Abstract
Objectives:
Tensiomyography (TMG) derived contraction time (Tc) and amplitude (Dm) are related to muscle fibre composition and to muscle atrophy/tone, respectively. However, the link between mobility and TMG-derived skeletal muscle contractile properties in older persons is unknown. The aim of the study was to correlate lower limb skeletal muscle contractile properties with balance and mobility measures in senior female residents of retirement homes in Austria.
Methods:
Twenty-eight female participants (aged from 67-99 years) were included in measurements of contractile properties (TMG) of four skeletal muscles: vastus lateralis, vastus medialis, biceps femoris and gastrocnemius medialis. Their balance and mobility performance was measured using a timed up and go test (TUG).
Results:
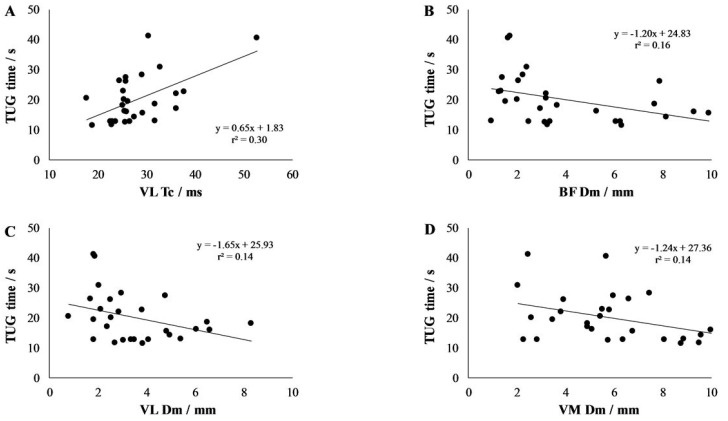
Time needed to complete TUG is negatively correlated to biceps femoris (r= -0.490; p= 0.008), vastus lateralis (r= -0.414; p=0.028) and vastus medialis (r= -0.353; p=0.066) Dm and positively correlated to vastus lateralis Tc (r=0.456; p=0.015). Overall, vastus lateralis Tc and vastus medialis Dm explained 37% of TUG time variance.
Conclusions:
Our study demonstrates that TMG-derived quadriceps muscle contractile parameters are correlated with the balance and mobility function in female nursing home residents.
Keywords: Aging, Body composition, Gait, Tensiomyography, Timed Up and Go Τest
Introduction
Skeletal muscle mass shows a progressive decline of up to 1-1.5% per year after the age of 50 as a consequence of loss and atrophy of muscle fibres[1-4]. Since many of the age-related changes in skeletal muscle are similar to those induced by disuse[4], it is likely that the decrease in physical activity levels in old age[5,6] is a major contributor to the muscle wasting during ageing[7]. As a result, master athletes that maintain high levels of physical activity[8-10] suffer from fewer morbidities[11] and muscle wasting[7,12-14].
On the other hand, various processes reduce the autonomy and independence of older persons to carry out their daily activities[15,16]. Decline in biological functional reserves, reduced stress resistance and changes in physiological systems highlight loss of independence of an individual due to illnesses, injuries, falls, and hospitalization, resulting in long-term care[17-22]. From the mobility and independence point of view, the most important indicators of the state of the older persons are muscle mass, strength, and dynamic balance with gait speed[18]. Sarcopenia and/or dynapenia are conditions that severely affect quality of life in elderly and also in young persons[23]. The European Working Group on Sarcopenia in Older People (EWGSOP) recently proposed a revised protocol for the definition of sarcopenia[24]: muscle performance and muscle quality take higher classification importance. The muscle performance could be easily assessed with various tests, whereas EWGSOP proposed the Timed up and go test (TUG). The TUG[25] is a clinical assessment tool widely used to assess balance and walking ability in elderly populations[26]. It includes basic everyday movements and daily life tasks (standing, walking, and turning) and is therefore very simple to perform and highly recommended. Several investigators have reported a significant positive association between the time taken to perform the TUG and history of falls[27] as well as it’s ability to predict sarcopenia[28]. However, the correlation between TUG values and muscle properties (TMG) has not yet been fully investigated[24].
