Abstract
The Covid-19 disease has recently become one of the biggest challenges globally, and there is still no specific medication. Findings showed the immune system in severe Covid-19 patients loses regulatory control of pro-inflammatory cytokines, especially IL-6 production, called the “Cytokine storm” process. This process can cause injury to vital organs, including lungs, kidneys, liver, and ultimately death if not inhibited. While many treatments have been proposed to reduce cytokine storm, but the safety and effectiveness of each of them are still in doubt. Mesenchymal stem cells (MSCs) are multipotent cells with self-renewal potential capable of suppressing overactive immune responses and leading to tissue restoration and repair. These immuno-modulatory properties of MSCs and their derivatives (like exosomes) can improve the condition of Covid-19 patients with serious infectious symptoms caused by adaptive immune system dysfunction. Many clinical trials have been conducted in this field using various MSCs around the world. Some of these have been published and summarized in the present article, while many have not yet been completed. Based on these available data, MSCs can reduce inflammatory cytokines, increase oxygen saturation, regenerate lung tissue and improve clinical symptoms in Covid-19 patients. The review article aims to collect available clinical data in more detail and investigate the role of MSCs in reducing cytokine storms as well as improving clinical parameters of Covid-19 patients for use in future clinical studies.
Keywords: Covid-19, Cytokine storm, Mesenchymal stem cells, Immune response
1. Introduction
The Covid-19 is the biggest challenge in the medical world in the first half of the twenty-first century, and all scientists around the world are trying to find a way to tackle this problem. The prevalence of new coronavirus (SARS-CoV-2) causing lung infection (Covid-19) started in December 2019 in China [1], and the virus spread rapidly around the world. SARS-CoV-2 is an enveloped virus, having a positive-sense, single-stranded RNA, as a member of the B lineage coronaviruses family. The most critical targets of the virus are epithelial cells, the airways, and alveoli [2]. This virus can access pulmonary alveolar cells through Angiotensin-Converting Enzyme 2 (ACE-2) receptors and enter the cell via endocytosis. Since the ACE-2 receptors exist on various cells, including epithelial lung cells and kidney, heart, and liver parenchymal cells, it can cause multi-organ failure in the final progressive stages of Covid-19 disease [3]. Covid-19 has a wide range of clinical symptoms, including cough, fever, lymphopenia, sore throat, fatigue, headache, and sense of taste or smells' loss [4]. One of the well-known facts about SARS-CoV-2 infection is its multi-organ downfall and Acute Respiratory Distress Syndrome (ARDS) in critical patients [5]. This infection is correlated with a destructive inflammatory response, including releasing many pro-inflammatory cytokines called “Cytokine storm.”. There is much evidence from the cytokine profile of Covid-19 patients, indicating that cytokine storm is directly linked to lung injury, multi-organ failure, and the disease's severity [[6], [7], [8]].
So far, many treatment strategies for this disease have been proposed [[9], [10], [11]], including hydroxychloroquine and combinations [[11], [12], [13]], neutralizing antibodies [14], repurposing various antiviral treatments [9,[15], [16], [17]], and passive antibody transfer from convalescent patients' sera [18,19]. There are still controversies about these treatments, and they have a complementary role; besides, they seem insufficient due to the rapid progression of the disease and the increase in the number of patients, and certain limitations of any treatment method. For example, using high doses of steroids is not only useful in treating Covid-19 patients but can also cause severe side effects like the increased risk of bacterial infections or diabetes [[20], [21], [22]]. On the other hand, considering differences in the severity of the disease and individual discrepancies in the incidence of immune responses to some existing treatments, the need to find new treatment strategies is felt. In addition to inhibiting virus replication, perhaps one of the strategies to improve critically Covid-19 patients with severe lung involvement is to prevent cytokine storms formation.
Mesenchymal Stem Cells (MSCs) and their derivatives may be able to prevent or mitigate this cytokine storm through their immunomodulatory capacity [23] and lack of expression of ACE2. Many clinical trials have been registered in line with MSCs therapy for Covid-19 patients (https://clinicaltrials.gov), many of which are still in the process, and only a few results have been published. Many review articles have also pointed to registered trials [[24], [25], [26], [27]], but the article that summarizes the published results is not yet available. This review paper aims to investigate the role of MSCs in reducing and managing cytokine storms in Covid-19 patients based on summarizing the available results from recent studies that can provide useful information for clinicians and researchers in this field.
2. Cytokine Storm
Cytokine storm is an inflammatory phenomenon which is marked by the systemic release of large numbers of cytokines including: Interleukin-6 (IL-6), Interferon-gamma (IFNγ), Tumor Necrosis Factor-alpha (TNFα), Interferon-alpha (IFNα), Monocyte Chemoattractant Protein-1 (MCP-1), Interleukin-1β (IL-1β), Interferon-Beta (IFNβ) , and Interleukin-2 (IL-2). The release of a large number of free radicals from immune cells is induced by these cytokines, which are the primary cause of Acute Respiratory Distress Syndrome (ARDS) and multiple organ failure [28].
It has been proven that the internalization of the virus in tissue and immune cells leads to activation of the nuclear factor-kappa B (NF-kB) pathway and secretion of a myriad of inflammatory factors leading to Cytokine storm [29]. Indeed, a complex set of interactions between cytokines, cell types, and signaling pathways are involved in cytokine storm formation. The most important cells of the innate immune system involved in cytokine storms' pathogenesis are neutrophils, macrophages, and Natural killer cells (NK). Also, some T-lymphocyte subsets are involved in the cytokine storm process among adaptive immune system cells, including T-helper 1 (TH1), Cytotoxic T Lymphocyte (CTL), and T-helper 17 (TH17). T regulatory cell (Treg) discharge leads to an intensification in the levels of IFN-γ, IL-17, and IL-6, and through the secretion of IL-8, decreases the neutrophils' clearance [30]. Taking together all these observations is responsible for lung damage in Covid-19 and ARDS [31]. B cells are rarely contributed to the cytokine storm pathogenesis [32]. Since cytokine storm is the main reason for morbidity in patients infected with SARS-CoV and MERS-CoV, recognizing it is vital in diagnosing and treating the disease by physicians. The medicinal problems of patients involved with cytokine storm can progress rapidly and have consequences such as hypoxemia, hemorrhages, anemia, thrombocytopenia, dyspnea, hypotension, vasodilatory shock, and death [33]. Immunosuppression is crucial in the treatment of cytokine storm in Covid-19 patients, especially those with severe conditions. Blocking IL-6, IL-1 and TNFα may prevent or inhibit the disease [34].
As immune-suppressive, steroids were widely used to treat SARS to ameliorate the intensity of inflammatory injuries [35]. However, high doses of steroids were not sufficient for treating extreme pulmonary injury in SARS and Covid-19 patients [20,21]. Instead, they may lead to destructive side effects, including an increased risk of diabetes, osteonecrosis, severe bacterial infections, and cardiovascular disease [22], dramatically affecting the prognosis. So finding new and safe treatment ways to suppress the immune system can be very helpful for patients.
