Abstract
Objectives:
To examine the effects of the regulation on IGF-1 by miR-26a on the serum of patients with osteoporosis (OP) and apoptosis and proliferation of chondrocytes of mice with OP.
Methods:
Totally 47 patients with OP treated in our hospital between July 2018 and November 2019 were selected as the research group, and 42 healthy individuals in physical examination over this period were selected as the control group. Serum was sampled from each participant in both groups, and miR-26a in the sampled serum was quantified and compared. In addition, chondrocytes were sampled from mice with OP. The changes of proliferation and apoptosis of the chondrocytes were analyzed via MTT and flow cytometry, and the levels of Caspase3, Caspase9, Bax, and Bcl-2 were quantified by western blot (WB) assay.
Results:
MiR-26a was expressed highly in the serum of patients with OP and chondrocytes of mice with OP, while IGF-1 was lowly expressed in them. According to the dual-luciferase reporter assay, there was a targeting correlation between miR-26a and IGF-1, and suppressing miR-26a significantly up-regulated the expression and protein level of IGF-1.
Conclusions:
MiR-26a can serve as a biological marker for the diagnosis of OP, and it can suppress the proliferation of chondrocytes and promote their apoptosis by regulating IGF-1.
Keywords: Apoptosis, MiR-26a, IGF-1, Mouse Cartilage, Proliferation
Introduction
Osteoporosis (OP) is one of the common clinical chronic metabolic bone diseases. Patients with OP are prone to fracture due to the damage of bone strength, reduction of bone mass and deterioration of microstructure[1,2]. Postmenopausal women are considered to face a high risk of developing OP because of the faster bone turnover due to the lack of estrogen and loss of net bone[3]. OP poses a colossal threat to the health worldwide, so it is particularly important to clarify the detailed molecular mechanism of OP and find a treatment strategy for it[4,5].
MicroRNAs (miRNAs) are single-stranded non-coding RNAs, which usually regulate gene expression by destabilizing mRNA or hindering translation of the mRNA[6]. Reportedly, they can be used to search for diagnostic biomarkers and new therapeutic targets for intervention of orthopedic diseases[7]. For example, miR-320a can promote the apoptosis of MC3T3-E1 cells in patients with OP by targeting MAP9 and affecting the PI3K/AKT signaling pathway and can also inhibit survival and differentiation of MC3T3-E1 cells[8]. In addition, miR-151a-3p contributes to the pathogenesis of postmenopausal OP, and can promote the progression of the disease by targeting SOCS5 and activating the transduction of JAK2/STAT3 signal[9]. Moreover, miR-26a is a crucial regulator of cell cycle and differentiation, which targetedly participates in the development of cells and plays a pivotal role in tumor development[10]. The molecular mechanism of the differentiation of human adipose tissue stem cells is essential in treating OP, and miR-26a can impact cell differentiation by targeting SMAD1[11]. Therefore, it can be inferred that miR-26a is also likely to play a role in the development of OP. Insulin-like growth factor-1 (IGF-1) is a primary growth factor involved in the synthesis and repair of cartilage matrix, which can promote the synthesis of type II collagen, proteoglycan, and other matrix components[12]. The cartilage is composed of chondrocytes[13]. Earlier studies have revealed that miR-101-3p, miR-211-5p, and miR-34a can all interfere with the biological behavior of chondrocytes by affecting its target genes, thus taking part in the pathogenesis of OP[14-16]. Therefore, we hypothesized that miR-26a could take part in the pathogenesis of OP by regulating IGF-1.
We have found potential targeting loci between miR-26a and IGF-1 based on an online website for target gene prediction, but there is little research on the regulation of miR-26a on IGF-1 in patients with OP at present. We hypothesized that miR-26a may affect the progression of OP in patients by regulating IGF-1, so we carried out the following studies for confirmation.
Materials and methods
Sample collection
Totally 47 patients with OP treated in the Rizhao People’s Hospital in China between July 2018 and November 2019 were included in a research group (res group), and 42 healthy individuals over this period were included in a control group (con group). Patients meeting the diagnostic criteria of OP[17] and those with complete general data were included in the study. Patients who dropped out of the study midway, patients with comorbid organ diseases in liver, kidney, heart, or other organs, patients accompanied by malignant tumors, and those who had taken drugs that may affect indexes adopted in this study within the last six months were excluded. The study was approved by the Ethics Committee of the orthopedics department of People’s Hospital of Rizhao, and written informed consent was obtained from all participants.