Currently, there are only a few methods available to non-invasively assess muscle quality in population studies. Tensiomyography (TMG) is a non-invasive tool to determine the twitch contractile properties (e.g. contraction time - Tc, in ms) of a single muscle. It can be used to estimate the percentage of type I myosin heavy chain proportion, at least, in the vastus lateralis (VL) muscle[29]. Recent TMG reports from large study cohorts provide evidence that regular sport exercise in adolescence decreases Tc in non-postural biceps femoris (BF) but not in postural VL[30]. TMG has also used in populations of varying ages (35-90 years) to provide evidence that anaerobic master athletes preserve lower Tc in the three muscles when compared to non-athletes, while aerobic master athletes have higher Tc than non-athletes[7]. In addition, 8 weeks of high-velocity plyometric training in young[31] and old persons[32] resulted in similar (~13%) improvements in explosive power and 8-26% shorter Tc in five lower limb skeletal muscles that could explain the ~30% improvement in explosive power. Furthermore, amplitude of TMG response (Dm, in mm) is sensitive to muscle atrophy: it increased by ~30% after 35-day of horizontal bed rest[33]. However, it was recently shown that the increase in Dm is even higher after 35-days of 6-degree head down bedrest. Specifically, the initial increases in the Dm were observed much before atrophy was evident from the anatomical measures of the muscles[34]. This suggests that Dm measurements could provide evidence of pre-atrophic changes, which is of great clinical importance in older persons who atrophy at higher rates, and in whom inactivity induced atrophy is less reversible than in young persons[35]. However, the question remains how Tc and Dm adapts as persons age (especially in older persons) and how this is linked to their balance and mobility function. The aim of the study was to correlate lower limb skeletal muscle contractile properties with balance and mobility measures in senior citizens. We hypothesized that TUG time is correlated with TMG-derived contractile properties in four different skeletal muscles in older female persons living in senior homes.
Materials and methods
Participants
A non-randomised paradigm was used due to availability of only four nursing homes in the Graz vicinity as well as due to needed consent of their residents. Female residents of four retirement homes in the city of Graz, Austria, were invited and enrolled in the study. Inclusion criteria included mobility (also with the help of a walking stick or a rollator walker); over 65 years of age; and the cognitive ability to participate in the study. The exclusion criteria were presence of tumours or malignancies, complete deterioration of mobility, muscle spasticity, and/or presence of a severe form of dementia.
Forty-two female participants were enrolled in the study. Only 28 participants finished all the measurements (average age: 85.6±7.6 years; height: 158.8±8.8 cm; mass: 64.4±11.7 kg). Participants signed written consent prior to the study. An ethical approval was obtained from the Ethics Committee of the Medical University of Graz before the study (EK: 30-051 ex 17/18).
Study and protocol
Participants did not perform any strenuous exercise 24 hours before the assessments. In this cross-sectional design study, participants performed anthropometric tests, TMG testing and TUG test, in that order.
Anthropometry
After determining body height and mass with standard tools, fat and muscle mass were assessed with multi frequency segmental body analyser Tanita MC-780-MA, which previously has shown satisfactory valid results for fat mass[36]. The participants stepped barefooted on the platform and held the grips in both hands (alongside their body) during the impedance measurements (hand to foot). A full analysis of fat and muscle mass in each participant was performed in less than 20 seconds.
Tensiomyography
Skeletal muscle mechanical contractile properties were assessed with a TMG device (TMG-ZD1, TMG-BMC d.o.o., Slovenia) in four muscles of the right leg: VL, vastus medialis (VM), biceps femoris (BF) and gastrocnemius medialis (GM). Participants were asked to relax on a medical bed in supine position (VL, VM) with their knee angle fixed at 30 degrees flexion or prone (GM, BF), with knee angle fixed at 5 degrees flexion and ankle in neutral position. Foam pads were used to support the joints. The measuring point for TMG assessment was the thickest part of each muscle belly, as described previously[7,37]. Briefly, two self-adhesive electrodes (PALS, Axelgaard) were positioned 5 cm distal (cathode) and 5 cm proximal (anode) to the thickest part of the muscle belly (a measuring point identified during voluntary isometric contraction). No skin preparation was carried out. Muscle contraction was triggered with a single maximal rectangular electrical stimulus of 1 ms duration. The linear displacement sensor detected the transverse radial enlargement as a response in time domain. Two contractile parameters were estimated: Dm as a maximal amplitude of the response and Tc as the time between 10 % and 90 % of Dm[29,34]. In each muscle, two TMG responses were recorded and an average of estimated parameters were taken for analysis. The absolute variability of Tc and Dm in all four muscles ranged from 2.3 to 4.7 %, (being highest in BF Dm at 4.7 %).
Timed up and go test
We used an instrumentalized TUG at a distance of three meters[38]. The TUG detector was placed on the back of the 45 cm chair and transmitted high-frequency ultrasonic waves at a frequency of 40 kHz over a period of 10 Hz, which, after a reflection of the target object, was detected by an ultrasonic sensor for detecting the distance to the target object with an accuracy of 3 cm within a range of 6 meters[38]. All participants wore their regular walking shoes, followed the same procedure in the same room and used the same chair. They started the test by sitting on a stable chair, then they stood up, walked around a marked pole, returned to the chair and sat on it. Each participant performed one test trial and two trials with or without walking aids. The trial with the shortest TUG time was considered for the final result[39].