3. Important cytokines involved in cytokine storm
3.1. IL-6
Interleukin-6 (IL-6), among all cytokines, is more suspected to be involved in the cytokine storm induced by a coronavirus. IL-6 is tightly linked to mediate various immunomodulatory and inflammatory pathways [36]. It is produced by approximately all stromal cells and competent immune cells, including macrophages, mast cells, T lymphocytes, dendritic cells (DC), monocytes, B lymphocytes, as well as some other non-lymphocytic cells, such as glomerular mesangial cells, endothelial cells, tumor cells, keratinocytes, and fibroblasts [37]. IL-6 performs as an alarming intermediary for the announcement of the occurrence of some urgent occasion. IL-6 is produced in infectious damage and transmits an alerting sign to the entire body [38]. IL-6 is responsible for activating TH17 cells in the interaction between T cell-dendritic cell [39]. In Covid-19 patients, an increased percentage of activated TH17 cells may be due to increased production of IL-6 in response to the virus's entry into the immune system [40]. It is recognized that IL-6 and D-Dimer levels dramatically increase in severe Covid-19 patients, and so they can be good diagnostic predictors for finding the severity of the disease [41].It has been proven that the viral nucleocapsid SARS-CoV N protein is responsible for the elevated IL-6 levels and lung injury in the severe stage of disease progression in SARS patients [42]. Besides, laboratory tests have indicated that IL-6 boosts macrophage activation syndrome (MAS), triggering mass manufacture of pro-inflammatory cytokines and inducing fibroblasts and neutrophils migration into the alveolar epithelium. This results in an enlarged deposition of fibrin and collagen, leading to harm to underlining lung tissue [43,44].
3.2. TNFα
As a dominant pro-inflammatory cytokine, Tumor Necrosis Factor-α (TNF-α) is applied pleiotropic impacts on distinct cell types and critically is involved in viral diseases associated with the pathogenesis of chronic inflammatory [45]. It is known to be released by activated monocytes and is cytotoxic to tumor cells [46]. It has been proven that TNF-α triggers macrophage-induced angiogenesis, which is a virtual event throughout inflammation, tumor growth, and wound repair [47].
As another mechanism, it is accepted that TNF and other cytokines in the TNF–TNF receptor superfamily are potent inducers of NF-κB, leading to multiple expression pro-inflammatory genes [32]. Since TNFα is one of the most critical hyaline-production inducer cytokines in alveolar lung cells and fibroblasts, its high un-controlled rate in Covid-19 patients can cause serious lung damage [48]. Also obtained data from several studies show that anti-TNF therapy reduces morbidity and mortality in Covid-19 patients [49,50].
3.3. Interferons (IFNs) family
The interferons (IFNs) are a set of cytokines secreted by cells over a specific situation like exposure to viruses, polypeptides, or double-stranded RNA and have crucial immunomodulatory, antiviral, antitumor, and anti-proliferative properties [51]. In mammals, this system is the first protective barrier against viral infection. This system's design is such that even at the cost of increasing the death rate of virus-infected cells, prevents the spread of the virus in the body [52]. Type I IFNs (IFN-α/β), as an essential part of the intrinsic antiviral innate immune system, are the fastest and most effective antiviral response as soon as the virus enters the body. Viral infections, including SARS-CoV-2, can suppress the production of IFN types 1 and 3 but induce the production of IL-6 and TNF. IFN type 1 might remarkably reduce viral replication [53]. Current studies show that IFN disorder is an important marker to discover Covid-19 pathogenesis. Efficient IFN stimulation or prophylactic direction of IFNs at the early stage before severe Covid-19 may bring about an autonomous antiviral state, limit the virus infection, and inhibit Covid-19 progression [54].
3.4. IL-1 family
The interleukin-1(IL-1) cytokine family is consists of 11 members. Some more important of them are: IL-1α, IL-1β, IL-1 receptor antagonist (IL-1Ra), IL-18, and IL-33 [55]. During the cytokine storm, IL-1 β, IL-18, and IL-33 are the three most essential cytokines from this family [56]. IL-1 family members boost the activity of the innate immune system cells. They also have crucial roles in provoking and amplifying the performance of polarized T cells. Regardless of some exceptions, as a general rule, IL-18 mainly affects T helper 1 (TH1) cells, IL-33 mainly affects TH2 cells, and IL-1 has a crucial role in TH17 cell differentiation and maintenance [55].
The benefits or disadvantages of IL-1 depends on the dosage. Although low doses may be protective, when it is generated in high doses in infectious diseases, it can be destructive; therefore, blocking it is necessary. When activating by SARS-CoV-2, IL-1 provokes the release of IL-6 and TNF-α, pro-inflammatory network that can cause cytokine storms and be detrimental both in the lung and systemically [57].
3.5. Mesenchymal stem cell (MSC) therapy
Mesenchymal stem cells (MSCs) have been used in laboratory and clinical cell therapies for many years. MSCs are adult multipotent cells with self-renewal potential and multi-lineage differentiation ability into specialized cells [58]. The long-term culture proficiency, easy accessibility, and control of how such cells specialize in forming the body's different tissues offer significant healthcare advances [59].MSCs are accessible using simple methods and have been isolated from multiple tissues such as bone marrow [60], adipose tissues [61], umbilical cord [62], the dental pulp [63], menstrual-blood [64], Wharton's Jelly [65] and periodontal Ligament [66].MSCs can reach the required volume for clinical trials at a suitable time [67].
The safety and efficacy of MSCs have been recorded in several clinical trials, especially in immune-mediated inflammatory diseases, like graft-versus-host disease (GVHD) and systemic lupus erythematosus (SLE) [68,69]. Mainly MSCs exert their beneficial therapeutic effects in two ways, including immunomodulatory and differentiation ability. MSCs can release various cytokines by paracrine method or make direct interactions with immune cells, including natural killer cells (NK), T cells, B cells, Dendritic cells, and macrophages leading to immune system regulation. MSC therapy can also theoretically suppress the over-activated immune systems and upgrade endogenous restore by improving the microenvironment [70]. Strong anti-inflammatory properties of MSCs are the main reasons for improving the health of Covid-19 patients after MSCs injection. Besides, as another beneficial mechanism, direct cell–cell transmission of mitochondria [71] from MSCs to respiratory epithelial and immune cells has also been described [72].
4. Effects of MSCs on ARDS and immune system of Covid-19 patients
As mentioned, in Covid-19 patients, in a positive feedback of the immune system, it produces many inflammatory factors that lead to cytokine storms, and again the resulting cytokine storm causes overproduction of inflammatory cytokines and immune cells [73].
Acute Respiratory Distress Syndrome (ARDS) is the main typical intricacy of Covid-19 disease-causing by various events, including the renin-angiotensin system dysregulation, cytokine storm, over-activation of neutrophils, and elevated coagulation [74]. The ARDS usually involves a general lung injury that is associated with characteristics such as pulmonary edema and damage to the endothelium of the lungs [75]. Unfortunately, the mortality rate caused by the 2019 coronavirus, leading to the induction of this severe lung damage (ARDS), is relatively high [76]. For example, in Huang et al. report, among critically covid-19 patients, 67–85% of them had ARDS, which is one of the leading causes of high mortality rates (61.5%) [4]. Many studies showed that MSCs are also useful in the treatment of ARDS [77,78].
