Abstract
Chondrocyte differentiation is a critical process for endochondral ossification, which is responsible for long bone development and fracture repair. Considerable progress has been made in understanding the transcriptional control of chondrocyte differentiation; however, epigenetic regulation of chondrocyte differentiation remains to be further studied. NSD1 is a H3K36 (histone H3 at lysine 36) methyltransferase. Here, we showed that mice with Nsd1 deficiency in Prx1+ mesenchymal progenitors but not in Col2+ chondrocytes showed impaired skeletal growth and fracture healing accompanied by decreased chondrogenic differentiation. Via combined RNA sequencing (RNA-seq) and chromatin immunoprecipitation sequencing (ChIP-seq) analysis, we identified sex determining region Y box 9 (Sox9), the key transcription factor of chondrogenic differentiation, as a functional target gene of NSD1. Mechanistically, NSD1 regulates Sox9 expression by modulating H3K36me1 and H3K36me2 levels in the Sox9 promoter region, constituting a novel epigenetic regulatory mechanism of chondrogenesis. Moreover, we found that NSD1 can directly activate the expression of hypoxia-inducible factor 1α (HIF1α), which plays a vital role in chondrogenic differentiation through its regulation of Sox9 expression. Collectively, the results of our study reveal crucial roles of NSD1 in regulating chondrogenic differentiation, skeletal growth, and fracture repair and expand our understanding of the function of epigenetic regulation in chondrogenesis and skeletal biology.
Subject terms: Bone, Pathogenesis
Introduction
Human stature depends mainly on the growth of long bones. Chondrocyte differentiation in the growth plate is a major factor for bone growth and is involved in endochondral ossification, the process that vertebrates use mainly to form the skeleton.1 Endochondral ossification begins with mesenchymal progenitor condensation to form chondroprogenitor cells, and chondrogenic differentiation, proliferation, and hypertrophy follow. Finally, blood vessels, osteoblasts, and osteoclasts invade the hypertrophic zone to generate cancellous bone.2 For chondrogenic differentiation, sex determining region Y box 9 (Sox9) is the key regulator and activates the chondrocyte-specific enhancer of Col2 (collagen II). Mice with Sox9 haploinsufficiency present defective primordial cartilage and abnormal skeletal mineralization.3 The transcriptional regulation of Sox9 expression has been extensively reported; for example, hypoxia-inducible factor 1α (HIF1α) directly binds to the promoter of Sox9 and activates Sox9 expression, affecting early skeletogenesis.4–6
Accumulating evidence indicates that epigenetic modifications play important roles during chondrogenic differentiation and longitudinal bone growth. The histone demethylase PHF2 can stimulate chondrogenesis by binding to the promoter region of chondrocyte-related genes and removing H3K9me2 from these genes.7 Both KDM4B, an H3K9me3 demethylase, and KDM6B, an H3K27me2/3 demethylase, play crucial roles in chondrogenesis.8,9 Mutations in KMT2D or KDM6A are causes of Kabuki syndrome, characterized by mild-to-moderate intellectual disability, typical facial features, and short stature.10,11 Combined loss of the H3K27 methyltransferases EZH1 and EZH2 in mice severely impairs skeletal growth by affecting chondrogenesis in the growth plate and chondrocyte proliferation and hypertrophy.12 Beyond these processes related to H3K9 and H3K27 regulation, epigenetic regulation of chondrocyte differentiation and skeletal development remains to be further studied.
Nuclear receptor binding SET domain-containing protein 1 (NSD1), encoded by the Nsd1 gene, catalyzes the mono- and dimethylation of histone H3 at lysine 36 (H3K36).13 In the clinic, deletion or mutation of the NSD1 gene are the major causes of Sotos syndrome (cerebral gigantism),14,15 a genetic disorder with increased bone growth during infancy and childhood and normal height after puberty,16 strongly indicating that NSD1 is associated with bone growth. Histone H3 lysine 36 to methionine (H3K36M) mutation leads to decreased H3K36 methylation levels, which impairs mesenchymal progenitor differentiation and leads to undifferentiated sarcoma in mice and chondroblastoma in clinical patients,17,18 suggesting that H3K36 methylation plays important roles during chondrogenic differentiation and cartilage development; however, the exact enzyme that plays the key role has not yet been identified.
To explore the role of NSD1 and H3K36 methylation in chondrogenic differentiation and skeletal growth, we conditionally deleted Nsd1 in mesenchymal progenitors by mating Nsd1f/f mice with Prx1-Cre mice and in chondrocytes by mating Nsd1f/f mice with Col2-Cre mice. Strikingly, we found that deletion of Nsd1 in Prx1+ mesenchymal progenitors but not in Col2+ chondrocytes led to impaired skeletal growth and fracture healing in mice. Mechanistically, NSD1 regulated chondrogenic differentiation by controlling the expression of Sox9 through direct regulation by modulating the occupancy of H3K36me1 and H3K36me2 on the Sox9 promoter and indirect regulation by binding to the Hif1α promoter. These findings identified NSD1 as a novel regulator of chondrogenic differentiation, skeletal growth, and fracture healing, providing new insights into epigenetic regulation of chondrogenic differentiation and bone growth.
Results
Mice with Nsd1 knockout in mesenchymal progenitors showed impaired cartilage development
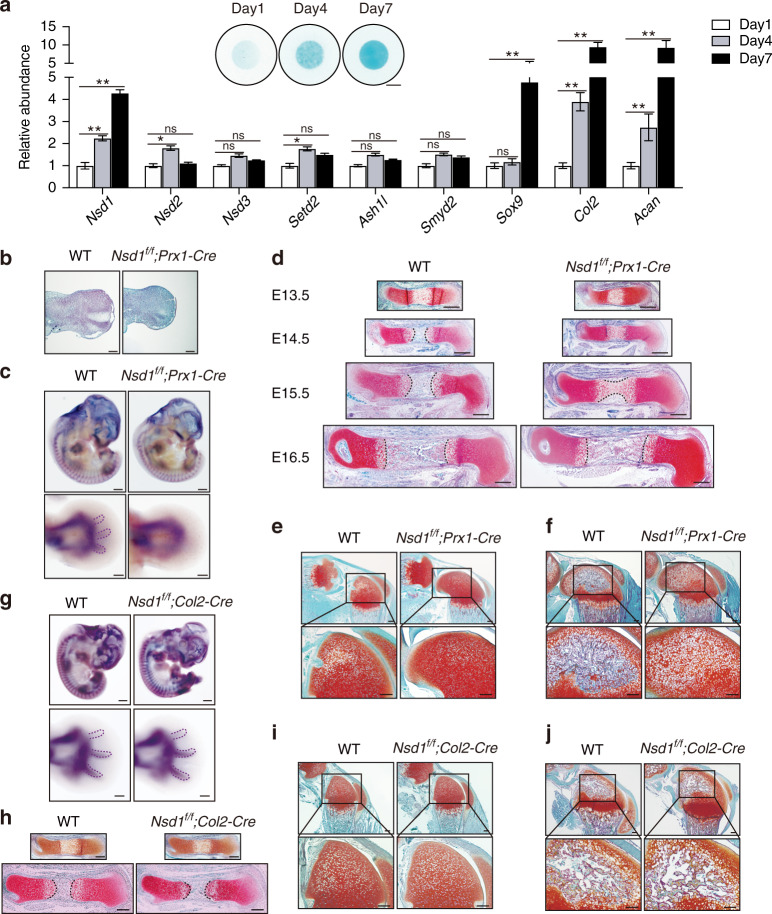
To explore the functions of NSD1 and histone methylation in chondrogenic differentiation and skeletal growth, we first examined the expression levels of H3K36 methyltransferases in a 3D chondrogenic differentiation system in vitro. Through micromass culture with chondroprogenitor cells,19 we collected micromasses at different differentiation times and determined the expression levels of H3K36 methyltransferases. Among the H3K36 methyltransferases, Nsd1 showed a significantly increased mRNA level (Figs. 1a and S1A), raising the possibility that NSD1 is correlated with chondrogenic differentiation. Then, we detected NSD1 in P7 cartilage and found that NSD1 was widely expressed in articular cartilage and the growth plate (Fig. S1B). Since Nsd1 germline knockout is embryonic lethal in mice,13 we conditionally knocked out Nsd1 in mesenchymal progenitors or chondrocytes by breeding Nsd1f/f mice with Prx1-Cre or Col2-Cre mice, respectively20,21 (Fig. S1C, D). The specificity and efficiency of Nsd1 knockout were verified by qRT-PCR analyses (Fig. S1E, F). In Nsd1f/f;Prx1-Cre mice, chondrogenesis lagged behind that in wild-type littermate mice (Fig. 1b), and whole-mount in situ hybridization staining of Col2 showed delayed autopod formation (Fig. 1c). Safranin O staining results showed retarded formation of both the primary and secondary ossification centers (Fig. 1d–f). However, in Nsd1f/f;Col2-Cre mice, chondrogenesis and ossification center formation were normal (Fig. 1g–j). These data indicate that NSD1 deletion in Prx1+ mesenchymal progenitors led to embryonic and postnatal limb development defects. However, the above defects were not observed in Nsd1f/f;Col2-Cre mice, indicating that NSD1 functions in the very early process of chondrogenesis.
