Abstract
Objectives:
To explore the expression and correlation of Omentin-1 and miR-502-3p in serum of patients with osteoporotic fracture (OPF).
Methods:
Sixty OPF patients diagnosed and treated in our hospital from June 2018 to December 2019 were included in group A. Fifty-six osteoporosis patients without fractures were included in group B. Omentin-1 and miR-502-3p levels were detected by enzyme-linked immunosorbent assay (ELISA) and real-time quantitative PCR (qRT-PCR). Their predictive value for diagnostic efficiency was assessed by ROC curve. Spearman’s rank correlation test was used for correlation analysis. The risk factors related to the prognosis of OPF were analyzed by Logistic univariate and multivariate analysis.
Results:
The expression of Omentin-1 and miR-502-3p in group A was markedly lower than in group B (P<0.001). Spearman correlation analysis showed that in OPF, there was a negative correlation between serum Omentin-1 and TNF-α (r=0.8579, P<0.001), a negative correlation between serum miR-502-3p and TNF-α (r= 0.8653, P<0.001), and a positive correlation between serum Omentin-1 and miR-502-3p (r= 0.8764, P<0.001).
Conclusions:
Omentin-1 and miR-502-3p were down-regulated in serum of patients with OPF, both of which could be used as potential biomarkers for the diagnosis and disease evaluation of OPF.
Keywords: miR-502-3p, Omentin-1, Osteoporotic Fracture, Prognosis
Introduction
Osteoporotic fractures (OPF) are a result of osteoporosis, a condition in which the bones become more fragile due to bone deterioration or low bone mass[1]. In recent years, osteoporotic fracture incidence has been increased, and there is a global trend towards younger ages[2,3]. The causes of OPF are complicated. At present, the complete cure of OPF is not clear, and the prognosis of patients with OPF is disappointed. The primary focus of current clinical orthopedic research is how to effectively treat OPF and restore patients’ normal functions while alleviating their pain[4-7]. Therefore, the research for more sensitive bio-diagnostic factors is a hotspot in osteoporosis research[8].
Inflammatory cytokines have been reported to enhance the progression of the pathogenesis of osteoporosis[9,10]. Evidence has shown that, during OPF, increased proinflammatory cytokines significantly aggravate bone loss[11], which inhibits osteoblast maturation and function by promoting bone resorption and impairing bone formation[12]. Omentin-1 is a newly discovered adipocytokine with anti-inflammatory effect, affecting osteoblast differentiation and osteoclast formation by inhibiting TNF-α and IL-8 proinflammatory cytokines[13,14]. Acting as an anti-inflammatory and anti-insulin resistance fat factor, Omentin-1 is negatively correlated with oxidative stress, and plays an essential role in tumorigenesis, cell differentiation, and accelerating apoptosis of cancer cells[14]. Currently, microRNAs (miRNAs) have attracted extensive attention from researchers in various fields as they are involved in multicellular signal regulation. Previous studies have reported that changes in circulating miRNAs are associated with OPF[15]. The potential signal pathways related to candidate miRNAs were searched through online tools, in which miR-502 family has been proved to play an important role in osteosarcoma. MiR-502-5p is up-regulated in osteosarcoma tissue and serum and is a novel potential diagnostic biomarker for osteosarcoma[16]. At present, there are only few studies on the clinicopathological characteristics of Omentin-1 and miR-502-3p in patients with OPF and the specific relationship remains unclear. Therefore, this study aims to explore the expression and clinical value of Omentin-1 and miR-502-3p in patients with OPF.
Materials and methods
General information
From June 2018 to December 2019, 60 patients with OPF diagnosed and treated in our hospital were included in group A, and 56 osteoporosis patients without fractures were included in group B. All the included participants were diagnosed as OPF by our hospital, and the clinical diagnosis was in accordance with the World Health Organization (WHO) criteria for OPF[17]. Patients with: infectious diseases; a history of blood system diseases such as hemolytic anemia and idiopathic thrombocytopenic purpura; bone tumor, kidney tumor, primary liver tumor, or other primary malignant tumors; complications that may affect the expression of Omentin-1 and miR-502-3p were excluded. None of the drugs used in the treatment of the participants in our hospital had any effect on the levels of Omentin-1 and miR-502-3p. Informed consent was obtained from all individual participants included in the study. This study was approved by the Medical Ethics Committee of Tai’an City Center Hospital (Approval No.T201766534). There were no significant differences in age, gender, and body mass index (BMI) between the two groups of patients (P>0.05), indicating comparability (Table 1).
Table 1.