Animal models
Female C57BL/6J mice (cs-005, Changsheng Biotechnology Co., Ltd., Liaoning, China) were raised in the pathogen-free environment at room temperature under a 12-hour light/dark cycle for 7 days for later analysis, during which all mice were allowed to eat and drink freely. After 7 days, the mice were assigned to an ovariectomized group and a sham operation group (each n=20). Operative methods: For mice in the ovariectomized group, each mouse was anesthetized with 30 mg/kg pentobarbital sodium through intraperitoneal injection, and after the abdominal position of the mouse was fixed, the mouse was regularly treated to make skin preparation on the middle and lower part of the back, and the skin part was disinfected with 3% iodophor. Under aseptic conditions, a longitudinal incision of about 0.5 cm was made about 1cm below the costal margin at the junction of about 1 cm on both sides of the spine, respectively. The skin was separated, and the dorsal muscle was cut to expose the ovary. The fat around the ovary was bluntly dissected, and the ovary was entirely removed after the fallopian tube and blood vessels were ligated separately. The uterus was retained in the abdominal cavity. The abdominal cavity was cut open and sutured, and the incision was then closed. The other ovary of the mouse was removed using the same method. For mice in the sham operation group, their ovaries were not removed, but they were treated using the same method used for the ovariectomized group except for ovary removal. Three days after the operation, 200,000 IU/kg penicillin was given to each mouse to prevent infection.
Establishment of mice models of osteoporotic fracture18
Six weeks after OP mice were modeled, 5 mice were taken from the ovariectomized group and the sham operation group, respectively, and HE staining was carried out to the distal femur of each mouse to verify the modeling results of OP.
Cell culturing and transfection
All soft tissues were removed from the right femur and incubated in DMEM (RY0146, JISSKANG Biotechnology Co., Ltd., Qingdao, China) supplemented with 10% fetal bovine serum (FBS; MP20001, Yuanye Biotechnology Co., Ltd., Shanghai, China) under 5%CO2 at 37oC, and cells in logarithmic growth phase were used for later analysis. Cell transfection: MiR-26a and IGF-1 overexpression and inhibition plasmids were constructed with pcDNA 3.1 plasmids as vectors and transfected into cells using a Lipofectamine™ 2000 kit (11668027, Gaochuang Chemical Technology Co., Ltd., Shanghai, China), separately, and the cells were continuously cultured.
qRT-PCR assay
MiR-26a in harvested serum and cells was quantified using a qRT-PCR assay as follows: Total RNA of serum and cells was acquired by a Trizol kit (5003050, Mingjing Biology Co., Ltd., Shanghai, China) according to the kit instructions, and its purity, concentration, and integrity were detected by an ultraviolet spectrophotometer and agarose gel electrophoresis. Total RNA (2 µg) was reversely transcribed to cDNA by a reverse transcription kit (BPI01030, Protein Innovation Co., Ltd., Beijing, China). Subsequently, amplification was conducted by a PrimeScript RT Master Mix kit (HRR036A-1, Yihui Biological Technology Co. Ltd., Shanghai, China) in an amplification system containing 20 µL total reaction volume supplemented with 10 µL SYBR qPCR Mix, 10 µL 2X TransScript® Tip Green qPCR SuperMix, 0.4 µL upstream and downstream primers, 0.4 µL Passive Reference Dye (50X), and RNase-free added for volume adjustment. PCR was conducted through 95oC for 60 s, followed by 40 cycles of 95oC for 30 s, and 60oC for 40 s. MiR-26a expression was calculated based on the 2-∆∆ct method[19].
Western blot (WB) assay
Cells to be determined were lysed with Radio Immunoprecipitation Assay (RIPA) buffer (121200, Chreagen Biotechnology Co., Ltd., Beijing, China), and protein concentration was detected via a bicinchoninic acid (BCA) assay kit (YX-C-C202, Yipu Biotechnology Co., Ltd., Wuhan, China), and regulated to 4 µg/µL. Subsequently, the protein was separated by SDS-PAGE (V100), and transferred to a PVDF membrane. The membrane was washed by TBST for 5 min, followed by 2-h blocking with 5% skim milk. Afterward, the membrane was added with RUNX2, ALP, OCN, and β-catenin primary antibodies (1247-1, Taize Jiaye Science and Technology Development Co., Ltd., Beijing, China) at a dilution rate of 1: 1000 for each item, incubated for one night at 4oC, followed by 3 times of washing with TBST. Then the membrane was added with sheep anti-mouse secondary antibody (LD-BJ-101891, Lvdu Bio-science& Technology Co., Ltd., Shangdong, China) at a dilution rate of 1:4000, incubated at 37oC for 2 h, and then washed with TBST three times. Finally, the membrane was developed in the dark and analyzed using the electrochemiluminescence method. The expression of proteins in every group was calculated using Quantity One.
Cell proliferation assay
Cell proliferation was detected through the MTT method as follows: Cells transfected for 24 hours were harvested and transferred to a 96-well plate at 4*106/well, and each well was added with CCK-8 solution (10 µL) and basic DMEM (90 µL) at 24, 48, 72, and 96 h after culturing, separately. After the addition of the solution each time, the plate was incubated at 37oC for 2 hours. Finally, the optical density of every well at 570 nm was determined by a microplate reader.
Cell apoptosis assay
The harvested transfected cells were trypsinized by 0.25% trypsin and then prepared into 1*106cells /mL suspension. AnnexinV-FITC/PI (10 µL, BJ-10153, Bangjing Industry Co., Ltd., Shanghai, China) was put into the suspension in order. Then the suspension was incubated at indoor temperature in the dark for 5 min, and finally measured by a flow cytometer, and the cell apoptosis was evaluated.