Fig. 1.
Mice with Nsd1 knockout in mesenchymal progenitors showed impaired cartilage development. a mRNA levels of H3K36 methyltransferases and chondrocyte differentiation marker genes were determined by qRT-PCR in micromasses at different differentiation time points. The values are presented as the means ± SEMs, n = 4. *P < 0.05, **P < 0.01, ns means not significant. The inset shows Alcian blue staining results of micromasses cultured for 1, 4, and 7 days with chondroprogenitor cells. Scale bar = 2 mm. b SO staining results of E11.5 limb buds. Scale bar = 100 μm. c Whole-mount in situ hybridization (WISH) results for Col2 in E12.5 embryos (top) and sections of forelimbs (bottom). The dashed purple lines show the digits already present. Scale bar (top) = 500 μm, scale bar (bottom) = 200 μm. d SO staining results of E13.5 (first line), E14.5 (second line), E15.5 (third line), and E16.5 (fourth line) femur sections from WT and Nsd1f/f;Prx1-Cre mice. The dashed black lines show the borders between hypertrophic chondrocytes and the primary ossification center. Scale bar = 200 μm. SO staining of P7 (e) and P14 (f) hindlimb sections from WT and Nsd1f/f;Prx1-Cre mice. Scale bar (top) = 200 μm. Scale bar (bottom) = 500 μm. g Whole-mount in situ hybridization (WISH) results for Col2 in E12.5 embryos (top) and sections of forelimbs (bottom). The dashed purple lines show the digits already present. Scale bar (top) = 500 μm, scale bar (bottom) = 200 μm. h SO staining results of E13.5 (top) and E15.5 (bottom) femur sections from WT, Nsd1f/f;Col2-Cre mice. The dashed black lines show the borders between hypertrophic chondrocytes and the primary ossification center. Scale bar = 200 μm. SO staining of P7 (i) and P14 (j) hindlimb sections from WT and Nsd1f/f;Col2-Cre mice. Scale bar (top) = 200 μm, Scale bar (bottom) = 500 μm
Nsd1 deletion in mesenchymal progenitors led to skeletal growth defects in mice
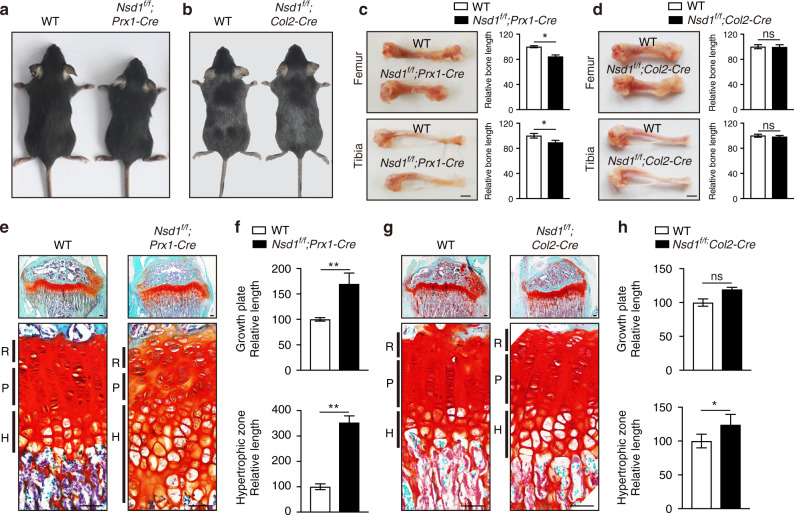
Chondrogenic differentiation is a prerequisite for endochondral bone formation, which supports long bone growth. Having seen chondrogenesis and cartilage development defects after NSD1 knockout, we next sought to determine whether these defects impair bone growth. One-month-old Nsd1f/f;Prx1-Cre mice showed smaller stature than control mice (Fig. 2a), while Nsd1f/f;Col2-Cre mice displayed normal stature (Fig. 2b). Nsd1f/f;Prx1-Cre mice displayed shorter hindlimb bone lengths than control mice, whereas the hindlimb bone lengths in Nsd1f/f;Col2-Cre mice were comparable to those in control mice (Fig. 2c, d). The growth plate is very important for postnatal bone growth and is the basis of endochondral bone formation. Examination of the growth plate revealed that Nsd1f/f;Prx1-Cre mice exhibited thicker growth plates with abnormal cellular morphology in the resting zone, shorter and disorganized columns in the proliferating zone and a strikingly thickened hypertrophic zone (Fig. 2e, f). However, Nsd1f/f;Col2-Cre mice only showed slight thickening in the hypertrophic zone of the growth plate (Fig. 2g, h). The bone mass in Nsd1f/f;Prx1-Cre mice was lower than that in control mice (Fig. S2A,B), and the bone mass in Nsd1f/f;Col2-Cre mice was comparable to that in control mice (Fig. S2C,D). These results show that Nsd1 knockout in Prx1+ mesenchymal progenitors leads to growth plate malformation and skeletal growth defects in mice, meaning that NSD1 plays an important role during endochondral bone formation.
Fig. 2.
Nsd1 deficiency in mesenchymal progenitors led to skeletal growth defects in mice. Gross images of 1-month-old WT, Nsd1f/f;Prx1-Cre (a) and Nsd1f/f;Col2-Cre (b) mice. Pictures of hindlimbs (left) and quantitative statistics (right) of hindlimb length in 1-month-old WT, Nsd1f/f;Prx1-Cre (c) and Nsd1f/f;Col2-Cre (d) mice. Scale bar = 2 mm. The values are presented as the means ± SEMs, n = 6. *P < 0.05, ns means not significant. Safranin O (SO) staining results (e, g) and growth plate quantification data (f, h) of tibia sections from 1-month-old WT, Nsd1f/f;Prx1-Cre (e, f) and Nsd1f/f;Col2-Cre (g, h) mice. Scale bar (top) = 100 μm. Scale bar (bottom) = 50 μm. The values are presented as the means ± SEMs, n = 6. *P < 0.05, **P < 0.01, ns means not significant
Nsd1 deletion in Prx1+ mesenchymal progenitors led to impaired fracture healing in mice
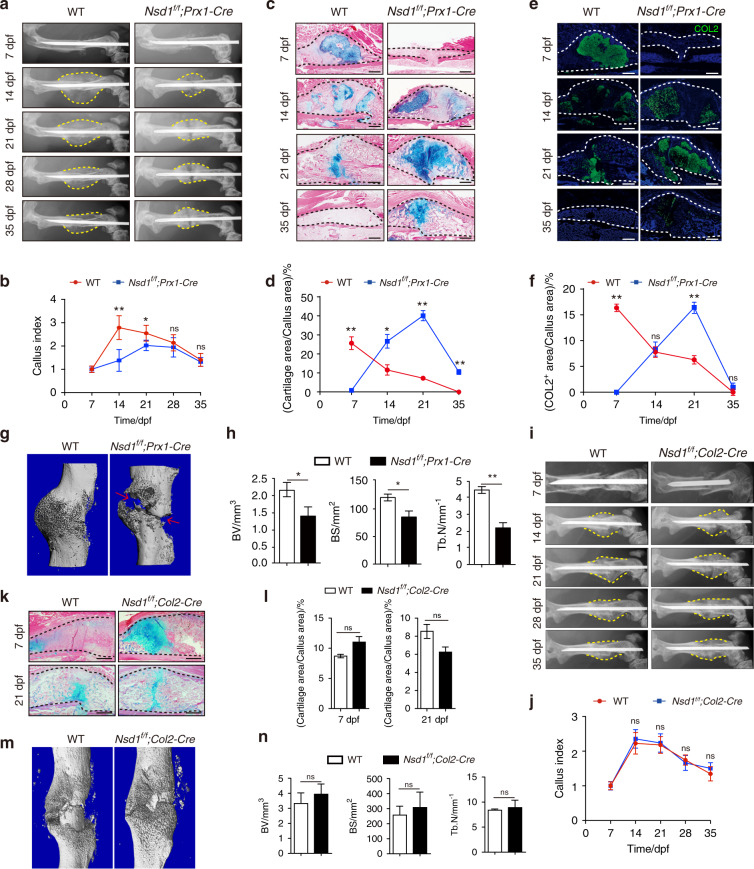
Bone fracture healing is a regenerative process that recapitulates many skeletal development events, including endochondral and intramembranous ossification.22 The chondrogenesis and skeletal growth defects in Nsd1f/f;Prx1-Cre mice prompted us to further explore whether the absence of NSD1 affects fracture repair. X-ray scan results showed that Nsd1f/f;Prx1-Cre mice had less callus formation than control mice at the same time point (Fig. 3a, b). Histological assessments showed that cartilage formation was delayed in Nsd1f/f;Prx1-Cre mice (Fig. 3c, d). Immunofluorescence staining of COL2 also showed delayed cartilage appearance in calluses in Nsd1f/f;Prx1-Cre mice during fracture healing (Fig. 3e, f). Micro-CT analysis at 18 days post fracture showed that cracks remained in the calluses only in Nsd1f/f;Prx1-Cre mice and not in control mice (Fig. 3g). Quantitative analysis of the micro-CT results showed that the bone volume and trabecular bone number in calluses in Nsd1f/f;Prx1-Cre mice were less than those in control mice (Fig. 3h). However, in Nsd1f/f;Col2-Cre mice, callus formation was comparable to that in control mice at the same time point (Fig. 3i, j). Alcian blue staining showed normal cartilage formation in Nsd1f/f;Col2-Cre mice (Fig. 3k, l). The union of fracture ends was synchronized with that in control mice (Fig. 3m), and the bone formed in the callus showed no difference from that in control mice (Fig. 3n).
