Abstract
Stem cell-based embryo models open an unprecedented avenue for modeling embryogenesis, cell lineage differentiation, tissue morphogenesis, and organogenesis in mammalian development. Experimentation on these embryo models can lead to a better understanding of the mechanisms of development and offers opportunities for functional genomic studies of disease-causing mechanisms, identification of therapeutic targets, and preclinical modeling of advanced therapeutics for precision medicine. An immediate challenge is to create embryo models of high fidelity to embryogenesis and organogenesis in vivo, to ensure that the knowledge gleaned is biologically meaningful and clinically relevant.
Keywords: stem cells, embryo models, blastoids, gastruloids, human embryo development, amniotic sac, primitive streak
In this article, Rossant and Tam review the current progress in stem cell-based embryo models in both mouse and human. They provide a classification of such models based on the presence or absence of early extraembryonic lineages. They provide a glimpse of the future where interdisciplinary approaches will be able to program development in vitro, leading to truly synthetic embryology.
Main Text
Modeling embryonic development
Embryonic development begins with a single cell, the zygote, and progresses through steps of establishing diverse cell lineages, assembling the cells into an embryonic architecture in which increasingly complex cellular interactions and developmental cues drive the generation and patterning of the tissues and organs that make up the whole body. Knowledge gleaned from experimental analysis of genetically modified mammalian embryos and in vitro models of cell and tissue differentiation has provided an understanding of many of the key transcriptional and signaling activities that underlie these processes. In the embryonic setting, individual cells and groups of cells respond to local and long-range signals that can be chemical in nature, such as growth factors, morphogens, ions, and metabolites, as well as mechanobiological signals, such as adhesion, stretch, and pressure. It was inferred that integration of these signals would somehow result in the generation of an embryo and its resultant tissues and organs in a consistently organized manner. However, we still have limited knowledge of how the size and shape of tissues and organs are regulated and how the assembly and proportionality of body parts can be achieved in the stereotypic and reproducible manner typical of normal development. While the early mammalian embryo itself is in principle amenable for elucidating these mechanistic attributes of embryogenesis in the biologically relevant setting, the inaccessibility of peri- and postimplantation stage embryos, the paucity of embryonic material, and the technical difficulties in direct experimental manipulation in vivo pose a significant barrier for undertaking the embryological investigation. A compelling imperative is to develop ex vivo embryo culture systems (see Nakamura et al. and Alberio et al. [this issue of Stem Cell Reports]) and in vitro embryo models that could replicate the events of in vivo mammalian early embryogenesis. A critical prerequisite of the experimental models is that they present an accurate reconstruction of the in vivo events of mammalian embryogenesis, particularly at the early stages when the basic body plan is laid down and when the embryo in vivo is at its most inaccessible for experimentation.
The availability of embryo-derived and reprogrammed pluripotent stem cells (PSCs) that capture the lineage propensity of early embryonic cell types and their later derivatives has allowed the development of innovative stem cell-based models to explore the underlying processes of lineage differentiation and embryonic morphogenesis (Baillie-Benson et al., 2020). PSCs of both mouse and human are deemed to be the surrogate of the epiblast of the blastocyst at peri-implantation stages and they can be driven to differentiate into multiple somatic cell types and the germline cells of the fetus. Conventionally, most differentiation protocols are designed to generate specific cell types that are of clinical relevance for developing regenerative medicine treatments, rather than organized models of normal development. It has long been known that PSCs, when grown in suspension, can form cellular aggregates (embryoid bodies) with some degree of tissue organization (Martin et al., 1977). However, the inconsistent tissue architecture and the variable admixture of cell types in embryoid bodies offer limited opportunity to gain insights into the mechanism of tissue morphogenesis and embryogenesis. Recent advances in the design of in vitro culture conditions have enabled the formation of organotypic structures—organoids or mini-organs—from PSCs (Sahu and Sharan, 2020). However, the formation of organoids seems to have bypassed the earlier phases of embryogenesis from implantation to gastrulation and beyond. Therefore, neither embryoid bodies nor organoids are appropriate experimental models for studying the initial events of embryogenesis and morphogenesis.
The quest to build embryo-like entities (Sahu and Sharan, 2020) from PSCs for in vitro experimentation has been facilitated by the availability of other embryo-derived stem cells of more restricted lineage potential, for example, trophoblast stem cells (TSCs) (Tanaka et al., 1998) and extraembryonic endoderm (XEN) cells (Kunath et al., 2005). PSCs alone or in combination with these other cell types can generate models to recapitulate the underlying developmental events of embryonic-extraembryonic axis formation and the establishment of the basic body plan at gastrulation as well as the kinetics of embryonic patterning at early organogenesis stages. These stem cell-based embryo models are the focus of this commentary and this special issue.
Nomenclature: what are these embryo-like structures?
The popular terms, such as “artificial embryos” or “synthetic embryos”, have been used to describe various forms of stem cell-based embryo models (see Matthews et al. [this issue of Stem Cell Reports]). However, there is nothing artificial about the aggregates of living stem cells that make up the models. Nor are they synthetic, since no new genetic switches or synthetic pathways have been programmed into the starting cells at this time. Synthetic biology and genome engineering approaches may well provide effective ways of generating novel/atypical cells, tissues, and structures from stem cells in the future (see Morsut [this issue of Stem Cell Reports]). Such experimental manipulations may deliberately disrupt the intrinsic gene regulatory networks to reconstruct the development of the embryo from component parts in a synthetic manner. However, current non-synthetic stem cell models are based on known properties of stem cells and embryos and are designed to accurately inform the cellular and tissue behavior and mechanistic attributes of the morphogenetic process in vivo.
