Abstract
Mammalian embryonic development is a complex process driven by self-organization. Understanding how a fertilized egg develops into an embryo composed of more than 200 cell types in precise spatial patterns remains one of the fundamental challenges in biology. Pluripotent stem cells have been used as in vitro models for investigating mammalian development, and represent promising building blocks for regenerative therapies. Recently, sophisticated stem cell-based models that recapitulate early embryonic fate patterning and morphogenesis have been developed. In this article, we review recent advances in stem cell models of embryos in particular focusing on signaling activities underpinning cell fate decisions in space and time.
Keywords: signaling, self-organization, embryonic stem cells, differentiation, gastrulation
In this article, Liu and Warmflash discuss recent advances in stem cell-based models of embryos in particular focusing on morphogen signaling pathways underpinning cell fate decisions. In-depth comparisons of signaling activities between in vitro 2D and 3D models of embryos and their in vivo counterparts highlight the value of these emerging models for understanding self-organized signaling dynamics during mammalian embryogenesis.
Introduction
A mammalian embryo begins as a single cell that divides to form a ball of isotropic cells. These cells then undergo polarization, lineage allocation, symmetry breaking, and body axis specification. During this process, individual cells adopt distinct fates according to their position in the embryo. Ex-vivo-cultured pre-implantation embryos can continue development in the absence of maternal tissues (Bedzhov et al., 2015; Deglincerti et al., 2016; Ma et al., 2019; Shahbazi et al., 2016; Xiang et al., 2020), indicating that early mammalian embryos possess a remarkable capacity for self-organization. During embryonic development, signaling molecules, known as morphogens, convey positional information within the embryo to orchestrate cell fate decisions. In the classic model, cells positioned in a gradient of a morphogen implement differential transcriptional programs according to the local concentration. Two crucial questions that have drawn long-standing interest are: (1) How are spatial patterns of morphogen molecules established within mammalian embryos? (2) How do the receiving cells interpret these signals? Several recent studies have shown that understanding signal interpretation requires understanding how cells process temporally changing signaling inputs. In addition to the amplitude of signaling, the duration of ligand exposure and the rate of change of ligand concentration also affect cellular responses, highlighting the importance of signaling dynamics (Dessaud et al., 2007; Dubrulle et al., 2015; Heemskerk et al., 2019; van Boxtel et al., 2015).
A number of factors make it difficult to address these questions. Mammalian embryos are small and difficult to obtain in large numbers, and development takes place in utero, which prevents perturbation and observation without interrupting normal development. Investigation of human embryonic development is even more complicated owing to additional ethical and practical issues in obtaining human embryos.
Pluripotent stem cells possess the potential to give rise to all the cell types of the embryo and can be stably maintained in vitro. They are either derived from embryos or somatic cells through reprogramming (Evans and Kaufman, 1981; Martin, 1981; Takahashi and Yamanaka, 2006; Thomson et al., 1998). By varying the culture environment, pluripotent stem cells can be maintained in distinct states that correspond to progressive development through several pluripotent stages in vivo. Differentiation studies using pluripotent stem cells have provided insights into developmental programs at the molecular and cellular levels; however, the resulting cultures of differentiated cells typically lack spatial ordering of cell fates. Recent studies have shown that appropriate physical or biochemical manipulation of the culture environment for 2D embryonic stem cell (ESC) colonies or 3D aggregates can harness the ability of PSCs to self-organize into a patterned ensemble of differentiated cell fates resembling key features of mammalian embryos, including signaling events, fate patterning, and morphogenesis (Beccari et al., 2018; van den Brink et al., 2020; Harrison et al., 2017; Moris et al., 2020; Poh et al., 2014; Turner et al., 2017; Warmflash et al., 2014; Zheng et al., 2019). These models present an opportunity to address questions which have proven difficult in vivo.
In this article, we discuss the developmental relevance of pluripotent stem cells and review recent progress in pluripotent stem cell-based embryo models. We focus in particular on the signaling activities involved in pre- and post-implantation embryonic development and how in vitro embryo models can provide unique opportunities for quantitative dissection of signaling responses and networks that mammalian embryos utilize to coordinate complex developmental events.
Signaling in early mammalian development
Development of early-stage embryos
The pre-implantation embryo possesses a remarkable ability for self-organization. Mammalian life starts as a fertilized egg that divides and undergoes lineage segregation, giving rise to three distinct subpopulations comprising the blastocyst. The first fate decision segregates the outer trophectoderm (TE) from the inner cell mass (ICM). Subsequently, the ICM separates into primitive endoderm (PrE) at the surface and epiblast (EPI) in the deeper portion. TE generates the placenta connecting the embryo proper with the mother, while the PrE will differentiate into the parietal endoderm and visceral endoderm (VE) of the yolk sac. The EPI cells give rise to all three germ layers of the embryo, while the TE- and PrE-derived extra-embryonic tissues serve as signaling hubs providing cues for embryonic patterning. The embryo is able to accomplish this entire process without external cues.