Fig. 3.
Mice with Nsd1 knockout in mesenchymal progenitors showed impaired fracture healing. a Radiographs of fractured femurs from WT and Nsd1f/f;Prx1-Cre mice at different days post fracture (dpf). b Quantitative analysis of formed calluses at different days post fracture (dpf). n = 5. Alcian blue/eosin staining (c) and quantitative results (d) of callus sections. The dashed black lines show the location of the callus. Scale bar = 500 μm. n = 5. Immunofluorescence staining (e) and quantitative results (f) of type II collagen in callus sections. The dashed white lines show the location of the callus. Scale bar= 50 µm. n = 5. g Micro-CT images of calluses in WT and Nsd1f/f;Prx1-Cre mice at 18 dpf. h Quantitative statistics of micro-CT results of calluses. n = 3. i Radiographs of fractured femurs in WT and Nsd1f/f;Col2-Cre mice at different days post fracture (dpf). j Quantitative analysis of formed calluses at different days post fracture (dpf). n = 5. Alcian blue/eosin staining (k) and quantitative results (l) of callus sections. The dashed black lines show the location of the callus. Scale bar = 500 μm. n = 5. m Micro-CT images of calluses in WT and Nsd1f/f;Col2-Cre mice at 21 dpf. n Quantitative statistics of micro-CT results of calluses. BV bone volume, BS bone surface, Tb.N trabecular bone number. n = 3. The values are presented as the means ± SEMs. *P < 0.05, **P < 0.01, ns means not significant
These findings suggest that Nsd1 deletion in Prx1+ mesenchymal progenitors leads to impaired fracture healing in mice. Therefore, NSD1 in Prx1+ mesenchymal progenitors is indispensable for fracture healing.
Nsd1-deficient chondroprogenitor cells showed decreased chondrogenic differentiation
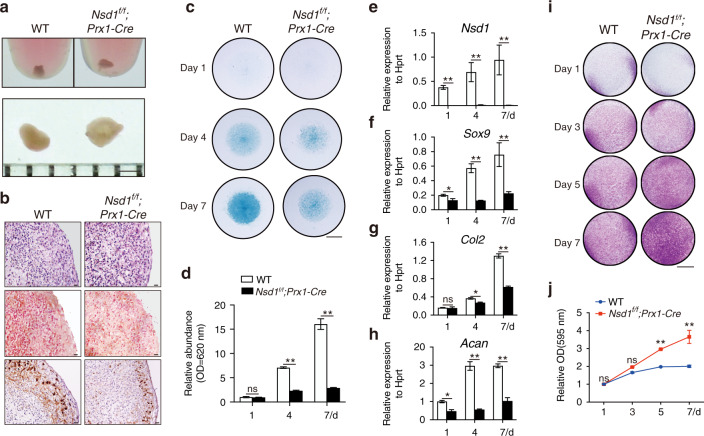
To investigate the role of NSD1 in chondrogenic differentiation, we performed 3D pellet culture with chondroprogenitor cells and found that pellets formed by chondroprogenitor cells from Nsd1f/f;Prx1-Cre mice were larger and looser (Fig. 4a), with less proteoglycan content and lower Col2 expression (Fig. 4b) than those formed by chondroprogenitor cells from control mice. Alcian blue staining showed that the proteoglycan content was decreased in micromasses formed by chondroprogenitor cells from Nsd1f/f;Prx1-Cre mice (Fig. 4c, d). qRT-PCR analyses confirmed the decreased expression of Sox9, Col2, and Acan in micromasses formed by Nsd1-deficient chondroprogenitor cells (Fig. 4e–h). In chondroprogenitor cells, there is a balance among cell differentiation, proliferation, and apoptosis.17,18 We next examined the proliferation and apoptosis abilities of NSD1-deficient chondroprogenitor cells. Crystal violet staining, quantification, and the MTT assay revealed increased cell proliferation (Figs. 4i, j and S3A), and the TUNEL assay showed no alterations in the apoptosis (Fig. S3B) of Nsd1-deficient chondroprogenitor cells. These data indicate that NSD1 is necessary for chondrogenic differentiation of chondroprogenitor cells.
Fig. 4.
