Abstract
Background
Good syndrome is a rare adult-onset immunodeficiency characterized by thymoma and hypogammaglobulinemia. Its clinical manifestations are highly heterogeneous, ranging from various infections to autoimmunity.
Objective
This study was to summarize patient characteristics, identify prognostic factors and define clinical subgroups of Good syndrome.
Methods
A systematic literature review was conducted to include patients with Good syndrome identified in PubMed, Embase and Cochrane databases between January 2010 and November 2020. Logistic and Cox regressions were used to identify prognostic factors impacting outcomes. Clinical subgroups were defined by multiple correspondence analysis and unsupervised hierarchical clustering. A decision tree was constructed to characterize the subgroup placement of cases.
Results
Of 162 patients included in the current study, the median age at diagnosis was 58 years and 51% were male. Type AB was the most common histological subtype of thymoma, and infections as well as concurrent autoimmune disorders were identified in 92.6% and 51.2% patients, respectively. Laboratory workup showed typical findings of combined immunodeficiency. Thymoma status (odds ratio [OR] 4.157, confidence interval [CI] 1.219-14.177, p = 0.023), infections related to cellular immunity defects (OR 3.324, 95% CI 1.100-10.046, p = 0.033), infections of sinopulmonary tract (OR 14.351, 95% CI 2.525-81.576, p = 0.003), central nerve system (OR 6.403, 95% CI 1.205-34.027, p = 0.029) as well as bloodstream (OR 6.917, 95% CI 1.519-31.505, p = 0.012) were independent prognostic factors. The 10-year overall survival was 53.7%. Cluster analysis revealed three clinical subgroups with distinct characteristics and prognosis (cluster 1, infections related to cellular immunity defects; cluster 2, infections related to other immunity defects; cluster 3, infections related to humoral and phagocytic immunity defects). A decision tree using infection types (related to humoral and cellular immunity defects) could place patients into corresponding clusters with an overall correct prediction of 72.2%.
Conclusions
Infection type and site were the main prognostic factors impacting survival of patients with Good syndrome. We identified three subgroups within Good syndrome associated with distinct clinical features, which may facilitate the study of underlying pathogenesis as well as development of targeted therapy.
Keywords: Good syndrome, immunodeficiency, prognosis, clinical subgroups, infections
Introduction
Good syndrome is an adult-onset acquired immunodeficiency, characterized by thymoma and hypogammaglobulinemia. Its underlying pathogenesis remains elusive. Patients always have low to absent peripheral B cells and impaired T-cell mediated immunity. Its clinical constellations are highly heterogeneous, ranging from various infections to concurrent autoimmune disorders. Recognition of the disease across a range of manifestations is challenging, commonly leading to diagnostic delay. Treatments are mainly supportive with antimicrobials and immunoglobulin replacement. Prognosis is believed to be worse compared with other adult immunodeficiencies (1, 2).
Data on Good syndrome are scarce due to its rarity. Most studies are case reports as well as small series, and the last systematic review was published in 2010 focusing on descriptions of clinical features (2). Therefore, the current systematic literature review aimed to summarize the clinical features, concurrent disorders, treatments, and outcomes of Good syndrome cases published since 2010. Independent prognostic factors impacting survival were explored. Given its spectrum of manifestations, we also sought to define disease subgroups sharing similar clinical features and prognosis, which may enable earlier diagnosis and more specific treatment.
Methods
Study Design, Search Strategy and Selection Criteria
Records were identified by searching PubMed, Embase and Cochrane databases between January 2010 and November 2020, with the terms “Good syndrome”, “Good’s syndrome”, “thymoma” AND “hypogammaglobulinemia”, “thymoma” AND “immunodeficiency”, as well as “thymoma” AND “infection”. We chose 2010 as the initial year because a previous systematic review already summarized cases published between 1956 and 2009 (2). In addition, cases reported in the recent decade were described in a more standardized manner, allowing better synthesis of the data. We further reviewed the reference lists of retrieved records to identify additional cases. Duplicated records were excluded first. Two authors (YS and CW) screened the title and abstract of each record independently, and full texts of the record deemed relevant were reviewed by both authors to reach a consensus for inclusion or exclusion. As there is no formal diagnostic consensus for Good syndrome, we used the presence of both thymoma and hypogammaglobulinemia as the minimal criteria to define Good syndrome, consistent with the previous systematic review (2). Cases with individual patient data were included. Exclusion criteria were an inappropriate type of record (review, conference abstract or non-English publication), an alternative diagnosis or a study of Good syndrome with aggregated data. Aggregated data of the Good syndrome cohort were summarized and reported separately ( Supplementary Table 1 ) to allow comparison (3–6).
Primary aims of the current systematic review were to record the baseline clinical features (especially infection histories and related pathogens ( Supplementary Table 2 ), laboratory findings, concurrent diseases, treatments, and mortality of patients with Good syndrome. Given the diversity of pathogens reported in these patients, they were grouped according to the major type of host immunity defect predisposing to the infection, including humoral, cellular, phagocytic and others (including pathogens not associated with a particular type of immunity defects or pathogen was not reported for the infection) (7–11). Details of pathogens in each category were shown in Supplementary Table 3 . More advanced immunological workups, including vaccination response, lymphocyte proliferation to mitogens and respiratory burst test were not available in most cases. Prognostic factors impacting outcomes (mortality and survival) were explored. Clinical findings were also used to identify disease subgroups with similar manifestations and outcomes.
Statistical Analysis
Data analysis was performed with SPSS version 26.0 (Armonk, NY, USA) and XLSTAT version 2020.1 version (New York, NY, USA). Continuous variables were summarized with median and interquartile range (IQR). Categorical variables were reported as numbers and percentages. Continuous variables were compared using Mann-Whitney test or Kruskal-Wallis test, and categorical variables were compared using Fisher’s exact test or chi-square test, as appropriate.
Binary logistic regression was used to identify prognostic factors impacting mortality for all patients (n = 162), and variables with both clinical and statistical relevance (p < 0.10) in univariate analysis were included in the multivariate model. For patients with clearly documented follow up duration (n = 109), overall survival was measured from the time of Good syndrome diagnosis until death or the last follow up. Survival curves were plotted using Kaplan-Meier method and compared with log-rank test. Cox proportional hazards model was further applied to test the significance of those prognostic factors regarding their survival impact.
To unravel homogeneous clinical subgroups within Good syndrome, a multiple correspondence analysis was first used to reduce the dimension of datasets. It cross-tabulated categorical variables and represented them graphically in a 2-dimensional Euclidean space by a multidimensional scaling technique (12). Input variables were chosen based on the defining criteria of Good syndrome, including thymoma, infection (type of pathogen and site of infection) and IgG level. After transformation of categorical variables into continuous variables (coordinates), we performed unsupervised hierarchical cluster analysis to determine clinical subgroups according to various characteristics. The clustering was constructed using Euclidean distance with the Ward agglomerative method. This method starts with each patient in its own cluster and the two most “similar” clusters based on Euclidean distance are combined at each step until the last two clusters are merged into a single cluster with all patients, as shown in a dendrogram (13, 14). Discriminative variables among clusters were further selected based on higher V test value with significant p value (15).
A decision tree was constructed with the use of chi-square automatic interaction detection technique to easily place the cases into different clusters (16). The p value was adjusted by Bonferroni correction. Ten-fold cross validation was applied to select the optimal tree. Performance of the decision tree was further evaluated by overall sensitivity and specificity of the cluster placement of cases.
All data were considered statistically significant at p < 0.05.
Results
General Characteristics
Our systematic literature review identified 162 patients from 121 records (17–137) ( Supplementary Figure 1 ). The demographics, clinical features and outcomes are shown in Table 1 . The median age of Good syndrome diagnosis was 58 years (IQR 51-67), and 51.0% were male. Because the ethnic origin of patient was not reported in most cases, geographic location of the corresponding author was recorded instead: Asia (n = 73, 45.1%), Europe (n = 58, 35.8%), North America (n = 22, 13.5%), Australia/Oceania (n = 5, 3.1%) and South America (n = 4, 2.5%). In 136 patients with available data regarding the chronological sequence of initial clinical presentation and subsequent Good syndrome diagnosis establishment, thymoma, infection and autoimmunity preceded the establishment of Good syndrome diagnosis in 44.9%, 29.4% and 25.7% patients, respectively. The median interval from initial clinical presentation to subsequent establishment of Good syndrome diagnosis was 24 months (IQR 4-72). Patients presented with infection (median interval 2 months, IQR 0-36) reached diagnosis earlier compared to those who presented with thymoma (median interval 36 months, IQR 6-82, p = 0.002) or autoimmunity (median interval 36 months, IQR 12-96, p = 0.001).
Table 1.