Dual-luciferase reporter (DLR) assay
The possible target gene of miR-26a was forecasted by the website miRDB (http://www.mirdb.org/). IGF-1-3’UTR wild type (Wt) and IGF-1-3’UTR mutant (Mut) were constructed by a Lipofectamine™ 2000 kit, respectively, and they were transfected into chondrocytes (American Type Culture Collection (ATCC)) with miR-26a-inhibitor and miR-NC. Afterward, the luciferase activity changes of transfected cells were determined by a DLR gene determination kit (Solarbio, CA, China).
Statistical analysis
The data were processed statistically and visualized into figures via the GraphPad 6. Inter-group comparison was conducted by the independent-samples T-test, and multi-group comparison by the one-way ANOVA and expressed as F. Post hoc pairwise comparison was conducted via the LSD-t-test, and expression comparison in different time points was conducted via the variance of repeated measures and expressed as F. Post-test was conducted by Bonferroni. In addition, receiver operating characteristic (ROC) curves of serum miR-26a and IGF-1 in diagnosing OP were drawn, and Pearson’s correlation analysis was conducted for correlation analysis between miR-26a and IGF-1. P<0.05 implies a statistically significant difference.
Results
MiR-26a is significantly up-regulated in the serum of patients with OP
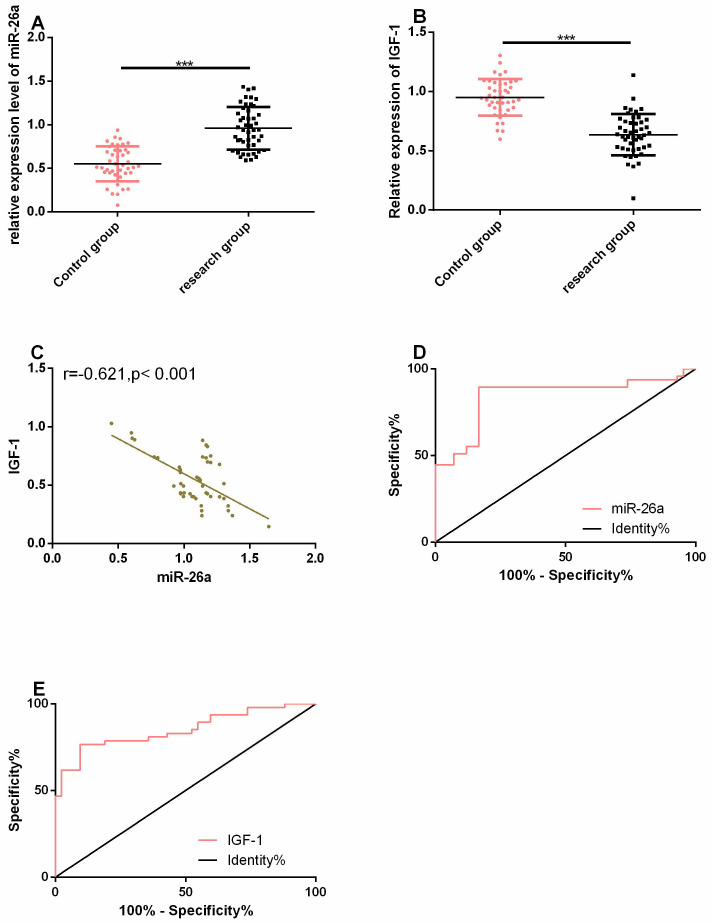
MiR-26a was considerably up-regulated in the serum of patients with OP, while IGF-1 was considerably down-regulated in it (both P<0.05). Pearson correlation coefficient was adopted for evaluation of the relation between miR-26a and IGF-1. It was found that miR-26a had a strong negative correlation with IGF-1 (r=-0.621, P<0.05). Additionally, ROC curves of miR-26a and IGF-1 in diagnosing OP were drawn, and it was found that the areas under the curves (AUCs) of miR-26a and IGF-1 in diagnosing OP were 0.841 and 0.851, respectively (Figure 1).
Figure 1.
MiR-26a was significantly up-regulated in the serum of patients with osteoporosis. A. MiR-26a was highly expressed in the serum of patients with OP. B. IGF-1 was lowly expressed in the serum of patients with OP. C. The expression of miR-26a was negatively correlated with that of IGF-1. D-E. ROC curves of miR-26a and IGF-1 in diagnosing OP. *** indicates that in terms of comparison of two items, P<0.001.
