Abstract
A novel coronavirus (SARS-CoV-2) has caused more than 150 million confirmed infections worldwide, while it is not clear whether it affects the coastal waters. This paper proposed a biophysical model based on 16 scenarios with different virus half-life parameters to assess potential viral contamination from 25 municipal sewage outfalls into the Bohai Sea. Viral concentration maps showing spatial and temporal changes are provided based on a biophysical model under multiple scenarios. Results demonstrate that adjacent sea areas can become exposed to SARS-CoV-2 via water-borne transport from outfalls, with a higher risk in winter, because SARS-CoV-2 can be highly stable at low temperature. As coastal waters are the ultimate sink for wastewater and the epidemic will last for long time, this work is of great importance to raise awareness, identify vulnerable areas for marine mammals, and avoid the risk of exposure of tourists at bathing beach.
Keywords: SARS-CoV-2, Bohai sea, Biophysical model, Spatial risk assessment
1. Introduction
According to the World Health Organization (WHO) Coronavirus Disease 2019 (COVID-19) situation report released on 3 May 2021, there have been over 152 million people infected and 3.2 million killed by severe acute respiratory syndrome coronavirus 2 (SARS-COVID-2) have been confirmed worldwide and the toll has been increasing (WHO, 2021). Epidemiology and virologic studies provide shreds of evidence that COVID-19 is primarily transmitted from symptomatic people to others who keep in close contact through respiratory droplets, by directly contacting with infected men, or by contacting with polluted objects and surfaces (Chan et al., 2020). Although direct droplet transmission is a key route of transmission, sewage from toilet-flushing, showering, and washing might contribute to viral transmission (Cahill and Morris, 2020). Considering the evidence of faecal excretion for both severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV), and their ability to remain active in conditions that could facilitate faecal-oral transmission, it is possible that SARS-CoV-2 could also be transmitted via this route.
The presence of SARS-CoV-2 in wastewater and rivers has been demonstrated in many countries with high COVID-19 caseloads (La Rosa et al., 2020a, La Rosa et al., 2020b, Rimoldi et al., 2020). There are few reports of faecal-oral transmission, however, this does not exclude the possibility of fecal-oral transmission. The Paris water authority’s laboratory detected tiny amounts of the virus in four of samples collected in Paris’s non-potable water. The coastal ocean is the ultimate sink for urban sewage. The risk of SARS-CoV-2 transmission in the receiving coastal water bodies should not be underestimated, although it has a lower stability in water than known non-enveloped human enteric viruses with waterborne transmission (La Rosa et al., 2020a, La Rosa et al., 2020b, Yeo et al., 2020). Traces of SARS-CoV-2 were found in water samples taken from Lake Superior beaches in America despite the detection level at 100–1000 copies per liter or 10,000 times lower than levels observed in wastewater.
Long survival time brings threatens to marine mammals. Aside from the probable bat origin, a four-year-old Malayan tiger at the Bronx Zoo tested positive for COVID-19. So far, there are few investigations for marine mammals whether inflected by SARS-CoV-2 (Mordecai and Hewson, 2020). Mathavarajah et al. (2020) adopted conservation of the virus host receptor ACE2 and determined that fifteen species of endangered marine mammals are susceptible to SARS-CoV-2. Viruses, which if present in high numbers in raw wastewater, are not likely to be removed through water treatment practices, consequently, they become environmental pollutants. Although the ocean provides a rapid dilution of sewage, however, the self-depuration capacity of ocean is finite, especially for the coastal ocean. Marine water may become a conduit for zoonotic transmission of SARS-CoV-2 to marine wildlife until the virus concentration decrease below the infection dose level. Although the range of infectious doses for direct seawater contact is not known and the degree of exposure is difficult to estimate, the potential impact assessment of virus pollution in coastal marine waters is advocated.