Chondroprogenitor cells with Nsd1 knockout showed impaired chondrocyte differentiation and increased proliferation. a Gross images of pellets formed by chondroprogenitor cells from neonatal mice. Scale bar = 1 mm. b HE staining (top), SO staining (middle), and Col2 in situ hybridization (bottom) results of sections from pellets formed by chondroprogenitor cells. Scale bar = 20 μm. c Alcian blue staining results of micromasses cultured for 1, 4, and 7 days with chondroprogenitor cells. Scale bar = 2 mm. d Quantitative analysis of Alcian blue staining. The values are presented as the means ± SEMs, n = 4. **P < 0.05, ns means not significant. qRT-PCR results for Nsd1 (e) and chondrocyte differentiation marker genes, including Sox9 (f), Col2 (g), and Acan (h), in micromasses cultured for 1, 4, and 7 days with chondroprogenitor cells. The values are presented as the means ± SEMs, n = 4. *P < 0.05, **P < 0.01, ns means not significant. i Crystal violet staining results of chondroprogenitor cells cultured for 1, 3, 5, and 7 days. Scale bar = 5 mm. j Quantification of crystal violet staining. The values are presented as the means ± SEMs, n = 6. **P < 0.01, ns means not significant
Sox9 was regulated by NSD1 through H3K36 methylation
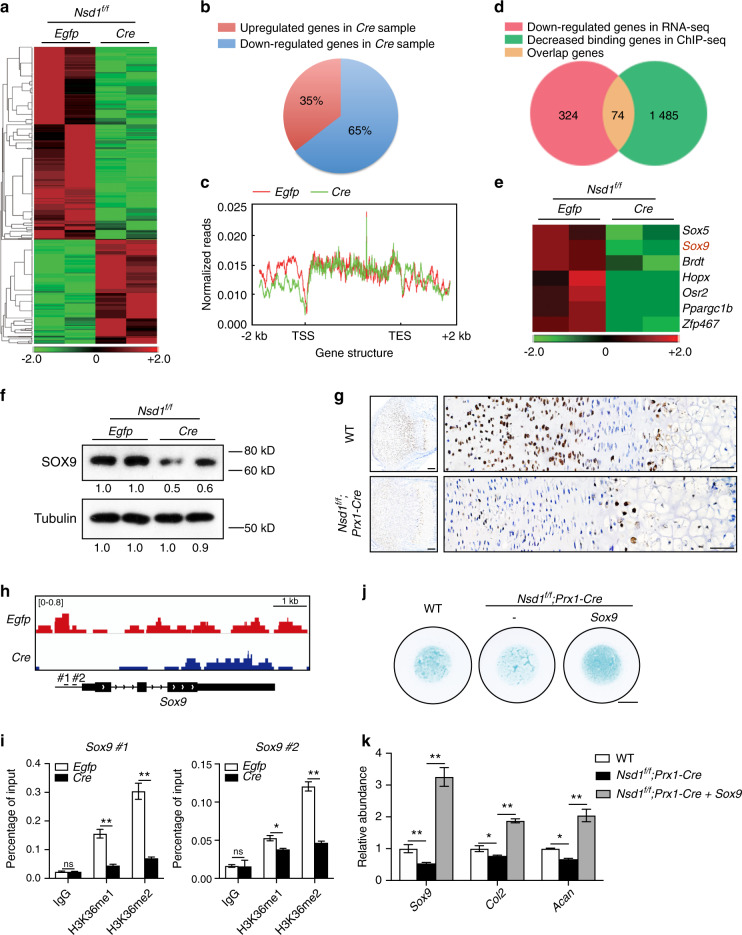
The skeletal growth and fracture healing defects and decreased chondrogenic differentiation in Nsd1f/f;Prx1-Cre mice prompted us to examine the underlying mechanisms by which NSD1 regulates chondrogenic differentiation. Nsd1f/f chondroprogenitor cells were immortalized and infected with lentivirus expressing Egfp or Cre recombinase. Western blot analysis showed that Cre induced depletion of NSD1 and decreased the H3K36me1/2 levels (Fig. S4A). Accordingly, chondrogenic differentiation was impaired in Cre-expressing cells (Fig. S4B). RNA sequencing (RNA-seq) data showed that more genes were downregulated (Fig. 5a)—~65% of the differentially expressed genes—than upregulated in Cre-expressing cells (Fig. 5b), indicating that H3K36 methylation is mainly linked to the active regulation of transcription.23,24 In addition, the H3K36me2 chromatin immunoprecipitation sequencing (ChIP-seq) assay revealed that differential H3K36me2 binding peaks mainly accumulated in promoter regions close to transcription start sites (TSSs) (Fig. 5c). After integration of the H3K36me2 ChIP-seq data with the RNA-seq data, 74 genes showed not only decreased expression levels but also decreased H3K36me2 occupancy in Cre-expressing cells (Fig. 5d). Gene Ontology (GO) analysis revealed that these genes were mainly involved in cell differentiation (Fig. S5). Among these genes were seven transcription factors, including Sox9, the key transcription factor for chondrogenic differentiation (Fig. 5e). SOX9 expression was decreased in both Cre-expressing cells and the growth plate of Nsd1f/f;Prx1-Cre mice (Fig. 5f, g). Mice with Nsd1 knockout in chondrocytes did not show a change in the SOX9 protein level (Fig. S6A). H3K36me2 ChIP-seq data showed decreased H3K36me2 levels on the promoter of Sox9 in Cre-expressing cells (Fig. 5h). ChIP-PCR assays confirmed the decreased occupancy of H3K36me1 and H3K36me2 in the promoter region of Sox9 in NSD1 knockout cells (Fig. 5i). Moreover, overexpression of Sox9 in chondroprogenitor cells rescued the chondrogenic differentiation defects of Nsd1-deficient cells, as demonstrated by Alcian blue staining and qRT-PCR analysis of chondrogenic differentiation marker genes (Fig. 5j, k). Collectively, the above data indicate that the regulation of gene expression by NSD1 occurs mainly through H3K36 methylation in the TSS region and that Sox9 is directly regulated by NSD1 through H3K36me1/2 occupancy in the Sox9 promoter region.
Fig. 5.
Sox9 was regulated by NSD1 through H3K36 methylation. a Heat map of RNA-seq results for Egfp- and Cre-expressing immortalized Nsd1f/f chondroprogenitor cells. b Pie chart showing the percentages of differentially expressed genes between Egfp and Cre samples. c Normalized reads of H3K36me2 ChIP-seq analyses in Egfp- and Cre-expressing immortalized Nsd1f/f chondroprogenitor cells from 2 kb upstream of the TSS to 2 kb downstream of the TSS in the genome. d Venn diagram showing the numbers of genes with decreased expression in RNA-seq data (pink), genes with decreased H3K36me2 occupancy in ChIP-seq data (green), and overlapping genes (yellow). e Heat map and annotation of transcription factors from the set of overlapping genes. f Western blot analysis of SOX9 in Egfp- and Cre-expressing immortalized Nsd1f/f chondroprogenitor cells. g Immunohistochemical assay of SOX9 in growth plate sections from P7 mice. Scale bar (left) = 100 μm, scale bar (right) = 5 μm. h H3K36me2 binding peaks on Sox9 in Egfp- and Cre-expressing immortalized Nsd1f/f chondroprogenitor cells from the H3K36me2 ChIP-seq assay. i ChIP-PCR assay of H3K36me1 (left) and H3K36me2 (right) occupancy of Sox9. The values are presented as the means ± SEMs, n = 3. *P < 0.05, **P < 0.01, ns means not significant. j Alcian blue staining results of micromass culture with chondroprogenitor cells without or with Sox9 overexpression. Scale bar = 2 mm. k qRT-PCR results of Sox9, Col2, and Acan in micromass culture. The values are presented as the means ± SEMs, n = 4. *P < 0.05, **P < 0.01
NSD1 showed direct regulation on Hif1α
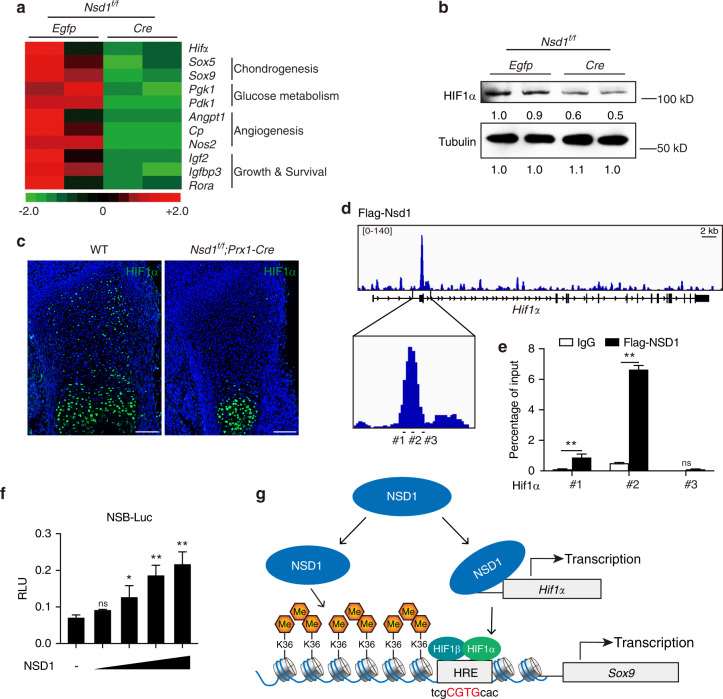
As the key regulator of chondrogenic differentiation, Sox9 is regulated by a number of factors, including HIF1α.4 When RNA-seq data were analyzed separately, we found that the levels of Hif1α and its target genes were decreased after Nsd1 deletion (Figs. 6a and S7A). Western blot analysis and immunofluorescence staining showed decreased protein levels of HIF1α after Nsd1 knockout in mesenchymal progenitors (Fig. 6b, c), and no change in the HIF1α protein level occurred after Nsd1 knockout in chondrocytes (Fig. S8A). The H3K36me2 ChIP-seq results showed no obvious binding peak differences in Hif1α (Fig. S9A); thus, we performed NSD1 ChIP-seq with an anti-Flag antibody after transfecting the Flag-NSD1 plasmid into ATDC5 cells, a chondrogenic cell line.25 From the Flag-NSD1 ChIP-seq results, we observed a specific NSD1 binding peak in the Hif1α promoter region (Fig. 6d). The ChIP-PCR assay results verified this binding (Fig. 6e). Next, we cloned the genomic sequence of the NSD1-specific-binding (NSB) peak into the pGL3 luciferase reporter (NSB-Luc) plasmid and assessed the effects of NSD1 on this reporter. The luciferase reporter assay showed that NSD1 can activate NSB-Luc (Fig. 6f), indicating positive regulation of Hif1α. Since Sox9 is a well-known target gene regulated by HIF1α, these data indicate that NSD1 directly regulates Hif1α and that the regulation of Sox9 by NSD1 can also be achieved indirectly through Hif1α (Fig. 6g).
