular quantum crystals. Rev. Mod. Phys. 2017;89:035003. doi: 10.1103/RevModPhys.89.035003. [DOI] [Google Scholar]
- 43.Sagotra AK, Chu D, Cazorla C. Influence of lattice dynamics on lithium-ion conductivity: A first-principles study. Phys. Rev. Mater. 2019;3:035405. doi: 10.1103/PhysRevMaterials.3.035405. [DOI] [Google Scholar]
- 44.Skripov AV, Soloninin AV, Ley MB, Jensen TR, Filinchuk Y. Nuclear magnetic resonance studies of BH reorientations and Li diffusion in LiLa(BH)Cl. J. Phys. Chem. C. 2013;117:14965. doi: 10.1021/jp403746m. [DOI] [Google Scholar]
- 45.Stern-Taulats E, Planes A, Lloveras P, Barrio M, Tamarit J-L, Pramanick S, Majumdar S, Yüce S, Emre B, Frontera C, Mañosa L. Tailoring barocaloric and magnetocaloric properties in low-hysteresis magnetic shape memory alloys. Acta Mater. 2015;96:324. doi: 10.1016/j.actamat.2015.06.026. [DOI] [Google Scholar]
- 46.Stern-Taulats E, Gracia-Condal A, Planes A, Lloveras P, Barrio M, Tamarit J-L, Pramanick S, Majumdar S, Mañosa L. Reversible adiabatic temperature changes at the magnetocaloric and barocaloric effects in FeRh. App. Phys. Lett. 2015;107:152409. doi: 10.1063/1.4933409. [DOI] [Google Scholar]
- 47.Matsunami D, Fujita A, Takenaka K, Kano M. Giant barocaloric effect enhanced by the frustration of the antiferromagnetic phase in MnGaN. Nat. Mater. 2015;14:73. doi: 10.1038/nmat4117. [DOI] [PubMed] [Google Scholar]
- 48.Boldrin D, Mendive-Tapia E, Zemen J, Staunton JB, Hansen T, Aznar A, Tamarit J-Ll, Barrio M, Lloveras P, Kim J, Moya X, Cohen LF. Multisite exchange-enhanced barocaloric response in MnNiN. Phys. Rev. X. 2018;8:041035. [Google Scholar]
- 49.Lloveras P, Stern-Taulats E, Barrio M, Tamarit J-L, Crossley S, Li W, Pomjakushin V, Planes A, Mañosa L, Mathur ND, Moya X. Giant barocaloric effects at low temperature in ferrielectric ammonium sulphate. Nat. Commun. 2015;6:8801. doi: 10.1038/ncomms9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bermúdez-García JM, et al. Giant barocaloric effect in the ferroic organic-inorganic hybrid [TPrA][Mn(dca)] perovskite under easily accessible pressures. Nat. Commun. 2017;8:15715. doi: 10.1038/ncomms15715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bermúdez-García JM, Sánchez-Andújar M, Señarís-Rodríguez MA. A new playground for organic-inorganic hybrids: Barocaloric materials for pressure-induced solid-state cooling. J. Phys. Chem. Lett. 2017;8:4419. doi: 10.1021/acs.jpclett.7b01845. [DOI] [PubMed] [Google Scholar]
- 52.Gorev M, Bogdanov E, Flerov IN, Laptash NM. Thermal expansion, phase diagrams and barocaloric effects in (NH)NbOF. J. Phys. Condens. Matter. 2010;22:185901. doi: 10.1088/0953-8984/22/18/185901. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez EL, Filisko FE. Thermoelastic temperature changes in poly(methyl methacrylate) at high hydrostatic pressure: Experimental. J. Appl. Phys. 1982;53:6536. doi: 10.1063/1.330081. [DOI] [Google Scholar]
- 54.Vallone SP, et al. Giant barocaloric effect at the spin crossover transition of a molecular crystal. Adv. Mater. 2019;31:1807334. doi: 10.1002/adma.201807334. [DOI] [PubMed] [Google Scholar]
- 55.Tang WS, et al. Unparalleled lithium and sodium superionic conduction in solid electrolytes with large monovalent cage-like anions. Energy Environ. Sci. 2015;8:3637. doi: 10.1039/C5EE02941D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim S, et al. A complex hydride lithium superionic conductor for high-energy-density all-solid-state lithium metal batteries. Nat. Commun. 2019;10:1081. doi: 10.1038/s41467-019-09061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verdal N, et al. Evidence of a transition to reorientational disorder in the cubic alkali-metal dodecahydro-closo-dodecaborates. J. Solid State Chem. 2011;184:3110. doi: 10.1016/j.jssc.2011.09.010. [DOI] [Google Scholar]
- 58.Christy AG. Isosymmetric structural phase transitions: Phenomenology and examples. Acta Cryst. 1995;B51:753. doi: 10.1107/S0108768195001728. [DOI] [Google Scholar]
- 59.Adams DM, Hatton PD, Heath AE, Russell DR. X-ray diffraction measurements on potassium nitrate under high pressure using synchrotron radiation. J. Phys. C Solid State Phys. 1988;21:505. doi: 10.1088/0022-3719/21/3/007. [DOI] [Google Scholar]
- 60.Frenkel D, Smit B. Understanding Molecular Simulation. Academic Press Inc.; 2001. [Google Scholar]
- 61.Plimpton, S. J. Fast parallel algorithms for short-range molecular dynamics. J. Comp. Phys.117, 1 (1995) http://lammps.sandia.gov.
- 62.Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996;54:11169. doi: 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- 63.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 64.Blöchl PE. Projector augmented-wave method. Phys. Rev. B. 1994;50:17953. doi: 10.1103/PhysRevB.50.17953. [DOI] [PubMed] [Google Scholar]
- 65.Pecharsky VK, Gschneidner KA., Jr Heat capacity near first order phase transitions and the magnetocaloric effect: An analysis of the errors, and a case study of Gd(SiGe) and Dy. J. Appl. Phys. 1999;86:6315. doi: 10.1063/1.371734. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (C.C.) upon reasonable request.