Primer sequences of Omentin-1 and internal controls.
| Gene | Upstream primer | Downstream primer |
|---|---|---|
| miR-502-3p | 5’-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGT GAATCCT-3’ | 5’-ACACTCCAGCTGGGAATGCACCTGGGCAAGGA-3’ |
| U6 | 5’-GCGCGTCGTGAAGCGTTC-3’ | 5’-GTGCAGGGTCCGAGGT-3’ |
Main reagents, instruments, and detection methods
Main reagents and instruments
Omentin-1 enzyme-linked immunosorbent assay (ELISA) kit (Jianglai Bio/Import, Shanghai, China, JL19866-48T); Trizol reagent (Applide Invitrogen, USA), real-time quantitative PCR (qRT-PCR) kit, and minScript reverse transcription kit (TaKaRa, Dalian, China), HBS-1096A microplate reader (Deutsche Tie Experimental Equipment Co., Ltd., Nanjing, China), real-time quantitative PCR instrument (BioRad, USA). The primer sequence of miR-502-3p, the internal reference U6, and miRNA negative control were all synthesized and designed by Shanghai GenePharma Co., Ltd. (Table 1).
Detection of Omentin-1 and miR-502-3p
Serum Omentin-1 and TNF-α were determined by double-antibody sandwich ELISA, and the test was carried out with reference to the manual of human ELISA detection kit. The OD value of each well was detected by a microplate reader at a wavelength of 450 nm, and the concentrations of Omentin-1 and TNF-α were calculated.
The serum expression of miR-502-3p of patients in the two groups was detected by qRT-PCR. According to the operation instructions of Trizol reagent, the total RNA was extracted and dissolved in 20 µL of DEPC, and then reversely transcribed using a reverse transcription kit. The reaction system was as follows: M-MLV: 1 µl, Olig (d T): 1 µl, RNase inhibitor: 0.5 µl, d NTPs: 1 µl, and RNAse free water was added to a total volume of 15 µl. After that, it was incubated for 60 min at 38°C before taking 1 µl of it and treating it at 85°C for 5s. The synthesized DNA was used as a template for qRT-PCR amplification, with the reaction system prepared as follows: 10×PCR buffer: 2.5 µl, d NTPs: 1 µl, upstream and downstream primers: 1 µl each, Taq DNA Polymerase: 0.25 µl, and dd H2O was added to make up for 25 µl. Reaction conditions: pre-denaturation 95°C, 15 min; denaturation 95°C, 15 s; annealing 58°C, 30 s, totaling 35 cycles; extension was performed at 72°C for 15 min at last. Three duplicate wells were set for each sample, and the experiment was repeated three times, with U6 as the internal reference for miR-502-3p. After the reaction, the amplification curve and fusion curve of real-time PCR were confirmed, and the relative quantities of target genes were calculated according to the result parameters. The relative quantification of target genes was worked out by 2-ΔCt.
Statistical methods
Statistical analysis was performed using SPSS 17.0 (Bizinsight Information Technology Co., Ltd., Beijing, China). The counting data between the two groups were tested by X[2] test. The measurement data are expressed as (x±s), and the inter-group comparison was conducted by the independent sample t-test. Receiver operating characteristic (ROC) curve was employed to evaluate the diagnostic value of serum Omentin-1 and miR-502-3p in OPF. A logistic regression model was established with Omentin-1 and miR-502-3p as independent variables. When P<0.05, the difference was statistically significant.
Results
General data of patients in the two groups
The general data of patients in the two groups were compared, and the differences in age, gender, smoking status, drinking status and medical history were not statistically significant (P>0.05) (Table 2).
Table 2.
General clinical data of patients in the two groups.