Once entering natural water systems, sewage carrying SARS-CoV-2 in high doses provides a potential fecal–oral transmission route (Ahmed et al., 2020). Shutler et al. (2021) evaluated the risk of SARS-CoV-2 fecal−oral transmission within freshwater systems, while its significance of transmission in the coastal oceans is vacant. When discharged into the sea, viruses are subjected to not only physical dilution and dispersion but also physicochemical conditions, such as temperature, salinity, sunlight radiation, pH, etc. The former determines where the virus will go, and the latter controls how long it will survive. It could be assumed that SARS-CoV-2 will suffer an aggressive treatment in the marine environment because of UV radiation and heat and will die-off within a short time (Efstratiou and Tzoraki, 2021). We focus on if these viruses can survive long enough and present in high enough concentration in polluted water to cause disease in individuals or marine mammals that got in contact with the water. Temperature is the decisive factor determining how long virus survives in the environment. Low temperature prolongs the presence of SARS-CoV-2 viral stability in cold seawaters. At 4 °C, there was only about a 0.7 log-unit reduction of infectious titre after 14 days (Reza and Mohammad, 2020). Little is known about the presence of the virus in marine waters, which brings potential risk for exposed human and resident animals. Surveillance and assessment for SARS-CoV-2 in marine environment should be considered as part of efforts to eliminate COVID-19.
The potential risk of effluent carried SARS-CoV-2 into the ocean has not aroused public concern, partly due to its vast dilution capability. Biophysical model simulating dispersion of virus in the marine environment after direct release can be a powerful tool providing with a reliable prediction of the spreading and path of the pathogens (Cantrell et al., 2020). In this study, we simulated the scope of SARS-COVID-2 from outfalls and estimated its potential threat in an inland sea, the Bohai Sea of China.
2. Materials and methods
2.1. Study area
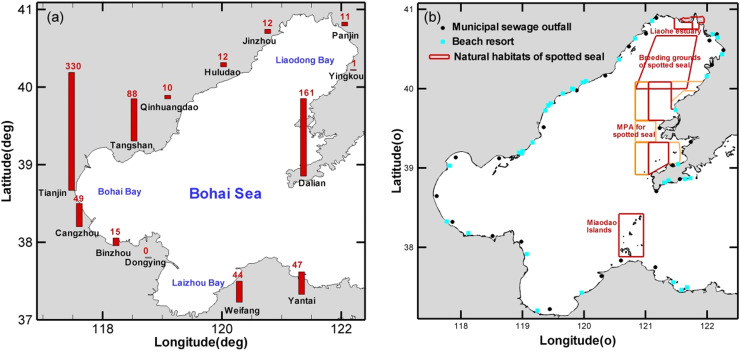
The Bohai Sea is a shallow, semi-enclosed marginal sea situated in the northern part of China (37–41°N, 117°35′–121°10′E). As of 13th January 2021, there have been 614 cases of COVID-19 confirmed from 13 cities around the Bohai Sea ( Fig. 1(a)). Although the vast majority of patients have been cured, these cities still face problems of patients coming from foreign countries and asymptomatic infections. So far, traces of the virus in sewage have been detected in the Netherlands, Sweden and America. Despite faecal-oral transmission process is not yet clear and there are few reports of SARS-CoV-2 in wastewater entering the Bohai Sea, potential health risk deserves attention.
Fig. 1.
(a) Cities with COVID-19 cases reported surrounding the Bohai Sea. (b) Geographical distribution of the municipal sewage outfall, beach resorts and natural habitats of spotted seal in the Bohai Sea.
Twelve among the thirteen cities with COVID-19 cases reported concentrates in the coastline and directly discharge over 1 million tonnes wastewater into the Bohai Sea through 25 municipal sewage outfalls annually (Fig. 1(b)). Daily sewage discharge of each outfall is estimated 50,000 tonnes. Through these outfalls, the coronavirus that are not effectively disinfected in domestic sewage treatment plants or from waste discharges without treatment can be a problem for visitors recreational swimming in coastal waters and marine mammals living in the sea.