Clinical characteristics, management, and outcomes of patients with Good syndrome.
| Variables | N = 162 |
|---|---|
| Demographics | |
| Age (median, IQR) | 58 (51–67) |
| Male (%) | 79 (51.0) (n = 155) |
| Country of cases (%) | |
| North America | 22 (13.5) |
| South America | 4 (2.5) |
| Asia | 73 (45.1) |
| Europe | 58 (35.8) |
| Australia/Oceania | 5 (3.1) |
| Index presentation | (n = 136) |
| Thymoma (%) | 61 (44.9) |
| Infections (%) | 40 (29.4) |
| Autoimmunity (%) | 35 (25.7) |
| Index to diagnosis (median, IQR; months) | (n = 116) |
| Overall | 24 (4–72) |
| Thymoma | 36 (6–82) (n = 52) |
| Infection | 2 (0–36) (n = 31) |
| Autoimmunity | 36 (12–96) (n = 33) |
| Thymoma classification* (%) | (n = 121) |
| WHO classification (%) | (n = 98) |
| Type A | 23 (23.5) |
| Type B1/2/3 | 26 (26.5) |
| Type AB | 49 (50.0) |
| Traditional classification (%) | (n = 6) |
| Spindle cell | 2 (33.3) |
| Lymphocytic rich | 1 (16.7) |
| Lymphoepithelial | 2 (33.3) |
| Epithelial | 1 (16.7) |
| Other classification (%) | (n = 17) |
| Benign | 8 (47.1) |
| Malignant | 9 (52.9) |
| Thymoma staging (Masaoka system) (%) | (n = 22) |
| Stage I | 7 (31.8) |
| Stage II | 8 (36.4) |
| Stage III | 4 (18.2) |
| Stage IV | 3 (13.6) |
| Thymoma status when Good syndrome is diagnosed (%) | (n = 146) |
| Disease free | 75 (51.4) |
| Disease active | 71 (48.6) |
| Infections (%) | (n = 150 with infection) |
| Classification | |
| Humoral defects | 34 (22.7) |
| Cellular defects | 86 (57.3) |
| Phagocytic defects | 41 (27.3) |
| Others/unknown | 72 (48.0) |
| Site | |
| Sinopulmonary tract | 101 (67.3) |
| Eye | 15 (10.0) |
| GI tract and liver | 41 (27.3) |
| Urinary tract | 11 (7.3) |
| Bone and joint | 4 (2.7) |
| Skin and soft tissue | 27 (18.0) |
| Central nerve system | 12 (8.0) |
| Complicated bloodstream (with organ involvement) | 13 (8.7) |
| Viremia without organ involvement | 10 (6.7) |
| Mucosa** | 21 (14.0) |
| Autoimmunity (%) | (n = 83 with autoimmunity) |
| Pure red cell aplasia | 26 (31.3) |
| Myasthenia gravis | 23 (27.7) |
| Lichen planus | 19 (22.9) |
| Diarrhea (%) | (n = 52 with diarrhea) |
| Infection | 30 (57.7) |
| Non-infection | 22 (42.3) |
| Second malignancy | (n = 14 with concurrent second malignancy)*** |
| Type | |
| Solid | 6 (42.9) |
| Hematological | 8 (57.1) |
| Timing of diagnosis | |
| Before Good syndrome | 5 (35.7) |
| Concurrent with Good syndrome | 3 (21.4) |
| After Good syndrome | 6 (42.9) |
| Laboratory findings (%) | |
| Immunoglobulin (Ig) | |
| Low IgG | 162 (100.0) |
| Low IgA | 117 (86.0) (n = 136) |
| Low IgM | 125 (92.6) (n = 135) |
| Complete blood counts | |
| Leukopenia | 33 (44.0) (n = 75) |
| Anemia | 45 (66.2) (n = 68) |
| Thrombocytopenia | 6 (14.3) (n = 42) |
| Peripheral B cells | (n = 124) |
| Absent | 55 (44.4) |
| Low | 63 (50.8) |
| Normal | 6 (4.8) |
| Peripheral T cells | |
| Low CD4 | 77 (71.3) (n = 108) |
| Elevated CD8 | 17 (20.5) (n = 83) |
| Inversed CD4/8 ratio | 94 (82.5) (n = 114) |
| HIV test negativity | 64 (100.0) (n = 64) |
| Management (%) | |
| Thymoma | (n = 150) |
| Thymectomy | 135 (90.0) |
| Radiotherapy | 10 (6.7) |
| Chemotherapy | 12 (8.0) |
| Immunoglobulin replacement | 127 (78.4) |
| Antimicrobial prophylaxis | 28 (17.3) |
| Concurrent immunosuppressant | 42 (50.6) (n = 83 with autoimmunity) |
| Outcomes | |
| Overall mortality | 25 (15.4) |
| Infection | 23 (92.0) |
| Others | 2 (8.0) |
| 10-year overall survival (%; 95% CI) | 53.7 (25.8-75.1) (n = 109) |
IQR, interquartile range; CI, confidence interval.
*Thymoma histological subtype was traditionally classified based on morphology alone. WHO classification was subsequently introduced, based on both the morphology of epithelial cells and the lymphocyte-to epithelial cell ratio.
**Mucosa included oral and vaginal mucosal infection, such as oral candidiasis.
***Fourteen pre-malignancy and malignancy were reported in 13 patients, including breast cancer (n = 1), nasopharyngeal cancer (n = 1), cutaneous Kaposi sarcoma (n = 1), lung cancer (squamous cell, n = 2; adenocarcinoma, n = 1), mucosa associated lymphoid tissue lymphoma (ocular adnexa, n = 1), monoclonal gammopathy of undetermined significance (n = 3), myelodysplastic syndrome (n = 3) and T cell large lymphocytic granular leukemia (n = 1).
Clinical Features: Thymoma, Infection, Concurrent Autoimmunity and Second Malignancy
In 121 patients with thymoma histological classification data, WHO classification was used in 98 patients and type AB was found in 50.0%. Staging with the Masaoka system was only reported in 22 patients, and 68.2% were localized disease (stage I and II). At the time of Good syndrome diagnosis, half of the cases (51.4%) were in remission from thymoma standpoint.
Infections were recorded in 150 patients (92.6%), and the leading site was sinopulmonary tract (67.3%). Other common sites of infections included gastrointestinal system (27.3%), skin and soft tissue (18.0%), mucosa (14.0%) and eye (10.0%). Recovered pathogens are shown in Supplementary Table 2 . The most frequently recorded bacterium, fungus and virus were Pseudomonas spp. (12.7%), Candida spp. (16.7%), and cytomegalovirus (24.7%), respectively. Given the diversity of pathogens reported, they were further categorized according to the major type of host defect predisposing to the infection (7–11) ( Supplementary Table 3 ). As a result, 22.7%, 57.3% and 27.3% patients had infections related to humoral, cellular, and phagocytic defects.
Concurrent autoimmunity was identified in 83 patients (51.2%). Pure red cell aplasia (31.3%), myasthenia gravis (27.7%) and lichen planus (22.9%) were the most frequent disorders in these patients. Fourteen second pre-malignancy and malignancy were diagnosed in 13 patients (8.0%), before (n = 5), concurrent with (n = 3) and after (n = 6) diagnosis of Good syndrome. Eight were hematological and six were solid tumors.
Laboratory Findings
All patients had hypogammaglobulinemia with a median IgG of 332 mg/dL (IQR 188-476) in those with reported levels (n = 126). Concurrent low IgA and IgM were noted in 86.0% (117/136) and 92.6% (125/135) patients with records. Absent or low peripheral B cells were shown in 118 of 124 patients (95.2%), as well as low absolute CD4 count in 77 of 108 patients (71.3%). Of 114 patients, 94 (82.5%) had an inverted CD4/8 ratio. The human immunodeficiency virus (HIV) was negative in all tested patients (n = 64).
There were no statistically significant differences in terms of immunoglobulin levels and peripheral B cell count between patients with and without infections related to humoral immunity defects. Regarding CD4 cell count and CD4/8 ratio, no significant differences were found between patients with and without infections related to cellular immunity defects ( Supplementary Table 4 ).
Overall Management
Of 150 patients with thymoma treatment data, 135 (90.0%) underwent thymectomy at the time of report. Chemotherapy and radiotherapy were given to 12 (8.0%) and 10 (6.7%) patients. In terms of immunoglobulin replacement, 127 patients (78.4%) received at least one dose. Antimicrobial prophylaxis was only used in 28 patients (17.3%). Of 83 patients with autoimmunity, 42 (50.6%) received concurrent immunosuppressive treatments (corticosteroid alone in 18 patients; immunosuppressants, such as cyclosporine, with or without corticosteroid in 24 patients).
Outcomes and Prognostic Factors
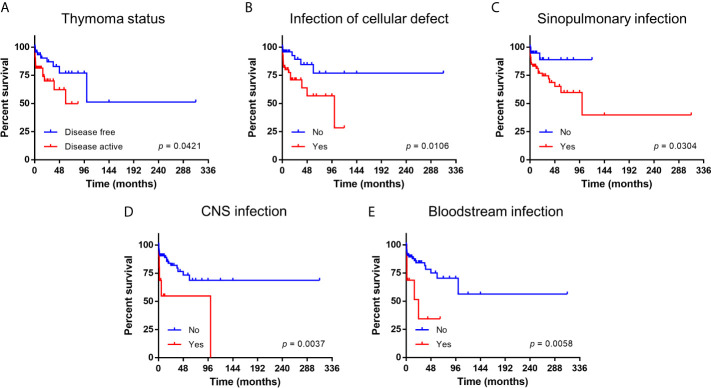
A total of 25 patients (15.4%) died at the time of report. The main cause of mortality was infection (n = 23, 92.0%). In the whole cohort, factors impacting mortality consistently in both univariate and multivariate logistic regression included thymoma status (active disease, odds ratio [OR] 4.157, 95% confidence interval [CI] 1.219-14.177, p = 0.023), infection associated with cellular immunity defect (OR 3.324, 95% CI 1.100-10.046, p = 0.033), infection of sinopulmonary tract (OR 14.351, 95% CI 2.525-81.576, p = 0.003), infection of central nerve system (OR 6.403, 95% CI 1.205-34.027, p = 0.029) and infection of bloodstream (OR 6.917, 95% CI 1.519-31.505, p = 0.012) ( Table 2 ).
Table 2.