MSCs therapy can theoretically suppress the overactive immune system, and by improving the microenvironment, assist endogenous restoration. After intravenously reaching the body, some of the MSCs accumulate in the lung and potentially prevent pulmonary fibrosis, protect lung pneumocytes, improve pulmonary microenvironment and enhance lung function [70,76]. MSCs with raising autophagy through the phosphoinositol 3-kinase/protein B pathway may also defend alveolar cells [79]. Furthermore, it has been revealed that MSCs can secret some soluble bioactive factors and interact with damaged tissue leading to an increase in the population of T-regulatory cells and inhibit of Th1 and Th17 proliferation.
Besides, because of ATP levels reduction in injured alveolar epithelial cells, as another mechanism, MSCs by transporting mitochondria to these locations and refilling depleted ATPs, improve their functional status [72].
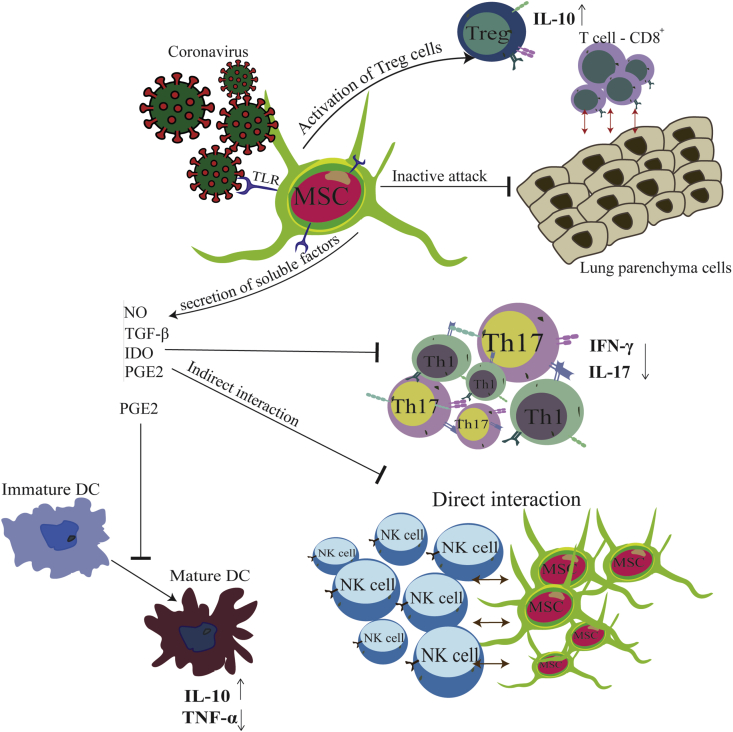
In the same direction, many clinical trials in the world were performed on the Covid-19 patients which stem cells were used to treat patients. Most recently, China, USA, Jordon, Iran (In this context, our research team is also conducting a cell therapy project for Covid-19 patients., IRCT20160809029275N1: 30/05/2020), and many other countries have started cell-based therapy clinical projects, and some reports have been published [70,76,80]. For example, on 2/5/2020, Beijing 302 Hospital register the first trial in this field. This project is accomplished to examine the safety and efficacy of Umbilical Cord-Mesenchymal Stem Cells (UC-MSCs) therapy for pneumonia in covid-19 patients [70]. The results of this project were auspicious, and no specific side effects were reported. MSCs can modulate the immune system by various mechanisms, which the most important ones are shown as follows and briefly in Fig. 1. The immunomodulatory effects of MSCs are triggered by the activation of TLR (Toll Like Receptor) in MSCs, stimulated by pathogen-associated molecules such as LPS or double-stranded RNA from the virus [81,82] like the HCoV-19. In response to the virus entering the body, mesenchymal stem cells secrete several soluble factors, including Nitric oxide (NO), Indoleamine2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), and Transforming growth factor β (TGF-β) [83,84], which affect the immune system in two ways. On the other hand, these soluble factors inhibit activation and proleiferation of Th1 and Th17 cells, which subsequently leads to a decrease in inflammatory cytokines IFNγ and interleukin-17 [85].Besides, with the help of these soluble factors MSCs exert immunosuppressive effects on DCs (the central antigen-presenting cells) by inhibiting DC activation, decreasing IL-12 production, decreasing endocytosis, and inhibiting dendritic formation cells from monocytes, and cell maturation arrest [84,85]. This, results in an increase in interleukin-10 and a decrease in TNF-α levels. Also, MSCs can suppress NK cells in two ways: direct contact with them and indirect interaction by releasing mentioned soluble factors [83,86,87]. It has also been proven that during the progression of COVID-19 disease, CD8+ T cells become overactive and attack lung parenchymal to remove the virus. Mesenchymal stem cells prevent serious lung damage by inhibiting these activated lymphocytes. Moreover, in COVID-19 patients, MSCs prevent pulmonary fibrosis by decreasing the pro-fibrotic factors' levels and improving the lung microenvironment [88]. Also, in another mechanism, MSCs cause re-modeling and shift the T-cell subsets to t-regulatory cells and subsequently, they increase the level of anti-inflammatory cytokine IL-10, which plays a crucial role in regulating immune responses.
Fig. 1.
Schematic diagram of how Mesenchymal stem cells (MSCs) can manage Cytokine storm in Covid-19 patients. Abbreviations (NO: Nitric oxide/TGF-β: Transforming growth factor β/IDO: Indoleamine2,3-dioxygenase/PGE2: prostaglandin E2/TNF-α: Tumor Necrosis Factor-alpha/IFN-γ: Interferon-gamma/T-reg: T regulatory cell/Th: T helper cells/NK cell: Natural Killer cell/IL-10: Interleukin-10).
To make these mechanisms of action more comfortable to understand, we have summarized the results from available studies in Fig. 1.
5. Effects of MSCs on clinical parameters of Covid-19 patients: results of recent clinical trials
So far, more than 90 clinical trials in the field of stem cell therapy have been registered for Covid-19 patients (https://clinicaltrials.gov). Most of them are in the process of being completed, and only a few have reached an end and published their results. The results are promising, and no serious side effects have been reported during the patients' treatment period. For example, Liang B et al. found that injection of 3 doses (50 × ) of Umbilical cord-derived MSC (UMSC) in a 65-year-old woman significantly improved the clinical symptoms of Covid-19 disease. Also, no side effects were observed from the mentioned intervention [89]. Another clinical experiment involving seven Covid-19 pneumonia patients revealed that injections of a single dose of mesenchymal stem cells (1 × Cell/Kg BW) led to the normalization of oxygen saturation (PaO2) and the number of inflammatory markers and lung tissue repair, as chest CT imaging showed improvement mainly on the 9th day after MSC transplantation. Also, as we know, with the help of ACE2 receptors widely distributed on human cells, including alveolar and capillary endothelium, hCoV-19 can access the cells.
Interestingly, using an RNA seq survey to identify 12,500 transplanted MSC in this study, the results showed that mesenchymal stem cells were ACE2 negative during treatment [76]. Results reported in a different study by Lanzoni et al. on 24 Covid −19 patients with ARDS symptoms revealed that intravenous injection of 2 doses (100 × Cells) of UMSC with an interval of 3 days dramatically improved clinical and immunological symptoms of patients [90]. Similarly, the results of MSCs therapy in 60 patients with moderate to severe ARDS, using a single dose of 1 × Cell/kg of bone marrow-derived MSCs revealed that the MSCs were well-tolerated, and a significant reduction in C-Reactive Protein (CRP), IL-6, and IL-8 levels was observed [77]. These successful outcomes in treating lung damage in these patients can be a hope for the treatment of pulmonary injuries in Covid-19 patients. The summary of the published results of recent trials on covid-19 patients is given in Table .1. Generally, in the counducted studies, the dose of injectable cell varies from 1 × (Cell/Kg BW) [91] to 4 × (Cell/Kg BW) [92] depending on the treatment protocol and there are still controversies in this regard. However, more research and a larger patient population with high precautions are needed to validate MSC therapeutic interventions further.