The surface water temperature in the Bohai Sea does not exceed 4 °C until April. Even in the hottest month of August, there are few parts with surface temperatures over 27 °C. Low water temperatures prolonged the presence of SARS-CoV-2, which presents potential affects to marine wildlife, especially for the spotted seal, the only fin-footed species that breeds in China. In November each year, spotted seals cross the Bohai Strait. After entering the Bohai Sea, the female seals will give birth in January-March of the following year. Most seals will leave the Bohai Sea in May, but some will live here all year around. To safeguard spotted seals, three Marine Protected Areas were established in the Shuangtaizi Estuary, Miaodao Island, Huping Island, and Changxing Island, and one reserve was zoned for breeding ground in the Liaodong Bay (Fig. 1(b)).
2.2. Model description
Biophysical model is a synthetic product of hydrodynamic model, scalar transport model, and biological model. First, the transport/dispersion processes of SARS-CoV-2 is simulated by an advection-diffusion equation under the action of sea currents from a high temporal and spatial wave-current coupled hydrodynamic model (Guo et al., 2016), with an unstructured grid containing 52,336 nodes and 125,690 elements. The open boundary condition is driven by water level from eight harmonic constituents (M 2, S 2, N 2, K 2, K 1, O 1, P 1, Q 1) (Matsumoto et al., 2000), and the upper boundary is forced by daily average wind velocities obtained from re-analysis data based numerical results provided by the Weather Research & Forecasting Model.
While viruses are generally perceived the ‘simplest’ to model, dispersion processes can still be quite sophisticated; inclusion of transfer by asymptomatic host species or sediment adsorption (Cantrell et al., 2020). For convenience of calculations, we assumed that viruses are passively moved by simulated currents and followed for a predetermined length of time. Equations describing the impacts of physicochemical conditions on the pathogen’s presence time are usually parameterized from observations and experiments. In order to consider the viral mortality in seawater, an exponential decay of the number of virus with time is prescribed, based on a Weibull model (González et al., 2018), in terms of the so-called inactivation rate T 50 (time lapse in which 50% of virus exposed to seawater die on average). It is still unknown how long SARS-CoV-2 will survive in the marine environment, but it is certain that temperature plays the most crucial role. For the purpose of understanding the pollution consequences as a result of different stabilities of SARS-CoV-2, sixteen simulations with types of T 50s were conducted. Fifteen ones were constant, from 1 to 15 days, and the rest was dependent on temperature (t) 1.7325 × 106 exp-0.1518t. Based on the infectious doses of > 400 copies per 1 L of sewage into environments within countries with high infection rates (hutler et al., 2021), the releases virus was estimated to be 1.0 × 105 SARS-CoV-2 most probable number (MPN) per m3 (MPN/m3) from twenty-five coastal outfalls, with daily emissions of 50,000 m3 municipal wastewater, and the continuous emissions last from January 2020 to December 2020.
3. Results
3.1. Potentially affected area
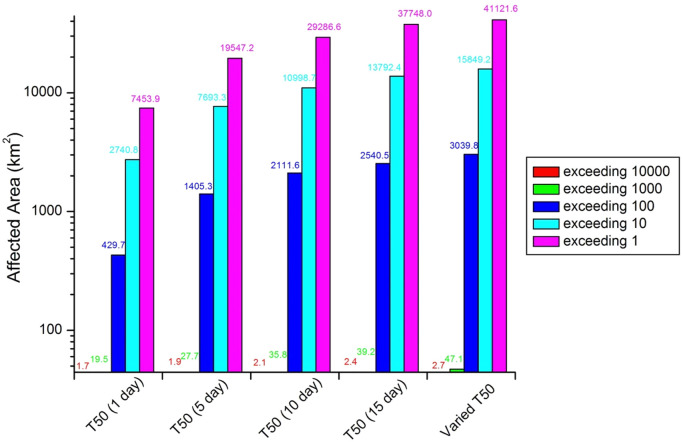
A hypothetical release of 105 MPN/m3 SARS-CoV-2 viruses in wastewater was assumed with an emission rate of 50,000 m3/d from twenty-five outfalls along the Bohai Sea. Fig. 2 presents the envelope area of maximal virus concentration corresponding to one year of continuous discharges into the sea. Because of powerful seawater disperse dilution, the areas with peak concentration over 10% of the initial discharge values do not exceed 3 km2 in any case. As the T 50 s used for SARS-CoV-2 varies from 1 to 15 days, the envelope range over 1 MPN/m3 rises from 7543.9 to 41121.6 km2, while the 10,000 MPN/m3 contour is nearly constantly located at distance of about 1 km from the sewage outfall. The potentially polluted area reaches the maximum 15849.2 km2 under the temperature-dependent T 50 scenario, because of the quite long survival time within the cold even frozen sea water in winter.