Statistics
Statistical analyses were conducted with the SPSS software package (version 23, IBM, USA). All data are presented with the average value and standard deviation. Normal distribution was checked with visual inspection of histogram and Q-Q plot and Shapiro-Wilk test and was not confirmed for TUG time. Therefore, for all correlation analyses, we used Spearman correlation coefficient (rho). However, for the purpose of Figure 1 we used the linear regression technique. After correcting TUG time to normal distribution using logarithmic transformation, we performed multivariate linear regression to predict log (TUG time). Four TMG-derived candidate predictors were used based on the high bivariate correlation with log(TUG time) (prho≤0.10) and low multicollinearity (Spearman rho≤0.5 and variance inflation factor <2), whereas only two predictors were used (Forward method) in the final regression model which was found to be appropriate as we only analysed/included 28 participants. Statistical decisions were confirmed at a risk level of α≤0.05.
Figure 1.
Correlation between Timed up and go (TUG) time and tensiomyographic-derived contraction time (Tc) and amplitude (Dm) in vastus lateralis (VL), vastus medialis (VM) and biceps femoris (BF) muscles.
Results
Twenty-eight female participants finished all measurements and were included in the statistical analysis. Tables 1 and 2 show the descriptive data of all participants.
Table 1.
Descriptive data of participants.
| Variable | Average ± standard deviation |
|---|---|
| N | 28 |
| Age (years) | 85.6±7.6 |
| Body height (cm) | 158.8±8.8 |
| Body mass (kg) | 64.4±11.7 |
| Body fat percentage (%) | 18.6±7.3 |
| Body fat mass (kg) | 28.4±8.07 |
| Timed Up and Go time (s) | 20.07±8.07 |
Table 2.
Descriptive tensiomyographic data of all measured muscles.
| Muscle | Contraction time (ms) | Maximum amplitude (mm) |
|---|---|---|
| Biceps femoris | 44.7±10.0 | 4.00±2.67 |
| Gastrocnemius medialis | 32.39±6.40 | 2.42±1.03 |
| Vastus lateralis | 32.46±6.83 | 3.54±1.81 |
| Vastus medialis | 48.54±10.10 | 5.88±2.46 |
Table 3 presents analysis of the Spearman’s correlation coefficients of TUG time with anthropometrics and TMG variables. We found that TUG time is negatively correlated to BF Dm (rho= -0.490; p=0.008) and VL Dm (rho= -0.414; p=0.028) and is positively correlated to VL Tc (rho=0.456; p=0.015) and body mass (rho=0.482; p=0.009) and further shows a tendency towards negative correlation to VM Dm (rho= -0.353; p=0.066) (Figure 1). As age was not correlated neither to Tc nor to Dm in any of four muscles (p>0.251) we only focused on correlations between TUG time and TMG-derived contractile properties.
Table 3.
Spearman’s correlation (rho) of Timed Up and Go time with individual anthropometric parameters and contractile properties of four skeletal muscles - contraction time (Tc) and maximum amplitude (Dm).
| Rho | p | |
|---|---|---|
| Age | -0.065 | 0.739 |
| Body mass (kg) | 0.482 | 0.009 |
| Body height | 0.328 | 0.082 |
| Fat mass (%) | 0.137 | 0.479 |
| Muscle mass (%) | 0.197 | 0.306 |
| Biceps femoris Tc | -0.103 | 0.602 |
| Biceps femoris Dm | -0.490 | 0.008 |
| Gastrocnemius medialis Tc | 0.224 | 0.251 |
| Gastrocnemius medialis Dm | -0.313 | 0.105 |
| Vastus lateralis Tc | 0.456 | 0.015 |
| Vastus lateralis Dm | -0.414 | 0.028 |
| Vastus medialis Tc | 0.191 | 0.329 |
| Vastus medialis Dm | -0.353 | 0.066 |
Multiple regression correlation confirmed 37% of explained TUG time variance (R=0.605; p=0.003) from two predictors: VL Tc and VM Dm (Table 4). Whereas, longer TUG times were explained by longer VL Tc and lower VM Dm.
Table 4.
Multivariate linear correlation between logarithmic transformation of Timed Up and Go time and two tensiomyographic predictors.
| B | β | Partial correlation | p | |
|---|---|---|---|---|
| Constant | 0.405 | |||
| Vastus lateralis Tc | 0.027 | 0.457 | 0.495 | 0.009 |
| Vastus medialis Dm | -0.058 | -0.348 | -0.399 | 0.039 |
Legend: Tc - contraction time; Dm - maximal amplitude.