Table 1.
A summary of published results in the field of stem cell therapy for COVID-19 patients.
| Source of MSCs | Autologous/ |
Times of injection | Population size | Follow-up period (day) | Main therapeutic effects | Ref |
|---|---|---|---|---|---|---|
| Allogenic | ||||||
| UCB-MSCs | Allogenic | Five times | N = 1 | 10 days | 1:Static pulmonary compliance ↑, lymphocyte number ↑ | [93] |
| (case report) | 2: improvement in (PaO2/FiO2) ratio. | |||||
| 3:Blood creatinine ↓, blood urea nitrogen ↓. | ||||||
| UMSCs | Allogenic | Once | N = 1 | 19 days | 1: The percentage and counts of CD3+ T cell, CD4+ T cell, and CD8+ T cell↑. | [91] |
| (case report) | 2:Inflammatory factors including CRP, TNF-α, and IL-6 ↓. | |||||
| UMSCs | Allogenic | Three times | N = 1 | 30 days | 1: CRP,ALT, AST, Bilirubin and D-dimer ↓. | [89] |
| (case report) | 2:T cell population (CD3+,CD4+ and CD8+ T cells) ↑. | |||||
| 3:CT image showed remission of the inflammation symptom | ||||||
| UMSCs | Allogenic | Once | N = 1 | 30 days | 1: Oxygen saturation ↑ | [94] |
| (case report) | 2:ALT, AST, Alb, BUN, CRP ↓ | |||||
| MB-MSCs | Allogenic | Three times | N = 2 | 24 days | 1:Improvement in (PaO2/FiO2) ratio & CT images | [95] |
| 2: CRP and IL-6 ↓. | ||||||
| NM | Allogenic | Once | N = 7 | 14 days | 1: Oxygen saturation ↑, lung tissue repair ↑, peripheral lymphocytes ↑and IL-10↑. | [76] |
| 2: CRP and TNF-α levels ↓. | ||||||
| UMSCs (6 casese) or PL-MSCs (5 cases) | Allogenic | Three times | N = 11 | 19 days | 1:Oxygen saturation ↑. | [96] |
| (Case Series) | 2: CRP, IL-8, TNF-α, IFN-γ, and IL-6 levels ↓. | |||||
| UMSCs | Allogenic | Three times | N = 18 | 23 days | 1:(PaO2/FiO2) ratio and CT images improvement ↑. | [98] |
| 2: IL-6, IFN-γ, TNF-α, CRP, ALT, serum creatinine, and serum ferritin ↓. | ||||||
| UMSCs | Allogenic | Two times | N = 24 | 90 days | 1: Dendritic cell population, IL-10 levels, and oxygen saturation ↑. | [90] |
| 2: CRP and TNF-α levels ↓. | ||||||
| Exosomes- derived from BM-MSCs | Allogenic | Once | N = 24 | 14 days | 1: lymphocyte counts↑. | [99] |
| 2: CRP, D-dimer, ferritin and neutrophil counts ↓. | ||||||
| NM | Allogenic | Case series, different for cases (one, two, or three times) | N = 25 | 3 days | 1: Inflammatory factors (IL-6, procalcitonin and CRP) and IgG and IgM were not significantly changed. | [100] |
| 2: Creatine kinase-MB (CK-MB),lactate (LAC), and cardiac troponin T (cTnT) ↑. | ||||||
| UMSCs | Allogenic | Once | N = 41 | 28 days | 1: CRP and IL-6 ↓ | [101] |
| 2: lymphocyte numbers and CT images improvement ↑. | ||||||
| UMSCs | Allogenic | Three times | N = 101 | 28 days | 1:CT image showed remission of the inflammation symptom. | [92] |
| 2: CRP ↓ and oxygen saturation ↑. |
Abbreviations (UMSCs: Umbilical cord-derived Mesenchymal Stem Cells/NM: Not Mentioned/CRP: C-Reactive Protein/ALT: Alanine aminotransferase/AST: Aspartate aminotransferase/MB-MSCs: Menstrual blood-derived MSCs/UCB-MSCs: Umbilical Cord Blood-derived Mesenchymal Stem Cells/AT-MSCs: Adipose Tissue derived mesenchymal Stem Cells/BM-MSCS: Bone Marrow-derived Mesenchymal Stem Cells/PL-MSCs: Placental derived mesenchymal stromal cells/IV: Intravenous/Alb: Albumin/BUN: Blood Urea Nitrogen).
Also, as mentioned in Table .1, there are many laboratory abnormalities such as elevated CRP, liver enzymes (Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Bilirubin, serum ferritin, D-dimer, and lower leukocyte and lymphocyte count, as well as some other parameters like Albumin and Urea, in Covid-19 patients, which are useful in the diagnosis of the onset and progression of the disease [102].
In the same direction, it should be briefly explained, C-Reactive Protein (CRP) is classified as one of the classical acute-phase proteins by its biological properties. It is synthesized and secreted to the blood by the liver after initiating signals from the body, for example, infection, trauma, or tissue damage, mediated by inflammatory cytokines [103].
CRP levels are directly related to inflammation levels, and situations like sex, age, and physical conditions cannot affect its concentration [104].Comparing the serum profile of Covid-19 patients with different disease severity levels suggested that CRP level is a good predictor of disease severity. In the early stage of Covid-19 disease progression, CRP levels are positively associated with the diameter of lung lesions and severe presentation [105]. Findings of many studies revealed that treatment with MSC decreased CRP levels and pro-inflammatory cytokines and chemokines, like IL-6 and TNF-α [76,95,101].
It has also been found that SARS-CoV-2 can affect liver cells by causing elevated levels of aminotransferase enzymes and liver impairment [106]. Indeed, like observation in coronavirus SARS, liver dysfunction is a common complication in Covid-19 patients. Based on prior results, up to 60% of patients had a liver dysfunction, with liver biopsy specimens suggesting viral nucleic acid and damage [107]. In Covid-19 patients, liver enzyme abnormalities have been reported to range from 20% to 50%, but there is still controversy about the correlation of these findings and the disease's prognosis or progression [108,109]. Two hypotheses have been proposed in different studies in liver dysfunction and infection with Coronaviridae family viruses: systemic inflammation and direct liver attack by the virus [110, 111]. For example, in a retrospective cohort study conducted by Cheuk-Fung et al., reporting data from 1040 Covid-19 patients showed that 22.5% of patients had elevated liver enzyme levels [112]. Several studies have demonstrated that MSCs can differentiate in vitro along the hepatogenic lineage, and so they can be useful in improving liver problems and hepatic tissue regeneration with different mechanisms. For example, in a study using bone marrow-derived MSCs, the results showed that MSCs transplantation significantly inhibited Reactive Oxygen Species (ROS) and improve liver injury [113]. The results in the above studies [91,94,98] also showed a decrease in the number of liver enzymes after MSCs injection, resulting from the anti-inflammatory and immunomodulatory properties of these cells. Besides, several studies have described renal involvement mainly due to Acute Kidney Injury (AKI), affecting up to 70% of Covid-19 patients [114,115]. The kidney is the second most affected organ in this disease, behind the lung and followed by the heart and the liver [116]. In the Diao et al. study, SARS-CoV-2 nucleocapsid protein was observed in the kidney's tubular structures of 6 patients involved in the study. Also, Cheng et al. have shown that among 710 hospitalized Covid-19 patients, elevated serum creatinine and urea (BUN) were 15.5% and 14.1%, respectively [117]. In this context, several studies revealed that administering in vitro expanded MSCs defends against acute renal injury and enhances renal restoration. In acute and chronic kidney injury models, intravenously injected MSCs have moved to tubulin, glomeruli, interstitium, and peritubular capillaries [118,119].