Fig. 2.
Envelope area corresponding to 366 days of continuous discharge in 2020.
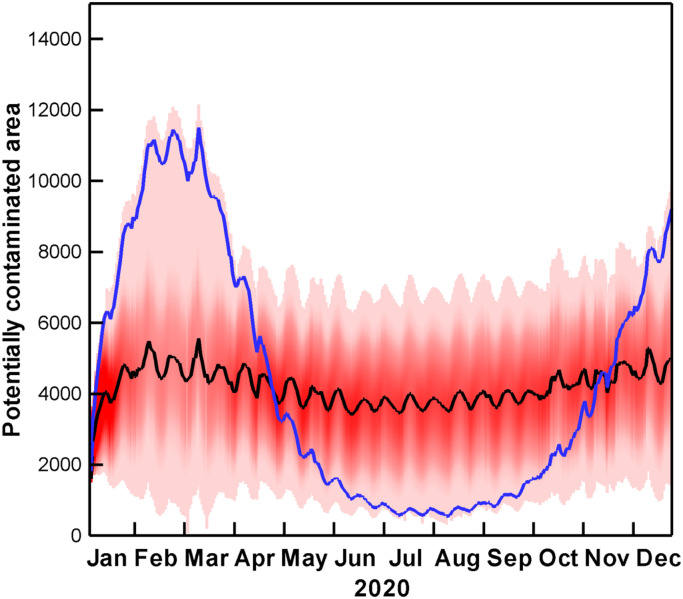
Temporal evolution of affected areas in the Bohai Sea is shown in Fig. 3. Once wastewater discharged into the sea, the polluted area increased rapidly. Owing to robust dilution capacity of sea water, it only took one month to reach the steady state, approximately 4000 km2. When the survival of SARS-CoV-2 is considered to be constant, estimations vary in magnitude, but not in structure. For the tidal current plays as a dominant dynamic factor in the Bohai Sea, the peak level even follows a four-week cycle of changing spring-neap tidal amplitude. Once temperature is involved in virus survival, the simulation is completely different. The monthly change of affected area in a year is consistent with the monthly change of water temperature reaching its peak in February and March. The affected range decreases during the warm-up process until August, then rises again with the falling temperature.
Fig. 3.
Potentially affected area (exceeding 1 MPN/m3) in the Bohai Sea during 2020. Black line is projection using point estimates (n = 16), and blue line accounts for the contaminated area considering temperature effects. Red shaded area is 95% confidence interval, and colour saturation indicates estimated likelihood.
3.2. Risk to the Bohai Sea
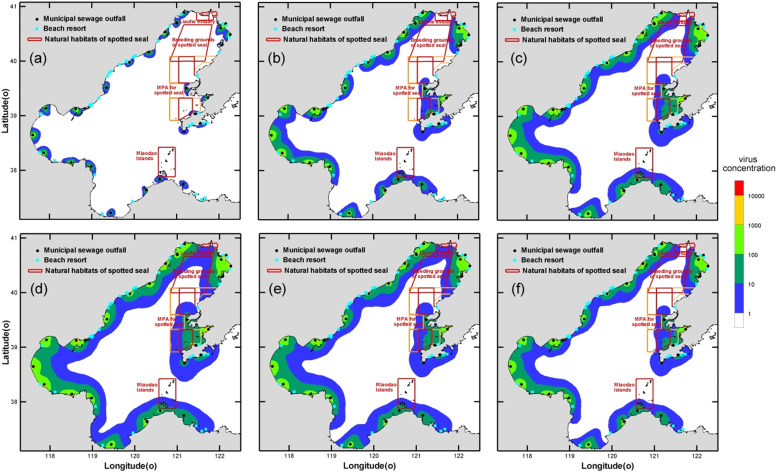
The maximum concentration envelope could suggest the invaded extent, while the annual mean concentrations reveal exposure effects ( Fig. 4). High-risk areas are concentrated near sewage outlets. The survival time of virus has a great influence on the exposure range. If the T 50 is 1 day, only 3226.1 km2 sea area is exposed to an average concentration higher than 1 MPN/m3. Furthermore, these high risk regions scattered separately along the coastline. They are connected as a whole as the virus survival period rises. While the T 50 increased to 15 days, up to 20182.9 km2 is contaminated by SARS-CoV-2 exceeding 1 MPN/m3. There are more viruses in nearshore waters than in open seas, indicating an effective dilution in the central basin attributed to energetic currents and deep bathymetry.