MiR-26a is considerably up-regulated in the chondrocytes of mice with OP
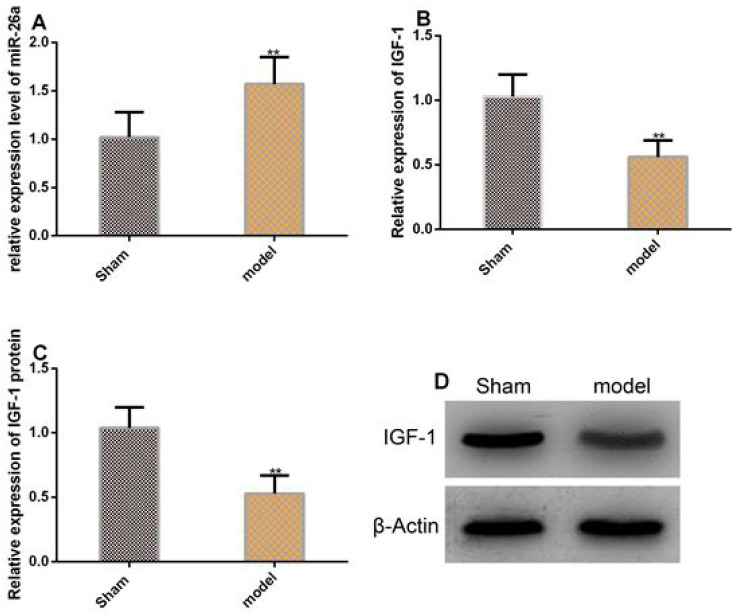
MiR-26a was significantly up-regulated in chondrocytes of OP mice, while the expression and protein level of IGF-1 was considerably down-regulated in them (both P<0.05, Figure 2).
Figure 2.
MiR-26a was significantly up-regulated in the chondrocytes of mice with OP. A. The expression of miR-26a was significantly up-regulated in the chondrocytes of mice with OP. B-C. The expression and protein level of IGF-1 were significantly down-regulated in the chondrocytes of mice with OP. D. Protein profiling. ** in terms of inter-group comparison, P<0.001.
MiR-26a can inhibit the proliferation of chondrocytes and promote apoptosis of them
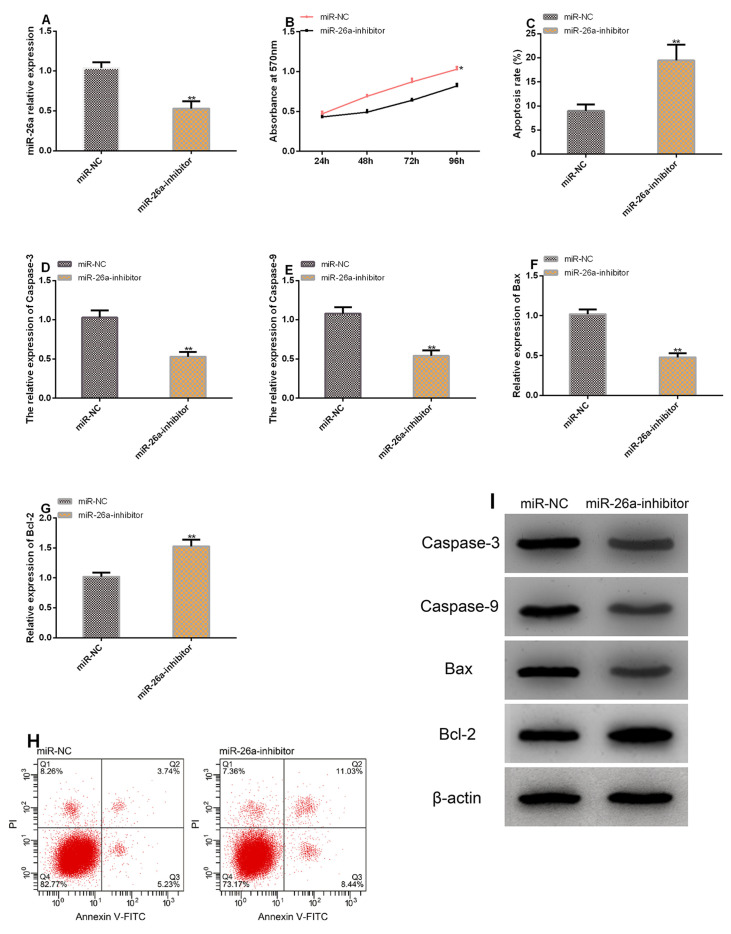
Transfecting miR-26a-inhibitor successfully inhibited miR-26a in chondrocytes. According to the MTT assay, cell proliferation was suppressed after transfection of miR-26a-inhibitor. According to the Flow cytometry, after transfection of miR-26a-inhibitor, cell apoptosis rate increased, and the levels of Caspase-3, Caspase-9, and Bax increased significantly, while the level of Bcl-2 decreased greatly (all P<0.05) (Figure 3).
Figure 3.
MiR-26a can inhibit proliferation of chondrocytes and promote the apoptosis of them. A. Transfection with miR-26a-inhibitor down-regulated miR-26a in chondrocytes. B. Cell proliferation after transfection with miR-26a-inhibitor. C. Cell apoptosis after transfection with miR-26a-inhibitor. D-G.Transfection of miR-26a-inhibitor ameliorated the levels of apoptosis-related factors. H. Cell apoptosis profiling. I. Protein profiling. ** in terms of inter-group comparison, P<0.001.