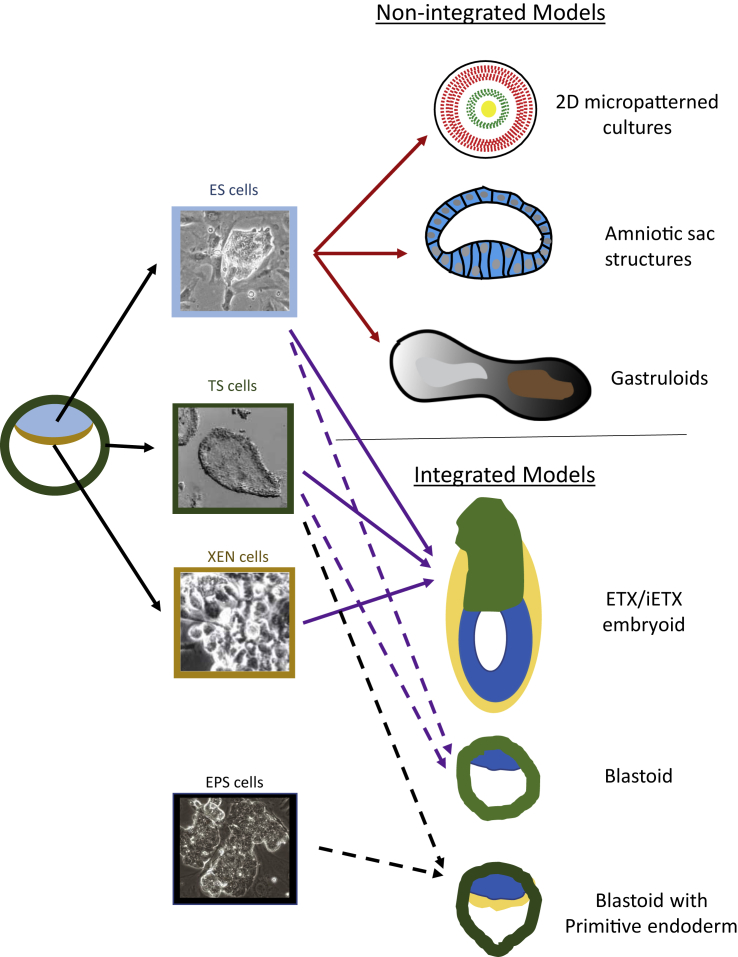
An emerging consensus in the field is that all stem cell-derived models aimed at modeling early embryonic development should be termed stem cell-based embryo models (Hyun et al., 2020; Rivron et al., 2018a; Sahu and Sharan, 2020) (Figure 1). Under this heading, a distinction should be made between models that contain all the integral parts of the whole conceptus, including its extraembryonic tissues, and models that use PSCs exclusively to focus on a selected/specific developmental process, such as gastrulation, development of the embryo axis and laterality, neural tube formation, or somitogenesis. Models that mimic the whole conceptus would be regarded as integrated stem cell-based embryo models. They would include the blastoid (Rivron et al., 2018b) and the ETX embryoid assembled from TSCs, PSCs, and XEN cells (Sozen et al., 2018). Models that shed light on selected morphogenetic events (e.g., gastrulation) or developmental processes (e.g., germ layer differentiation) would be non-integrated stem cell-based embryo models, and would include gastruloids (Beccari et al., 2018; Martyn et al., 2018) and amniotic sac models (Zheng et al., 2019). This distinction is important because of the ethical and regulatory concerns that integrated stem cell-based embryo models could in future be endowed with the potential to form a viable embryo with full developmental potential, in contrast to the non-integrated models that are restricted to the formation of specific body parts of the embryo (Hyun et al., 2020). Currently, integrated models displaying extended potential for postimplantation development have not been generated for laboratory mice or humans.
Figure 1.
Non-integrated And Integrated Stem Cell-Based Embryo Models Generated from Embryo-Derived Stem Cells and Extended Potential Stem Cells
ES cells, embryonic stem cells; TS cells, trophectoderm stem cells; XEN cells, extraembryonic endoderm cells; EPS cells, extended/expanded potential stem cells (image courtesy of Jiang Yang and Pentao Liu).
Non-integrated PSC-based models of embryonic patterning and morphogenesis
Recent advances in the optimization of culture media and customization of the physiological and biophysical properties of the extracellular matrix and niche have enabled the generation of complex and sophisticated embryonic structures from human and mouse PSCs in vitro. Plating human and mouse PSCs on a micro-structured matrix substrate and treating the culture with signaling factors led to the generation of 2D regionalized patterns of germ layer derivatives reminiscent of the primitive streak of the gastrulating embryo. These cultures can recapitulate the local delamination of the mesodermal cells and the specification of germ layer derivatives (see Liu and Warmflash [this issue of Stem Cell Reports]) (Britton et al., 2019; Deglincerti et al., 2016b; Martyn et al., 2018; Morgani et al., 2018; Muncie et al., 2020; Tewary et al., 2017; Xue et al., 2018). PSCs can also be forced to initiate morphogenetic processes downstream of gastrulation by modulation of the property of the scaffold matrix and the extrinsic signaling activity to generate 3D elongated “gastruloids” that partially resemble the posterior elongating body axis. In the mouse, these structures have been shown to display the posterior patterning of the neural tube (Beccari et al., 2018) and the meristic morphogenesis of paraxial mesoderm derived from the posterior neuromesodermal progenitors (van den Brink et al., 2020). Similar but less well-defined structures can also be derived from human PSCs (Moris et al., 2020). By blocking WNT signaling activity, gastruloids comprising anterior structures can be generated (Girgin and Lutolf, 2020; Rossi et al., 2020). Control of the niche factors (e.g., matrix materials) and the dynamic and spatial distribution of signaling molecules in a microfluidic setting can replicate the graded pattern of morphogenetic cues for the formation of neural structures spanning the whole cranio-caudal axis (Rifes et al., 2020). Another microfluidic-controlled environment can generate amniotic sac-like structures from human PSCs with evident polarization of the amniotic epithelium and the embryonic tissues (embryonic disc) that is characteristic of human and non-human primate embryos (Zheng et al., 2019). Transcriptome data indicate the possible presence of mesoderm and germ cell lineages in these structures.