As mentioned in Table 1, in many studies [93,94], the amount of serum creatinine, Blood Urea Nitrogen (BUN), and renal parameters after injection of MSCs returned to normal, which indicates the beneficial therapeutic effects of these cells in the regeneration of kidney tissue and reduce inflammation in it.
6. Discussion
Since the Covid-19 pandemic continues and the number of patients is increasing worldwide, understanding the mechanisms involved in the onset and treatment of the disease is one of the essential medical needs today. Many treatments have been suggested so far for COVID-19, which, while effective in some of them, are still inadequate to the increasing trend of disease. Many studies have emphasized that loss of immune system control (both innate and adaptive) and over-production of inflammatory cytokines called Cytokine storm respond to the virus's arrival in critical Covid-19 patients are the leading causes of vital tissue damage and subsequently death [43,120]. Therefore, finding and using new therapeutic strategies based on immune system regulatory properties can effectively improve patients' clinical status. In this context, Mesenchymal Stem Cells (MSCs) have been approved due to their safety and efficacy and the immune system's regulatory properties in many diseases; for the Covid-19 patients, treatment has also been considered by many researchers and clinicians.MSCs are accessible from various tissues and possess immunomodulatory functions as well as multipotency and self–renewal properties.
The question that arises here is: do mesenchymal stem cells have useful effects on cytokine storm and disorders involved in covid-19 disease? What clinical evidence is available in this regard? In response to these questions, we carefully summeraized available clinical data from recent studies in this review article.
The results obtained from several clinical trials show that MSCs with various mechanisms are able to reduce cytokine storm in Covid-19 patients. For example, as, mentioned in Table 1, in a recent study by Shu et al., on 41 Covid-19 patients, all hospitalized patients showed elevated levels of IL-6 and CRP. Intravenous injection of single dose of Umbilical cord-derived Mesenchymal Stem Cells (UMSCs) significantly reduced the amount of these inflammatory markers in patients involved in this study [101]. Also, serum profiles analysis of 13 Covid-19 patients in Sánchez-Guijo et al.'s experiment showed that allogenic injection of 3 doses of Adipose Tissue derived mesenchymal Stem Cells (AT-MSCs) significantly reduced IL-6 which is one of the most important inflammatory cytokines involved in the cytokine storm formation [97]. The results of another study also showed a significant increase in anti-inflammatory cytokine (IL-10) and decrease in inflammatory cytokine (TNF-α) levels after receiving 2 doses of UMSCs [90].
Moreover, it has been reported that exosomes, which are derivatives of MSCs, also have beneficial therapeutic effects in the treatment of Covid-19 patients and reduction of cytokine storm. Exosomes are extra-cellular nanoparticles releasing by all cells and act through paracrine pathways. Exosomes have been proven to be mediators of immune regulation function of MSCs. MSC-Exos could shift macrophages from the M1 to the M2 phenotype, further suppressing pro-inflame-matory states [121]. Using a single dose of 15 ml ExoFlo from Bone Marrow-derived Mesenchymal Stem Cells (BM-MSCS) in a study performing by Sengupta et al. reaveled that exosomes can significantly increase the number of lymphocytes and decrease inflammatory markers, as well as the number of neutrophils in Covid-19 patients [99]. The effective role of MSCs in cytokine storm reduction in Covid-19 patients has also been proven in several other clinical trials [[96], [97], [98]].
Briefly and as reflected in this article, MSCs, due to their immunomodulatory, anti-inflammatory, and regenerative properties, can control immune dysfunction and inflammation in Covid-19 patients. Interestingly, MSCs are not affected by the Covid-19 infection for not expressing the ACE2 receptor [76]. Besides, MSCs can help in remodeling the CD4 and CD8 T Cells depleting function in Covid-19 and thus improve pulmonary function. Most importantly, MSCs can decrease cytokine storms by reducing the proliferation of immune cells as well as regulating the balance of pro-inflammatory and anti-inflammatory cytokines.
7. Summary
The pandemic of Covid-19 infection caused by the highly pathogenic virus SARS-CoV-2 has prompted an urgent need for novel therapies. It is proved that SARS-CoV-2 in some patients leads to induction of excessive and prolonged inflammatory responses leading to hyper-inflammation called Cytokine storm. In Covid-19 patients, especially those with severe status, cytokine storm inhibition by immune-suppressives is urgent. Pre-clinical and preliminary clinical data suggest that Mesenchymal Stem Cells (MSCs), through their anti-inflammatory and immunomodulatory actions, can significantly inhibit this process, heal tissues, thereby enhancing recovery. Many clinical trials have been conducted in this field have reported significant results, and no specific side effects have also been reported in any of them. Of course, further clinical investigations with a larger sample size are still needed in this area.
Declaration of competing interest
All authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Najmeh Kaffash Farkhad, Email: kaffash.immunology2018@gmail.com.
Hamidreza Reihani, Email: Reihanihr@mums.ac.ir.
Alireza sedaghat, Email: Sedaghatar@mums.ac.ir.
Amir Adhami Moghadam, Email: Amir.adhami58@yahoo.com.
Ahmad Bagheri Moghadam, Email: BagheriA@mums.ac.ir.
Jalil Tavakol-Afshari, Email: Tavakolaj@mums.ac.ir.