Discussion
We observed that TUG times are correlated to body mass and contractile properties of specific muscles (BF, VL and a tendency also in VM) but not to GM in older females. Furthermore, using a multiple correlation, we could explain up to 37% of TUG time variance. However, only VL and VM contractile properties significantly contributed to the model. In addition, our participants demonstrated very low mobility, with an average TUG time of 19.94±7.96 seconds, which is higher than the predictive time (>12.6 seconds) for future falls[40]. TUG times above 12 seconds represent the clinical cut-off value for reduced physical function[41]. Furthermore, TUG times of our participants are far longer than those of a reference values of young men (3.9±0.4 seconds), women (4.2±0.3 seconds), older men (5.2±0.7 seconds) and women (5.8±1.0 seconds)[42]. Maden-Wilkinson and colleagues found a correlation between the TUG times and muscle mass only at group level (younger men and women, older men and women), whereas at individual levels this was found not to be the case[42]. This is also in agreement with our results. Our study is the first to report skeletal muscle TMG responses of immobile older populations. Specifically, we report TMG reference values for four important muscles in the population with limited mobility: postural VM, VL and GM; and non-postural BF. More specifically, lower Dm of VM and BF (a tendency also in VM), as well as longer Tc of VL, were correlated to longer TUG times. Higher Dm has been previously shown to be associated with muscle atrophy after disuse[33]. However, it was found that Dm initially increased already after 1-4 days of disuse (depending on the muscle studied) before anatomical atrophy was confirmed[34]. Initial increase in Dm was explained by fluid redistribution from legs to torso and diminished muscle tone/stiffness. However, later increase in Dm was associated with atrophy of muscle fibres, adaptation of muscle architecture towards lower thickness, pennation angle and fascicle length[34]. In this current study, we found increased Dm as a potential risk factor for lower mobility, specifically, for lower balance and walking ability[26]. We believe that our study is the first study to show a negative correlation between Dm and mobility measure (TUG time). This information is of clinical relevance, especially in aspect related to the mobility of older ambulatory populations. Therefore, future studies should evaluate whether this could be caused by fat and connective tissue infiltration within the muscles[43] and could therefore contribute as a marker of sarcopenia confirmation[24].
We had actually expected to see higher values of Dm in our study. Higher Dm levels are seen during physical inactivity exposures in the young[33,34] and aging is associated with increases in physical inactivity[44]. However, we observed that Dm values were lower than those reported for healthy and mobile older participants (discussed in the next paragraph). Furthermore, we found that lower Dm (in BF, VL and tendency in VM) was correlated to lower TUG times. Reduction of Dm values have been reported following local muscle fatigue[45-47], general fatigue[48] and/or following chronic adaptation to high-velocity exercise[31,32]. However, the exact mechanisms underlying the lowering of Dm values, for example during fatigue, remain unexplained.
It is important is to mention that our participants showed lower Dm values than those found in healthy and very mobile older participants[32] in VL (3.56±1.78 vs. 5.4±1.8 mm) and in BF (4.12±2.61 vs. 6.5±2.8 mm). Lower Dm in less mobile populations, as in our study, could be attributed to accompanying adaptation of the muscle tissue to disuse and ageing, e.g. lower muscle (contractile) quality due to: infiltration of fat deposits within the muscle tissue; impaired muscle junction yielding impaired excitation-contraction coupling; and fibrosis due to the excessive formation of fibrous bands of scar tissue in between muscle fibres[49]. It is also known that older people have lower elasticity of muscle fibers[50] that could arise due to selective atrophy, loss and remodelling of muscle fibres towards type I (less elastic than the fast-twitch fibres)[51]. This is also seen in our study, as we found higher VL Tc values than those reported in normal aged population or master athletes[7]: VL Tc in our participants 32.46±6.83 ms vs. in mobile older participants 28.4±3.5 ms vs. in power master athletes 25.9±2.5 ms vs. in endurance master athletes 32.7±8.5 ms. Longer VL Tc values could be interpreted as a high proportion of type I muscle fibres[29] found in our participants, which are not able to develop powerful and fast contractions, as required for TUG. Indeed, we found significant non-parametrical bivariate correlation between VL Tc and TUG time as well as multiple linear correlation when we transformed TUG time results to normal distribution. Indeed, multiple linear correlations showed that contractile properties of VM and VL muscle - but not contractile properties of other muscles (BF and GM) - were predictive of TUG time. Although BF and VL Dm were also considered as potential predictors of TUG time using bivariate correlations, it did not appear as a meaningful predictor in multivariate models. These results suggest that knee extensors (VL and VM) are the most important muscles (of those muscles tested) for TUG performance. VL and VM are knee extensor muscles, postural muscles, which are activated in concentric mode (sit-to-stand), stretch-shortening cycle (walking), and eccentric mode (stand-to-sit) during TUG performance[52]. Indeed, both VL and VM have a similar role but it has not found to be equally predictive of TUG time. VL contributed with higher proportion of fast-twitch fibres (longer Tc) while VM with higher muscle tone (smaller Dm) contributed to longer TUG time. It is well known that VL is more activated in lower knee flexions and open kinetic chain tasks, while VM is being more activated during squat and closed kinetic chain tasks[53,54].