How the different stem cell-based embryo models are generated through the constrained biological activities presented in vitro, however, remains unclear. The presumption that they are formed by the same “self-organization” principles involved in generating specific body parts and fetal organs in vivo reflects our present ignorance of the intrinsic mechanisms of morphogenesis. However, these models can be powerful tools for both validating prior knowledge of the molecular mechanisms of organogenesis and for testing the functional impact of the topological constraint of molecular and mechanobiological signals on cell lineage development and organogenesis. These bioengineering-based approaches may provide an entry point to elucidate the black box of “self-organizing” activity in morphogenesis (see Raspopovic and Marcon [this issue of Stem Cell Reports]). While there is much to be learnt from studying tissue morphogenesis in these non-integrated models, they do not replicate the full range of cell lineage interactions seen in the preimplantation and immediate postimplantation embryo. This has limited their utility for modeling in vivo embryogenesis, the first steps of lineage development and implantation (see Posfai et al. [this issue of Stem Cell Reports]). Therefore, it is crucial to validate and interrogate the findings from these experimental models, especially those related to “non-canonical” morphogenetic events and “novel” function of molecular drivers of lineage differentiation and morphogenesis in settings that are of closest approximation to the embryo in vivo, such as the integrated stem cell-based embryo models.
Integrated stem cell-based embryo models for modeling early embryogenesis
PSC-based embryo models have generally not been able to recapitulate all the features of the implanting embryo, most notably the formation of the trophoblast and the yolk sac derivatives. This is related to the restricted ability of pluripotent embryonic stem cells (ESCs) to generate these two tissue types. The trophectoderm of the blastocyst is essential for attachment and invasion of the conceptus into the uterus at implantation. In addition, extraembryonic cell types, both trophectoderm and primitive endoderm, are key sources of signals to drive patterning and differentiation of the epiblast at gastrulation. Thus, current PSC-based models are not ideal for modeling peri- and postimplantation events in the intact embryo. These are the time points where we still know least about normal human development and yet where problems of early embryo loss in humans are prevalent. While advances have been made in extended in vitro culture of human blastocysts (Deglincerti et al., 2016a; Shahbazi et al., 2016; Xiang et al., 2020; Zhou et al., 2019), and there are new initiatives to collect data directly from early postimplantation human embryos (Li et al., 2020; Tyser et al., 2020), there are still practical and ethical issues in generating enough data from these restricted and limited sources. Thus, it would be particularly empowering to generate integrated stem cell-based embryo models in the human system.
Most work on such models has been performed to date in the mouse. Three lineage-specific stem cell lines have been derived that represent the three lineages of the blastocyst: trophectoderm, epiblast, and primitive endoderm. ESCs originate from the epiblast of the blastocyst and represent the pluripotent lineage. When placed back in a chimera, they contribute to the fetus but usually not to the trophoblast of the placenta or to the yolk sac endoderm (Beddington and Robertson, 1989). TSCs arise from the trophectoderm and have a different growth factor requirement that enables them to self-renew in culture; they contribute only to placental tissues later in development (Tanaka et al., 1998). Primitive endoderm-derived (XEN) cell lines can be passaged in culture and contribute only to yolk sac endoderm (Kunath et al., 2005). These three cell types can be assembled into 3D aggregates, the so-called ETX embryoids, in which the three cell types sort out to generate structures resembling those present in the peri-implantation embryo, but without the outer layers that are required for implantation (Sozen et al., 2018) (Figure 1). The ETX aggregates have been used for in vitro experimentation to model early interactions between the lineages that are critical in later development, particularly up to the onset of gastrulation and germline formation (Sozen et al., 2018). Assembling ESCs and TSCs in a more controlled manner allows them to form an aggregate, the blastoid, in which TSCs form an outer trophectoderm layer enclosing the ESCs and a blastocoel-like cavity indicating a close resemblance to the mouse blastocyst (Rivron et al., 2018b) (Figure 1). Blastoids can potentially form all the later embryonic and extraembryonic lineages and they can elicit a decidual response when transferred to the uterus of a recipient mouse. However, to date there was no further development of the epiblast into an embryo.