Fig. 6.
NSD1 directly regulated Hif1α. a Heat map of Hif1α and its target genes from the RNA-seq results of Egfp- and Cre-expressing immortalized Nsd1f/f chondroprogenitor cells. b Western blot analysis of the HIF1α level in Egfp- and Cre-expressing immortalized Nsd1f/f chondroprogenitor cells. c Immunofluorescence analysis of HIF1α in limb buds of E15.5 mice. Scale bar = 100 μm. d NSD1 binding peaks on Hif1α in ATDC5 cells from the Flag-NSD1 ChIP-seq assay. e ChIP-PCR assay of NSD1 binding on Hif1α. The values are presented as the means ± SEMs, n = 3. **P < 0.01, ns means not significant. f Luciferase assay of the NSD1-specific binding (NSB) region in the Hif1α promoter in C3H10 cells treated with NSD1. The values are presented as the means ± SEMs, n = 3. *P < 0.05, **P < 0.01, ns means not significant. g Model that summarizes our findings on the role of NSD1 in regulating Sox9 directly and indirectly. On the one hand, NSD1 can directly promote Sox9 expression by regulating the levels of H3K36me1/2 in the Sox9 promoter region. On the other hand, NSD1 directly binds to the promoter region of Hif1α, activating Hif1α transcription and ultimately promoting Sox9 expression
Discussion
In this study, we found that the histone methyltransferase NSD1 plays a key role in chondrogenic differentiation. We observed increased Nsd1 mRNA levels during chondrogenic differentiation. Nsd1f/f;Prx1-Cre mice showed delayed chondrogenesis, delayed primary and secondary ossification center formation, shorter stature, and malformation of the growth plate, but these phenotypes were not seen in Nsd1f/f;Col2-Cre mice, meaning that NSD1 mainly functions in the stage before Col2+ chondrocyte formation. From single-cell RNA-seq data of E11.5 limb buds, we found that the distribution of Nsd1 was more overlapped with that of Prrx1 and broader than that of Col2a1 (Fig. S10A).26 The skeletal growth defects in Nsd1f/f;Prx1-Cre mice were due to aberrant growth plate formation, especially the abnormal resting zone and disorganized proliferating zone, consistent with a previous finding that chondrocyte progenitors in the resting zone can supply cells for longitudinal bone growth in postnatal mice.27 In addition to participating in bone formation and elongation under physiological conditions, chondrogenic differentiation also participates in fracture healing under pathological conditions.28 In the fracture model, mice with NSD1 deletion showed impaired fracture healing, delayed appearance of cartilage, and decreased endochondral bone formation. Further study showed that NSD1 deletion disrupted the balance between the proliferation and differentiation of chondroprogenitor cells.
In Nsd1f/f;Prx1-Cre mice, we observed shorter stature and decreased bone length, inconsistent with the pre- and postnatal overgrowth in Sotos syndrome patients.16 In NSD1 heterozygous mice, the growth rate was normal, and the Sotos phenotype was only observed with careful analysis of the growth pattern, which was more subtle than that in humans.13 This inconsistency is also observed in Df(13)Ms2Dja (+/−) mice, a chromosome-engineered mouse model of Sotos syndrome; most of the Sotos phenotypes, except for overgrowth, are replicated in these mice, and Df(13)Ms2Dja (+/−) mice show reduced gestational and postnatal growth.29 In this study, the inconsistency in bone growth between mice and humans may be attributed to the deletion of NSD1 within a specific cell population at a particular stage of development in our mouse model and the observation that NSD1 may play divergent roles in regulating bone growth in mice and humans. Overgrowth-related genes identified in patients do not always cause overgrowth in mice. For example, mice carrying DNMT3A mutations show postnatal growth retardation, which is different from the phenotype of DNMT3A overgrowth syndrome patients.30,31 In addition, deletion of EZH1 and EZH2 in chondrocytes causes severe skeletal growth impairment in mice, which is due to reduced growth plate chondrogenesis rather than longitudinal bone overgrowth.12,32
Over the past decades, the study of NSD1 has mainly focused on its function in tumorigenesis, including in head and neck squamous cell carcinomas,33 laryngeal tumors,34 myelodysplastic syndromes,35 and so on. H3K36 methylation is also related to tumor formation, and H3K36M mutation impairs the differentiation potential of mesenchymal progenitors and leads to undifferentiated sarcoma generation.18 H3K36M leads to decreased H3K36 di- and trimethylation, activating cancer pathways and resulting in chondroblastoma.17 A recent study found that NSD1-mediated H3K36me2 is required for the maintenance of DNA methylation at intergenic regions, which is crucial for the regulation of downstream gene expression.36 Collectively, these findings indicate that NSD1-mediated histone modification plays important roles in various pathophysiological processes. In our study, Nsd1 knockout led to a decrease in H3K36me1 and H3K36me2, leading to defects in chondrogenesis and growth plate formation. However, our previous study demonstrated that there was no cartilage phenotype in Setd2f/f;Prx1-Cre mice.37 As SET domain-containing protein 2 (SETD2) is the only methyltransferase for H3K36me3, our current study suggested the different functions of different forms of H3K36 methylation. We performed RNA-seq and ChIP-seq analysis and found that NSD1 and H3K36 methylation regulate the transcription of different sets of genes. Among these genes, SOX9 can promote chondrogenic differentiation. It has been proven that SOX9 is indispensable for skeletogenesis, especially for growth plate formation.2,3,38,39 The expression of Sox9 was regulated by NSD1 through H3K36me1 and H3K36me2 occupancy of the promoter (Fig. 5), and overexpression of Sox9 rescued the chondrogenic differentiation impairment (Fig. 5), suggesting that NSD1 is a key epigenetic regulator of chondrogenesis, at least partially through the regulation of Sox9 expression. NSD1 deficiency in Prx1-positive MSCs affected limb formation, with abnormal Col2-positive chondrocyte formation and abnormal Sox9 expression (Figs. 1c and 5g). However, Nsd1f/f;Col2-Cre mice had normal limb formation (Fig. 1g) with normal Sox9 expression (Fig. S6A), suggesting that NSD1 functions before the activation of Col2-cre or the expression of collagen II. In summary, we believe that NSD1 functions as an epigenetic regulator of Sox9 expression mainly in Col2-cre-negative chondroprogenitor cells but not in Col2-cre-positive chondrocytes.
Moreover, we found from the transcriptome analysis that the expression of Hif1α and its target genes was reduced when Nsd1 was depleted. HIF1α plays a crucial role during chondrogenic differentiation and limb development. Mice with limb bud mesenchyme-specific Hif1a knockout show significantly shorter hindlimbs with abnormal cartilage formation and decreased differentiation of prechondrogenic cells through direct regulation of Sox9.4 It has been known for years that histone lysine methyltransferases (KMTs) can promote or inhibit gene expression by targeting the enhancer or promoter regions of different genes.40 In addition, some KMTs can regulate target gene expression independent of HMT activity. EZH2 can promote cyclin D1 expression directly in natural killer cells independent of its enzymatic activity.41 G9a, another histone KMT, inhibits adipogenesis by repressing Pparγ expression in a manner dependent on its HMT activity and promoting Wnt10a expression in an enzymatic activity-independent manner.42 Here, H3K36me2 occupancy on Hif1α showed no difference after NSD1 knockout, and among the seven transcription factors found by combined analysis of the RNA-seq and H3K36me2 ChIP-seq data, only Sox9, Hopx, Osr2, and Zfp467 showed obvious NSD1 binding peaks (Fig. S9B–H), revealing that NSD1 binding and H3K36me2 occupancy on target genes are not entirely synchronous. In this study, NSD1 bound to the Hif1α promoter directly and activated Hif1α transcription, raising the possibility that NSD1 may also function independent of HMT activity.