There is a targeting control correlation between miR-26a and IGF-1
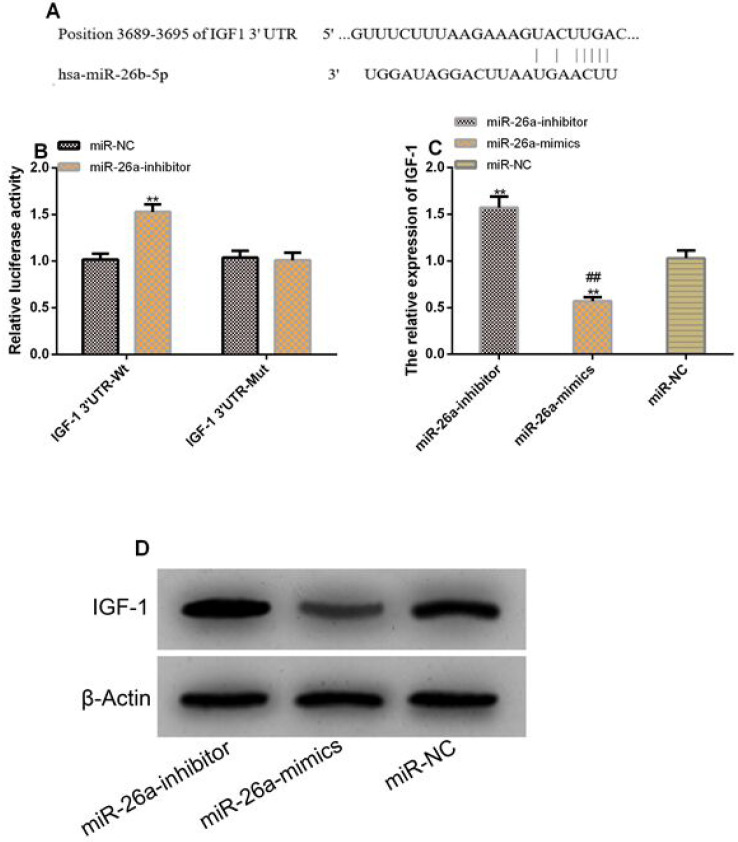
We discovered targeted binding loci between IGF-1 and miR-26a by Targetscan7.2. The DLR assay results showed that down-regulating miR-26a significantly increased the luciferase activity of IGF-1 3’UTR-Wt (P<0.05) but exerted no influence on that of IGF-1 3’UTR-Mut (P>0.05). The WB assay showed transfection with inhibitor significantly elevated the protein level of IGF-1 in cells (P<0.05) (Figure 4).
Figure 4.
There was a targeting control relationship between miR-26a and IGF-1. A. There were targeted binding loci between miR-26a and IGF-1. B. Relative luciferase activity - DLR assay. C. Protein level of IGF-1 in transfected cells. D. Protein profiling. ** indicates P<0.01 vs. NC; ## indicates P<0.01 vs. inhibitor.
Increasing IGF-1 can weaken the influence of miR-26a on the proliferation and apoptosis of chondrocytes
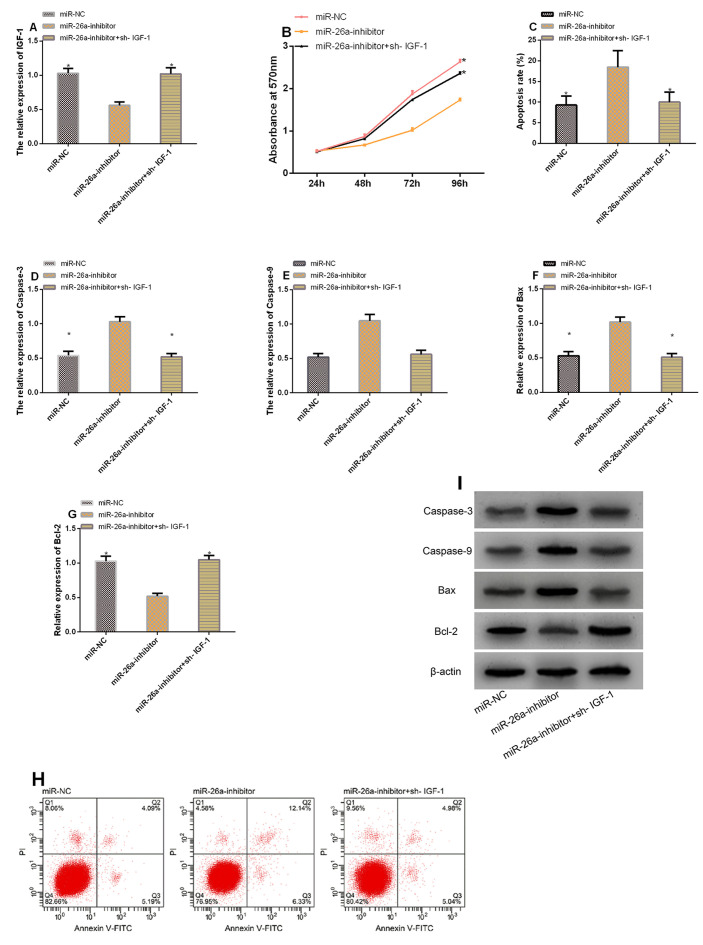
To explore whether miR-26a can impact chondrocytes’ biological behaviors by regulating IGF-1, we transfected chondrocytes with miR-26a-inhibitor and IGF-1 meantime for analyzing whether such treatment can reverse or weaken the changes of chondrocytes in proliferation, apoptosis, as well as differentiation due to transfection of miR-26a-inhibitor. The results showed that in contrast to cells transfected with miR-26a-inhibitor, those transfected with miR-26a-inhibitor+sh- IGF-1 showed increased IGF-1 (P<0.05), intensified proliferation (P<0.05), decreased apoptosis rate (P<0.05), significantly down-regulated Caspase-3, Caspase-9, as well as Bax (all P<0.05), and significantly up-regulated Bcl-2 (P<0.05) (Figure 5).