Univariate and multivariate analyses of factors impacting outcomes.
| Variables | Logistic regression | Cox regression | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| OR (95% CI) | P | OR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Thymoma status: active disease | 2.243 (0.886-5.667) | 0.088 | 4.157 (1.219-14.177) | 0.023 | 2.474 (1.000-6.119) | 0.050 | 4.173 (1.584-10.990) | 0.004 |
| Infection: cellular immunity defects | 2.609 (1.024-6.647) | 0.044 | 3.324 (1.100-10.046) | 0.033 | 3.172 (1.243-8.095) | 0.016 | 2.895 (1.074-7.802) | 0.036 |
| Infection: sinopulmonary tract | 5.384 (1.538-18.848) | 0.008 | 14.351 (2.525-81.576) | 0.003 | 3.439 (1.016-11.633) | 0.047 | 4.312 (1.175-15.820) | 0.028 |
| Infection: central nerve system | 4.643 (1.343-16.051) | 0.015 | 6.403 (1.205-34.027) | 0.029 | 4.051 (1.456-11.273) | 0.007 | 3.424 (1.099-10.669) | 0.034 |
| Infection: bloodstream | 4.031 (1.199-13.554) | 0.024 | 6.917 (1.519-31.505) | 0.012 | 3.707 (1.361-10.101) | 0.010 | 3.832 (1.342-10.944) | 0.012 |
OR, odds ratio; HR, hazard ratio; CI, confidence interval.
Of 109 patients with documented follow up duration (median 24 months, 95% CI 19-29 months), the 10-year overall survival was 53.7% (95% CI 25.8-75.1). The five prognostic factors identified by logistic regression in the whole cohort remained significant in these patients by both univariate and multivariate Cox regression ( Table 2 and Supplementary Table 5 ). Survival outcomes are depicted in Figure 1 .
Figure 1.
Kaplan-Meier survival curve of patients with Good syndrome stratified according to prognostic factors. (A) Thymoma status. (B) Infection related to cellular immunity defects. (C) Infection of sinopulmonary tract. (D) Infection of central nervous system (CNS). (E) Infection of bloodstream.
Identification of Subgroups
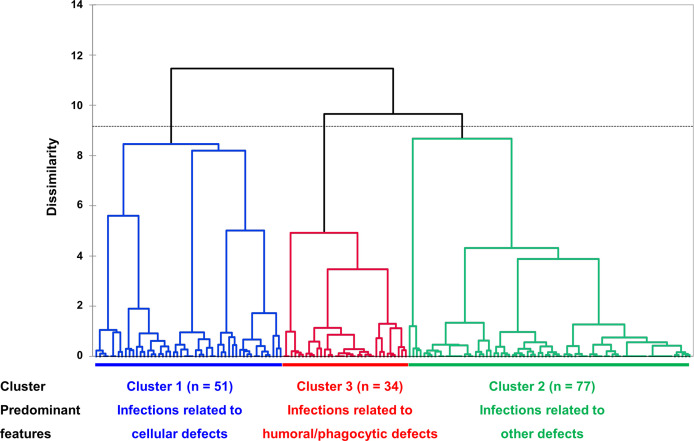
Using multiple correspondence analysis and hierarchical clustering, we identified three clusters within the whole cohort ( Figure 2 and Supplementary Figure 2 ). Comparisons of clinical features among the three clusters are shown in Table 3 . Cluster 1 (n = 51, 31.5%) was best characterized by more frequent infections related to cellular immunity defect (94.1%, p < 0.001; V test, 7.071), affecting mucosa (37.3%, p < 0.001; V test, 6.220) and central nerve system (23.5%, p < 0.001; V test, 5.295). The most discriminative variables of cluster 2 (n = 77, 47.5%) included infections related to other immunity defect (61.0%, p < 0.001; V test, 4.033) and more frequent IgG < 500 mg/dL (88.9%, p = 0.041; V test, 2.269). In terms of cluster 3 (n = 34, 21.0%), patients had more frequent infections related to humoral (67.7%, p < 0.001; V test, 7.493) and phagocytic defects (58.8%, p < 0.001; V test, 5.041), as well as involving sinopulmonary tract (91.2%, p < 0.001; 3.891). The outcomes were statistically different between patients of cluster 1 and 2 (OR 0.325, 95% CI 0.118-0.893, p = 0.029; hazard ratio 0.382, 95% CI 0.150-0.974, p = 0.044 for those who with follow up duration) ( Supplementary Figure 3 ).
Figure 2.
Dendrogram of unsupervised hierarchical clustering. Three clusters, based on similarity of cases, were identified, and represented in different colors (cluster 1, blue, infections related to cellular immunity defects; cluster 2, green, infections related to other immunity defects; cluster 3, red, infections related to humoral and phagocytic immunity defects). The vertical axis represents a measure of dissimilarity. In the horizontal axis, each patient is represented by a vertical line starting at the bottom and progressively merge with other patients to form clusters. Dashed line indicates the cut-off point for the three clusters.
Table 3.
Comparison of clinical characteristics among the three subgroups of patients with Good syndrome.
| Variables (N, %) | Cluster 1 (n = 51) | Cluster 2 (n = 77) | Cluster 3 (n = 34) | P* |
|---|---|---|---|---|
| Age > 60 years | 24 (47.1) | 33 (42.9) | 17 (50.0) | 0.394 |
| Male | 25 (50.0) (n = 50) | 35 (46.7) (n = 75) | 19 (63.3) (n = 30) | 0.300 |
| Asian | 21 (41.2) | 38 (49.4) | 14 (41.2) | 0.580 |
| Thymoma status: active | 28 (58.3) (n = 48) | 40 (56.3) (n = 71) | 3 (11.1) (n = 27) | < 0.001 |
| Infection: humoral defects | 7 (13.7) | 4 (5.2) | 23 (67.6) | < 0.001 |
| Infection: cellular defects | 48 (94.1) | 22 (28.6) | 16 (47.1) | < 0.001 |
| Infection: phagocytic defects | 11 (21.6) | 10 (13.0) | 20 (58.8) | < 0.001 |
| Infection: others | 17 (33.3) | 47 (61.0) | 8 (23.5) | < 0.001 |
| Infection: sinopulmonary | 24 (47.1) | 46 (59.7) | 31 (91.2) | < 0.001 |
| Infection: eye | 12 (23.5) | 0 (0.0) | 3 (8.8) | < 0.001 |
| Infection: GI and liver | 11 (21.6) | 20 (26.0) | 10 (29.4) | 0.705 |
| Infection: urinary tract | 8 (15.7) | 2 (2.6) | 1 (2.9) | 0.010 |
| Infection: bone and joint | 0 (0.0) | 4 (5.2) | 0 (0.0) | 0.104 |
| Infection: skin and soft tissue | 10 (19.6) | 16 (20.8) | 1 (2.9) | 0.053 |
| Infection: central nerve system | 12 (23.5) | 0 (0.0) | 0 (0.0) | < 0.001 |
| Infection: bloodstream | 5 (9.8) | 0 (0.0) | 8 (23.5) | < 0.001 |
| Infection: viremia | 10 (19.6) | 0 (0.0) | 0 (0.0) | < 0.001 |
| Infection: mucosa | 19 (37.3) | 0 (0.0) | 2 (5.9) | < 0.001 |
| Autoimmunity: lichen planus | 5 (9.8) | 13 (16.9) | 1 (2.9) | 0.096 |
| Autoimmunity: MG | 8 (15.7) | 9 (11.7) | 6 (17.6) | 0.663 |
| Autoimmunity: PRCA | 9 (17.6) | 10 (13.0) | 7 (20.6) | 0.562 |
| Autoimmunity: others | 6 (11.8) | 14 (18.2) | 1 (2.9) | 0.084 |
| IgG < 500 mg/dL | 29 (69.0) (n = 42) | 56 (88.9) (n = 63) | 20 (80.0) (n = 25) | 0.041 |
| Low IgA | 34 (77.3) (n = 44) | 57 (89.1) (n = 64) | 26 (92.9) (n = 28) | 0.112 |
| Low IgM | 39 (90.7) (n = 43) | 61 (96.8) (n = 63) | 25 (86.2) (n = 29) | 0.166 |
| Absent peripheral B cells | 18 (47.4) (n = 38) | 22 (38.6) (n = 57) | 15 (51.7) (n = 29) | 0.462 |
| Low CD4 | 26 (70.3) (n = 37) | 30 (63.8) (n = 47) | 21 (87.5) (n = 24) | 0.112 |
| Inverted CD4/CD8 ratio | 31 (83.8) (n = 37) | 39 (78.0) (n = 50) | 24 (88.9) (n = 27) | 0.471 |
| Thymectomy | 43 (86.0) (n = 50) | 65 (90.3) (n = 72) | 27 (96.4) (n = 28) | 0.336 |
| Radio- and/or chemotherapy | 11 (22.0) (n = 50) | 8 (11.1) (n = 72) | 3 (10.7) (n = 28) | 0.199 |
| Immunoglobulin replacement | 33 (64.7) | 62 (80.5) | 32 (94.1) | 0.004 |
| Antimicrobial prophylaxis | 10 (19.6) | 10 (13.0) | 8 (23.5) | 0.347 |
| Immunosuppressant (n = 83)** | 15 (60.0) (n = 25) | 19 (43.2) (n = 44) | 8 (57.1) (n = 14) | 0.351 |
GI, gastrointestinal; MG, myasthenia gravis; PRCA, pure red cell aplasia; Ig, immunoglobulin.
*Global p value.