| Group | Group A (n=60) | Group B (n=56) | t/X2 | P |
|---|---|---|---|---|
| Age (years old) | 68.00±1.00 | 68.10±1.00 | ||
| Gender | 0.148 | 0.701 | ||
| Male | 30 (50.00) | 30 (53.57) | ||
| Female | 30 (50.00) | 26 (46.43) | ||
| BMI (kg/m2) | 18.20±1.50 | 19.00±1.50 | ||
| Smoking | 0.112 | 0.738 | ||
| Yes | 34 (56.67) | 30 (53.57) | ||
| No | 26 (43.33) | 26 (46.43) | ||
| Drinking | 0.000 | 0.990 | ||
| Yes | 31 (51.67) | 29 (51.79) | ||
| No | 29 (48.33) | 27 (48.21) | ||
| Family history of osteoporosis | 0.091 | 0.763 | ||
| Yes | 37 (61.67) | 33 (58.93) | ||
| No | 23 (38.33) | 23 (41.07) | ||
| History of diabetes | 0.290 | 0.590 | ||
| No | 33 (55.00) | 28 (50.00) | ||
| Yes | 27 (45.00) | 28 (50.00) | ||
| History of hypertension | 0.744 | 0.389 | ||
| No | 40 (66.67) | 33 (58.93) | ||
| Yes | 20 (33.33) | 23 (41.07) | ||
| BMD (g/cm2) | 428.20±52.08 | 587.13±62.25 | 14.950 | <0.001 |
Expression of Omentin-1, miR-502-3p, and TNF-α in Group A and Group B
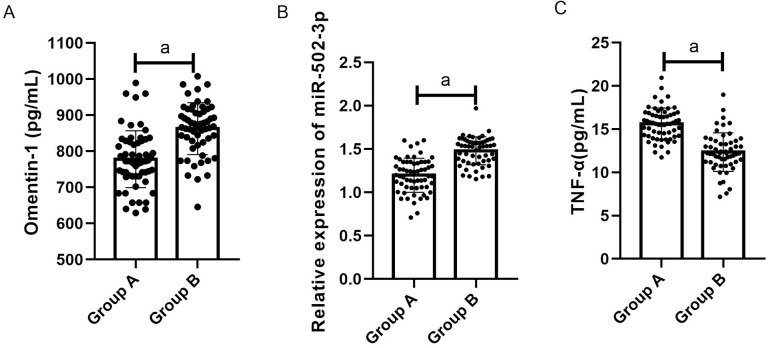
QRT-PCR results showed that the Omentin-1 levels in group A and group B were (795.10±70.20) pg/mL and (860.10±75.20) pg/mL respectively, the miR-502-3p levels in group A and group B were (1.20±0.20) and (2.00±0.20), respectively, and the TNF-α levels in group A and group B was (15.20±2.20) pg/mL and (12.60±2.20) pg/mL, respectively. Compared with the two groups, the expression levels of Omentin-1 and miR-502-3p in group A were significantly lower than those in low group (both P<0.001), and the TNF-α level in group A was markedly higher than in group B (P<0.001) (Figure 1).
Figure 1.
Comparison of the relative expression levels of Omentin-1, miR-502-3p and TNF-α in group A and group B. A. The Omentin-1 level in group A was significantly lower than that in group B (P<0.001). B. The miR-502-3p level in group A was markedly higher than that in group B (P<0.001). C. The TNF-α level in group A was remarkably higher than that in group B (P<0.001). Note: a indicates P<0.001.
Correlation between the expression levels of Omentin-1 and miR-502-3p and the clinicopathological characteristics of OPF patients
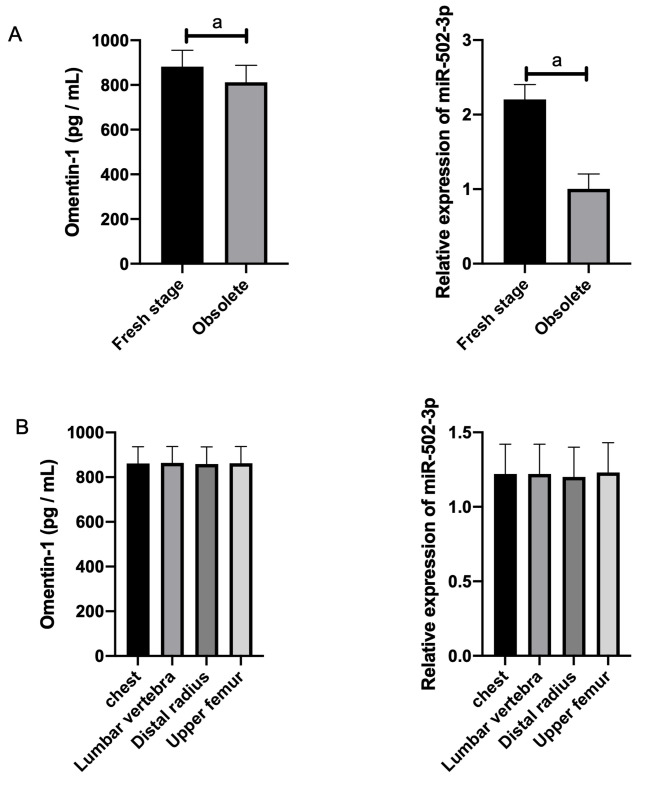
The expression of Omentin-1 and miR-502-3p had no correlation with the age, gender, or fracture site of the patients with OPF, but was related to OVF (osteoporotic vertebral fractures) staging (Table 3 and Table 4, Figure 2).
Table 3.