Fig. 4.
Mean exposure of water surface exposed SARS-CoV-2 during 2020 with respect to different virus persistence in sea water. Panel (a) T50 1 day, (b) T50 5 days, (c) T50 10 days, (d) T50 15 days, (e) variable T50 with temperature, (e) average of all sixteen scenarios.
There are three habitats in the Bohai Sea, i.e. the Shuangtaizi Estuary, Miaodao Island, and Huping Island (Fig. 1(b)). All the three primarily habitats of spotted seals are contacting the concentration contour and threatened by varying degrees of contamination. The diluted level is 10,000 times lower than levels in wastewater, however, SARS-CoV-2 could possibly cause infection in marine mammals. The risk particularly acute in winter when the habitats become breeding ground for spotted seals. Plenty of sewage outfalls around some bays together with their weaker water exchange ability lead to higher viral concentrations, on the other hand they undergo dense human activity and provide important coastal habitats.
4. Discussion
The impact of virus SARS-CoV-2 discharge in the marine environment on humans/mammals is difficult to assess. Three primary factors are responsible for the occurrence of infection: (a) the pathogenic microorganism fluxes, (b) the prevalence of the virus in the marine environment, and (c) least probable number of cytopathic units. It is a pity that related findings of the three factors is still scarce or uncertain.
Mathavarajah et al. (2020) considered that many marine mammal species are susceptible to SARS-CoV-2 based on conservation of the virus host receptor ACE2, while Maal-Bared et al. (2020) argued that it is becoming increasingly common to overemphasize the risk from wastewater. In principle, we agree with Maal-Bared et al. (2020) statement. On the other hand, Maal-Bared et al. (2020) stating does not violate the findings of Mathavarajah et al. (2020). It is important to note that dose determines toxicity. Levels of SARS-CoV-2 in water samples taken from Lake Superior dropped 10,000 times lower than levels observed in wastewater, similar with what our model simulates. Concentrations of viable SARS-CoV-2 are highly variable and typically low in water environments, so exposure is (Maal-Bared et al., 2020). This further increases the difficulty of risk assessment.
Our model suggests that continuous domestic sewage carrying SARS-CoV-2 can pollute tens of thousands of square kilometers of sea area. The influence range depends on the concentration of virus in sewage and its survival time in sea water. If the virus concentration increases by an order of magnitude, the affected area will increase by thousands of square kilometers. According to the model simulation in the Bohai Sea, most sea-bathing resorts are contaminated. Millions of residents and visitors play in the water at the beach every July and August. Fortunately, there has been no case of infection through exposure to seawater.
While there is limited data on the persistence of SARS-CoV-2 in marine environment, temperature probably plays the most critical role. The virus concentration reduces rapidly at high temperatures but its long persistence in cold waters may result in long distance migration beyond the geographical boundaries. Persistent survival at low temperatures could lead to ecological disaster for marine mammals. Just as persons in contact with seawater, exposed marine mammals can also be infected by SARS-CoV-2, which can not even respond in effective ways to avoid toxic effects. Although least probable number of cytopathic units for marine mammals are not clear, it is identified that many species of marine mammals susceptible to infective SARS-CoV-2. Similar tragedy has been reported to cause deaths in a number of marine mammal deaths by viral infection, including deaths of over 1000 common seals during 1918 in Iceland and deaths of hundreds of harbor seals along the New England seacoast during 1979–1980 (Nabi and Khan, 2020).