Figure 5.
Increasing IGF-1 can weaken the effect of miR-26a on the proliferation and apoptosis of chondrocytes. A. Expression of IGF-1 in each group. B. Comparison of cell proliferation between different groups. C. Comparison of apoptosis rate between different groups. D-G. Comparison of apoptosis-related factors between different groups. H. Cell apoptosis profiling. I. Protein profiling. * indicates P<0.001 vs. the miR-26a-inhibitor group.
Discussion
Osteoporosis is a disease characterized by a decrease in bone mass and a deterioration in the bone architecture, which leads to an enhanced fragility and, therefore, to an increased risk of fracture[20]. Due to the rapid aging of the world’s population, the prevalence rate of OP is continuously increasing, and the incidence and mortality of osteoporotic fractures are also increasing[21,22]. Thus, it is of great value to study the pathological process of OP. Based on studies, miR-26a and IGF-1 potentially take part in the regulation of the OP. For instance, a study by Anastasilakis AD et al.[23] has shown that the dynamic up-regulation of miR-26a in patients with OP is the most significant.
Moreover, IGF-1, as a crucial anabolic hormone, can regulate bone homeostasis and maintain the bone structure of individuals throughout adult life. The decrease of serum IGF-1 level is one of the important causes of OP fracture in postmenopausal women[24,25]. In some studies, IGF-1 gene is considered as a candidate gene for osteoporotic fracture risk[26,27]. However, through research, we found that targeted regulation of miR-26a on IGF-1 assists to regulate the disease state of patients with OP.
In our study, miR-26a was significantly up-regulated in the serum of patients with OP, while IGF-1 was significantly down-regulated, which indicated that miR-26a and IGF-1 may play important roles in patients with OP. Then, we explored the relationship between the expression of miR-26a and IGF-1, and concluded that the expression of these two was negatively related, suggesting that the two may play an antagonistic role in the procession of OP. We further analyzed the potential of miR-26a and IGF-1 in diagnosing OP. We found that the AUCs of them in diagnosing OP were 0.841 and 0.851, respectively, which implies that the two can serve as high sensitivity diagnostic indicators for OP. Also, we further confirmed the above results through in vitro and in vivo experiments. We developed mouse models of OP, and found that the expression of miR-26a and IGF-1 in the chondrocytes of mice were in accordance with the previous results: miR-26a was highly expressed, while IGF-1 was lowly expressed. A previous study has shown that weakened proliferation of cartilages and enhanced apoptosis of them are crucial reasons for the development of OP[28]. In our study, we analyzed the effects of miR-26a on OP by evaluating the changes of chondrocyte cell biological behaviors, including proliferation and apoptosis after inhibition of miR-26a. The MTT assay and flow cytometry revealed that in chondrocytes, inhibiting miR-26a strongly inhibited cell proliferation, increased cell apoptosis rate and led to high levels of apoptosis-related factors such as Caspase-3, Caspase-9, and Bax except for Bcl-2, which indicates that inhibiting the expression of miR-26a in chondrocytes can effectively improve cell survival.
MiR can take part in biological processes by regulating its downstream target genes[29]. By understanding the mechanism through which miR-26a affects the biological behaviors of chondrocytes, we predicted the possible target genes of miR-26a via the biological prediction website miRDB, and acquired that IGF-1 and miR-26a had targeted binding loci. IGF-1 is an effective growth hormone-dependent serum factor, which can stimulate sulfate incorporation through cartilage in vitro and can inhibit the degradation of chondrocytes and prevent death of them by stimulating chondrocytes to produce matrix protein[30]. In our study, we analyzed the correlation between IGF-1 and miR-26a. We found that knock-down of miR-26a in chondrocytes up-regulated IGF-1 in them, and miR-26a-inhibitor inhibited the fluorescent activity of IGF-1-3’UTR Wt, but did not strongly affect that of IGF-1-3’UTR Mut, which implied that the effect of miR-26a on the proliferation and apoptosis of chondrocytes may be linked to its negative regulation on IGF-1. Afterward, we inhibited miR-26a in chondrocytes and up-regulated IGF-1 in them, separately, and found that up-regulating IGF-1 could reverse the impact of miR-26a on the biological behaviors of chondrocytes, which implied that miR-26a could affect the proliferation and apoptosis of chondrocytes through regulating IGF-1.