**In patients with concurrent autoimmune disorders.
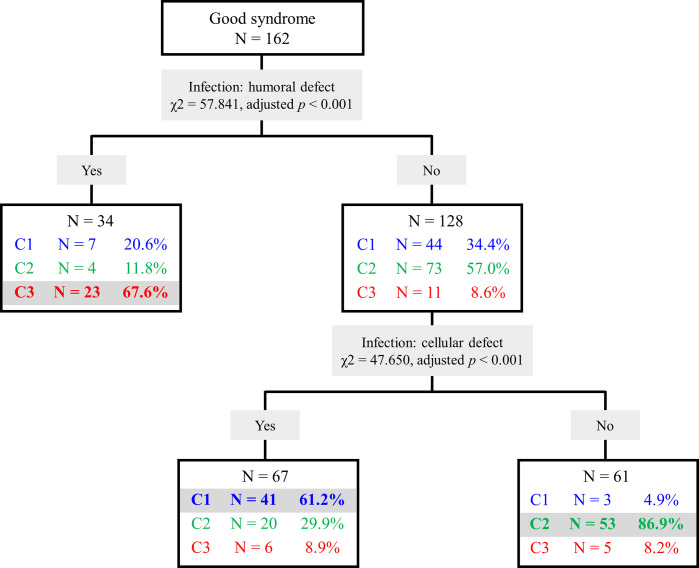
To easily classify cases into the three clusters, a decision tree was created by screening discriminative variables among the three clusters ( Figure 3 ). Using infections related to humoral (χ2 = 57.841, adjusted p < 0.001) and cellular defects (χ2 = 47.650, adjusted p < 0.001), the tree showed 72.2% correct estimation with an overall sensitivity of 72.3% and specificity of 86.2%.
Figure 3.
Decision tree of Good syndrome classification. Cluster 1 (C1), infections related to cellular immunity defects; Cluster 2 (C2), infections related to other immunity defects; Cluster 3 (C3), infections related to humoral and phagocytic immunity defects.
Discussion
The current systematic review summarized clinical data of a recent and large series of patients with Good syndrome. To the best of our knowledge, this is the first study to comprehensively explore the independent prognostic factors and define clinical subgroups within this rare adult-onset immunodeficiency. We found thymoma status and infection type as well as site impacting the outcome. Distributions of these features varied significantly among subgroups identified via hierarchical clustering, supporting the relevance to define these subgroups in clinical practice. Otherwise, the findings of current study were comparable to those of a previous systematic review including cases published between 1956 and 2009 (2) as well as four case series reported in the recent decade (3–6) ( Supplementary Table 1 ), in terms of demographics, thymoma classification, infection sites and pathogens, concurrent autoimmunity and laboratory findings of immunodeficiency, reinforcing our understanding of this rare disorder.
Notably, we introduced thymoma status and infection classification in our study, both turned to be critical in the subsequent prognostic and clustering analyses. First, although the exact role of thymoma in Good syndrome development remains unclear, it likely disrupts the balance between host-defense and self-tolerance. Given the crucial physiological role of thymus in T cell education, concurrent autoimmunity and immunodeficiency observed in the context of thymoma reflects both over-reactivity to self-antigens and hypo-reactivity to pathogens (138). Patients with thymoma requiring treatment (e.g., thymectomy, chemotherapy and/or radiotherapy) at Good syndrome diagnosis showed inferior outcomes, which may suggest a different underlying immunological status but could also be attributed to more complicated clinical needs, especially in the context of active infections. It is noteworthy that hypogammaglobulinemia did not improve in all patients of the current series with IgG level measured after thymectomy (n = 92), indicating thymoma management alone is not sufficient to resolve this disorder.
Second, we classified infection into different subtypes according to the pathogens, given its huge diversity and limited cases of each pathogen making statistical analysis less feasible in this rare disease. The classification is based on our current knowledge of predisposing immunological factors related to infection of each pathogen, considering the typical infections observed in patients with X-linked agammaglobulinemia (humoral defect) (7), acquired immunodeficiency syndromes (AIDS, cellular defect) (8) and neutropenia (such as chemotherapy induced) (9) as well as neutrophil dysfunction in chronic granulomatous disease (phagocytic defect) (10) as prototypes. Of note, infection related to cellular immunity defects was the predominant type, emphasizing Good syndrome is a combined immunodeficiency. Although low CD4 cell count and inverted CD4/8 ratio were commonly found in these patients, both were not prognostic and their association with the onset of opportunistic infection was not established. Indeed, these infections occurred even in those who had preserved CD4 cell count, as noted in a recent French Good syndrome series (3). In addition, all patients had hypogammaglobulinemia, but the level of IgG was neither prognostic nor differed in terms of infections related to humoral defects. Even in patients with common variable immunodeficiency, the prognostic value of baseline IgG remains controversial (139, 140). Overall, the type of infections discriminates the three clusters defined in our study and could be used to easily place a patient into the corresponding cluster (cluster 1, infections related to cellular immunity defects; cluster 2, infections related to other immunity defects; cluster 3, infections related to humoral and phagocytic immunity defects). The enrichment of clinical features suggests a shared underlying pathogenesis in each cluster, which was not well reflected with the use of routine immunological workups, including serum immunoglobulin levels and lymphocyte subset enumeration of peripheral blood. These observations indicate the requirement of further workups to particularly assess functions of different immune cell subsets. Unfortunately, more advanced immunological workups to evaluate B (e.g., vaccination response), T (e.g., lymphocyte proliferation to mitogens) and phagocytic cell (e.g., respiratory burst) functions were not available in most cases. Moreover, anti-cytokine autoantibodies seem to be crucial links between thymoma and cellular immunity defects, predisposing to various opportunistic infections (141). Their roles in Good syndrome pathogenesis and disease evolution need further studies. In addition, whether there is underlying molecular defect predisposing to Good syndrome also remains underexplored.
Although infection remained the leading cause of death, overall mortality of Good syndrome showed significant improvement in the current series (15.4%) compared to the previous one (46.1%) (2), which could be multifactorial and related to improved recognition of this rare disorder, more frequent use of immunoglobulin replacement, expanded availability of various antimicrobial agents as well as better supportive cares in the recent decade. Majority of patients underwent thymectomy, which unfortunately did not resolve the hypogammaglobulinemia. Immunoglobulin replacement was used in 78.4% patients for at least one dose, although it did not impact prognosis in the current series. Given the description in most case reports, it is hard to know whether these patients received a regular long-term replacement. Moreover, infection related to cellular immunity defects, the main prognostic factor, is not affected by immunoglobulin replacement. Antimicrobial prophylaxis was less commonly used in this series, which deserves further studies to address its potential significance, given the efficacy and safety of low dose antibiotics prophylaxis have been demonstrated in patients with primary antibody deficiencies (142).
Our study has limitations given its design. Although the current series included the largest number of cases to date, analyses were restricted to what was reported in the literature (i.e., unavoidable reporting bias and missing data), and limited events as well as incomplete follow up may also underpower our analyses. Therefore, the results should not be over-interpreted. Independent prognostic factors as well as the subgroup clustering requires external validations. More investigational studies are needed to unravel the unique pathogenesis of each cluster, such as functions of different immune cell subsets and the presence of a specific set of anti-cytokine antibodies.
In conclusion, Good syndrome is a rare combined immunodeficiency in adults that has subgroups with different clinical features. Infection type and site impact its overall survival. Future studies should explore whether each subgroup has unique disease pathogenesis that may be amenable to more specific treatments.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Author Contributions
YS conceived the study, collected the records, analyzed the data, and contributed to the manuscript. CW conceived the study, collected the records, analyzed the data, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.679556/full#supplementary-material
References