Overall it appears that TMG-derived contractile parameters could be a good candidate markers of non-invasive muscle quality assessment in population-based studies to fulfil the gaps identified by EWGSOP[24].
Limitations
This is the first study being performed in older female participants with low mobility. Due to their poor mobility, TUG performances were tested with different mobility aids (e.g. walking stick, rollator). We, however, believe that the TUG results are valid as the TUG test was designed, and its results previously validated, to be used with - or without - mobility aids (none, cane, walker, supervision)[25].
The older participants were also tested in one season and fixed times of the day. Therefore, the effects of circadian rhythms and circ-annual rhythms, which are known to influence the risk of falls an falls-related injuries, were not included. Future studies should monitor these parameters related to TUG and muscle function in different seasons[55,56].
Finally, we did not include males in the study. However, this is the strength of this study, as data related to females is very scanty. As sex-based differences have been reported in risk of falls, future studies should include both males and females[57].
Conclusions
Apart from higher body mass, we also observed that TMG-derived contractile properties of BF, VL and VM muscle are correlated to TUG balance and walking ability. That is, longer Tc (VL), lower Dm (BF, GM and VM) and higher body mass are associated with TUG performance. Our results provide an important link between TMG and functional outcomes in aged population. As the risk of orthostatic intolerance and falls increases as person age[20,58], and falls and falls-related injuries are associated with increased morbidity and mortality, it is important to understand the underlying mechanisms that can lead to falls in older persons[59,60]. Better knowledge of the etiology of falls can go a long way in development of preventive measures to prevent falls[61-63], reduce costs associated with hospitalization that occurs due to falls and falls-related injuries[27], and lead to general improvement in quality of our senior citizens.
Acknowledgements
We thank the nurses from the Retirement homes Aigner Rollett, Peter Rosegger, Robert Stolz and Erica Horn of Graz for their contribution in the research. Finally, we thank the participants for their time, enthusiasm and patience.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
Funding
This work was partly funded by the “Zukunftsfonds des Landes Steiermark” (GZ: ABT08-182942j2016 PN:8011).
References
- 1.Janssen I, Heymsfield SB, Wang Z, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol. 2000;89(1):81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 2.Sato T, Akatsuka H, Kito K, Tokoro Y, Tauchi H, Kato K. Age changes in size and number of muscle fibers in human minor pectoral muscle. Mech Ageing Dev. 1984;28(1):99–109. doi: 10.1016/0047-6374(84)90156-8. [DOI] [PubMed] [Google Scholar]
- 3.Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? J Neurol Sci. 1988;84(2-3):275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 4.Degens H, Alway SE. Control of Muscle Size During Disuse, Disease, and Aging. Int J Sports Med. 2006;27(2):94–99. doi: 10.1055/s-2005-837571. [DOI] [PubMed] [Google Scholar]
- 5.McPhee JS, French DP, Jackson D, Nazroo J, Pendleton N, Degens H. Physical activity in older age:perspectives for healthy ageing and frailty. Biogerontology. 2016;17(3):567–580. doi: 10.1007/s10522-016-9641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wullems JA, Verschueren SMP, Degens H, Morse CI, Onambélé GL. A review of the assessment and prevalence of sedentarism in older adults, its physiology/health impact and non-exercise mobility counter-measures. Biogerontology. 2016;17(3):547–565. doi: 10.1007/s10522-016-9640-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Šimunič B, Pišot R, Rittweger J, Degens H. Age-Related Slowing of Contractile Properties Differs Between Power, Endurance, and Nonathletes:A Tensiomyographic Assessment. Journals Gerontol Ser A. 2018;73(12):1602–1608. doi: 10.1093/gerona/gly069. [DOI] [PubMed] [Google Scholar]
- 8.Rittweger J, Kwiet A, Felsenberg D. Physical performance in aging elite athletes - Challenging the limits of physiology. J Musculoskelet Neuronal Interact. 2004;4(2):159–160. [PubMed] [Google Scholar]