References
- 1.Rajarshi K., Chatterjee A., Ray S. Combating COVID-19 with mesenchymal stem cell therapy. Biotechnology Reports. 2020 doi: 10.1016/j.btre.2020.e00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang Y., Liu H., Huang H., Li H., Saqi A., Qiang L. Distinct stem/progenitor cells proliferate to regenerate the trachea, intrapulmonary airways and alveoli in COVID-19 patients. Cell Res. 2020;30(8):705–707. doi: 10.1038/s41422-020-0367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irmak D.K., Darıcı H., Karaöz E. Stem cell based therapy option in COVID-19: is it really promising? Aging and disease. 2020;11(5):1174. doi: 10.14336/AD.2020.0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miao Y., Fan L., Li J.-Y. Potential treatments for COVID-19 related cytokine storm-beyond corticosteroids. Front Immunol. 2020:11. doi: 10.3389/fimmu.2020.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun D., Li H., Lu X.-X., Xiao H., Ren J., Zhang F.-R. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World Journal of Pediatrics. 2020:1–9. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. 2020. Features, evaluation and treatment coronavirus (COVID-19) Statpearls [internet]: StatPearls Publishing. [PubMed] [Google Scholar]
- 10.Li H., Wang Y., Xu J., Cao B. Potential antiviral therapeutics for 2019 novel coronavirus. Journal of tuberculosis and respiratory diseases. 2020;43:E002. doi: 10.3760/cma.j.issn.1001-0939.2020.0002. Zhonghua jie he he hu xi za zhi= Zhonghua jiehe he huxi zazhi= Chinese. [DOI] [PubMed] [Google Scholar]
- 11.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jie Z., He H., Xi H., Zhi Z. Multicenter collaboration group of department of science and technology of guangdong province and health commission of guangdong province for chloroquine in the treatment of novel coronavirus pneumonia. Expert Consensus on Chloroquine Phosphate for the Treatment of Novel Coronavirus Pneumonia. 2020;10:1001–1939. [in Chinese]. 2020. [Google Scholar]
- 14.Tortorici M.A., Veesler D. Structural insights into coronavirus entry. Advances in virus research. 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. Elsevier. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai C.-H., Lee P.-Y., Stollar V., Li M.-L. Antiviral therapy targeting viral polymerase. Curr Pharmaceut Des. 2006;12(11):1339–1355. doi: 10.2174/138161206776361156. [DOI] [PubMed] [Google Scholar]
- 16.Dyall J., Coleman C.M., Hart B.J., Venkataraman T., Holbrook M.R., Kindrachuk J. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrobial agents and chemotherapy. 2014;58(8):4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruse R.L. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Research. 2020;9 doi: 10.12688/f1000research.22211.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marano G., Vaglio S., Pupella S., Facco G., Catalano L., Liumbruno G.M. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfusion. 2016;14(2):152. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mire C.E., Geisbert J.B., Agans K.N., Thi E.P., Lee A.C., Fenton K.A. Passive immunotherapy: assessment of convalescent serum against Ebola virus Makona infection in nonhuman primates. J Infect Dis. 2016;214(suppl_3):S367–S374. doi: 10.1093/infdis/jiw333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang S.-C. Clinical findings, treatment and prognosis in patients with severe acute respiratory syndrome (SARS) J Chin Med Assoc. 2005;68(3):106–107. doi: 10.1016/S1726-4901(09)70229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King T., Faiman B. Steroid-associated side effects. Clin J Oncol Nurs. 2017;21(2) doi: 10.1188/17.CJON.240-249. [DOI] [PubMed] [Google Scholar]
- 23.Tang L., Jiang Y., Zhu M., Chen L., Zhou X., Zhou C. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front Med. 2020:1–10. doi: 10.1007/s11684-020-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoury M., Cuenca J., Cruz F.F., Figueroa F.E., Rocco P.R., Weiss D.J. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J. 2020;55(6) doi: 10.1183/13993003.00858-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barros I., Silva A., de Almeida L.P., Miranda C.O. Mesenchymal stromal cells to fight SARS-CoV-2: taking advantage of a pleiotropic therapy. Cytokine Growth Factor Rev. 2020;58:114–133. doi: 10.1016/j.cytogfr.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yen B.L., Yen M.L., Wang L.T., Liu K.J., Sytwu H.K. Current status of mesenchymal stem cell therapy for immune/inflammatory lung disorders: gleaning insights for possible use in COVID-19. Stem cells translational medicine. 2020;9(10):1163–1173. doi: 10.1002/sctm.20-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canham M.A., Campbell J.D., Mountford J.C. The use of mesenchymal stromal cells in the treatment of coronavirus disease 2019. J Transl Med. 2020;18(1):1–15. doi: 10.1186/s12967-020-02532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korth M., Simmons C., Farrar J., Martin T., Katze M. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Military Medical Research. 2020;7(1):1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin S., Wu H., Wang C., Xiao Z., Xu F. Regulatory T cells and acute lung injury: cytokines, uncontrolled inflammation, and therapeutic implications. Front Immunol. 2018;9:1545. doi: 10.3389/fimmu.2018.01545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar P., Saini S., Khan S., Lele S.S., Prabhakar B.S. Restoring self-tolerance in autoimmune diseases by enhancing regulatory T-cells. Cell Immunol. 2019;339:41–49. doi: 10.1016/j.cellimm.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fajgenbaum D.C., June C.H. Cytokine storm. N Engl J Med. 2020;383(23):2255–2273. doi: 10.1056/NEJMra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dose P., Lee D.W., Gardner R., Porter D.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X. 2020. COVID-19 infection: the perspectives on immune responses. Nature Publishing Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. Jama. 2003;289(21):2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 36.Brocker C., Thompson D., Matsumoto A., Nebert D.W., Vasiliou V. Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum Genom. 2010;5(1):30. doi: 10.1186/1479-7364-5-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheller J., Rose-John S. Interleukin-6 and its receptor: from bench to bedside. Med Microbiol Immunol. 2006;195(4):173–183. doi: 10.1007/s00430-006-0019-9. [DOI] [PubMed] [Google Scholar]
- 38.Hirano T., Matsuda T., Turner M., Miyasaka N., Buchan G., Tang B. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol. 1988;18(11):1797–1802. doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- 39.Kimura A., Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40(7):1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 40.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell B., Moss C., George G., Santaolalla A., Cope A., Papa S. Associations between immune-suppressive and stimulating drugs and novel COVID-19—a systematic review of current evidence. ecancermedicalscience. 2020;14 doi: 10.3332/ecancer.2020.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X., Wu K., Wang D., Yue X., Song D., Zhu Y. Nucleocapsid protein of SARS-CoV activates interleukin-6 expression through cellular transcription factor NF-κB. Virology. 2007;365(2):324–335. doi: 10.1016/j.virol.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pearce L., Davidson S.M., Yellon D.M. The cytokine storm of COVID-19: a spotlight on prevention and protection. Expert Opin Ther Targets. 2020;24(8):723–730. doi: 10.1080/14728222.2020.1783243. [DOI] [PubMed] [Google Scholar]
- 44.McGonagle D., Sharif K., O'Regan A., Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feldmann M., Maini R.N. Anti-TNFα therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol. 2001;19(1):163–196. doi: 10.1146/annurev.immunol.19.1.163. [DOI] [PubMed] [Google Scholar]
- 46.Rock C.S., Lowry S.F. Tumor necrosis factor-α. J Surg Res. 1991;51(5):434–445. doi: 10.1016/0022-4804(91)90146-d. [DOI] [PubMed] [Google Scholar]