- 1. Kelleher P, Misbah SA. What Is Good’s Syndrome? Immunological Abnormalities in Patients With Thymoma. J Clin Pathol (2003) 56:12–6. 10.1136/jcp.56.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kelesidis T, Yang O. Good’s Syndrome Remains a Mystery After 55 Years: A Systematic Review of the Scientific Evidence. Clin Immunol (2010) 135:347–63. 10.1016/j.clim.2010.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malphettes M, Gérard L, Galicier L, Boutboul D, Asli B, Szalat R, et al. Good Syndrome: An Adult-Onset Immunodeficiency Remarkable for its High Incidence of Invasive Infections and Autoimmune Complications. Clin Infect Dis (2015) 61:e13–19. 10.1093/cid/civ269 [DOI] [PubMed] [Google Scholar]
- 4. Jansen A, van Deuren M, Miller J, Litzman J, de Gracia J, Sáenz-Cuesta M, et al. Prognosis of Good Syndrome: Mortality and Morbidity of Thymoma Associated Immunodeficiency in Perspective. Clin Immunol (2016) 171:12–7. 10.1016/j.clim.2016.07.025 [DOI] [PubMed] [Google Scholar]
- 5. Dong J-P, Gao W, Teng G-G, Tian Y, Wang H-H. Characteristics of Good’s Syndrome in China: A Systematic Review. Chin Med J (Engl) (2017) 130:1604–9. 10.4103/0366-6999.208234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zaman M, Huissoon A, Buckland M, Patel S, Alachkar H, Edgar JD, et al. Clinical and Laboratory Features of Seventy-Eight UK Patients With Good’s Syndrome (Thymoma and Hypogammaglobulinaemia). Clin Exp Immunol (2019) 195:132–8. 10.1111/cei.13216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Winkelstein JA, Marino MC, Lederman HM, Jones SM, Sullivan K, Burks AW, et al. X-Linked Agammaglobulinemia: Report on a United States Registry of 201 Patients. Med (Baltimore) (2006) 85:193–202. 10.1097/01.md.0000229482.27398.ad [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention (CDC) . Revised Surveillance Case Definition for HIV Infection–United States, 2014. MMWR Recomm Rep (2014) 63:1–10. [PubMed] [Google Scholar]
- 9. Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical Practice Guideline for the Use of Antimicrobial Agents in Neutropenic Patients With Cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis (2011) 52:e56–93. 10.1093/cid/cir073 [DOI] [PubMed] [Google Scholar]
- 10. Marciano BE, Spalding C, Fitzgerald A, Mann D, Brown T, Osgood S, et al. Common Severe Infections in Chronic Granulomatous Disease. Clin Infect Dis (2015) 60:1176–83. 10.1093/cid/ciu1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aguilar C, Malphettes M, Donadieu J, Chandesris O, Coignard-Biehler H, Catherinot E, et al. Prevention of Infections During Primary Immunodeficiency. Clin Infect Dis (2014) 59:1462–70. 10.1093/cid/ciu646 [DOI] [PubMed] [Google Scholar]
- 12. Dion J, Costedoat-Chalumeau N, Sène D, Cohen-Bittan J, Leroux G, Dion C, et al. Relapsing Polychondritis can Be Characterized by Three Different Clinical Phenotypes: Analysis of a Recent Series of 142 Patients. Arthritis Rheumatol (2016) 68:2992–3001. 10.1002/art.39790 [DOI] [PubMed] [Google Scholar]
- 13. Ward JH, Jr. Hierarchical Grouping to Optimize an Objective Function. J Am Stat Assoc (2012) 58(301):236–44. [Google Scholar]
- 14. Bartoloni E, Baldini C, Ferro F, Alunno A, Carubbi F, Cafaro G, et al. Application of Artificial Neural Network Analysis in the Evaluation of Cardiovascular Risk in Primary Sjögren’s Syndrome: A Novel Pathogenetic Scenario? Clin Exp Rheumatol (2019) 37(Suppl 118):133–9. [PubMed] [Google Scholar]
- 15. Mariampillai K, Granger B, Amelin D, Guiguet M, Hachulla E, Maurier F, et al. Development of a New Classification System for Idiopathic Inflammatory Myopathies Based on Clinical Manifestations and Myositis-Specific Autoantibodies. JAMA Neurol (2018) 75:1528–37. 10.1001/jamaneurol.2018.2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kass GV. An Exploratory Technique for Investigating Large Quantities of Categorical Data. J R Stat Soc Ser C (Applied Statistics) (1980) 29:119–27. 10.2307/2986296 [DOI] [Google Scholar]
- 17. Kuribayashi K, Fujimi A, Kobune M, Takimoto R, Kikuchi S, Iyama S, et al. Pure Red Cell Aplasia Associated With Good’s Syndrome Accompanied by Decreased Stem Cell Factor Production in the Bone Marrow. Intern Med (2010) 49:377–82. 10.2169/internalmedicine.49.2811 [DOI] [PubMed] [Google Scholar]
- 18. Hamadani M, Awan F, Villalona-Calero MA. Malignant Thymoma With Immunodeficiency (Good Syndrome) Associated With Mucormycosis. Am J Clin Oncol (2010) 33:109. 10.1097/COC.0b013e31802c5430 [DOI] [PubMed] [Google Scholar]
- 19. Hanafusa T, Umegaki N, Yamaguchi Y, Katayama I. Good’s Syndrome (Hypogammaglobulinemia With Thymoma) Presenting Intractable Opportunistic Infections and Hyperkeratotic Lichen Planus. J Dermatol (2010) 37:171–4. 10.1111/j.1346-8138.2009.00781.x [DOI] [PubMed] [Google Scholar]
- 20. Del Pozo N, Sarmiento E, Lanio N, Gallego A, Largo J, Carbone J. Immunophenotypic Abnormalities of CD8+ T-Cell Subsets in a Patient With Unusual Good’s Syndrome. Allergologia Immunopathologia (2010) 38:102–5. 10.1016/j.aller.2009.07.002 [DOI] [PubMed] [Google Scholar]
- 21. Mateo-Montoya A, Stanescu D, Wolff B, Sahel J-A, Bonnel S. Cytomegalovirus Retinitis Associated With Good’s Syndrome. Eur J Ophthalmol (2010) 20:479–80. 10.1177/112067211002000238 [DOI] [PubMed] [Google Scholar]
- 22. Park DH, Kim SY, Shin JP. Bilateral Cytomegalovirus Retinitis With Unilateral Optic Neuritis in Good Syndrome. Jpn J Ophthalmol (2010) 54:246–8. 10.1007/s10384-009-0795-z [DOI] [PubMed] [Google Scholar]
- 23. Squintani G, Ferrari S, Bazzoli E, Eleopra R, La Monaca C, Cagliari E, et al. Progressive Multifocal Leukoencephalopathy in a Patient With Good’s Syndrome. Int J Infect Dis (2010) 14:e444–7. 10.1016/j.ijid.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 24. Yang OO, Kelesidis T. Interleukin-2-Unresponsive Immune Defects in Good Syndrome: Letter to the Editor. Clin Immunol (2010) 135:496–8. 10.1016/j.clim.2010.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ho JKY, Wong MML, Tai TKF, Tse DMW. A Rare Combination of Recurrent Pneumonia, Diarrhoea, and Visual Loss in a Patient After Thymectomy: Good Syndrome. Hong Kong Med J (2010) 16:493–6. [PubMed] [Google Scholar]
- 26. Kikuchi R, Mino N, Okamoto T, Matsukura T, Hirai T. A Case of Good’s Syndrome: A Rare Acquired Immunodeficiency Associated With Thymoma. Ann Thorac Cardiovasc Surg (2011) 17:74–6. 10.5761/atcs.cr.09.01505 [DOI] [PubMed] [Google Scholar]
- 27. Nitta H, Harada Y, Okikawa Y, Fujii M, Arihiro K, Kimura A, et al. Good’s Syndrome-Associated Pure Red Cell Aplasia With Myelodysplastic Syndrome. Intern Med (2011) 50:2011–4. 10.2169/internalmedicine.50.5709 [DOI] [PubMed] [Google Scholar]
- 28. Kaku Y, Shimamoto N, Matsunaga H, Makiura M, Fujisawa A, Morita K. Oral Erosive Lichen Planus and Alopecia Areata With Good’s Syndrome (Thymoma With Hypogammaglobulinemia). Eur J Dermatol (2011) 21:124–5. 10.1684/ejd.2010.1183 [DOI] [PubMed] [Google Scholar]
- 29. Chen J, Yang Y, Zhu D, Chen G, Wei S, Qiu X, et al. Thymoma With Pure Red Cell Aplasia and Good’s Syndrome. Ann Thoracic Surg (2011) 91:1620–2. 10.1016/j.athoracsur.2010.10.010 [DOI] [PubMed] [Google Scholar]
- 30. Jethava Y, Alamelu J, Rangarajan S, Lang-Lazdunski L, van der Walt J, Fields P. Acquired Agranulocytosis and Factor XI Deficiency in Association With Thymoma. JCO (2011) 29:e604–6. 10.1200/JCO.2010.34.3707 [DOI] [PubMed] [Google Scholar]
- 31. Ternavasio-de la Vega H-G, Velasco-Tirado V, Pozo-Rosado L, Soler-Fernández M-C, Pérez-Andres M, Orfao A, et al. Persistence of Immunological Alterations After Thymectomy in Good’s Syndrome: A Clue to its Pathogenesis. Cytometry (2011) 80B:339–42. 10.1002/cyto.b.20595 [DOI] [PubMed] [Google Scholar]
- 32. Fahim A, Abuzakouk M, Hart SP. Haemophilus Influenzae Pneumonia and Immunodeficiency in Association With Thymoma—A Presentation of Good’s Syndrome. Rev Portuguesa Pneumologia (2011) 17:272–4. 10.1016/j.rppneu.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 33. Furtado AK. Giardia Infection: Protein-losing Enteropathy in an Adult With Immunodeficiency. WJG (2012) 18:2430. 10.3748/wjg.v18.i19.2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ogoshi T, Ishimoto H, Yatera K, Oda K, Akata K, Yamasaki K, et al. A Case of Good Syndrome With Pulmonary Lesions Similar to Diffuse Panbronchiolitis. Intern Med (2012) 51:1087–91. 10.2169/internalmedicine.51.7028 [DOI] [PubMed] [Google Scholar]
- 35. Chen L-P, Tsai J-S, Lai W-M, Yen L-J, Yu M-S, Lin S-J. Myelodysplasia Followed by Good’s Syndrome: A Unique Manifestation Associated With Thymoma. Kaohsiung J Med Sci (2012) 28:236–40. 10.1016/j.kjms.2011.10.012 [DOI] [PubMed] [Google Scholar]
- 36. Nakagawa Y, Murakami K, Hirashita Y, Ogawa R, Hisamatsu A, Mizukami K, et al. A Case of Good Syndrome With Refractory Gastrointestinal Ulcers. Endoscopy (2012) 44:E246–7. 10.1055/s-0032-1309749 [DOI] [PubMed] [Google Scholar]