Collectively, we identified NSD1 as a novel regulator of chondroprogenitor cell fate and suggested that epigenetic regulation of SOX9 by NSD1 is an important process for chondrogenesis. These findings suggest that modulation of NSD1 and H3K36 methylation would have therapeutic potential for skeletal growth defects and fracture healing disorders resulting from chondrogenic differentiation impairment. Revealing the function of NSD1 in chondrogenic differentiation and bone growth is helpful to understand the overgrowth of Sotos syndrome patients with NSD1 mutations and to expand our understanding of the function of epigenetic regulation in chondrogenesis and skeletal biology.
Materials and methods
Ethics statement
All animal experiments were conducted in accordance with a protocol approved by the Animal Care and Use Committee of Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (approval number: SIBCB-NAF-14-001-S350-019). Animals were bred and maintained under specific pathogen-free conditions in the institutional animal facility of the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences.
Mice
Nsd1f/f mice were purchased from the Jackson Laboratory. The Prx1-Cre mouse strain was a gift from Andrew McMahon. The Col2-Cre mice were kindly provided by Dr. Xiao Yang. All mice analyzed were maintained on the C57BL/6 background.
Assessment of Nsd1 knockout efficiency
The Nsd1 gene knockout efficiency assay was performed in three tissues: cartilage, bone, and liver. Cartilage was taken from the tibial plateau, and bone was obtained by cutting out the ends of the tibial growth plate and flushing out the bone marrow. Liver tissue was used as the negative control.
Mouse femoral fracture
The fracture model was established as described previously with 6-week-old mice.43 Weekly radiographs were performed on mice with fractures to measure the repair process with a Faxitron Model MX-20 instrument (Faxitron, America). The callus index was defined as the maximum diameter of the callus divided by the diameter of the bone.
X-ray analysis
Prior to X-ray analysis, mice were anesthetized with 2% chloral hydrate solution (10 μL·g−1 body weight) injected intraperitoneally. Fractures were confirmed and monitored weekly under anesthesia using a Faxitron MX-20 Cabinet X-ray System (Faxitron X-ray Corp.).
Micro-CT analysis
For micro-CT analysis, soft tissue was removed from fractured femurs from age- and sex-matched mice, and the femurs were fixed with 70% ethanol. Fractured femurs from Nsd1f/f;Prx1-Cre mice and 1-month-old Nsd1f/f;Col2-Cre mice were scanned with a Scanco Micro CT80 instrument (SCANCO Medical, Switzerland) at a resolution of 10 μm. Fractured femurs from Nsd1f/f;Col2-Cre mice and 1-month-old Nsd1f/f;Prx1-Cre mice were scanned with a Skyscan 1176 scanner (Bruker, Kartuizersweg, Belgium) at a spatial resolution of 9 μm. For statistical analysis of trabecular bone in the callus, the whole region of the callus with a threshold of 85–255 was used. A Gaussian noise filter optimized for murine bones was applied to reduce the noise in the thresholded 2D image, and 3D images were reconstructed.44 Indices of trabecular and cortical bone are shown according to the guidelines.45
Cell culture
Chondroprogenitor cells were obtained from the femoral condyles and tibial plateau of newborn mice. The cartilage was digested with 1 mg·mL−1 collagenase II (Sigma, C6885) for 2 h at 37 °C, and the digests were discarded. The remaining tissue was digested with half the concentration of collagenase II overnight at 37 °C, and the digests were filtered through a 70 μm cell strainer (Falcon, 352350) the next day. Cells were plated in α-MEM (Corning, 10-022-CVR) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. ATDC5 cells were cultured in DMEM:F12 (1:1) supplemented with 5% FBS and 1% penicillin/streptomycin. C3H10 cells were cultured in α-MEM (low glucose) supplemented with 10% FBS and 1% penicillin/streptomycin.
Micromass culture
Micromass culture was performed when chondroprogenitor cells were 80%–90% confluent. Chondroprogenitor cells were digested, resuspended at 1 × 107 cells per cell, and plated in a 12.5 μL droplet of cell suspension in the center of a 12-well-plate; the plate was placed at 37 °C for 2 h, and chondrogenic differentiation medium, which contained DMEM (Corning, 10-013-CVR), 10 ng·mL−1 TGFβ3 (Peprotech, 100-36E), 100 nmol·L−1 dexamethasone (Sigma, D1756), 50 μg·mL−1 L-ascorbic acid 2-phosphate (Sigma, A8960), 1 mmol·L−1 sodium pyruvate (Sigma, 25-000-CIR), 40 μg·mL−1 proline (Sigma, P5607), and 1% ITS (Cyagen, ITSS-10201-10), was then gently added. At different time points, micromasses were acidified with 0.1 N HCl and were then stained with 1% Alcian blue (Sigma, A5268). Quantification of Alcian blue staining was performed by measuring the absorbance at 620 nm after dissolving the stained micromass with 6 M guanidine hydrochloride solution.
Pellet culture
Pellet culture was performed when chondroprogenitor cells were 80%–90% confluent. Chondroprogenitor cells were digested and resuspended at 1 × 107 cells per mL, 12.5 μL of cell suspension was added to 500 μL of chondrogenic differentiation medium in a 15 mL tube, the tube was centrifuged at 400 × g for 4 min to pellet the cells in the bottom of tube, the tube was allowed to stand, and cells were cultured at 37 °C. The culture medium was replaced with fresh medium every 3 days in the first week and weekly thereafter.
Immortalization of chondroprogenitor cells
Chondroprogenitor cells were infected with pLenti-CMV-SV40 lentivirus expressing simian virus 40 (SV40) T antigen to achieve immortalization.
Lentiviruses and infection
Lentiviral vectors expressing Egfp and Cre were constructed by inserting the genes’ CDSs into the pLenti vector. Virus packaging was conducted according to the VSVG-delta 8.9 system. Mouse chondroprogenitor cells were cultured for 2 days, infected with lentivirus for 24 h, and treated with puromycin for 48 h.
Histology and immunohistochemistry
Hindlimbs and fractured femurs from mice were fixed with 4% paraformaldehyde for 48 h at 4 °C, decalcified in 10% EDTA, and embedded in paraffin. Each sample was sectioned sagittally at a thickness of 8 μm for staining. HE staining and Safranin O staining were performed. Immunohistochemical staining was conducted using a standard protocol. The in situ hybridization probe for Col2 was a gift from the Laurie H. Glimcher Laboratory.
Immunofluorescence
Sections were blocked in PBS with 10% horse serum for 1 h and were then stained overnight with a specific antibody at 4 °C. Secondary antibodies were used according to the species of the primary antibody. DAPI (Sigma, D8417) was used for counterstaining. Slides were mounted with anti-fluorescence quenching mounting medium (Dako, S3023), and images were acquired with an Olympus BX51 microscope.
Antibodies
Antibodies specific for the following molecules were used: NSD1 (Bioss, bs-8170R), COL2 (Abcam, ab34712), H3K36me1 (Abcam, ab9048), H3K36me2 (Abcam, ab9049), H3K36me3 (Abcam, ab9050), SOX9 (Millipore, AB5535), HIF1α (WB: Novus, NB100-134; IF: Bioss, bs-0737R), and Flag (Sigma, F1804).
Western blot analysis
Cells were harvested and lysed with EBC buffer (1% NP-40, 10% glycerol, 135 nmol·L−1 NaCl, 20 mmol·L−1 Tris (pH 8.0)) containing a protease inhibitor (MCE, HY-K0010). Then, lysates were separated through SDS-PAGE and transferred to a PVDF membrane (Bio-Rad, 1620177). After incubation with specific antibodies, we used an enhanced chemiluminescence kit (Millipore, P90720) to detect protein signals. Quantitative data were analyzed by ImageJ software (Bethesda, MD, USA).
RNA-seq and data processing
Egfp- and Cre-expressing immortalized Nsd1f/f chondroprogenitor cells were collected, and total RNA was extracted with TRIzol Reagent (Sigma, T9424). High-throughput sequencing was performed by the Computational Biology Omics Core, CAS-MPG Partner Institute for Computer Biology (PICB), Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. Raw reads were mapped to the mm10 genome using the TopHat program. We assigned each gene an expression value in fragments per kilobase per million using Cufflinks software. Then, Cuffdiff software was used to identify differentially expressed genes between Egfp- and Cre-expressing samples. Differentially expressed gene heat maps were clustered by k-means clustering using the Euclidean distance as the distance and visualized using Heml software. GO analysis was carried out with the DAVID Functional Annotation Bioinformatics Microarray Analysis tool.