Relationship between Omentin-1 and clinicopathological characteristics of OPF.
| Groups | n | Omentin-1 (pg/mL) | t/F | P |
|---|---|---|---|---|
| Age (years old) | 0.104 | 0.918 | ||
| ≤68 | 28 | 857.10±75.20 | ||
| >68 | 32 | 859.10±74.20 | ||
| Gender | 0.155 | 0.878 | ||
| Male | 30 | 865.10±75.20 | ||
| Female | 30 | 868.10±75.20 | ||
| OVF staging | 3.590 | 0.001 | ||
| Fresh stage | 35 | 882.00±73.20 | ||
| Obsolete stage | 25 | 812.00±76.20 | ||
| Fracture site | 0.015 | 0.997 | ||
| Chest | 1 | 861.10±75.20 | ||
| Lumbar vertebrae | 40 | 864.10±73.20 | ||
| Distal radius | 13 | 859.10±76.20 | ||
| Upper femur | 6 | 862.10±75.20 |
Table 4.
Relationship between miR-502-3p and clinicopathological characteristics of OPF.
| Groups | n | miR-502-3p | t/F | P |
|---|---|---|---|---|
| Age (years old) | 0.386 | 0.701 | ||
| ≤68 | 28 | 1.24±0.20 | ||
| >68 | 32 | 1.22±0.20 | ||
| Gender | 0.387 | 0.700 | ||
| Male | 30 | 1.21±0.20 | ||
| Female | 30 | 1.23±0.20 | ||
| OVF staging | 22.910 | <0.001 | ||
| Fresh stage | 35 | 2.20±0.20 | ||
| Obsolete stage | 25 | 1.00±0.20 | ||
| Fracture site | 0.043 | 0.988 | ||
| Chest | 1 | 1.22±0.20 | ||
| Lumbar vertebrae | 40 | 1.22±0.20 | ||
| Distal radius | 13 | 1.20±0.20 | ||
| Upper femur | 6 | 1.23±0.20 |
Figure 2.
Expression of Omentin-1 and miR-502-3p in different OVF stages and fracture sites. A. Expression of Omentin-1 and miR-502-3p in different OVF stages. B. Expression of Omentin-1 and miR-502-3p at different fracture sites. Note: a indicates P<0.05.
Correlation between Omentin-1, miR-502-3p and inflammatory factor TNF-α
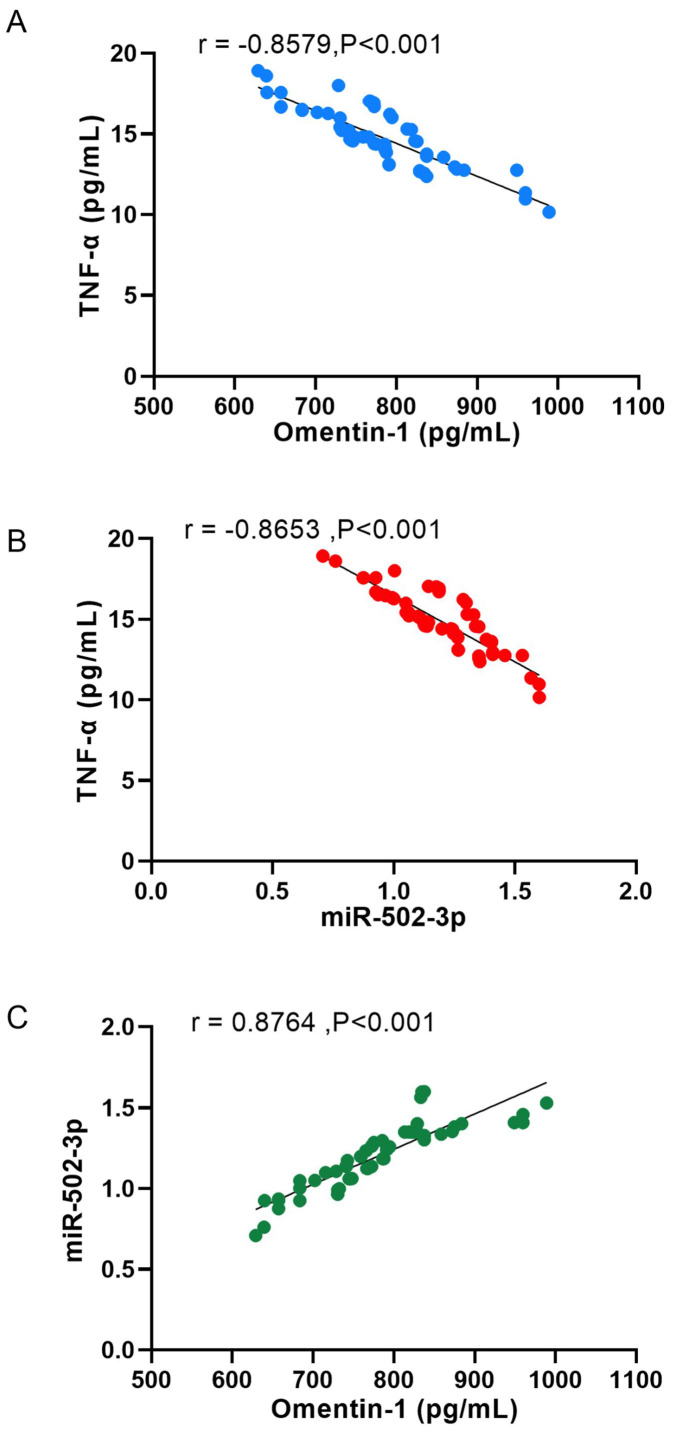
Spearman correlation analysis showed that in OPF, serum Omentin-1 and TNF-α were negatively correlated (r= -0.8579, P<0.001), serum miR-502-3p and TNF-α were negatively correlated (r= -0.8653, P<0.001), and serum Omentin-1 and miR-502-3p were positively correlated (r=0.8764, P<0.001) (Figure 3).