- 37. Zabsonré JT, Laoubi K, Kemiche F, Cerf-Payrastre I, Pertuiset E. Streptococcus B Septic Polyarthritis Revealing Good’s Syndrome. Joint Bone Spine (2012) 79:412–4. 10.1016/j.jbspin.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 38. Sáenz-Cuesta M, Martínez-Pomar N, de Gracia J, Echaniz P, Villegas E, Prada Á, et al. TACI Mutation in Good’s Syndrome: in Search of a Genetic Basis. Clin Immunol (2012) 145:27–30. 10.1016/j.clim.2012.07.014 [DOI] [PubMed] [Google Scholar]
- 39. Noska A, Nasr R, Williams DN. Closed Trauma, Mycoplasma Hominis Osteomyelitis, and the Elusive Diagnosis of Good’s Syndrome. Case Rep (2012) 2012. 10.1136/bcr-2012-007056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oehler E, Heuberger L, Ghawche F, Valour F. Good’s Syndrome and IgA Monoclonal Gammopathy of Undetermined Significance. Case Rep (2012) 2012. 10.1136/bcr-2012-007601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Adamczyk-Korbel M, Kieszko R, Krawczyk P, Homa I, Ramlau R, Milanowski J. Good’s Syndrome Twelve Years After Thymectomy Due to Thymoma. A Case Study. Cent Eur J Immunol (2013) 4:505–10. 10.5114/ceji.2013.39769 [DOI] [Google Scholar]
- 42. Briones J, Iruretagoyena M, Galindo H, Ortega C, Zoroquiain P, Valbuena J, et al. Thymoma Associated With Hypogammaglobulinaemia and Pure Red Cell Aplasia. E Can Med Sci (2013) 7:364. 10.3332/ecancer.2013.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Idress A, Wahla AS. Good Syndrome, A Rare Disease That Physicians Cannot Afford to Overlook. Case Rep Rev Lit (2013) 63:3. [PubMed] [Google Scholar]
- 44. Joven MH, Palalay MP, Sonido CY. Case Report and Literature Review on Good’s Syndrome, a Form of Acquired Immunodeficiency Associated With Thymomas. Public Health (2013) 72:7. [PMC free article] [PubMed] [Google Scholar]
- 45. Margraf RL, Coonrod EM, Durtschi JD, Augustine NH, Voelkerding KV, Hill HR, et al. TACI Mutation p.Lys154Ter Identified in Good Syndrome. Clin Immunol (2013) 146:10–2. 10.1016/j.clim.2012.10.006 [DOI] [PubMed] [Google Scholar]
- 46. Wan CK, Teoh SC. Autoimmune Retinopathy in Benign Thymoma After Good Syndrome-Associated Cytomegalovirus Retinitis. Ocular Immunol Inflammation (2013) 21:79–81. 10.3109/09273948.2012.730652 [DOI] [PubMed] [Google Scholar]
- 47. Mancuso A, Gentiluomo M, Vangeli M, Torre MD, Belli LS. Diarrhea as Sole Presentation of Good’s Syndrome Mimicking Crohn’s Disease. Clin Immunol (2013) 147:9–10. 10.1016/j.clim.2013.01.012 [DOI] [PubMed] [Google Scholar]
- 48. Qu J, Lü X, Gao Q, Zhang Y. Good Syndrome, a Rare Cause of Refractory Chronic Diarrhea and Recurrent Pneumonia in a Chinese Patient After Thymectomy. Clin Vaccine Immunol (2013) 20:1097–8. 10.1128/CVI.00141-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sveinsson O, Matell H, Herrman L. Progressive Multifocal Leukoencephalopathy in a Patient With Good’s Syndrome. Case Rep (2013) 2013. 10.1136/bcr-2013-009763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kelly A, Merlin C, Trouillier S, Cachin F, Guettrot-Imbert G. Thymoma and Immunodeficiency: (18)F-FDG-PET/CT Imaging of Good Syndrome. Hell J Nucl Med (2013) 16:140–1. 10.1967/s002449910086 [DOI] [PubMed] [Google Scholar]
- 51. Hunt WR, Allam JS, Sica G, Little BP, Veeraraghavan S. A 22-Year-Old Woman With Bronchiectasis and a Mediastinal Mass. Chest (2013) 144:1406–9. 10.1378/chest.13-0647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu K, Cowlishaw JL. Beware of the Patient With Thymectomy: Good’s Syndrome in a Patient Presenting With Diarrhea. ACG Case Rep J (2013) 1:33–5. 10.14309/crj.2013.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stoeckle M, Holbro A, Arnold A, Neumayr A, Weisser M, Blum J. Treatment of Mucosal Leishmaniasis (L. Infantum) With Miltefosine in a Patient With Good Syndrome. Acta Tropica (2013) 128:168–70. 10.1016/j.actatropica.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 54. Kristiansen HA, Spetalen S, Fløisand Y, Heldal D. Eltrombopag in Good’s Syndrome. Case Rep Hematol (2014) 2014:1–3. 10.1155/2014/172139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nagoya A, Kanzaki R, Nakagiri T, Inoue M, Susaki Y, Inoue S, et al. Ectopic Cervical Thymoma Accompanied by Good’s Syndrome. ATCS (2014) 20:531–4. 10.5761/atcs.cr.12.02027 [DOI] [PubMed] [Google Scholar]
- 56. Akinosoglou K, Melachrinou M, Siagris D, Koletsis E, Marangos M, Gogos CA, et al. Good’s Syndrome and Pure White Cell Aplasia Complicated by Cryptococcus Infection: A Case Report and Review of the Literature. J Clin Immunol (2014) 34:283–8. 10.1007/s10875-014-0014-7 [DOI] [PubMed] [Google Scholar]
- 57. Frith J, Toller-Artis E, Tcheurekdjian H, Hostoffer R. Good Syndrome and Polymyositis. Ann Allergy Asthma Immunol (2014) 112:478. 10.1016/j.anai.2014.03.001 [DOI] [PubMed] [Google Scholar]
- 58. Lougaris V, Vitali M, Baronio M, Tampella G, Plebani A. BAFF-R Mutations in Good’s Syndrome. Clin Immunol (2014) 153:91–3. 10.1016/j.clim.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 59. Thurneysen C, Boggian K. Legionella Pneumophila Serogroup 1 Septic Arthritis With Probable Endocarditis in an Immunodeficient Patient. JCR: J Clin Rheumatol (2014) 20:297–8. 10.1097/RHU.0000000000000128 [DOI] [PubMed] [Google Scholar]
- 60. Rawat A, Dhir V, Gupta A, Suri D, Burad DK, Nada R, et al. Good’s Syndrome Presenting With Recurrent Giardiasis. J Clin Immunol (2014) 34:751–2. 10.1007/s10875-014-0080-x [DOI] [PubMed] [Google Scholar]
- 61. Macdonald JB, Mangold AR, Connolly SM. Good Syndrome and Lichen Planus: Case and Review. J Eur Acad Dermatol Venereol (2014) 28:1828–30. 10.1111/jdv.12378 [DOI] [PubMed] [Google Scholar]
- 62. Tachdjian R, Keller JJ, Pfeffer M. A Bad Case of Good’s Syndrome. Infect Dis Ther (2014) 3:333–7. 10.1007/s40121-014-0045-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pu C, Sukhal S, Fakhran S. Humoral Immunity in Bronchiectasis: Finding Good’s Syndrome. Case Rep Pulmonology (2015) 2015:1–3. 10.1155/2015/531731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tadic D, Markovic O, Kraguljac-Kurtovic N, Drobnjak-Tomasek O. Good’s Syndrome With Increasing γδ T-Lymphocyte Subpopulation: A Case Report. VSP (2015) 72:1039–43. 10.2298/VSP140609102T [DOI] [PubMed] [Google Scholar]
- 65. Arnold SJ, Hodgson T, Misbah SA, Patel SY, Cooper SM, Venning VA. Three Difficult Cases: The Challenge of Autoimmunity, Immunodeficiency and Recurrent Infections in Patients With Good Syndrome. Br J Dermatol (2015) 172:774–7. 10.1111/bjd.13293 [DOI] [PubMed] [Google Scholar]
- 66. Jack KL, Kula M, Flint JD, Mezei MM. A Case of Good Syndrome Presumed Secondary to Metastatic Pancreatic Thymoma in a Patient Presenting With a Myasthenic Crisis Postthymectomy. J Clin Neuromuscular Dis (2015) 16:159–63. 10.1097/CND.0000000000000070 [DOI] [PubMed] [Google Scholar]
- 67. Maehara T, Moriyama M, Kawano S, Hayashida J-N, Furukawa S, Ohta M, et al. Cytokine Profiles Contribute to Understanding the Pathogenic Difference Between Good Syndrome and Oral Lichen Planus: Two Case Reports and Literature Review. Medicine (2015) 94:e704. 10.1097/MD.0000000000000704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang C-H, Chan ED, Perng C-L, Chian C-F, Chen C-W, Perng W-C, et al. Intravenous Immunoglobulin Replacement Therapy to Prevent Pulmonary Infection in a Patient With Good’s Syndrome. J Microbiol Immunol Infect (2015) 48:229–32. 10.1016/j.jmii.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 69. Bahal S, McKain L, Moore J, Jones J, Clifford H, Krishna MT, et al. Absent AB Isoagglutinins: A Clue to Immunodeficiency. Transfusion Med (2015) 25:201–3. 10.1111/tme.12213 [DOI] [PubMed] [Google Scholar]
- 70. Wargo JJ, Kim AH, Hart A, Berg A. It Took a Village: Good’s Syndrome. Am J Med (2015) 128:699–701. 10.1016/j.amjmed.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 71. van Luijk CM, Missotten T, Smit EF, Stallaert RALM, Baarsma GS, van Velthoven MEJ. Acquired Ocular Toxoplasmosis in a Patient With Thymoma. Acta Ophthalmol (2015) 93:488–9. 10.1111/aos.12638 [DOI] [PubMed] [Google Scholar]
- 72. Jensen ML, Bendstrup E, Hilberg O. Granulomatous-Lymphocytic Interstitial Lung Disease and Recurrent Sinopulmonary Infections in a Patient With Good’s Syndrome. BMJ Case Rep (2015). 10.1136/bcr-2014-205635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fidias PM, Long AA, Fintelmann FJ, Zukerberg LR. Case 31-2015: A 29-Year-Old Man With Thymoma, Diarrhea, and Weight Loss. N Engl J Med (2015) 373:1458–67. 10.1056/NEJMcpc1406663 [DOI] [PubMed] [Google Scholar]