Previous studies have shown that cold and dry environments can help the virus survive and spread, while warm and humid environments can reduce virus transmission. However, based on the existing research and reports, the suitable temperature range of the survival of the new coronavirus is relatively wide, and its adaptability to the environment is relatively strong. The local transmission cases in the tropical countries mean that the new coronavirus may be more resistant to high temperatures than the past influenza and other respiratory viruses. Even worse, SARS-CoV-2 is continually evolving to be well adapted to habitable temperatures. A new variant due to triple mutation enhances its resistance to extreme variations in temperature and leads to India’s second wave becoming the worst COVID-19 surge in the world. SARS-CoV-2 loads within wastewater are consistent with population infection rates. Coupled with extreme thermostability, it can even pose secondary pollution threat in tropical and subtropical seas.
It should be noticed that sea ice acts as long-distance transport devices for SARS-CoV-2. When the wastewater is discharged in extreme cold weather, it freezes instantly and becomes a carrier of pathogenic virus. The prolonged retention time in combination with non-dilution by sorption of virus to ice would be expected to positively impact virus infectivity. The high latitude cold-temperate regions, providing favorable habitats polar bears, pinnipeds, and cetaceans, especially during the breeding season, are highly vulnerable for the absent protection measures.
5. Conclusion
Even if there has been few evidence showing the existence of SARS-CoV-2 in Bohai Sea waters, the risk of its transmission in coastal waterbodies is worthy of our attention. The initial assessment of the potential threats for the Bohai Sea coastal areas associated with the discharge of treated and untreated sewage has been presented. To identify high-risk sea area for virus spillover in the Bohai Sea, we calculate the concentration distribution and present predictions on exposure with respect to varied survival time of SARS-CoV-2. Special attention is required to protect some sensitive areas or susceptible organisms. Although the source input is subjectively set, our model is very meaningful for virus risk assessment. Susceptibility areas are identified by means of the presented numerical model. Once the virus concentration in the discharged sewage is determined, it can be used to determine the virus spread range.
According to the available information, temperature is the only well-defined factor with a consistent effect on SARS-CoV-2. Viruses may remain infectious for a month at near-freezing temperature, indicating they may cause ecological disaster for marine mammals in cold regions, especially it is estimated that the recurrent wintertime outbreaks of COVID-19 will probably occur (Kissler et al., 2020). Our model is applied in the Bohai Sea, and its principles could be applied to other coastal countries and districts, suffering from serious COVID-19 Pandemic, as an early-warning tool for SARS-CoV-2 infection risk assessment.
Although the model still has major limitations (for example, SARS-CoV-2 persistence in seawaters is artificially assumed; moreover, viral fluxes are not yet well determined), it is useful to understand the dispersion and the behavior of pathogen contamination in the marine environment. Estimation of the waterborne virus amounts into the sea is essential to better understand how the new coronavirus spread in the marine environment, so the following priority is to acquire concentrations of virus by testing water samples. A time-dependant decay rate of SARS-CoV-2 considering light, salinity, and temperature is also necessary for a prediction model. Several new variants as a result of continual evolution, which seem to be more infectious and thermostable than the original strain, make this task more difficult.
CRediT authorship contribution statement
Weijun Guo: Writing - original draft, Writing - review & editing. Yimeng Cao: Investigation, Writing - Original draft, Preparation. Xiangpeng Kong: Visualization, Investigation. Shujun Kong: Software, Data curation. Tiaojian Xu: Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This research is sponsored by National Key R&D Program of China (2019YFC1407700), and the National Natural Science Foundation of China (No. 51879019 and 51979037). The authors would also like to acknowledge the help of Qiao Ma and from Dalian Maritime University and Cheng Liu from Dalian Dongtai Xiajiahe Sludge Treatment Plant for providing information of microbiology and writing assistance.