Further studies on larger sample size need to be carried out in order to validate our results. Also, it would be interesting to elucidate the contribution of miR-26a and IGF-1 during fracture repair and to explore the impact of miR-26a and other downstream target genes on the biological behaviors of chondrocytes.
To conclude, miR-26a can be served as a diagnostic biological marker for OP, and it can inhibit the proliferation of chondrocytes and induce their apoptosis by regulating IGF-1.
Authors’ contributions
FY designed the study and drafted the manuscript. HC and PH were responsible for the collection and analysis of the experimental data. ZW and XG revised the manuscript. All authors read and approved the final manuscript.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Yan G, Huang Y, Cao H, Wu J, Jiang N, Cao X. Association of breastfeeding and postmenopausal osteoporosis in Chinese women:a community-based retrospective study. BMC Women's Health. 2019;19:110. doi: 10.1186/s12905-019-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao H, Li X, Zhang D, Chen H, Chao Y, Wu K, Dong X, Su J. Integrative Bone Metabolomics-Lipidomics Strategy for Pathological Mechanism of Postmenopausal Osteoporosis Mouse Model. Sci Rep. 2018;8:16456. doi: 10.1038/s41598-018-34574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang YR, Li CW, Wang JH, Huang XS, Yuan YF, Hu J, Liu K, Liang BC, Liu Z, Shi XL. Ubiquitylomes Analysis of the Whole blood in Postmenopausal Osteoporosis Patients and healthy Postmenopausal Women. Orthop Surg. 2019;11:1187–1200. doi: 10.1111/os.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu XD, Han WX, Liu YX. Suppression of miR-451a accelerates osteogenic differentiation and inhibits bone loss via Bmp6 signaling during osteoporosis. Biomed Pharmacother. 2019;120:109378. doi: 10.1016/j.biopha.2019.109378. [DOI] [PubMed] [Google Scholar]
- 5.Kharroubi A, Saba E, Smoom R, Bader K, Darwish H. Serum 25-hydroxyvitamin D and bone turnover markers in Palestinian postmenopausal osteoporosis and normal women. Arch Osteoporos. 2017;12:13. doi: 10.1007/s11657-017-0306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Z, Moore BT, Wang Y, Peng XH, Lappe JM, Recker RR, Xiao P. MiR-422a as a potential cellular microRNA biomarker for postmenopausal osteoporosis. PLoS One. 2014;9:e97098. doi: 10.1371/journal.pone.0097098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C, Ren S, Zhao S, Wang Y. LncRNA MALAT1/MiR-145 Adjusts IL-1beta-Induced Chondrocytes Viability and Cartilage Matrix Degradation by Regulating ADAMTS5 in Human Osteoarthritis. Yonsei Med J. 2019;60:1081–1092. doi: 10.3349/ymj.2019.60.11.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong Y, Nie ZK, Li F, Guo HM, Yang XL, Ding SF. MiR-320a was highly expressed in postmenopausal osteoporosis and acts as a negative regulator in MC3T3E1 cells by reducing MAP9 and inhibiting PI3K/AKT signaling pathway. Exp Mol Pathol. 2019;110:104282. doi: 10.1016/j.yexmp.2019.104282. [DOI] [PubMed] [Google Scholar]
- 9.Fu Y, Xu Y, Chen S, Ouyang Y, Sun G. MiR-151a-3p Promotes Postmenopausal Osteoporosis by Targeting SOCS5 and Activating JAK2/STAT3 Signaling. Rejuvenation Res. 2019 doi: 10.1089/rej.2019.2239. [DOI] [PubMed] [Google Scholar]
- 10.Jiang F, Zong Y, Ma X, Jiang C, Shan H, Lin Y, Xia W, Yin F, Wang N, Zhou L, Zhou Z, Yu X. miR-26a Attenuated Bone-Specific Insulin Resistance and Bone Quality in Diabetic Mice. Mol Ther Nucleic Acids. 2020;20:459–467. doi: 10.1016/j.omtn.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luzi E, Marini F, Sala SC, Tognarini I, Galli G, Brandi ML. Osteogenic differentiation of human adipose tissue-derived stem cells is modulated by the miR-26a targeting of the SMAD1 transcription factor. J Bone Miner Res. 2008;23:287–295. doi: 10.1359/jbmr.071011. [DOI] [PubMed] [Google Scholar]