- 74. DeBoard ZM, Taylor BJW. Good’s Syndrome: Successful Management of Thymoma With Hypoimmunoglobulinemia. Ann Thoracic Surg (2015) 100:1903–5. 10.1016/j.athoracsur.2014.12.108 [DOI] [PubMed] [Google Scholar]
- 75. Motegi S, Uchiyama A, Yamada K, Toki S, Amano H, Ishikawa O. Lichen Planus Complicated With Thymoma: Report of Three Japanese Cases and Review of the Published Work. J Dermatol (2015) 42:1072–7. 10.1111/1346-8138.12987 [DOI] [PubMed] [Google Scholar]
- 76. Sun X, Shi J, Wang M, Xu K, Xiao Y.Goodʼs Syndrome Patients Hospitalized for Infections: A Single-Center Retrospective Study. Medicine (2015) 94:e2090. 10.1097/MD.0000000000002090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Caperton C, Agrawal S, Gupta S. Good Syndrome Presenting With CD8+ T-Cell Large Granular Lymphocyte Leukemia. Oncotarget (2015) 6:36577–86. 10.18632/oncotarget.5369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chaudhuri AD, Tapadar SR, Dhua A, Dhara PN, Nandi S, Choudhury S. A Case of Good’s Syndrome Presenting With Pulmonary Tuberculosis. Indian J Chest Dis Allied Sci (2015) 57:247–50. [PubMed] [Google Scholar]
- 79. Ueno S, Sekimoto-Tsuboi S, Ishiguro Y, Koinuma T, Eguchi H, Machida Y, et al. Good’s Syndrome With Opportunistic Infection of the Central Nervous System: A Case Report. BMC Neurol (2015) 15:150. 10.1186/s12883-015-0406-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Disselhorst MJ, Dickhoff C, Alhan C. Good’s Syndrome: An Uncommon Cause of Therapy-Resistant Diarrhoea. Netherlands J Med (2016) 74:4. [PubMed] [Google Scholar]
- 81. Okusu T, Sato T, Ogata Y, Nagata S, Kozumi K, Kim S-H, et al. Good’s Syndrome Accompanied by Agranulocytosis Following a Rapid Clinical Course. Intern Med (2016) 55:537–40. 10.2169/internalmedicine.55.5542 [DOI] [PubMed] [Google Scholar]
- 82. Zdziarski P, Dworacki G. Role of Chemokine Signalling in the Pathogenesis of Good’s Syndrome-Case Reports, Clinical Characterization From Single-Centre Perspective. Immunome Res (2016) 12. 10.4172/1745-7580.10000119 [DOI] [Google Scholar]
- 83. Buyantseva L, Brooks JP, Craig T. A 73-Year-Old Woman With Persistent Diarrhea and Onychomycosis. Allergy Asthma Proc (2016) 37:76–9. 10.2500/aap.2016.37.3896 [DOI] [PubMed] [Google Scholar]
- 84. Aydintug YS, Bayar GR, Ozkan A, Gunhan O, Musabak U. Thymoma With Immunodeficiency With Multiple Recurrent Oral Herpetic Infections. J Dental Sci (2016) 11:103–6. 10.1016/j.jds.2012.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Colin GC, Ghaye B, Pieters T, Knoops L, Coche E. Good’s Syndrome: Clinical and Imaging Presentation. Diagn Intervent Imaging (2016) 97:487–9. 10.1016/j.diii.2015.11.004 [DOI] [PubMed] [Google Scholar]
- 86. Sobieszczańska M, Tubek S, Spychała S. Good’s Syndrome and Hypoparathyroidism Combined With Hypocalcaemia, Hypokalemia, Hypomagnesemia, and Hypophosphatemia—Case Report. Immunol Lett (2016) 172:132–3. 10.1016/j.imlet.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 87. Matsuura-Otsuki Y, Hanafusa T, Igawa K, Sato H, Nishizawa A, Yokozeki H. Macrophage Activation Syndrome Triggered by Disseminated Tuberculosis With Tuberculous Gumma in a Patient With Adult-Onset Still’s Disease and Good’s Syndrome. Eur J Dermatol (2016) 26:309–11. 10.1684/ejd.2016.2745 [DOI] [PubMed] [Google Scholar]
- 88. Antar AI, Otrock ZK, Kharfan-Dabaja MA, Mahfouz RA, Alameddine RS, El-Majzoub NMW, et al. Thymoma With Concomitant Pure Red Cell Aplasia, Good’s Syndrome and Myasthenia Gravis Responding to Rituximab. Indian J Hematol Blood Transfus (2016) 32:219–22. 10.1007/s12288-014-0478-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Aguilar-Company J, Los-Arcos I, Pigrau C, Rodríguez-Pardo D, Larrosa MN, Rodríguez-Garrido V, et al. Potential Use of Fosfomycin-Tromethamine for Treatment of Recurrent Campylobacter Species Enteritis. Antimicrob Agents Chemother (2016) 60:4398–400. 10.1128/AAC.00447-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Figueira Gonçalves JM, Palmero Tejera JM, Eiroa González L. Recurrent Respiratory Infections in a Patient With Chronic Diarrhea. Archivos Bronconeumología (English Edition) (2016) 52:399–400. 10.1016/j.arbr.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 91. Glück J, Rymarczyk B, Paluch U, Rogala B, Brzoza Z. A Case of Good’s Syndrome Diagnosed After More Than 20 Years Since Onset of Myasthenia in a Patient With Psoriasis. Neurol Sci (2016) 37:1179–80. 10.1007/s10072-016-2526-9 [DOI] [PubMed] [Google Scholar]
- 92. Luo L, Zhang W, Li C, Tang X. Tuberculous Lymphadenitis in a Patient With Good Syndrome. Br J Hosp Med (2016) 77:486–7. 10.12968/hmed.2016.77.8.486 [DOI] [PubMed] [Google Scholar]
- 93. Habib AM, Thornton H, Sewell WC, Loubani M. Good’s Syndrome: Is Thymectomy the Solution? Case Report and Literature Review. Asian Cardiovasc Thorac Ann (2016) 24:712–4. 10.1177/0218492316655641 [DOI] [PubMed] [Google Scholar]
- 94. Narahari NK, Gongati PK, Uppin SG, Kapoor A, Kakarla B, Tella RD. A 66-Year-Old Man With Mediastinal Mass and Dyspnea. Chest (2016) 150:e109–15. 10.1016/j.chest.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 95. Downes KM, Tarasewicz D, Weisberg LJ, Cunningham ET. Good Syndrome and Other Causes of Cytomegalovirus Retinitis in HIV-negative Patients—Case Report and Comprehensive Review of the Literature. J Ophthal Inflammation Infect (2016) 6:3. 10.1186/s12348-016-0070-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sasson SC, Davies S, Chan R, Davies L, Garsia R. Cerebral Toxoplasmosis in a Patient With Myasthenia Gravis and Thymoma With Immunodeficiency/Good’s Syndrome: A Case Report. BMC Infect Dis (2016) 16:457. 10.1186/s12879-016-1801-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Narahari NK, Gongati PK, Kakarla B, Nizami MI, Boddula RP, Sattavarapu LR. Thymoma-Associated Immunodeficiency: A Diagnostic Challenge for the Clinician. Asian Cardiovasc Thorac Ann (2017) 25:146–9. 10.1177/0218492316687934 [DOI] [PubMed] [Google Scholar]
- 98. Ferguson L, Chong H, Singh M. Ecthyma Gangrenosum Without Bacteraemia: Evidence in Favour of a Broader Definition. Clin Exp Dermatol (2017) 42:324–7. 10.1111/ced.13064 [DOI] [PubMed] [Google Scholar]
- 99. Okui M, Yamamichi T, Asakawa A, Harada M, Horio H. Pure Red Cell Aplasia Associated With Good Syndrome. Korean J Thorac Cardiovasc Surg (2017) 50:119–22. 10.5090/kjtcs.2017.50.2.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fukushima A, Ichimura Y, Obata S, Kinoshita-Ise M, Fujio Y, Takeno M, et al. Thymoma-Associated Multi-Organ Autoimmunity: A Case of Graft-Versus-Host Disease-Like Erythroderma Complicated by Good Syndrome Successfully Treated by Thymectomy. J Dermatol (2017) 44:830–5. 10.1111/1346-8138.13777 [DOI] [PubMed] [Google Scholar]
- 101. Chen X, Zhang J, Shang W, Xie W, Jin S, Wang F. Aberrant Peripheral Immune Function in a Good Syndrome Patient. J Immunol Res (2018) 2018:1–10. 10.1155/2018/6212410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Furukawa R, Yanagiya M, Matsumoto J, Hashimoto H, Horiuchi H, Usui K. Good’s Syndrome With Clinical Manifestation After Thymectomy: A Case Report. Respir Med Case Rep (2018) 24:89–91. 10.1016/j.rmcr.2018.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hu F, Du Y, Peng X-Y. Successively Bilateral Cytomegalovirus Retinitis in Immunocompromised Patient With Good’s Syndrome. Int J Ophthalmol (2018) 11:2021–3. 10.18240/ijo.2018.12.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tavakol M, Mahdaviani SA, Ghaemi MR, Vaezi M, Dorudinia A, Jamaati H, et al. Good’s Syndrome-Association of the Late Onset Combined Immunodeficiency With Thymoma. Rev Lit Case Rep (2018) 17:9. [PubMed] [Google Scholar]
- 105. Bhargava A, Eisenstadt R, Shih JA, Sueblinvong V. A Good Case of Recurrent Pneumonia. J Invest Med High Impact Case Rep (2018) 6:232470961880286. 10.1177/2324709618802869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lee SW, Lee YW, Bae JH. Cytomegalovirus Retinitis as the First Manifestation of Good Syndrome. Ocular Immunol Inflammation (2018) 26:122–4. 10.1080/09273948.2016.1199713 [DOI] [PubMed] [Google Scholar]
- 107. Inomata T, Honda M, Murakami A. Atypical VZV Retinitis in a Patient With Good Syndrome. Ocular Immunol Inflammation (2018) 26:194–8. 10.1080/09273948.2016.1201518 [DOI] [PubMed] [Google Scholar]
- 108. Lee AYS, Chockalingam G. Good Syndrome: Immunodeficiency Associated With Thymoma. Intern Med J (2018) 48:891–2. 10.1111/imj.13950 [DOI] [PubMed] [Google Scholar]