Figure 3.
Correlation between Omentin-1, miR-502-3p and inflammatory factor TNF-α. A. Serum Omentin-1 and TNF-α were negatively correlated in OPF (r= -0.8579, P<0.001). B. Serum miR-502-3p and TNF-α were negatively correlated in OPF (r= -0.8653, P<0.001). C. Serum Omentin-1 and miR-502-3p were positively correlated in OPF (r= 0.8764, P<0.001).
Diagnostic value of Omentin-1, miR-502-3p, and TNF-α in the prognosis of OPF
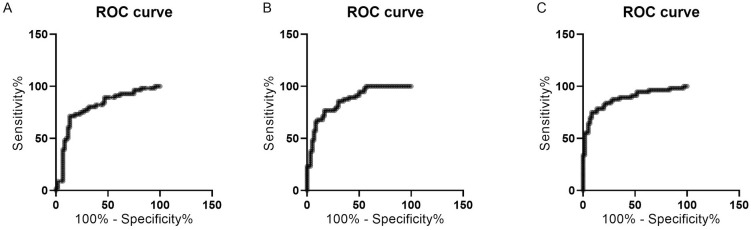
In the diagnosis of OPF, the sensitivity, specificity, and AUC values of Omentin-1, miR-502-3p and TNF-α were not significantly different in single diagnosis, all of which had good diagnostic efficacy (Table 5, Figure 4).
Table 5.
Diagnostic value of Omentin-1, miR-502-3p and TNF-α in patients with OPF before treatment.
| Indicators | Omentin-1 | miR-502-3p | TNF-α |
|---|---|---|---|
| AUC | 0.8000 | 0.8652 | 0.882 |
| 95%CI | 0.7162-0.8838 | 0.8010-0.9294 | 0.8180-0.9457 |
| Std. Error | 0.0427 | 0.0328 | 0.0326 |
| Cut-off value | 801.200 | 1.334 | 13.890 |
| Sensitivity (%) | 80.36 | 78.57 | 80.36 |
| Specificity (%) | 68.33 | 71.67 | 80.00 |
Figure 4.
ROC curves of Omentin-1, miR-502-3p and TNF-α in the diagnosis of OPF. A. ROC curve of O mentin-1 in the diagnosis of OPF. B. ROC curve of miR-502-3p in the diagnosis of OPF. C. ROC curve of TNF-α in the diagnosis of OPF.
Predictive value of Omentin-1 and miR-502-3p in the prognosis of patients with OPF
Univariate analysis of prognosis and related factors of OPF
The attending physicians classified patients into a good prognosis group (35 cases) and a poor prognosis group (25 cases) according to the prognosis statistics of patients with OPF after treatment. Logistic univariate analysis of the risk factors of OPF showed significant differences in age, OVF staging, Omentin-1, and miR-502-3p levels between the two groups (P<0.001). The patient’s age, OVF staging, Omentin-1, and miR-502-3p were all associated with the prognosis of OPF, and were the risk factors for the prognosis of OPF (Table 6 and Table 7).
Table 6.
Assignment description of prognostic factors of OPF.
| Related factors | Assignment description |
|---|---|
| Age (years old) | 68±0.1 |
| Gender | Male=0, female=1 |
| OVF staging | Fresh stage=0, obsolete stage=1 |
| Fracture site | Chest, distal radius and upper femur=0, Lumbar vertebra=1 |
| Treatment method | Drug therapy= 0, others= 1 |
| Omentin-1 (pg/mL) | <860.00=0: >860.00=1 |
| miR-502-3p | <1.20=0;>1.20=1 |
Table 7.