One underpinning factor for the developmental deficiencies of the blastoid may be that the ESC, TSC, and XEN cell types are not completely equivalent to the three cell types of the blastocyst. TSCs that express higher levels of Cdx2 can be isolated from TSC cultures and may be closer to the polar trophectoderm than postimplantation trophoblasts. They are thus more likely to contribute cells that reconstitute a blastoid (Aldeguer et al., 2019). This may not be the case for XEN cells, which predominantly give rise to the parietal endoderm and rarely to the visceral endoderm in the chimera. In this regard, a way forward may be to incorporate XEN cells that have been modified by extrinsic signaling activity (Julio et al., 2011) or nEND cells (from ESCs) that may be closer to the primitive endoderm than XEN cells (Anderson et al., 2017). Several laboratories have derived extended potential stem cells (EPSCs) that may have enhanced capacity to generate extraembryonic tissues directly (Li et al., 2019; Yang et al., 2017a, 2017b), although their full potential to generate trophectoderm lineages has been challenged (Posfai et al., 2021). When combined with TSCs they can generate both epiblast and primitive endoderm to make a blastoid that may be more comparable with the in vivo blastocyst (Sozen et al., 2019) (Figure 1). EPSCs have also been claimed to make complete blastoids without need for additional cell types (Li et al., 2019). Monitoring the early steps in the generation of these blastoids has revealed that the EPSC descendants display morphogenetic behavior of compaction, segregation of inside and outside, inner cell mass formation, and blastocyst cavitation. It has also been reported that mouse ESCs alone could make cyst-like structures with some properties similar to the blastocyst (Kime et al., 2019). At this time, none of these blastoid models can develop far after embryo transfer, so caution should be exercised in the interpretation of their in vivo equivalence (see Posfai et al. [this issue of Stem Cell Reports]).
Human integrated stem cell-based embryo models
Extension of the approaches of using naive ESC-, TSC-, and XEN-type cells for generating the mouse models into the human system would provide a powerful avenue to explore the key stages of human development around the time of implantation and immediate postimplantation. Utility of these stem cell-based models may overcome the ethical and logistical limitations of working directly with human embryos. To date, no such integrated models have been reported with human cell lines. In part this reflects that, until recently, stem cell lines reflecting all three lineages of the blastocyst were not available in the human. It is also a consequence of uncertainties about the regulatory principles for scientific research on human embryos and stem cell models and the funding environment for such models (Hyun et al., 2020). In particular, there are potential legal, regulatory, and ethical concerns posed by the integrated stem cell-based embryo models, with regard to the possibility that they could be endowed with the potential to form a whole organism (Pereira Daoud et al., 2020). While these concerns may not apply to those models that are restricted to the formation of specific parts of the embryo, the developmental attributes of the integrated models may place them under the regulatory framework for human embryo research in some jurisdictions (Matthews and Moralí, 2020).
Although the morphological events leading up to blastocyst formation are not dissimilar between mouse and human, there are differences in the timing of the different stages as well as both similarities and differences in gene expression trajectories (Rossant and Tam, 2018; Shahbazi, 2020). Experimental investigation directly in early human embryos has shown conservation of Hippo signaling function in establishing the separation of inner cell mass and trophectoderm (Gerri et al., 2020). In contrast, CRISPR-based knockout of the key pluripotency factor, OCT4, showed an earlier phenotype in human versus mouse embryos (Fogarty et al., 2017) and fibroblast growth factor (FGF) signaling is required for segregation of epiblast and primitive endoderm in mouse but apparently not in human (Kuijk et al., 2012; Roode et al., 2012). These and other differences have been reflected in differences reported in the properties of embryo-derived stem cells between mouse and human.
The ability of stem cells to generate functional ETX embryos or blastoids will depend on their developmental equivalence. Human PSCs in standard culture systems are more like mouse primed ESCs and early postimplantation epiblast and not the naive ESCs derived from the epiblast of the blastocyst (Nichols and Smith, 2009; Rossant, 2008). However, naive human ESCs (hESCs) have been derived and are transcriptionally closer to the epiblast of the blastocyst (Guo et al., 2016; Theunissen et al., 2014), although epigenetically distinct (Pastor et al., 2016). They could be a good starting material for generating human integrated stem cell-based embryo models. Permanent XEN-like cell lines have not been reported in human, but human nEND cells can be derived from ESCs in customized culture conditions. These cells are proposed to mimic the primitive endoderm and can be cultured indefinitely (Linneberg-Agerholm et al., 2019). Human TSCs cannot be derived from blastocysts in humans under the active FGF signaling conditions used for mouse TSCs (Kunath et al., 2014). However, potent TSCs can be isolated from human blastocysts and early villus structures using different signaling pathways and they display many properties of early cytotrophoblast cells (Okae et al., 2018). Human expanded potential cells that are reported to produce trophectoderm and primitive endoderm have been reported, using culture conditions similar to the mouse (Gao et al., 2019). Several pieces of evidence suggest that hESCs themselves may have more potential to generate trophectoderm than their mouse equivalents, beginning with early evidence of trophoblast gene expression in hES cultures (Xu et al., 2002). Recently several groups have shown that human naive ESCs have the potential to generate TS-like cells in vitro (Castel et al., 2020; Cinkornpumin et al., 2020; Dong et al., 2020; Guo et al., 2020). Furthermore, it has been shown that the pathway to reprogramming human fibroblasts to iPSC may pass through a window where trophectoderm-like gene expression can be discerned and TS-like cells can be isolated (Liu et al., 2020). It is plausible that other stem cell types, such as naive PSCs and XEN cells, may be present at intermediate stages of cellular reprogramming. Stem cells representing the three blastocyst lineages, iPSCs, iTSCs, and iXEN cells can be induced directly from the mouse fibroblasts (Benchetrit et al., 2019). Direct induction of these stem cells from human somatic cells may provide a ready cellular recipe for the generation of blastoids, without the need to isolate or assemble different stem cell types. The stage may be set soon for advances in both mouse and human integrated stem cell-based embryo models.