Real-time PCR analysis
Total RNA was isolated from different tissues and cells with TRIzol Reagent (Sigma, T9424) and reverse-transcribed with a PrimeScript RT Reagent Kit (Takara, RR037A). Real-time reverse transcription-PCR was performed in a Bio-Rad CFX Connect Real-Time System. The primer sets used were Nsd1: sense AAACTCGGAGGGTGCT, anti-sense CCTGAGGCGTTTCTTCT; Nsd2: sense TGCCAAAAAGGAGTACGTGTG, anti-sense CTTCGGGAAAGTCCAAGGCAG; Nsd3: sense TCCACTGGTGTTAAGTTCCAGG, anti-sense GGCACCTCTTGTGTTAATTTTGG; Setd2: sense AAATCAGGTACTGGGGCTACA, anti-sense GGCCCATTTCATTAGATCAGGGA; Ash1l: sense CCTCGGTGGACTAAAGTGGTG, anti-sense CGCTGGCTCAGAACTATTTGA; Smyd2: sense AAGGATTGTCAAAATGTGGACGG, anti-sense ATGGAGGAGCATTCCAGCTTG; Col2: sense CGGTCCTACGGTGTCAGG, anti-sense GCAGAGGACATTCCCAGTGT; Sox9: sense TTCCTCCTCCCGGCATGAGTG, anti-sense CAACTTTGCCAGCTTGCACG; Acan: sense AATCCCCAAATCCCTCATAC, anti-sense CTTAGTCCACCCCTCCTCAC; Hif1α: sense AGATCTCGGCGAAGCAAAGAGT, anti-sense CGGCATCCAGAAGTTTTCTCACAC; Sox5: sense CCCGTGATCCAGAGCACTTAC, anti-sense CCGCAATGTGGTTTTCGCT; Pgk1: sense ATGTCGCTTTCCAACAAGCTG, anti-sense GCTCCATTGTCCAAGCAGAAT; Pdk1: sense GGACTTCGGGTCAGTGAATGC, anti-sense TCCTGAGAAGATTGTCGGGGA; Angpt1: sense CACATAGGGTGCAGCAACCA, anti-sense CGTCGTGTTCTGGAAGAATGA; Cp: sense CTTAGCCTTGGCAAGAGATAAGC, anti-sense GGCCTAAAAACCCTAGCCAGG; Nos2: sense GTTCTCAGCCCAACAATACAAGA, anti-sense GTGGACGGGTCGATGTCAC; Igf2: sense GTGCTGCATCGCTGCTTAC, anti-sense ACGTCCCTCTCGGACTTGG; Igfbp3: sense CCAGGAAACATCAGTGAGTCC, anti-sense GGATGGAACTTGGAATCGGTCA; Rora: sense GTGGAGACAAATCGTCAGGAAT, anti-sense TGGTCCGATCAATCAAACAGTTC; and Hprt: sense GTTAAGCAGTACAGCCCCAAA, anti-sense AGGGCATATCCAACAACAAACTT.
ChIP-seq and ChIP-PCR
Cells were fixed with 1% formaldehyde for 10 min, and the crosslinking reaction was terminated with glycine for 5 min (final concentration = 0.125 mol·L−1). After two washes with precooled PBS (containing a protease inhibitor), the cells were removed by scraping and resuspended in SDS lysis buffer (50 mmol·L−1 Tris-HCl (pH 7.5), 10 mmol·L−1 EDTA, 1% SDS, and protease inhibitor) and sonicated. Cells were centrifuged to obtain cell extracts, which were then added to precleaning protein G agarose and rotated for 1 h at 4 °C. Extracts were centrifuged, and supernatants were harvested into new tubes. ChIP assays were performed using H3K36me1/2 or Flag antibodies. Normal IgG was used as negative control. ChIP-PCR was used to amplify various genomic regions of the target gene, and the primers used were Sox9 #1: sense GACTCCAGGCGCAGAAGCCC, anti-sense CCGGGACTTCGCTGGCGTTT; Sox9 #2: sense CACATCGGTTCACACGGAGA, anti-sense GTGGGGTGAGGGGACTTGGA. Hif1α #1: sense CTCGGCTTTTCCCTCCCC, anti-sense AGTCCTCGCGTCCCCTCA; Hif1α #2: sense GGGCAGTGTCTAGCCAGGC, anti-sense AAGTCCAGAGGCGGGGTG; and Hif1α #3: sense CGGTCCACGTCGCCATC, anti-sense CGGGAGCTAGAGGCGTAC. For the ChIP-PCR assay of micromasses harvested at different time points, the collected micromasses were cut into very small pieces after fixation and termination. Sonication was carried out with a QSONICA Q800R with a 30% sonicator amplitude, a schedule of 10 s on and 10 s off, and a total sonication (“on”) time of 30 min. Subsequent steps were consistent with those used for the ChIP-PCR assay of cells.
ChIP-seq data processing
High-throughput sequencing was performed by the Computational Biology Omics Core, CAS-MPG PICB, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. The SOAP alignment tool was used to align the ChIP-seq reads to the mouse genome build mm10. Reads with fewer than two mismatches that uniquely mapped to the genome were used in subsequent analyses. We calculated the distance from the peak centers to the annotated TSSs and then defined the nearest genes as peak-related genes.
Transient transfections and reporter gene assays
For transient transfections, C3H10 cells were seeded overnight in a 12-well plate at a concentration of 5 × 104 cells per well. Cells were then transfected with the Hif1α-Luc or HRE-Luc reporter plasmid and various combinations of NSD1 and HIF1A expression constructs as indicated. Forty-eight hours after transfection, luciferase assays were performed using the Dual-Luciferase Reporter Assay System (Promega). The Hif1α-Luc plasmid was constructed by inserting KpnI/XhoI-digested PCR products, which were amplified using the forward primer 5′-GGggtaccGGGCAGTGTCTAGCCAGGC-3′ and reverse primer 5′-CCGctcgagAAGTCCAGAGGCGGGGTG-3′, into the KpnI/XhoI-digested pGL3-Basic luciferase reporter plasmid.
Statistical analysis
Quantitative data are presented as the mean ± SEM values as indicated. The statistical significance of differences between WT and CKO mice was evaluated with GraphPad using unpaired two-tailed Student’s t tests, and one-way ANOVA was used to detect the effects of Sox9 treatment. P < 0.05 was considered statistically significant. The number of samples shown in each figure legend is the number of biological replicates. Three technical replicates were used for each experiment.
MTT assay
The MTT cell viability assay was conducted following the instructions provided in the MTT Cell Proliferation Assay Kit (Sangon Biotech, E606334).
TUNEL assay
The TUNEL apoptosis assay was conducted on paraffin sections following the instructions provided in the DeadEnd™ Fluorometric TUNEL System (Promega, G3250).
Supplementary information
Acknowledgements
The authors would like to thank Dr. Andrew McMahon (Harvard University, Boston) for providing the Prx1-Cre mouse line and Dr. Xiao Yang (Affiliated Hospital of Academy of Military Medical Sciences, Beijing) for providing the Col2-Cre mouse line. The authors would like to thank members of the Zou Lab for helpful discussions. This work was supported by grants from the National Natural Science Foundation of China (NSFC) [81902212, 81725010, 81672119, 81991512], Strategic Priority Research Program of the Chinese Academy of Sciences [XDB19000000], Major Program of Development Fund for Shanghai Zhangjiang National Innovation Demonstration Zone [ZJ2018-ZD-004].
Author contributions
W.Z. designed the research; R.S., Z.Z., Z.X., L.W., and H.O. performed the research; R.S., Z.Z., and W.Z. analyzed the data and wrote the paper; and H.O., X.C., and M.G. contributed new reagents/analytic tools.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Rui Shao, Zhong Zhang.
Change history
7/26/2021
A Correction to this paper has been published: 10.1038/s41413-021-00160-2
Supplementary information
The online version contains supplementary material available at 10.1038/s41413-021-00148-y.