- 12.Nazli SA, Loeser RF, Chubinskaya S, Willey JS, Yammani RR. High fat-diet and saturated fatty acid palmitate inhibits IGF-1 function in chondrocytes. Osteoarthritis Cartilage. 2017;25:1516–1521. doi: 10.1016/j.joca.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurer B, Cabuk S, Karakus O, Yilmaz N, Yilmaz C. In vivo cartilage tissue engineering. J Orthop Surg Res. 2018;13:107. doi: 10.1186/s13018-018-0823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang X, Zhang L, Cheng D, Liang X. [MiR-101-3p alleviates IL-1beta-induced chondrocyte injury by targeting stanniocalcin 1] Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2019;44:976–984. doi: 10.11817/j.issn.1672-7347.2019.180374. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Luo J. miR-211-5p contributes to chondrocyte differentiation by suppressing Fibulin-4 expression to play a role in osteoarthritis. J Biochem. 2019;166:495–502. doi: 10.1093/jb/mvz065. [DOI] [PubMed] [Google Scholar]
- 16.Yang B, Ni J, Long H, Huang J, Yang C, Huang X. IL-1beta-induced miR-34a up-regulation inhibits Cyr61 to modulate osteoarthritis chondrocyte proliferation through ADAMTS-4. J Cell Biochem. 2018;119:7959–7970. doi: 10.1002/jcb.26600. [DOI] [PubMed] [Google Scholar]
- 17.Ruzickova O, Killinger Z, Kasalicky P, Hamilton L, Tyl R, Tomkova S, Kalouche-Khalil L. Real-world Management of Women with Postmenopausal Osteoporosis Treated with Denosumab:A Prospective Observational Study in the Czech Republic and Slovakia. Adv Ther. 2018;35:1713–1728. doi: 10.1007/s12325-018-0779-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Z, Li Z, Zhang W, Yang Y, Han B, Liu W, Peng Y. Dietary Natural N-Acetyl-d-Glucosamine Prevents Bone Loss in Ovariectomized Rat Model of Postmenopausal Osteoporosis. Molecules. 2018;23:2302. doi: 10.3390/molecules23092302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.Akkawi I, Zmerly H. Osteoporosis:Current Concepts. Joints. 2018;6:122–127. doi: 10.1055/s-0038-1660790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramaniam S, Ima-Nirwana S, Chin KY. Performance of Osteoporosis Self-Assessment Tool (OST) in Predicting Osteoporosis-A Review. Int J Environ Res Public Health. 2018;15:1445. doi: 10.3390/ijerph15071445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29:441–464. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anastasilakis AD, Makras P, Pikilidou M, Tournis S, Makris K, Bisbinas I, Tsave O, Yovos JG, Yavropoulou MP. Changes of Circulating MicroRNAs in Response to Treatment With Teriparatide or Denosumab in Postmenopausal Osteoporosis. J Clin Endocrinol Metab. 2018;103:1206–1213. doi: 10.1210/jc.2017-02406. [DOI] [PubMed] [Google Scholar]
- 24.Gao ST, Lv ZT, Zhou CK, Mao C, Sheng WB. Association between IGF-1 polymorphisms and risk of osteoporosis in Chinese population:a meta-analysis. BMC Musculoskelet Disord. 2018;19:141. doi: 10.1186/s12891-018-2066-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yun-Kai L, Hui W, Xin-Wei Z, Liang G, Jin-Liang Z. The polymorphism of Insulin-like growth factor-I (IGF-I) is related to osteoporosis and bone mineral density in postmenopausal population. Pak J Med Sci. 2014;30:131–135. doi: 10.12669/pjms.301.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim SY, Zalilah MS, Chin YS, Ramachandran V, Chan YM. Dietary Acid Load, IGF-1 Single Nucleotide Polymorphism and Bone Resorption among Postmenopausal Chinese Women. Nutrients. 2018;10:915. doi: 10.3390/nu10070915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li F, Xing WH, Yang XJ, Jiang HY, Xia H. Influence of polymorphisms in insulin-like growth factor-1 on the risk of osteoporosis in a Chinese postmenopausal female population. Int J Clin Exp Pathol. 2015;8:5727–5732. [PMC free article] [PubMed] [Google Scholar]
- 28.Chen GQ, Wang S, Hu SY. Osteoporosis increases chondrocyte proliferation without a change in apoptosis during fracture healing in an ovariectomized rat model. Mol Med Rep. 2012;5:202–206. doi: 10.3892/mmr.2011.596. [DOI] [PubMed] [Google Scholar]
- 29.Zhu L, Gao J, Huang K, Luo Y, Zhang B, Xu W. miR-34a screened by miRNA profiling negatively regulates Wnt/beta-catenin signaling pathway in Aflatoxin B1 induced hepatotoxicity. Sci Rep. 2015;5:16732. doi: 10.1038/srep16732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Ceuninck F, Caliez A, Dassencourt L, Anract P, Renard P. Pharmacological disruption of insulin-like growth factor 1 binding to IGF-binding proteins restores anabolic responses in human osteoarthritic chondrocytes. Arthritis Res Ther. 2004;6:R393–403. doi: 10.1186/ar1201. [DOI] [PMC free article] [PubMed] [Google Scholar]