The future
There is still an ongoing requirement to investigate the human embryo per se to establish the benchmarks for assessing developmental milestones and the elucidation of the identity and trajectory of cell lineages in the stem cell-based models. Knowledge of the developmental profile of non-human primate embryos, such as the cynomolgus monkey and the marmoset, can provide some useful indicators of embryonic development based on the premise of conservation of mechanism of development among the primates (see Nakamura and Saitou and Alberio et al. [this issue of Stem Cell Reports]). However, there are differences in the details of the developmental processes among the embryos of different primate species that may have to be considered for cross-species extrapolation of information. The knowledge of the developmental potential of the different embryo models is critical for the consideration of their compliance with the ethical and legal framework of human embryo research.
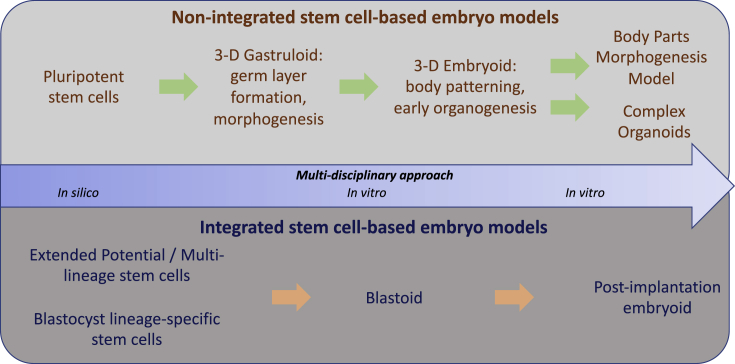
To make the best models in both mouse and human, there is an ongoing need to enhance the lineage relatedness of the starting stem cells to the lineage events or interactions of interest and to improve the development of integrated stem cell-based embryo models (Figure 2). Modeling the early phases of body patterning and organogenesis in non-integrated embryo models will ensure that later organoid models are high-fidelity reflections of in vivo organogenesis. The experimental approaches need not be limited to modulation of the in vitro culture conditions to enhance normal morphogenesis. Bioengineering approaches will be key to improving the development of embryo models by providing engineered in vitro niches that mimic the mechanobiological input as well as the chemical signaling environment of the embryo (Liu and Warmflash and Sonnen et al. [this issue of Stem Cell Reports]). In the longer term, an interdisciplinary approach that encompasses systems biology, cell and developmental biology, biophysics, mechanobiology, bioengineering, machine learning, data science, and computational modeling will enable the redirection of stem cells into new functional forms. The integrated knowledge will be used to design an embryo model in silico (Levin et al., 2020; Libby et al., 2019), followed by the experimental generation of this digital twin in reality (Figure 2). This then will launch truly the era of synthetic embryogenesis and morphogenesis.
Figure 2.
The Path to the Generation of Stem Cell-Based Embryo Models
The production of the embryo models would require the integration of multi-disciplinary knowledge to build an in silico model, followed by translating the digital information to the generation of the in vitro model. The generation of non-integrated stem cell-based embryo models may involve the stepwise development through the gastruloid and embryoid to the morphogenesis models and organoid models. The integrated stem cell-based embryo model requires the assembly of blastocyst lineage stem cells into the blastoid and the blastoid-derived postimplantation embryoid.
Conflicts of interests
J.R. is a member of the Editorial Board of Stem Cell Reports.
Contributor Information
Janet Rossant, Email: janet.rossant@gairdner.org.
Patrick P.L. Tam, Email: ptam@cmri.org.au.
References
- Aldeguer J.F., Kip M., Vivie J., Li L., Alemany A., Korving J., Darmis F., van Oudenaarden A., Geijsen N., Rivron N.C. Embryonic signals perpetuate polar-like trophoblast stem cells and pattern the blastocyst axis. bioRxiv. 2019 doi: 10.1101/510362. [DOI] [Google Scholar]
- Anderson K.G., Hamilton W.B., Roske F.V., Azad A., Knudsen T.E., Canham M.A., Forrester L.M., Brickman J.M. Insulin fine-tunes self-renewal pathways governing naive pluripotency and extra-embryonic endoderm. Nat. Cell Biol. 2017;19:1164–1177. doi: 10.1038/ncb3617. [DOI] [PubMed] [Google Scholar]
- Baillie-Benson P., Moris N., Martinez Arias A. Pluripotent stem cell models of early mammalian development. Curr. Opin. Cell Biol. 2020;66:89–96. doi: 10.1016/j.ceb.2020.05.010. [DOI] [PubMed] [Google Scholar]
- Beccari L., Moris N., Girgin M., Turner D.A., Baillie-Johnson P., Cossy A.C., Lutolf M.P., Duboule D., Arias A.M. Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature. 2018;562:272–276. doi: 10.1038/s41586-018-0578-0. [DOI] [PubMed] [Google Scholar]
- Beddington R.S.P., Robertson E.J. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development. 1989;105:733–737. doi: 10.1242/dev.105.4.733. [DOI] [PubMed] [Google Scholar]
- Benchetrit H., Jaber M., Zayat V., Sebban S., Pushett A., Makedonski K., Zakheim Z., Radwan A., Maoz N., Lasry R. Direct induction of the three pre-implantation blastocyst cell types from fibroblasts. Cell Stem Cell. 2019;24:983–994.e7. doi: 10.1016/j.stem.2019.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton G., Heemskerk I., Hodge R., Qutub A.A., Warmflash A. A novel self-organizing embryonic stem cell system reveals signaling logic underlying the patterning of human ectoderm. Development. 2019;146:dev179093. doi: 10.1242/dev.179093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel G., Meistermann D., Bretin B., Firmin J., Blin J., Loubersac S., Bruneau A., Chevolleau S., Kilens S., Chariau C. Induction of human trophoblast stem cells from somatic cells and pluripotent stem cells. Cell Rep. 2020;33:108419. doi: 10.1016/j.celrep.2020.108419. [DOI] [PubMed] [Google Scholar]