- 47.Leibovich S.J., Polverini P.J., Shepard H.M., Wiseman D.M., Shively V., Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-α. Nature. 1987;329(6140):630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- 48.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahil S.K., Dand N., Mason K.J., Yiu Z.Z., Tsakok T., Meynell F. Factors associated with adverse COVID-19 outcomes in patients with psoriasis—insights from a global registry–based study. J Allergy Clin Immunol. 2021;147(1):60–71. doi: 10.1016/j.jaci.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodríguez-Lago I., de la Piscina P.R., Elorza A., Merino O., de Zárate J.O., Cabriada J.L. Characteristics and prognosis of patients with inflammatory bowel disease during the SARS-CoV-2 pandemic in the Basque Country (Spain) Gastroenterology. 2020;159(2):781–783. doi: 10.1053/j.gastro.2020.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pestka S., Langer J.A., Zoon K.C., Samuel C.E. Interferons and their actions. Annu Rev Biochem. 1987;56(1):727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- 52.Sen G.C. Viruses and interferons. Annu Rev Microbiol. 2001;55(1):255–281. doi: 10.1146/annurev.micro.55.1.255. [DOI] [PubMed] [Google Scholar]
- 53.Blanco-Melo D., Nilsson-Payant B.E., Liu W.-C., Uhl S., Hoagland D., Møller R. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181(5):1036–1045. doi: 10.1016/j.cell.2020.04.026. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopez L., Sang P.C., Tian Y., Sang Y. Dysregulated interferon response underlying severe COVID-19. Viruses. 2020;12(12):1433. doi: 10.3390/v12121433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sims J.E., Smith D.E. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10(2):89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 56.Ye Q., Wang B., Mao J. The pathogenesis and treatment of theCytokine Storm'in COVID-19. J Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conti P., Caraffa A., Gallenga C., Ross R., Kritas S., Frydas I. Coronavirus-19 (SARS-CoV-2) induces acute severe lung inflammation via IL-1 causing cytokine storm in COVID-19: a promising inhibitory strategy. J Biol Regul Homeost Agents. 2020;34 doi: 10.23812/20-1-E. [DOI] [PubMed] [Google Scholar]
- 58.Farkhad N.K., Mahmoudi A., Mahdipour E. How similar are human mesenchymal stem cells derived from different origins? A review of comparative studies. Curr Stem Cell Res Ther. 2021 doi: 10.2174/1574888X16666210302151823. [DOI] [PubMed] [Google Scholar]
- 59.Tavakol Afshari J. Stem cell therapy: the ethical issues. Int J Pediatr. 2014;2(2.3):9. [Google Scholar]
- 60.Aboushady I.M., Salem Z.A., Sabry D., Mohamed A. Comparative study of the osteogenic potential of mesenchymal stem cells derived from different sources. Journal of clinical and experimental dentistry. 2018;10(1):e7. doi: 10.4317/jced.53957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Waldner M., Zhang W., James I.B., Allbright K., Havis E., Bliley J.M. Characteristics and immunomodulating functions of adipose-derived and bone marrow-derived mesenchymal stem cells across defined human leukocyte antigen barriers. Front Immunol. 2018;9:1642. doi: 10.3389/fimmu.2018.01642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ishige I., Nagamura-Inoue T., Honda M.J., Harnprasopwat R., Kido M., Sugimoto M. Comparison of mesenchymal stem cells derived from arterial, venous, and Wharton's jelly explants of human umbilical cord. Int J Hematol. 2009;90(2):261–269. doi: 10.1007/s12185-009-0377-3. [DOI] [PubMed] [Google Scholar]
- 63.Ren H., Sang Y., Zhang F., Liu Z., Qi N., Chen Y. Comparative analysis of human mesenchymal stem cells from umbilical cord, dental pulp, and menstrual blood as sources for cell therapy. Stem Cell Int. 2016;2016 doi: 10.1155/2016/3516574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Manley H., Sprinks J., Breedon P. Menstrual blood-derived mesenchymal stem cells: women's attitudes, willingness, and barriers to donation of menstrual blood. J Wom Health. 2019;28(12):1688–1697. doi: 10.1089/jwh.2019.7745. [DOI] [PubMed] [Google Scholar]
- 65.Alizadeh R., Bagher Z., Kamrava S.K., Falah M., Hamidabadi H.G., Boroujeni M.E. Differentiation of human mesenchymal stem cells (MSC) to dopaminergic neurons: a comparison between Wharton's Jelly and olfactory mucosa as sources of MSCs. J Chem Neuroanat. 2019;96:126–133. doi: 10.1016/j.jchemneu.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 66.Khurana R., Kudva P.B., Husain S.Y. Comparative evaluation of the isolation and quantification of stem cells derived from dental pulp and periodontal ligament of a permanent tooth and to assess their viability and proliferation on a platelet-rich fibrin scaffold. J Indian Soc Periodontol. 2017;21(1):16. doi: 10.4103/jisp.jisp_182_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Golchin A., Seyedjafari E., Ardeshirylajimi A. Mesenchymal stem cell therapy for COVID-19: present or future. Stem cell reviews and reports. 2020:1–7. doi: 10.1007/s12015-020-09973-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iyer S., Co C., Rojas M. Mesenchymal stem cells and inflammatory lung diseases. Panminerva Med. 2009;51(1):5–16. [PubMed] [Google Scholar]
- 69.Centeno C.J. Bone marrow concentrate (BMC) therapy in musculoskeletal disorders: evidence-based policy position statement of American Society of Interventional Pain Physicians (ASIPP) Pain Physician. 2020;23:E85–E131. [PubMed] [Google Scholar]
- 70.Orleans L., is Vice H., Manchikanti L. Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically ill COVID-19 patients: the case for compassionate use. Pain Physician. 2020;23:E71–E83. [PubMed] [Google Scholar]
- 71.Court A.C., Le-Gatt A., Luz-Crawford P., Parra E., Aliaga-Tobar V., Bátiz L.F. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020;21(2) doi: 10.15252/embr.201948052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Islam M.N., Das S.R., Emin M.T., Wei M., Sun L., Westphalen K. Mitochondrial transfer from bone-marrow–derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18(5):759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mehta P., Mcauley D., Brown M., Sanchez E., Tattersall R., Manson J. Correspondence COVID-19: consider cytokine storm syndromes and. Lancet. 2020;6736(20):19–20. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Magro G. SARS-CoV-2 and COVID-19: is interleukin-6 (IL-6) the'culprit lesion'of ARDS onset? What is there besides Tocilizumab? SGP130Fc. Cytokine X. 2020:100029. doi: 10.1016/j.cytox.2020.100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kao K.-C., Hu H.-C., Chang C.-H., Hung C.-Y., Chiu L.-C., Li S.-H. Diffuse alveolar damage associated mortality in selected acute respiratory distress syndrome patients with open lung biopsy. Crit Care. 2015;19(1):1–10. doi: 10.1186/s13054-015-0949-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q. Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging and disease. 2020;11(2):216. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matthay M.A., Calfee C.S., Zhuo H., Thompson B.T., Wilson J.G., Levitt J.E. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. The Lancet Respiratory Medicine. 2019;7(2):154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Loy H., Kuok D.I., Hui K.P., Choi M.H., Yuen W., Nicholls J.M. Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating influenza A (H5N1) virus–associated acute lung injury. J Infect Dis. 2019;219(2):186–196. doi: 10.1093/infdis/jiy478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J., Zhou J., Zhang D., Song Y., She J., Bai C. Bone marrow-derived mesenchymal stem cells enhance autophagy via PI 3K/AKT signalling to reduce the severity of ischaemia/reperfusion-induced lung injury. J Cell Mol Med. 2015;19(10):2341–2351. doi: 10.1111/jcmm.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sleem A., Saleh F. Mesenchymal stem cells in the fight against viruses: face to face with the invisible enemy. Curr Res Transl Med. 2020;68(3):105–110. doi: 10.1016/j.retram.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bunnell B.A., Betancourt A.M., Sullivan D.E. New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell Res Ther. 2010;1(5):1–8. doi: 10.1186/scrt34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li W., Ren G., Huang Y., Su J., Han Y., Li J. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 2012;19(9):1505–1513. doi: 10.1038/cdd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Le Blanc K., Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 84.Zhang W., Ge W., Li C., You S., Liao L., Han Q. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cell Dev. 2004;13(3):263–271. doi: 10.1089/154732804323099190. [DOI] [PubMed] [Google Scholar]