Multivariate analysis of prognosis and related factors of OPF.
| Factors | β | SE | Wald | P | Exp(β) | 95%CI |
|---|---|---|---|---|---|---|
| Age (years old) | 0.874 | 0.217 | 15.003 | 0.000 | 2.631 | 2.30-12.685 |
| OVF staging | 0.655 | 0.200 | 9.700 | 0.002 | 0.865 | 0.203-4.624 |
| Omentin-1 | 0.500 | 0.198 | 8.154 | 0.028 | 3.442 | 1.002-16.920 |
| miR-502-3p | 0.692 | 0.155 | 7.538 | 0.004 | 0.266 | 0.149-1.649 |
Multivariate analysis of prognosis and related factors of OPF
The risk factors related to the prognosis of OPF were further analyzed by multivariate conditional logistic regression. The results showed that age, OVF staging, Omentin-1, and miR-502-3p were independent risk factors for the prognosis of OPF (Table 7).
Discussion
Fractures are the most common complication of osteoporosis. At present, the primary treatment for osteoporotic fractures is reduction based on fracture displacement and the administration of drugs that promote bone formation and inhibit bone resorption (18,19). However, most of the drugs are anti-resorbers and cannot recover from the severe bone loss that has already occurred. Therefore, better treatment strategies for osteoporosis are needed[20].
In this study, we first detected the expression differences of Omentin-1 and miR-502-3p in OPF patients and osteoporosis patients without fractures by qRT-PCR. Based on our results, the expression levels of Omentin-1 and miR-502-3p in group A were significantly lower than in group B. Although the pathogenesis of osteoporosis is multifactorial, excess local and systemic production of proinflammatory cytokines is believed to be essential in the occurrence and development of osteoporosis[21]. Omentin-1 participates in inflammatory reaction and bone metabolism and plays a functional role in inflammatory and metabolic disorders. In related inflammatory diseases, Omentin-1 affects the biological function of proinflammatory cells, thus affecting the development of osteoporosis[22]. In addition, studies in animals have shown that miRNAs exert marked biological effects on bone development and metabolism[23]. It has been reported that new vectors of miRNA can be delivered to treat osteoporosis[24]. Ormseth et al. confirmed that chronic joint tissue inflammation was associated with miR-502-3p[25]. In our study, we found that the serum TNF-α level of patients with OPF was significantly higher compared to osteoporotic patients without fractures. Previous studies have demonstrated that inflammatory cytokines such as TNF-α can result in osteoblasts and osteoclasts damage. Also, TNF-α can induce osteoclasts formation, thereby degrading adjacent bones[26]. While Alizadeh et al. showed that Omentin-1 can down-regulate TNF-α, thereby activating the anti-osteoblast and osteoclast-promoting ability of macrophages[27]. Therefore, we presumed that Omentin-1 and miR-502-3p were down-regulated in patients with OPF. Our findings are in line with the study of Yang L et al in which it has been shown that the more serious the condition, the lower the level of Omentin-1 in patients with osteoporotic fracture, indicating that Omentin-1 is related to the severity of OPF[28]. Further, we analyzed the correlation between Omentin-1, miR-502-3p, and TNF-α. The expression of Omentin-1 has been confirmed to be up-regulated with the deterioration of osteoporosis, while Rao et al. revealed that the overexpression of Omentin-1 had an inhibitory effect on IL-1β and TNF-α in mouse bone tissue[29]. Finally, according to our results the single use of Omentin-1 and miR-502-3p showed good sensitivity and specificity in the diagnosis of OPF. Both of them were independent risk factors for the prognosis of patients with OPF. There are no previous studies on the diagnostic efficacy and predictive value of Omentin-1 and miR-502-3p in OPF patients. Still, in this study, we verified that Omentin-1 and miR-502-3p had some value in the diagnosis and prognosis of patients with metastatic OPF.
Although, this study confirmed the expression and predictive value of Omentin-1 and miR-502-3p in patients with OPF, further studies are still needed. First, there is no specific analysis of the regulation of Omentin-1 and miR-502-3p expression on OPF diseases and no further explanation of their biological function. Moreover, we have not analyzed the correlation of Omentin-1, miR-502-3p, and conventional clinical OPF markers, which impact the improvement of the study design. Therefore, in the follow-up studies, we will refer to the latest research in real-time and add corresponding research solutions to make up for the design flaws to improve our research.
To sum up, Omentin-1 and miR-502-3p are underexpressed in OPF, both of which can be used as potential biomarkers for the diagnosis and disease evaluation of OPF.
Authors’ contributions
JZ, YH, ZW and LW substantially contributed to the conception and design of this study. JZ, PeZ, PiZ, JW and ZS were responsible for the data collection and analysis. YH and ZW contributed to the interpretation of the data and drafted the manuscript. JZ, PeZ and LW revised it critically for important intellectual content. The final version was read and approved by all the authors.
Footnotes
The authors have no conflict of interest.