- Cinkornpumin J.K., Kwon S.Y., Guo Y., Hossain I., Sirois J., Russett C.S., Tseng H.W., Okae H., Arima T., Duchaine T.F. Naive human embryonic stem cells can give rise to cells with a trophoblast-like transcriptome and methylome. Stem Cell Reports. 2020;15:198–213. doi: 10.1016/j.stemcr.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deglincerti A., Croft G.F., Pietila L.N., Zernicka-Goetz M., Siggia E.D., Brivanlou A.H. Self-organization of the in vitro attached human embryo. Nature. 2016;533:251–254. doi: 10.1038/nature17948. [DOI] [PubMed] [Google Scholar]
- Deglincerti A., Etoc F., Guerra M.C., Martyn I., Metzger J., Ruzo A., Simunovic M., Yoney A., Brivanlou A.H., Siggia E. Self-organization of human embryonic stem cells on micropatterns. Nat. Protoc. 2016;11:2223–2232. doi: 10.1038/nprot.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C., Beltcheva M., Gontarz P., Zhang B., Popli P., Fischer L.A., Khan S.A., Park K.-m., Yoon E.-J., Xing X. Derivation of trophoblast stem cells from naïve human pluripotent stem cells. Elife. 2020;9:e52504. doi: 10.7554/eLife.52504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty N.M.E., McCarthy A., Snijders K.E., Powell B.E., Kubikova N., Blakeley P., Lea R., Elder K., Wamaitha S.E., Kim D. Genome editing reveals a role for OCT4 in human embryogenesis. Nature. 2017;550:67–73. doi: 10.1038/nature24033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Ruan D., Wu J., Liu P., Gao X., Chen X., Ryan D., Yang J., Antunes L., Campos L.S. Establishment of porcine and human expanded potential stem cells. Nat. Cell Biol. 2019;21:687–699. doi: 10.1038/s41556-019-0333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerri C., McCarthy A., Alanis-Lobato G., Demtschenko A., Bruneau A., Loubersac S., Fogarty N.M.E., Hampshire D., Elder K., Snell P. Initiation of a conserved trophectoderm program in human, cow and mouse embryos. Nature. 2020;587:443–447. doi: 10.1038/s41586-020-2759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgin M.U., Lutolf M.P. Gastruloids generated without exogenous Wnt activation develop anterior neural tissues. bioRxiv. 2020 doi: 10.1101/2020.2010.2010.334326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G., Stirparo G.G., Strawbridge S., Spindlow D., Yang J., Clarke J., Dattani A., Yanagida A., Li M.A., Myers S. Human naïve epiblast cells possess unrestricted lineage potential. bioRxiv. 2020 doi: 10.1101/2020.02.04.933812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G., von Meyenn F., Santos F., Chen Y., Reik W., Bertone P., Smith A., Nichols J. Naive pluripotent stem cells derived directly from isolated cells of the human inner cell mass. Stem Cell Reports. 2016;6:437–446. doi: 10.1016/j.stemcr.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun I., Munsie M., Pera M.F., Rivron N.C., Rossant J. Toward guidelines for research on human embryo models formed from stem cells. Stem Cell Reports. 2020;14:169–174. doi: 10.1016/j.stemcr.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julio M.K.-d., Alvarez M.J., Galli A., Chu J., Price S.M., Califano A., Shen M.M. Regulation of extra-embryonic endoderm stem cell differentiation by Nodal and Cripto signaling. Development. 2011;138:3885–3895. doi: 10.1242/dev.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kime C., Kiyonari H., Ohtsuka S., Kohbayashi E., Asahi M., Yamanaka S., Takahashi M., Tomoda K. Induced 2C expression and implantation-competent blastocyst-like cysts from primed pluripotent stem cells. Stem Cell Reports. 2019;13:485–498. doi: 10.1016/j.stemcr.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijk E.W., van Tol L.T., Van de Velde H., Wubbolts R., Welling M., Geijsen N., Roelen B.A. The roles of FGF and MAP kinase signaling in the segregation of the epiblast and hypoblast cell lineages in bovine and human embryos. Development. 2012;139:871–882. doi: 10.1242/dev.071688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunath T., Arnaud D., Uy G.D., Okamoto I., Chureau C., Yamanaka Y., Heard E., Gardner R.L., Avner P., Rossant J. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development. 2005;132:1649–1661. doi: 10.1242/dev.01715. [DOI] [PubMed] [Google Scholar]
- Kunath T., Yamanaka Y., Detmar J., MacPhee D., Caniggia I., Rossant J., Jurisicova A. Developmental differences in the expression of FGF receptors between human and mouse embryos. Placenta. 2014;35:1079–1088. doi: 10.1016/j.placenta.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Levin M., Bongard J., Lunshof J.E. Applications and ethics of computer-designed organisms. Nat. Rev. Mol. Cell Biol. 2020;21:655–656. doi: 10.1038/s41580-020-00284-z. [DOI] [PubMed] [Google Scholar]