References
- 1.Baron J, et al. Short and tall stature: a new paradigm emerges. Nat. Rev. Endocrinol. 2015;11:735–746. doi: 10.1038/nrendo.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 3.Bi W, et al. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc. Natl Acad. Sci. USA. 2001;98:6698–6703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amarilio R, et al. HIF1alpha regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. Development. 2007;134:3917–3928. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- 5.Bentovim L, Amarilio R, Zelzer E. HIF1alpha is a central regulator of collagen hydroxylation and secretion under hypoxia during bone development. Development. 2012;139:4473–4483. doi: 10.1242/dev.083881. [DOI] [PubMed] [Google Scholar]
- 6.Regan JN, et al. Up-regulation of glycolytic metabolism is required for HIF1alpha-driven bone formation. Proc. Natl Acad. Sci. USA. 2014;111:8673–8678. doi: 10.1073/pnas.1324290111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hata K, et al. Arid5b facilitates chondrogenesis by recruiting the histone demethylase Phf2 to Sox9-regulated genes. Nat. Commun. 2013;4:2850. doi: 10.1038/ncomms3850. [DOI] [PubMed] [Google Scholar]
- 8.Zhang F, et al. JMJD3 promotes chondrocyte proliferation and hypertrophy during endochondral bone formation in mice. J. Mol. Cell Biol. 2015;7:23–34. doi: 10.1093/jmcb/mjv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai J, et al. Kdm6b regulates cartilage development and homeostasis through anabolic metabolism. Ann. Rheum. Dis. 2017;76:1295–1303. doi: 10.1136/annrheumdis-2016-210407. [DOI] [PubMed] [Google Scholar]
- 10.Adam, M.P., Hudgins, L. & Hannibal, M. In Kabuki Syndrome, (eds Adam, M.P. et al.) GeneReviews((R)), (Seattle WA, 1993).
- 11.Schott DA, et al. Growth pattern in Kabuki syndrome with a KMT2D mutation. Am. J. Med. Genet. A. 2016;170:3172–3179. doi: 10.1002/ajmg.a.37930. [DOI] [PubMed] [Google Scholar]
- 12.Lui JC, et al. EZH1 and EZH2 promote skeletal growth by repressing inhibitors of chondrocyte proliferation and hypertrophy. Nat. Commun. 2016;7:13685. doi: 10.1038/ncomms13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rayasam GV, et al. NSD1 is essential for early post-implantation development and has a catalytically active SET domain. EMBO J. 2003;22:3153–3163. doi: 10.1093/emboj/cdg288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sotos JF, et al. Cerebral gigantism in childhood—syndrome of excessively rapid growth with acromegalic features and a nonprogressive neurologic disorder. N. Engl. J. Med. 1964;271:109–116. doi: 10.1056/NEJM196407162710301. [DOI] [PubMed] [Google Scholar]
- 15.Tatton-Brown, K., Cole, T. R. P. & Rahman, N. In Sotos syndrome, (eds Adam, M. P. et al.) GeneReviews (University of Washington, Seattle, 1993).
- 16.Agwu JC, et al. Growth in Sotos syndrome. Arch. Dis. Child. 1999;80:339–342. doi: 10.1136/adc.80.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang D, et al. The histone H3.3K36M mutation reprograms the epigenome of chondroblastomas. Science. 2016;352:1344–1348. doi: 10.1126/science.aae0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu C, et al. Histone H3K36 mutations promote sarcomagenesis through altered histone methylation landscape. Science. 2016;352:844–849. doi: 10.1126/science.aac7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosset M, et al. Primary culture and phenotyping of murine chondrocytes. Nat. Protoc. 2008;3:1253–1260. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- 20.Logan M, et al. Expression of Cre recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 21.Hao ZM, et al. Generation and characterization of chondrocyte specific Cre transgenic mice. Yi Chuan Xue Bao. 2002;29:424–429. [PubMed] [Google Scholar]
- 22.Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat. Rev. Rheumatol. 2015;11:45–54. doi: 10.1038/nrrheum.2014.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner EJ, Carpenter PB. Understanding the language of Lys36 methylation at histone H3. Nat. Rev. Mol. Cell Biol. 2012;13:115–126. doi: 10.1038/nrm3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu L, et al. Di- and tri- but not monomethylation on histone H3 lysine 36 marks active transcription of genes involved in flowering time regulation and other processes in Arabidopsis thaliana. Mol. Cell Biol. 2008;28:1348–1360. doi: 10.1128/MCB.01607-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucio-Eterovic AK, et al. Role for the nuclear receptor-binding SET domain protein 1 (NSD1) methyltransferase in coordinating lysine 36 methylation at histone 3 with RNA polymerase II function. Proc. Natl Acad. Sci. USA. 2010;107:16952–16957. doi: 10.1073/pnas.1002653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsushita, Y. et al. Notch effector Hes1 marks an early perichondrial population of skeletal progenitor cells at the onset of endochondral bone development. 10.1101/2020.03.13.990853 (2020).
- 27.Newton PT, et al. A radical switch in clonality reveals a stem cell niche in the epiphyseal growth plate. Nature. 2019;567:234–238. doi: 10.1038/s41586-019-0989-6. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, et al. Loss of Dnmt3b in chondrocytes leads to delayed endochondral ossification and fracture repair. J. Bone Min. Res. 2018;33:283–297. doi: 10.1002/jbmr.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Migdalska AM, et al. Generation of the Sotos syndrome deletion in mice. Mamm. Genome. 2012;23:749–757. doi: 10.1007/s00335-012-9416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sendzikaite G, et al. A DNMT3A PWWP mutation leads to methylation of bivalent chromatin and growth retardation in mice. Nat. Commun. 2019;10:1884. doi: 10.1038/s41467-019-09713-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tatton-Brown K, et al. Mutations in the DNA methyltransferase gene DNMT3A cause an overgrowth syndrome with intellectual disability. Nat. Genet. 2014;46:385–388. doi: 10.1038/ng.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tatton-Brown K, Rahman N. The NSD1 and EZH2 overgrowth genes, similarities and differences. Am. J. Med. Genet. C Semin. Med. Genet. 2013;163C:86–91. doi: 10.1002/ajmg.c.31359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cancer Genome Atlas, N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peri S, et al. NSD1- and NSD2-damaging mutations define a subset of laryngeal tumors with favorable prognosis. Nat. Commun. 2017;8:1772. doi: 10.1038/s41467-017-01877-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thol F, et al. Analysis of NUP98/NSD1 translocations in adult AML and MDS patients. Leukemia. 2013;27:750–754. doi: 10.1038/leu.2012.249. [DOI] [PubMed] [Google Scholar]
- 36.Weinberg DN, et al. The histone mark H3K36me2 recruits DNMT3A and shapes the intergenic DNA methylation landscape. Nature. 2019;573:281–286. doi: 10.1038/s41586-019-1534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, et al. H3K36 trimethylation mediated by SETD2 regulates the fate of bone marrow mesenchymal stem cells. PLoS Biol. 2018;16:e2006522. doi: 10.1371/journal.pbio.2006522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright E, et al. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat. Genet. 1995;9:15–20. doi: 10.1038/ng0195-15. [DOI] [PubMed] [Google Scholar]
- 39.Akiyama H, et al. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc. Natl Acad. Sci. USA. 2005;102:14665–14670. doi: 10.1073/pnas.0504750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Husmann D, Gozani O. Histone lysine methyltransferases in biology and disease. Nat. Struct. Mol. Biol. 2019;26:880–889. doi: 10.1038/s41594-019-0298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan J, et al. EZH2 overexpression in natural killer/T-cell lymphoma confers growth advantage independently of histone methyltransferase activity. Blood. 2013;121:4512–4520. doi: 10.1182/blood-2012-08-450494. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, et al. Histone H3K9 methyltransferase G9a represses PPARgamma expression and adipogenesis. EMBO J. 2013;32:45–59. doi: 10.1038/emboj.2012.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuasa M, et al. Fibrinolysis is essential for fracture repair and prevention of heterotopic ossification. J. Clin. Investig. 2015;125:3117–3131. doi: 10.1172/JCI80313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun J, et al. Histone demethylase LSD1 regulates bone mass by controlling WNT7B and BMP2 signaling in osteoblasts. Bone Res. 2018;6:14. doi: 10.1038/s41413-018-0015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouxsein ML, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Min. Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.