Edited by: Richard Handy
References
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728 doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill N., Morris D. Recreational waters–A potential transmission route for SARS-CoV-2 to humans? Sci. Total Environ. 2020;740 doi: 10.1016/j.scitotenv.2020.140122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D.L., Groner M.L., Ben-Horin T., Grant J., Revie C.W. Modeling pathogen dispersal in marine fish and shellfish. Trends Parasitol. 2020;36(3):239–249. doi: 10.1016/j.pt.2019.12.013. [DOI] [PubMed] [Google Scholar]
- Chan J., Yuan S., Kok K., To K., Chu H., Yang J., Xing F., Liu J., ip C., Poon R., Tsoi H., Lo S., Chan K., Poon V., Chan W., Ip J., Cai J., Cheng V., Chen H., Hui C., Yuen K. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet (Br. Ed.) 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiou M.A., Tzoraki O. Coronavirus survival on beach sand: Sun vs COVID-19. Mar. Pollut. Bull. 2021;167 doi: 10.1016/j.marpolbul.2021.112270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González C., Izquierdo A., Álvarez Ó., Bruno M., Mañanes R., Czerwinski I., Zurita F. Hazard assessment of bacterial contamination in coastal waters using a metocean modeling system: application to bivalve mollusk harvesting areas in Cadiz Bay (SW Spain) Ocean Coast Manag. 2018;166:31–39. [Google Scholar]
- Guo W., Wu G., Liang B., Xu T., Chen X., Yang Z., Xie M., Jiang M. The influence of surface wave on water exchange in the Bohai Sea. Cont. Shelf Res. 2016;118:128–142. [Google Scholar]
- La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods-A scoping review. Water Res. 2020;179 doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736 doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler S., Tedijanto C., Goldstein E., Grad Y., Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):eabb5793–eabb5868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathavarajah S., Stoddart A.K., Gagnon G.A., Dellaire G. Pandemic danger to the deep: the risk of marine mammals contracting SARS-CoV-2 from wastewater. Sci. Total Environ. 2021;760 doi: 10.1016/j.scitotenv.2020.143346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maal-Bared R., Sobsey M., Bibby K., Sherchan S.P., Fitzmorris K.B., Munakata N., Gerba C., Schaefer S., Swift J., Gary L., Babatola A., Bastian R., Olabode L., Reimers R., Rubin A., Kester G., Casson L. Letter to the Editor regarding Mathavarajah et al. (2020) Pandemic danger to the deep: the risk of marine mammals contracting SARS-CoV-2 from wastewater. Sci. Total Environ. 2021;773 doi: 10.1016/j.scitotenv.2020.144855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Takanezawa T., Ooe M. Ocean tide models developed by assimilating TOPEX/POSEIDON altimeter data into hydrodynamical model: a global model and a regional model around Japan. J. Oceano. 2000;56(5):567–581. [Google Scholar]
- Mordecai G., Hewson I. Coronaviruses in the Sea. Front. Microbiol. 2020;249(11) doi: 10.3389/fmicb.2020.01795. 1795–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi G., Khan S. Risk of COVID-19 pneumonia in aquatic mammals. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reza Dehbandi, Mohammad Ali Zazouli. Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe. 2020;1(4) doi: 10.1016/S2666-5247(20)30093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S.G., Stefani F., Gigantiello A., Polesello S., Comandatore F., Mileto D., Maresca M., Longobardi C., Mancon A., Romeri F., Pagani C., Cappelli F., Roscioli C., Moja L., Gismondo M.R., Salerno F. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutler J.D., Zaraska K., Holding T., Machnik M., Uppuluri K., Ashton I.G., Migdal L., Dahiya R.S. Rapid assessment of SARS-CoV-2 transmission risk for fecally contaminated river water. ACS Es&t. Water. 2021;1(4):949–957. doi: 10.1021/acsestwater.0c00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, 2021. Coronavirus disease (COVID-2019) situation reports. 〈https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports〉. (Last access on 3rd May).
- Yeo C., Kaushal S., Yeo D. Enteric involvement of coronaviruses: is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]