- 9.Hawkins SA, Wiswell RA, Marcell TJ. Exercise and the Master Athlete - A Model of Successful Aging? Journals Gerontol - Ser A Biol Sci Med Sci. 2003;58(11):1009–11. doi: 10.1093/gerona/58.11.m1009. [DOI] [PubMed] [Google Scholar]
- 10.Suominen H, Korhonen M. Sport Performance in Master Athletes:Age-Associated Changes and Underlying Neuromuscular Factors. In: Komi P V, editor. Neuromuscular Aspects of Sport Performance. Oxford, UK: Wiley-Blackwell; 2010. pp. 270–282. [Google Scholar]
- 11.Kettunen JA, Kujala UM, Kaprio J, Sarna S. Health of Master Track and Field Athletes. Clin J Sport Med. 2006;16(2):142–148. doi: 10.1097/00042752-200603000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Korhonen MT, Cristea A, Alén M, et al. Aging, muscle fiber type, and contractile function in sprint-trained athletes. J Appl Physiol. 2006;101(3):906–917. doi: 10.1152/japplphysiol.00299.2006. [DOI] [PubMed] [Google Scholar]
- 13.Arampatzis A, Degens H, Baltzopoulos V, Rittweger J. Why Do Older Sprinters Reach the Finish Line Later? Exerc Sport Sci Rev. 2011;39(1):18–22. doi: 10.1097/JES.0b013e318201efe0. [DOI] [PubMed] [Google Scholar]
- 14.Degens H. Determinants of skeletal muscle hypertrophy and the attenuated hypertrophic response at old age. J Sports Med Doping Stud. 2012;S1(003):2–9. [Google Scholar]
- 15.Šimunič B, Pišot S, Pišot R. In:Active and Quality Aging in Home Environment (A-Qu-A):Norwegian Financial Mechanism 2009-2014. Ljubljana: Solos; 2016. Kako obvladovati staranje? pp. 131–139. [Google Scholar]
- 16.Martins WR, Safons MP, Bottaro M, et al. Effects of short term elastic resistance training on muscle mass and strength in untrained older adults:a randomized clinical trial. BMC Geriatr. 2015;15(1):99. doi: 10.1186/s12877-015-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandarillas MÁ, Goswami N. Merging current healthcare trends:innovative perspective in aging care. Clin Interv Aging. 2018;13:2083–2095. doi: 10.2147/CIA.S177286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bousquet J, Bewick M, Cano A, et al. Building bridges for innovation in ageing:Synergies between action groups of the EIP on AHA. J Nutr Health Aging. 2017;21(1):92–104. doi: 10.1007/s12603-016-0803-1. [DOI] [PubMed] [Google Scholar]
- 19.Blain H, Masud T, Dargent-Molina P, et al. A comprehensive fracture prevention strategy in older adults:The European Union Geriatric Medicine Society (EUGMS) statement. J Nutr Health Aging. 2016;20(6):647–652. doi: 10.1007/s12603-016-0741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goswami N. Falls and Fall-Prevention in Older Persons:Geriatrics Meets Spaceflight! Front Physiol. 2017;8:603. doi: 10.3389/fphys.2017.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grote V, Unger A, Böttcher E, et al. General and Disease-Specific Health Indicator Changes Associated with Inpatient Rehabilitation. J Am Med Dir Assoc. 2020;21(12):2017. doi: 10.1016/j.jamda.2020.05.034. e10-2017.e27. [DOI] [PubMed] [Google Scholar]
- 22.Goswami N, Blaber AP, Hinghofer-Szalkay H, Montani J-P. Orthostatic Intolerance in Older Persons:Etiology and Countermeasures. Front Physiol. 2017;8:803. doi: 10.3389/fphys.2017.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr. 2002;75(4):683–688. doi: 10.1093/ajcn/75.4.683. [DOI] [PubMed] [Google Scholar]
- 24.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia:revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Podsiadlo, D, Richardson S. The Timed Up and Go:A Test of Basic Functional Mobility for Frail Elderly Persons. J Am Geriatr Soc. 1991;39(2):142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 26.Soubra R, Chkeir A, Novella J-L. A Systematic Review of Thirty-One Assessment Tests to Evaluate Mobility in Older Adults. Biomed Res Int. 2019;2019:1–17. doi: 10.1155/2019/1354362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beauchet O, Fantino B, Allali G, Muir SW, Montero-Odasso M, Annweiler C. Timed up and go test and risk of falls in older adults:A systematic review. J Nutr Health Aging. 2011;15(10):933–938. doi: 10.1007/s12603-011-0062-0. [DOI] [PubMed] [Google Scholar]
- 28.Martinez B, Gomes I, Oliveira C, et al. Accuracy of the Timed Up and Go test for predicting sarcopenia in elderly hospitalized patients. Clinics. 2015;70(5):369–372. doi: 10.6061/clinics/2015(05)11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Šimunič B, Degens H, Rittweger J, Narici M, Mekjavić IB, Pišot R. Noninvasive Estimation of Myosin Heavy Chain Composition in Human Skeletal Muscle. Med Sci Sport Exerc. 2011;43(9):1619–1625. doi: 10.1249/MSS.0b013e31821522d0. [DOI] [PubMed] [Google Scholar]