Edited by: G. Lyritis
References
- 1.Alarkawi D, Bliuc D, Tran T, Ahmed LA, Emaus N, Bjørnerem A, Jørgensen L, Christoffersen T, Eisman JA, Center JR. Impact of osteoporotic fracture type and subsequent fracture on mortality:the Troms Study. Osteoporos Int. 2020;31:119–130. doi: 10.1007/s00198-019-05174-5. [DOI] [PubMed] [Google Scholar]
- 2.Hsu YH, Farber CR, Kiel DP. Genetic determinants of bone mass and osteoporotic fracture. In:Principles of Bone Biology. Academic Press. 2020:1615–1630. [Google Scholar]
- 3.Zakroyeva A, Lesnyak O, Cazac V, Groppa L, Russu E, Chislari L, Rotaru L, Johansson H, Harvey NC, McCloskey E, et al. Epidemiology of osteoporotic fracture in Moldova and development of a country-specific FRAX model. Arch Osteoporos. 2020;15:13. doi: 10.1007/s11657-019-0669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu YH, Xu X, Jeong S. Genetic Determinants and Pharmacogenetics of Osteoporosis and Osteoporotic Fracture. In:Osteoporosis. Humana, Cham. 2020:485–506. [Google Scholar]
- 5.Søgaard AJ, Magnus JH, Bjørnerem Å, Holvik K, Ranhoff AH, Emaus N, Meyer HE, Strand BH. Grip strength in men and women aged 50–79 years is associated with non-vertebral osteoporotic fracture during 15 years follow-up:The Tromsø Study 1994–1995. Osteoporos Int. 2020;31:131–140. doi: 10.1007/s00198-019-05191-4. [DOI] [PubMed] [Google Scholar]
- 6.Arnold EL, Clement J, Rogers KD, Garcia-Castro F, Greenwood C. The use of µCT and fractal dimension for fracture prediction in osteoporotic individuals. J Mech Behav Biomed Mater. 2020;103:103585. doi: 10.1016/j.jmbbm.2019.103585. [DOI] [PubMed] [Google Scholar]
- 7.Zhang N, Chim YN, Wang J, Wong RMY, Chow SKH, Cheung WH. Impaired Fracture Healing in Sarco-Osteoporotic Mice Can Be Rescued by Vibration Treatment Through Myostatin Suppression. J Orthop Res. 2020;38:277–287. doi: 10.1002/jor.24477. [DOI] [PubMed] [Google Scholar]
- 8.Leder BZ, Clarke BL, Shane E, Khosla S, Kiel DP. A Lot of Progress With More to Be Done:A Response to NIH Pathways to Prevention Report “Research Gaps for Long-Term Drug Therapies for Osteoporotic Fracture Prevention”. J Bone Miner Res. 2019;34:1549–1551. doi: 10.1002/jbmr.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khera A, Kanta P, Kalra J, Dumir D, M T. Resveratrol restores the level of key inflammatory cytokines and RANKL/OPG ratio in the femur of rat osteoporosis model. J Women Aging. 2019;31:540–552. doi: 10.1080/08952841.2018.1522126. [DOI] [PubMed] [Google Scholar]
- 10.Wang T, Yu X, He C. Proinflammatory cytokines:cellular and molecular drug targets for glucocorticoid-induced-osteoporosis via osteocyte. Curr Drug Targets. 2019;20:1–15. doi: 10.2174/1389450119666180405094046. [DOI] [PubMed] [Google Scholar]
- 11.Fang H, Zhang H, Wang Z, Zhou Z, Li Y, Lu L. Systemic immune-inflammation index acts as a novel diagnostic biomarker for postmenopausal osteoporosis and could predict the risk of osteoporotic fracture. J Clin Lab Anal. 2020;34:e23016. doi: 10.1002/jcla.23016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, He M, Wang G, Fu Q. Organic gallium treatment improves osteoporotic fracture healing through affecting the OPG/RANKL ratio and expression of serum inflammatory cytokines in ovariectomized rats. Biol Trace Elem Res. 2018;183:270–279. doi: 10.1007/s12011-017-1123-y. [DOI] [PubMed] [Google Scholar]
- 13.Kenawi MZ, Akl EM, Sabry JH, Mostafa ST. Evaluation of serum level of omentin-1 in females with hirsutism. J Cosmet Dermatol. 2020;19:535–539. doi: 10.1111/jocd.13043. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Gao Y, Lin F, Han K, Wang X. Omentin-1 attenuates lipopolysaccharide (LPS)-induced U937 macrophages activation by inhibiting the TLR4/MyD88/NF-κB signaling. Arch Biochem Biophys. 2020;679:108187. doi: 10.1016/j.abb.2019.108187. [DOI] [PubMed] [Google Scholar]