- 109. Esvan R, Fontanelli Šuleková L, Gabrielli S, Biliotti E, Palazzo D, Spaziante M, et al. Severe Diarrhoea Due to Cystoisospora Belli Infection in a Good Syndrome Patient. Parasitol Int (2018) 67:413–4. 10.1016/j.parint.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 110. Jimura N, Fujii K, Higashi Y, Kanekura T. Alopecia Areata Complicated With Good’s Syndrome. Australas J Dermatol (2018) 59:e214–5. 10.1111/ajd.12725 [DOI] [PubMed] [Google Scholar]
- 111. Shankar Kikkeri N, Beladakere Ramaswamy S, Bhagavan SM, Govindarajan R. Recurrent Opportunistic Infections in a Thymectomised Patient With Myasthenia Gravis and Good’s Syndrome. Cureus (2018). 10.7759/cureus.3130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Markovic O, Colakovic N, Marisavljevic D. Agranulocytosis and Good Syndrome in Patient With Thymoma—The Role of Immunosuppressive Treatment After Thymectomy. J Thoracic Oncol (2018) 13:e177–8. 10.1016/j.jtho.2018.04.037 [DOI] [PubMed] [Google Scholar]
- 113. de Felice E, Rutgers A, van den Bergh WM, Lansink-Hartgring AO. Use of Veno-Venous Extracorporeal Life Support in a Patient With Cytomegalovirus and Pneumocystis Jiroveci Related Respiratory Failure Due to Thymoma Associated Immunodeficiency: A Case Report. J Thorac Dis (2018) 10:E736–8. 10.21037/jtd.2018.09.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hagiwara H, Iwata Y, Saito K, Watanabe S, Arima M, Ono Y, et al. Herpes Vegetans Accompanied by Good’s Syndrome. J Dermatol (2018) 45:e269–71. 10.1111/1346-8138.14323 [DOI] [PubMed] [Google Scholar]
- 115. Ueno T, Sato N, Kon T, Haga R, Nunomura J, Nakamichi K, et al. Progressive Multifocal Leukoencephalopathy Associated With Thymoma With Immunodeficiency: A Case Report and Literature Review. BMC Neurol (2018) 18:37. 10.1186/s12883-018-1041-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Barrios Recio J, Perez Rodriguez A, Callero A, Martinez Tadeo JA. Immunodeficiency Associated With Tumour Pathology: Good’s Syndrome. BMJ Case Rep (2019) 12:e227970. 10.1136/bcr-2018-227970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. del Pino Molina L, Wentink M, van Deuren M, van Hagen PM, Smith CIE, van der Burg M. Precursor B-cell Development in Bone Marrow of Good Syndrome Patients. Clin Immunol (2019) 200:39–42. 10.1016/j.clim.2018.11.009 [DOI] [PubMed] [Google Scholar]
- 118. Shi T-Y, Wen X-H, Shi X-H, Lu Y-W. Thymic Epithelial Tumor Complicated by Immunological Abnormalities: Results From a Single-Center Retrospective Study in China. J Thorac Dis (2019) 11:1580–8. 10.21037/jtd.2019.02.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Tajima Y, Yaguchi H, Mito Y. Non-Motor Comorbidity of Myasthenia Gravis: Myasthenia Gravis as a Systemic Immunological Disorder Involving Non-motor Systems. Intern Med (2019) 58:1341–7. 10.2169/internalmedicine.1990-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Thongngarm T, Boonyasiri A, Pradubpongsa P, Tesavibul N, Anekpuritanang T, Kreetapirom P, et al. Features and Outcomes of Immunoglobulin Therapy in Patients With Good Syndrome At Thailand’s Largest Tertiary Referral Hospital. Asian Pac J Allergy Immunol (2019) 37:109–15. 10.12932/AP-131117-0196 [DOI] [PubMed] [Google Scholar]
- 121. Tamburello A, Castelnovo L, Faggioli P, Bompane D, Brando B, Gatti A, et al. Good’s Syndrome, a Rare Form of Acquired Immunodeficiency Associated With Thymomas. Clin Pract (2019) 9(2):51–4. 10.4081/cp.2019.1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jagessar SAR, Long C, Cui B, Zhang F. Improvement of Good’s Syndrome by Fecal Microbiota Transplantation: The First Case Report. J Int Med Res (2019) 47:3408–15. 10.1177/0300060519854913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Glick LR, Wilson WW, Fletcher M. A Case of Good’s Syndrome Complicated by Erythema Multiforme. BMJ Case Rep (2019) 12:e229999. 10.1136/bcr-2019-229999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Campos-Murguía A, Marfil-Garza BA, León-Lara X, Jiménez Gutiérrez JM, Botello-Partida SL. A Patient With Good Syndrome Complicated With Phlegmonous Gastritis. ACG Case Rep J (2019) 6:e00246. 10.14309/crj.0000000000000246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Uy K, Levin E, Mroz P, Li F, Shah S. A Rare Complication of Thymoma: Pure White Cell Aplasia in Good’s Syndrome. Case Rep Hematol (2019) 2019:1–4. 10.1155/2019/1024670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chiatamone Ranieri S, Trasarti S, Arleo MA, Bizzoni L, Bonanni L, Di Battista V, et al. Aplastic Anemia and Good Syndrome in a Heavily Treated Stage Iv Thymoma Patient: A Case Report and Review of the Literature. Case Rep Hematol (2019) 2019:1–6. 10.1155/2019/1910923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Paranavitane S, Handagala S, De Silva R, Chang T. Thymoma Complicated With Myasthenia Gravis and Good Syndrome – a Therapeutic Conundrum: A Case Report. J Med Case Rep (2019) 13:348. 10.1186/s13256-019-2289-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Sena A, Ferreira R, Gonçalves J, Nobre Â. Surgical Treatment of an Advanced Stage Thymoma in a Good’s Syndrome Patient - Case Report. Rev Port Cir Cardiotorac Vasc (2019) 26:269–71. [PubMed] [Google Scholar]
- 129. Lai YW, Tan T-C. Atypical Presentation of Good Syndrome: Acute Hepatitis From Hepatitis B Virus Reactivation. Asia Pac Allergy (2020) 10:e37. 10.5415/apallergy.2020.10.e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Thuraisingam A. An Intrathoracic Mass Behind Severe Immunodeficiency: A Case Report of Good’s Syndrome and Large Type AB Thymoma. J Surg Case Rep (2020) 2020:rjz394. 10.1093/jscr/rjz394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Gereige JD, Maglione PJ, Guenechea-Sola M. Delayed Diagnosis of Good Syndrome. J Allergy Clin Immunol: In Pract (2020) 8:1396–7. 10.1016/j.jaip.2019.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Matta L, Ramírez-Velasco MC, Zea-Vera AF. Herpes Simplex Virus Type 2 Meningitis as a Manifestation of Good’s Syndrome. J Neurovirol (2020) 26:429–32. 10.1007/s13365-019-00819-x [DOI] [PubMed] [Google Scholar]
- 133. Tolomeo M, Bonura S, Abbott M, Anastasia A, Colomba C, Cascio A. Good’s Syndrome and Recurrent Leishmaniasis: A Case Report and Review of Literature. Heliyon (2020) 6:e05061. 10.1016/j.heliyon.2020.e05061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chastagner M, Durupt F, Hicks J, Bruyas A, Sève P, Jamilloux Y. Kaposi’s Sarcoma Associated With Good Syndrome. Médecine Mal Infectieuses (2020) 50:752–4. 10.1016/j.medmal.2020.07.006 [DOI] [PubMed] [Google Scholar]
- 135. Koning MT, van Rossum AP, Tiren-Verbeet NL, Burgers JA, Karim AF. Two Independent Hematological Malignancies in a B-Cell Deficient Good Syndrome Patient. Rheumatology (2020) 60(4):e126–e8. 10.1093/rheumatology/keaa666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Imoto W, Yamada K, Hajika Y, Okamoto K, Myodo Y, Niki M, et al. Disseminated Mycobacterium Abscessus Subsp. Massiliense Infection in a Good’s Syndrome Patient Negative for Human Immunodeficiency Virus and Anti-Interferon-γ Autoantibody: A Case Report. BMC Infect Dis (2020) 20:431. 10.1186/s12879-020-05136-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Isobe S, Sano A, Otsuka H, Azuma Y, Koezuka S, Makino T, et al. Good Syndrome With Cytomegalovirus Hepatitis: Successful Resection of Thymoma: A Case Report. J Cardiothorac Surg (2020) 15:141. 10.1186/s13019-020-01187-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Martinez B, Browne SK. Good Syndrome, Bad Problem. Front Oncol (2014) 4:307. 10.3389/fonc.2014.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, et al. Common Variable Immunodeficiency Disorders: Division Into Distinct Clinical Phenotypes. Blood (2008) 112:277–86. 10.1182/blood-2007-11-124545 [DOI] [PubMed] [Google Scholar]
- 140. Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and Mortality in Common Variable Immune Deficiency Over 4 Decades. Blood (2012) 119:1650–7. 10.1182/blood-2011-09-377945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Burbelo PD, Browne SK, Sampaio EP, Giaccone G, Zaman R, Kristosturyan E, et al. Anti-Cytokine Autoantibodies are Associated With Opportunistic Infection in Patients With Thymic Neoplasia. Blood (2010) 116:4848–58. 10.1182/blood-2010-05-286161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Milito C, Pulvirenti F, Cinetto F, Lougaris V, Soresina A, Pecoraro A, et al. Double-Blind, Placebo-Controlled, Randomized Trial on Low-Dose Azithromycin Prophylaxis in Patients With Primary Antibody Deficiencies. J Allergy Clin Immunol (2019) 144:584–593.e7. 10.1016/j.jaci.2019.01.051 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.