- 85.Jiang X.-X., Zhang Y., Liu B., Zhang S.-X., Wu Y., Yu X.-D. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 86.Regmi S., Pathak S., Kim J.O., Yong C.S., Jeong J.-H. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. Eur J Cell Biol. 2019;98(5–8):151041. doi: 10.1016/j.ejcb.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 87.Wang Y., Chen X., Cao W., Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15(11):1009. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 88.Li Z., Niu S., Guo B., Gao T., Wang L., Wang Y. Stem cell therapy for COVID-19, ARDS and pulmonary fibrosis. Cell Prolif. 2020;53(12) doi: 10.1111/cpr.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liang B., Chen J., Li T., Wu H., Yang W., Li Y. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: a case report. Medicine. 2020;99(31) doi: 10.1097/MD.0000000000021429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lanzoni G., Linetsky E., Correa D., Alvarez R., Marttos A., Hirani K. Umbilical cord-derived mesenchymal stem cells for COVID-19 patients with acute respiratory distress syndrome (ARDS) Cell. 2020;8:e2839. doi: 10.32113/cellr4_20204_2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y., Ding J., Ren S., Wang W., Yang Y., Li S. Intravenous infusion of human umbilical cord Wharton's jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res Ther. 2020;11(1):1–6. doi: 10.1186/s13287-020-01725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi L., Huang H., Lu X., Yan X., Jiang X., Xu R. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal transduction and targeted therapy. 2021;6(1):1–9. doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tao J., Nie Y., Wu H., Cheng L., Qiu Y., Fu J. Umbilical cord blood-derived mesenchymal stem cells in treating a critically ill COVID-19 patient. Journal of Infection in Developing Countries. 2020;14(10) doi: 10.3855/jidc.13081. [DOI] [PubMed] [Google Scholar]
- 94.Chen H., Zhang L., He Z., Wang D., Liu L., Zhang W. Systemic administration of human umbilical cord-derived mesenchymal stem cells effectively ameliorates the outcomes of a critically ill elderly patient with COVID-19 with multiple comorbidities: a case report. World Academy of Sciences Journal. 2020;2(6):1. [Google Scholar]
- 95.Tang L., Jiang Y., Zhu M., Chen L., Zhou X., Zhou C. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front Med. 2020;14(5):664–673. doi: 10.1007/s11684-020-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hashemian S.-M.R., Aliannejad R., Zarrabi M., Soleimani M., Vosough M., Hosseini S.-E. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther. 2021;12(1):1–12. doi: 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sánchez-Guijo F., García-Arranz M., López-Parra M., Monedero P., Mata-Martínez C., Santos A. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine. 2020;25:100454. doi: 10.1016/j.eclinm.2020.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meng F., Xu R., Wang S., Xu Z., Zhang C., Li Y. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal transduction and targeted therapy. 2020;5(1):1–7. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cell Dev. 2020;29(12):747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen X., Shan Y., Wen Y., Sun J., Du H. Mesenchymal stem cell therapy in severe COVID-19: a retrospective study of short-term treatment efficacy and side effects. J Infect. 2020;81(4):647–679. doi: 10.1016/j.jinf.2020.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shu L., Niu C., Li R., Huang T., Wang Y., Huang M. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):1–11. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lippi G., Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin Chem Lab Med. 2020;58(7):1131–1134. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 103.Janeway C.A., Jr., Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20(1):197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 104.Bilgir O., Bilgir F., Calan M., Calan O.G., Yuksel A. Comparison of pre-and post-levothyroxine high-sensitivity c-reactive protein and fetuin-a levels in subclinical hypothyroidism. Clinics. 2015;70(2):97–101. doi: 10.6061/clinics/2015(02)05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aloisio E., Chibireva M., Serafini L., Pasqualetti S., Falvella F.S., Dolci A. A comprehensive appraisal of laboratory biochemistry tests as major predictors of COVID-19 severity. Arch Pathol Lab Med. 2020;144(12):1457–1464. doi: 10.5858/arpa.2020-0389-SA. [DOI] [PubMed] [Google Scholar]
- 106.Chau T.N., Lee K.C., Yao H., Tsang T.Y., Chow T.C., Yeung Y.C. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39(2):302–310. doi: 10.1002/hep.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao X.-Y., Xu X.-X., Yin H.-S., Hu Q.-M., Xiong T., Tang Y.-Y. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis. 2020;20:1–8. doi: 10.1186/s12879-020-05010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shi H., Han X., Jiang N., Cao Y., Alwalid O., Gu J. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guan W-j, Ni Z-y, Hu Y., Liang W-h, Ou C-q, He J-x. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang C., Shi L., Wang F.-S. Liver injury in COVID-19: management and challenges. The lancet Gastroenterology & hepatology. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Omrani-Nava V., Maleki I., Ahmadi A., Moosazadeh M., Hedayatizadeh-Omran A., Roozbeh F. Evaluation of hepatic enzymes changes and association with prognosis in COVID-19 patients. Hepat Mon. 2020;20(4) [Google Scholar]
- 112.Yip T.C.-F., Lui G.C.-Y., Wong V.W.-S., Chow V.C.-Y., Ho T.H.-Y., Li T.C.-M. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut. 2020;70(4):733–742. doi: 10.1136/gutjnl-2020-321726. [DOI] [PubMed] [Google Scholar]
- 113.Cho K.A., Woo S.Y., Seoh J.Y., Han H.S., Ryu K.H. Mesenchymal stem cells restore CCl4-induced liver injury by an antioxidative process. Cell Biol Int. 2012;36(12):1267–1274. doi: 10.1042/CBI20110634. [DOI] [PubMed] [Google Scholar]
- 114.Qian J.-Y., Wang B., Liu B.-C. Acute kidney injury in the 2019 novel coronavirus disease. Kidney diseases. 2020;6(5):318–323. doi: 10.1159/000509086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brienza N., Puntillo F., Romagnoli S., Tritapepe L. Acute kidney injury in coronavirus disease 2019 infected patients: a meta-analytic study. Blood Purif. 2020:1–7. doi: 10.1159/000509274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li Y., Hu Y., Yu J., Ma T. Retrospective analysis of laboratory testing in 54 patients with severe-or critical-type 2019 novel coronavirus pneumonia. Lab Invest. 2020:1–7. doi: 10.1038/s41374-020-0431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Herrera M., Bussolati B., Bruno S., Morando L., Mauriello-Romanazzi G., Sanavio F. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007;72(4):430–441. doi: 10.1038/sj.ki.5002334. [DOI] [PubMed] [Google Scholar]
- 119.Ninichuk V., Gross O., Segerer S., Hoffmann R., Radomska E., Buchstaller A. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 2006;70(1):121–129. doi: 10.1038/sj.ki.5001521. [DOI] [PubMed] [Google Scholar]
- 120.Sun X., Wang T., Cai D., Hu Z., Liao H., Zhi L. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38–42. doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Domenis R., Cifù A., Quaglia S., Pistis C., Moretti M., Vicario A. Pro inflammatory stimuli enhance the immunosuppressive functions of adipose mesenchymal stem cells-derived exosomes. Sci Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-31707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]