- 15.Pickering ME, Millet M, Rousseau JC, Croset M, Szulc P, Borel O, Sornay Rendu E, Chapurlat R. Selected serum microRNA, abdominal aortic calcification and risk of osteoporotic fracture. PLoS One. 2019;14:e0216947. doi: 10.1371/journal.pone.0216947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C, Wang Q, Ma S, Sun Y, Vadamootoo AS, Jin C. A four serum-miRNA panel serves as a potential diagnostic biomarker of osteosarcoma. Int J Clin Oncol. 2019;24:976–982. doi: 10.1007/s10147-019-01433-x. [DOI] [PubMed] [Google Scholar]
- 17.Saleh A, Collins M. Osteoporotic Vertebral Compression Fractures. In:Vertebral Compression Fractures in Osteoporotic and Pathologic Bone. Springer, Cham. 2020:57–62. [Google Scholar]
- 18.Lewiecki EM. Denosumab:Mechanisms and Therapeutic Effects in the Treatment of Osteoporosis. In:Osteoporosis. Humana, Cham. 2020:309–322. [Google Scholar]
- 19.Tabacco G, Bilezikian JP. PTH and PTHrP Analogs:Treatment of Osteoporosis. In:Osteoporosis Humana, Cham. 2020:349–362. [Google Scholar]
- 20.Lloyd AA, Gludovatz B, Riedel C, Luengo EA, Saiyed R, Marty E, Lorich DG, Lane JM, Ritchie RO, Busse B, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proc Natl Acad Sci U S A. 2017;114:8722–8727. doi: 10.1073/pnas.1704460114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardoso PRG, Fragoso TS, Barbosa AD. Cytokine measurements in Brazilian postmenopausal osteoporosis patients reveal high levels of IL-8. Scientia Medica. 2016;26:9. [Google Scholar]
- 22.Dikker O, Bekpinar S, Ozdemirler G, Uysal M, Vardar M, Atar S, Usta M, Huner B. Evaluation of the Relation Between Omentin-1 and Vitamin D in Postmenopausal Women With or Without Osteoporosis. Exp Clin Endocrinol Diabetes. 2018;126:316–320. doi: 10.1055/s-0043-120110. [DOI] [PubMed] [Google Scholar]
- 23.Ramírez-Salazar EG, Carrillo-Patiño S, Hidalgo-Bravo A, Rivera-Paredez B, Quiterio M, Ramírez-Palacios P, Patiño N, Valdés-Flores M, Salmerón J, Velázquez-Cruz R. Serum miRNAs miR-140-3p and miR-23b-3p as potential biomarkers for osteoporosis and osteoporotic fracture in postmenopausal Mexican-Mestizo women. Gene. 2018;679:19–27. doi: 10.1016/j.gene.2018.08.074. [DOI] [PubMed] [Google Scholar]
- 24.De-Ugarte L, Yoskovitz G, Balcells S, Güerri-Fernández R, Martinez-Diaz S, Mellibovsky L, Urreizti R, Nogués X, Grinberg D, García-Giralt N, et al. MiRNA profiling of whole trabecular bone:identification of osteoporosis-related changes in MiRNAs in human hip bones. BMC Med Genomics. 2016;8:75. doi: 10.1186/s12920-015-0149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ormseth MJ, Solus JF, Sheng Q, Ye F, Wu Q, Guo Y, Oeser AM, Allen RM, Vickers KC, Stein CM. Development and validation of a MicroRNA panel to differentiate between patients with rheumatoid arthritis or systemic lupus erythematosus and controls. J Rheumatol. 2020;47:188–196. doi: 10.3899/jrheum.181029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raehtz S, Bierhalter H, Schoenherr D, Parameswaran N, McCabe LR. Estrogen Deficiency Exacerbates Type 1 Diabetes–Induced Bone TNF-αExpression and Osteoporosis in Female Mice. Endocrinology. 2017;158:2086–2101. doi: 10.1210/en.2016-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alizadeh S, Mirzaei K, Mohammadi C, Keshavarz SA, Maghbooli Z. Circulating omentin-1 might be associated with metabolic health status in different phenotypes of body size. Arch Endocrinol Metab. 2017;61:567–574. doi: 10.1590/2359-3997000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L, Zhao XL, Liao B, Qin AP. Relationships between serum Omentin-1 levels and bone mineral density in older men with osteoporosis. Chronic Dis Transl Med. 2016;2:48–54. doi: 10.1016/j.cdtm.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao SS, Hu Y, Xie PL, Cao J, Wang ZX, Liu JH, Yin H, Huang J, Tan YJ, Luo J, et al. Omentin-1 prevents inflammation-induced osteoporosis by downregulating the proinflammatory cytokines. Bone Res. 2018;6:9. doi: 10.1038/s41413-018-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]