- Li L.-C., Wang X., Xu Z.-R., Wang Y.-C., Feng Y., Yang L., Qiu W.-L., Yang L., Yu X.-X., Gu J. Single-cell patterning and axis characterization in the murine and human definitive endoderm. Cell Res. 2020 doi: 10.1038/s41422-020-00426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Zhong C., Yu Y., Liu H., Sakurai M., Yu L., Min Z., Shi L., Wei Y., Takahashi Y. Generation of blastocyst-like structures from mouse embryonic and adult cell cultures. Cell. 2019;179:687–702.e18. doi: 10.1016/j.cell.2019.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby A.R.G., Briers D., Haghighi I., Joy D.A., Conklin B.R., Belta C., McDevitt T.C. Automated design of pluripotent stem cell self-organization. Cell Syst. 2019;9:483–495.e10. doi: 10.1016/j.cels.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linneberg-Agerholm M., Wong Y.F., Romero Herrera J.A., Monteiro R.S., Anderson K.G.V., Brickman J.M. Naive human pluripotent stem cells respond to Wnt, Nodal and LIF signalling to produce expandable naive extra-embryonic endoderm. Development. 2019;146:dev180620. doi: 10.1242/dev.180620. [DOI] [PubMed] [Google Scholar]
- Liu X., Ouyang J.F., Rossello F.J., Tan J.P., Davidson K.C., Valdes D.S., Schröder J., Sun Y.B.Y., Chen J., Knaupp A.S. Reprogramming roadmap reveals route to human induced trophoblast stem cells. Nature. 2020;586:101–107. doi: 10.1038/s41586-020-2734-6. [DOI] [PubMed] [Google Scholar]
- Martin G.R., Wiley L.M., Damjanov I. The development of cystic embryoid bodies in vitro from clonal teratocarcinoma stem cells. Dev. Biol. 1977;61:230–244. doi: 10.1016/0012-1606(77)90294-9. [DOI] [PubMed] [Google Scholar]
- Martyn I., Kanno T.Y., Ruzo A., Siggia E.D., Brivanlou A.H. Self-organization of a human organizer by combined Wnt and Nodal signalling. Nature. 2018;558:132–135. doi: 10.1038/s41586-018-0150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews K.R., Moralí D. National human embryo and embryoid research policies: a survey of 22 top research-intensive countries. Regener. Med. 2020;15:1905–1917. doi: 10.2217/rme-2019-0138. [DOI] [PubMed] [Google Scholar]
- Morgani S.M., Metzger J.J., Nichols J., Siggia E.D., Hadjantonakis A.K. Micropattern differentiation of mouse pluripotent stem cells recapitulates embryo regionalized cell fate patterning. eLife. 2018;7:e32839. doi: 10.7554/eLife.32839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moris N., Martinez Arias A., Steventon B. Experimental embryology of gastrulation: pluripotent stem cells as a new model system. Curr. Opin. Genet. Dev. 2020;64:78–83. doi: 10.1016/j.gde.2020.05.031. [DOI] [PubMed] [Google Scholar]
- Muncie J.M., Ayad N.M.E., Lakins J.N., Xue X., Fu J., Weaver V.M. Mechanical tension promotes formation of gastrulation-like nodes and patterns mesoderm specification in human embryonic stem cells. Dev. Cell. 2020;55:679–694.e11. doi: 10.1016/j.devcel.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J., Smith A. Naive and primed pluripotent states. Cell Stem Cell. 2009;4:487–492. doi: 10.1016/j.stem.2009.05.015. [DOI] [PubMed] [Google Scholar]
- Okae H., Toh H., Sato T., Hiura H., Takahashi S., Shirane K., Kabayama Y., Suyama M., Sasaki H., Arima T. Derivation of human trophoblast stem cells. Cell Stem Cell. 2018;22:50–63.e6. doi: 10.1016/j.stem.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Pastor W.A., Chen D., Liu W., Kim R., Sahakyan A., Lukianchikov A., Plath K., Jacobsen S.E., Clark A.T. Naive human pluripotent cells feature a methylation landscape devoid of blastocyst or germline memory. Cell Stem Cell. 2016;18:323–329. doi: 10.1016/j.stem.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira Daoud A.M., Popovic M., Dondorp W.J., Trani Bustos M., Bredenoord A.L., Chuva de Sousa Lopes S.M., van den Brink S.C., Roelen B.A.J., de Wert G.M.W.R., Heindryckx B. Modelling human embryogenesis: embryo-like structures spark ethical and policy debate. Hum. Reprod. Update. 2020;26:779–798. doi: 10.1093/humupd/dmaa027. [DOI] [PubMed] [Google Scholar]
- Posfai E., Schell J.P., Janiszewski A., Rovic I., Murray A., Bradshaw B., Yamakawa T., Pardon T., El Bakkali M., Talon I. Evaluating totipotency using criteria of increasing stringency. Nat. Cell Biol. 2021;23:49–60. doi: 10.1038/s41556-020-00609-2. [DOI] [PubMed] [Google Scholar]
- Rifes P., Isaksson M., Rathore G.S., Aldrin-Kirk P., Møller O.K., Barzaghi G., Lee J., Egerod K.L., Rausch D.M., Parmar M. Modeling neural tube development by differentiation of human embryonic stem cells in a microfluidic WNT gradient. Nat. Biotechnol. 2020;38:1265–1273. doi: 10.1038/s41587-020-0525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivron N., Pera M., Rossant J., Martinez Arias A., Zernicka-Goetz M., Fu J., van den Brink S., Bredenoord A., Dondorp W., de Wert G. Debate ethics of embryo models from stem cells. Nature. 2018;564:183–185. doi: 10.1038/d41586-018-07663-9. [DOI] [PubMed] [Google Scholar]
- Rivron N.C., Frias-Aldeguer J., Vrij E.J., Boisset J.C., Korving J., Vivie J., Truckenmuller R.K., van Oudenaarden A., van Blitterswijk C.A., Geijsen N. Blastocyst-like structures generated solely from stem cells. Nature. 2018;557:106–111. doi: 10.1038/s41586-018-0051-0. [DOI] [PubMed] [Google Scholar]