- 30.Šimunič B, Degens H, Završnik J, Koren K, Volmut T, Pišot R. Tensiomyographic Assessment of Muscle Contractile Properties in 9- to 14-Year Old Children. Int J Sports Med. 2017;38(09):659–665. doi: 10.1055/s-0043-110679. [DOI] [PubMed] [Google Scholar]
- 31.Zubac D, Šimunič B. Skeletal Muscle Contraction Time and Tone Decrease After 8 Weeks of Plyometric Training. J Strength Cond Res. 2017;31(6):1610–1619. doi: 10.1519/JSC.0000000000001626. [DOI] [PubMed] [Google Scholar]
- 32.Zubac D, Paravlić A, Koren K, Felicita U, Šimunič B. Plyometric exercise improves jumping performance and skeletal muscle contractile properties in seniors. J Musculoskelet Neuronal Interact. 2019;19(1):38–49. [PMC free article] [PubMed] [Google Scholar]
- 33.Pišot R, Narici M V, Šimunič B, et al. Whole muscle contractile parameters and thickness loss during 35-day bed rest. Eur J Appl Physiol. 2008;104(2):409–414. doi: 10.1007/s00421-008-0698-6. [DOI] [PubMed] [Google Scholar]
- 34.Šimunič B, Koren K, Rittweger J, et al. Tensiomyography detects early hallmarks of bed-rest-induced atrophy before changes in muscle architecture. J Appl Physiol. 2019;126(4):815–822. doi: 10.1152/japplphysiol.00880.2018. [DOI] [PubMed] [Google Scholar]
- 35.Pišot R, Marusic U, Biolo G, et al. Greater loss in muscle mass and function but smaller metabolic alterations in older compared with younger men following 2 wk of bed rest and recovery. J Appl Physiol. 2016;120(8):922–929. doi: 10.1152/japplphysiol.00858.2015. [DOI] [PubMed] [Google Scholar]
- 36.Verney J, Schwartz C, Amiche S, Pereira B, Thivel D. Comparisons of a Multi-Frequency Bioelectrical Impedance Analysis to the Dual-Energy X-Ray Absorptiometry Scan in Healthy Young Adults Depending on their Physical Activity Level. J Hum Kinet. 2015;47(1):73–80. doi: 10.1515/hukin-2015-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Šimunič B. Two-dimensional spatial error distribution of key tensiomyographic parameters. J Biomech. 2019;92:92–97. doi: 10.1016/j.jbiomech.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 38.Ziegl A, Hayn D, Kastner P, et al. Quantitative falls risk assessment in elderly people:results from a clinical study with distance based timed up-and-go test recordings. Physiol Meas. 2020;41(11):115006. doi: 10.1088/1361-6579/abc352. [DOI] [PubMed] [Google Scholar]
- 39.Bloch ML, Jønsson LR, Kristensen MT. Introducing a Third Timed Up &Go Test Trial Improves Performances of Hospitalized and Community-Dwelling Older Individuals. J Geriatr Phys Ther. 2017;40(3):121–126. doi: 10.1519/JPT.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kojima G, Kendrick D, Skelton DA, Morris RW, Gawler S, Iliffe S. Frailty predicts short-term incidence of future falls among British community-dwelling older people:a prospective cohort study nested within a randomised controlled trial. BMC Geriatr. 2015;15(1):155. doi: 10.1186/s12877-015-0152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bischoff HA. Identifying a cut-off point for normal mobility:a comparison of the timed “up and go”test in community-dwelling and institutionalised elderly women. Age Ageing. 2003;32(3):315–320. doi: 10.1093/ageing/32.3.315. [DOI] [PubMed] [Google Scholar]
- 42.Maden-Wilkinson TM, McPhee JS, Jones DA, Degens H. Age-related loss of muscle mass, strength, and power and their association with mobility in recreationally-active older adults in the United Kingdom. J Aging Phys Act. 2015;23(3):352–360. doi: 10.1123/japa.2013-0219. [DOI] [PubMed] [Google Scholar]
- 43.Hamrick MW, McGee-Lawrence ME, Frechette DM. Fatty Infiltration of Skeletal Muscle:Mechanisms and Comparisons with Bone Marrow Adiposity. Front Endocrinol (Lausanne) 2016;7:69. doi: 10.3389/fendo.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gomes M, Figueiredo D, Teixeira L, et al. Physical inactivity among older adults across Europe based on the SHARE database. Age Ageing. 2017 doi: 10.1093/ageing/afw165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macgregor LJ, Ditroilo M, Smith IJ, Fairweather MM, Hunter AM. Reduced Radial Displacement of the Gastrocnemius Medialis Muscle After Electrically Elicited Fatigue. J Sport Rehabil. 2016;25(3):241–247. doi: 10.1123/jsr.2014-0325. [DOI] [PubMed] [Google Scholar]
- 46.Carrasco L, Sañudo B, de Hoyo M, Pradas F, Da Silva ME. Effectiveness of low-frequency vibration recovery method on blood lactate removal, muscle contractile properties and on time to exhaustion during cycling at VO2max power output. Eur J Appl Physiol. 2011;111(9):2271–2279. doi: 10.1007/s00421-011-1848-9. [DOI] [PubMed] [Google Scholar]