- Roode M., Blair K., Snell P., Elder K., Marchant S., Smith A., Nichols J. Human hypoblast formation is not dependent on FGF signalling. Dev. Biol. 2012;361:358–363. doi: 10.1016/j.ydbio.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J. Stem cells and early lineage development. Cell. 2008;132:527–531. doi: 10.1016/j.cell.2008.01.039. [DOI] [PubMed] [Google Scholar]
- Rossant J., Tam P.P.L. Exploring early human embryo development. Science. 2018;360:1075–1076. doi: 10.1126/science.aas9302. [DOI] [PubMed] [Google Scholar]
- Rossi G., Broguiere N., Miyamoto M., Boni A., Guiet R., Girgin M., Kelly R.G., Kwon C., Lutolf M.P. Capturing cardiogenesis in gastruloids. Cell Stem Cell. 2020;28:230–240.e6. doi: 10.1016/j.stem.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu S., Sharan S.K. Translating embryogenesis to generate organoids: novel approaches to personalized medicine. iScience. 2020;23:101485. doi: 10.1016/j.isci.2020.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi M.N. Mechanisms of human embryo development: from cell fate to tissue shape and back. Development. 2020;147:dev190629. doi: 10.1242/dev.190629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi M.N., Jedrusik A., Vuoristo S., Recher G., Hupalowska A., Bolton V., Fogarty N.M., Campbell A., Devito L.G., Ilic D. Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol. 2016;18:700–708. doi: 10.1038/ncb3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozen B., Amadei G., Cox A., Wang R., Ellen, Czukiewska S., Chappell L., Voet T., Michel G., Jing N. Self-assembly of embryonic and two extra-embryonic stem cell types into gastrulating embryo-like structures. Nat. Cell Biol. 2018;20:979–989. doi: 10.1038/s41556-018-0147-7. [DOI] [PubMed] [Google Scholar]
- Sozen B., Cox A.L., De Jonghe J., Bao M., Hollfelder F., Glover D.M., Zernicka-Goetz M. Self-organization of mouse stem cells into an extended potential blastoid. Dev. Cell. 2019;51:698–712. doi: 10.1016/j.devcel.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Kunath T., Hadjantonakis A.K., Nagy A., Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- Tewary M., Ostblom J., Prochazka L., Zulueta-Coarasa T., Shakiba N., Fernandez-Gonzalez R., Zandstra P.W. A stepwise model of reaction-diffusion and positional information governs self-organized human peri-gastrulation-like patterning. Development. 2017;144:4298–4312. doi: 10.1242/dev.149658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen T.W., Powell B.E., Wang H., Mitalipova M., Faddah D.A., Reddy J., Fan Z.P., Maetzel D., Ganz K., Shi L. Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell. 2014;15:471–487. doi: 10.1016/j.stem.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyser R.C.V., Mahammadov E., Nakanoh S., Vallier L., Scialdone A., Srinivas S. A spatially resolved single cell atlas of human gastrulation. bioRxiv. 2020 doi: 10.1101/2020.07.21.213512. [DOI] [Google Scholar]
- van den Brink S.C., Alemany A., van Batenburg V., Moris N., Blotenburg M., Vivie J., Baillie-Johnson P., Nichols J., Sonnen K.F., Martinez Arias A. Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Nature. 2020;582:405–409. doi: 10.1038/s41586-020-2024-3. [DOI] [PubMed] [Google Scholar]
- Xiang L., Yin Y., Zheng Y., Ma Y., Li Y., Zhao Z., Guo J., Ai Z., Niu Y., Duan K. A developmental landscape of 3D-cultured human pre-gastrulation embryos. Nature. 2020;577:537–542. doi: 10.1038/s41586-019-1875-y. [DOI] [PubMed] [Google Scholar]
- Xu R.H., Chen X., Li D.S., Li R., Addicks G.C., Glennon C., Zwaka T.P., Thomson J.A. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat. Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- Xue X., Sun Y., Resto-Irizarry A.M., Yuan Y., Aw Yong K.M., Zheng Y., Weng S., Shao Y., Chai Y., Studer L. Mechanics-guided embryonic patterning of neuroectoderm tissue from human pluripotent stem cells. Nat. Mater. 2018;17:633–641. doi: 10.1038/s41563-018-0082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Ryan D.J., Wang W., Tsang J.C., Lan G., Masaki H., Gao X., Antunes L., Yu Y., Zhu Z. Establishment of mouse expanded potential stem cells. Nature. 2017;550:393–397. doi: 10.1038/nature24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Liu B., Xu J., Wang J., Wu J., Shi C., Xu Y., Dong J., Wang C., Lai W. Derivation of pluripotent stem cells with in vivo embryonic and extraembryonic potency. Cell. 2017;169:243–257.e25. doi: 10.1016/j.cell.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Xue X., Shao Y., Wang S., Esfahani S.N., Li Z., Muncie J.M., Lakins J.N., Weaver V.M., Gumucio D.L. Controlled modelling of human epiblast and amnion development using stem cells. Nature. 2019;573:421–425. doi: 10.1038/s41586-019-1535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Wang R., Yuan P., Ren Y., Mao Y., Li R., Lian Y., Li J., Wen L., Yan L. Reconstituting the transcriptome and DNA methylome landscapes of human implantation. Nature. 2019;572:660–664. doi: 10.1038/s41586-019-1500-0. [DOI] [PubMed] [Google Scholar]