- 47.García-Manso JM, Rodríguez-Matoso D, Sarmiento S, et al. Effect of high-load and high-volume resistance exercise on the tensiomyographic twitch response of biceps brachii. J Electromyogr Kinesiol. 2012;22(4):612–619. doi: 10.1016/j.jelekin.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Wiewelhove T, Raeder C, Meyer T, Kellmann M, Pfeiffer M, Ferrauti A. Tauler P, editor. Markers for Routine Assessment of Fatigue and Recovery in Male and Female Team Sport Athletes during High-Intensity Interval Training. PLoS One. 2015;10(10):e0139801. doi: 10.1371/journal.pone.0139801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2018;9(1):3–19. doi: 10.1002/jcsm.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ochala J, Frontera WR, Dorer DJ, Hoecke J V, Krivickas LS. Single Skeletal Muscle Fiber Elastic and Contractile Characteristics in Young and Older Men. Journals Gerontol Ser A Biol Sci Med Sci. 2007;62(4):375–381. doi: 10.1093/gerona/62.4.375. [DOI] [PubMed] [Google Scholar]
- 51.Mutungi G, Ranatunga KW. The viscous, viscoelastic and elastic characteristics of resting fast and slow mammalian (rat) muscle fibres. J Physiol. 1996;496(3):827–836. doi: 10.1113/jphysiol.1996.sp021730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuesta-Vargas AI, Cano-Herrera C, Formosa D, Burkett B. Electromyographic responses during time get up and go test in water (wTUG) Springerplus. 2013;2(1):217. doi: 10.1186/2193-1801-2-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang SFT, Chen C-K, Hsu R, Chou S-W, Hong W-H, Lew HL. Vastus medialis obliquus and vastus lateralis activity in open and closed kinetic chain exercises in patients with patellofemoral pain syndrome:An electromyographic study. Arch Phys Med Rehabil. 2001;82(10):1441–1445. doi: 10.1053/apmr.2001.26252. [DOI] [PubMed] [Google Scholar]
- 54.Lee T, Park S, Yun S, Lee A, Lee Y, Yong M. Analysis of vastus lateralis and vastus medialis oblique muscle activation during squat exercise with and without a variety of tools in normal adults. J Phys Ther Sci. 2016;28(3):1071–1073. doi: 10.1589/jpts.28.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trozic I, Platzer D, Fazekas F, et al. Postural hemodynamic parameters in older persons have a seasonal dependency. Z Gerontol Geriatr. 2020;53(2):145–155. doi: 10.1007/s00391-019-01525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goswami N, Abulafia C, Vigo D, Moser M, Cornelissen G, Cardinali D. Falls Risk, Circadian Rhythms and Melatonin:Current Perspectives. Clin Interv Aging. 2020;15:2165–2174. doi: 10.2147/CIA.S283342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sachse C, Trozic I, Brix B, Roessler A, Goswami N. Sex differences in cardiovascular responses to orthostatic challenge in healthy older persons:A pilot study. Physiol Int. 2019;106(3):236–249. doi: 10.1556/2060.106.2019.16. [DOI] [PubMed] [Google Scholar]
- 58.Xu D, Tremblay MF, Verma AK, Tavakolian K, Goswami N, Blaber AP. Cardio-postural interactions and muscle-pump baroreflex are severely impacted by 60-day bedrest immobilization. Sci Rep. 2020;10(1):12042–12042. doi: 10.1038/s41598-020-68962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao Y, Arfat Y, Wang H, Goswami N. Muscle Atrophy Induced by Mechanical Unloading:Mechanisms and Potential Countermeasures. Front Physiol. 2018;9:235. doi: 10.3389/fphys.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Broadbent J, Reichmuth J, Trozic I, et al. Adrenomedullin and galanin responses to orthostasis in older persons. Eur J Clin Invest. 2017;47(11):812–818. doi: 10.1111/eci.12803. [DOI] [PubMed] [Google Scholar]
- 61.Goswami N, Kovacic V, Marucic U, et al. Effect of computerized cognitive training with virtual spatial navigation task during bed rest immobilization and recovery on vascular function:A pilot study. Clin Interv Aging. 2015;10:453–459. doi: 10.2147/CIA.S76028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White O, Babič J, Trenado C, Johannsen L, Goswami N. The Promise of Stochastic Resonance in Falls Prevention. Front Physiol. 2019;9:1865. doi: 10.3389/fphys.2018.01865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marusic U, Kavcic V, Pisot R, Goswami N. The Role of Enhanced Cognition to Counteract Detrimental Effects of Prolonged Bed Rest:Current Evidence and Perspectives. Front Physiol. 2019;9:1864. doi: 10.3389/fphys.2018.01864. [DOI] [PMC free article] [PubMed] [Google Scholar]