The two cells resulting from the first cell division are believed to be developmentally identical (Tarkowski, 1959). As development proceeds, morphological changes occur, and differential gene expression patterns emerge. Cells that acquire apical-basal polarity and remain at the embryo surface, become TE (Cdx2+/Oct4–), and continue to establish tight cell-cell junctions to form an epithelial layer ensuring the formation of blastocoel cavity. Concomitantly, a group of inside apolar cells, are compressed to one side of the cavity to become ICM (Cdx2–/Oct4+), resulting in broken spherical symmetry (Johnson and Ziomek, 1981; Lim et al., 2020; Maître et al., 2016; Zhang and Hiiragi, 2018). Transformation of the embryo from a spherically symmetric ball of isotropic cells to a hollow structure comprising two distinct cell types requires symmetry-breaking mechanisms. Unlike embryos of other model organisms, for example, fly and amphibian, which rely on maternal determinants to break symmetry, an early mammalian embryo appears to require no pre-patterning for symmetry breaking. Although genetic perturbations in animal models have identified genes that play roles in symmetry breaking of mammalian embryos (Hirate et al., 2013; Korotkevich et al., 2017), understanding the mechanisms will require quantitative and temporal measurements of signaling and cell fate specification. In vitro embryo models can circumvent the challenges in obtaining such data, especially when it comes to human embryonic development. It is worth noting that stochastic gene expression may contribute to symmetry-breaking events in early-stage embryos, and we refer readers to recent reviews on this topic (Chen et al., 2018; Eling et al., 2019).
BMP, Wnt, FGF, and Nodal signaling in early embryos
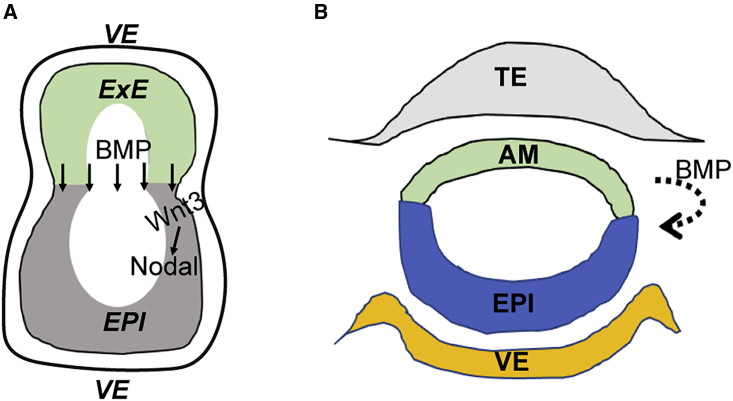
In early embryos, cells communicate with each other by using a handful of conserved signaling pathways, including the transforming growth factor β (TGF-β) superfamily (BMP and Nodal), Wnt, and fibroblast growth factor (FGF) signaling pathways. A hierarchy of BMP-Wnt-Nodal signaling dictates axis formation and cell fate allocation in peri-gastrulation embryos (Figure 1A). It is worth noting that these same pathways are involved in both earlier and later developmental events so that each signaling molecule plays multiple distinct roles that depend on the context.
Figure 1.
Schematic of signaling activities in peri-gastrulation embryos
(A) In mouse embryos (approximately E5.5–E6.5), BMP4 emanating from the ExE signals to the adjacent epiblast and visceral endoderm to enhance Wnt3 expression, which in turn upregulates Nodal expression.
(B) In non-human primate embryos (approximately days post-fertilization 13–14), BMP4 is suggested to be expressed in amnion and induces primitive streak formation in the adjacent epiblast (Yang et al., 2020).
BMP signaling is active at pre-implantation stages. Differential expression of BMP pathway ligands and receptors between inner (future ICM) and outer (future TE) cells is detected in 16-cell-stage mouse embryos. For example, Bmp4 and Bmp7 are exclusively produced by inner cells, while the receptor Bmpr2 is exclusively expressed in outer cells (Graham et al., 2014). Although it was thought that detectable phenotypes for loss-of-function mutations of pathway components do not emerge before implantation (Beppu et al., 2000; Chu et al., 2004; Sirard et al., 1998; Yang et al., 1998), recent studies suggest that loss of BMP signaling leads to impaired development of the TE and PrE lineages (Graham et al., 2014), and that its role in the TE lineage is to maintain the stem cell population (Sozen et al., 2021).
FGF/ERK signaling plays a variety of important roles in multiple tissues during early development (Rossant, 2018). Signaling through Fgfr1 is required for TE differentiation but not for specification (Kurowski et al., 2019). FGF signaling is essential for PrE specification (Kang et al, 2013, 2017; Krawchuk et al., 2013) and, through this role, FGF signaling has been suggested to maintain appropriate proportions of cell types in the blastocyst (Saiz et al., 2020). Recently, FGF signaling dynamics have been observed in early mouse embryos, and shown to be cell type specific. For example, high levels are observed in the PrE and sporadic bursts in the EPI. How these dynamics relate to fate decisions remains an open question (Simon et al., 2020). At gastrulation stage, loss of FGF signaling impairs the epithelial to mesenchymal transition associated with gastrulation and causes defects in maturation of the mesendoderm (Arnold and Robertson, 2009).
The Wnt pathway is likely active in early embryos as the β-catenin-dependent enhancer of the Nodal gene is active even before implantation (Granier et al., 2011). Nonetheless, the removal of all Wnt signaling does not have a clear phenotype before the onset of gastrulation (Biechele et al., 2013); however, a number of studies show that Wnt is absolutely required for gastrulation initiation and mesoderm differentiation (Biechele et al., 2013; Huelsken et al., 2000; Liu et al., 1999).
Nodal signaling is a key player in post-implantation development, it is required for the stability of the post-implantation EPI (Brennan et al., 2001; James et al., 2005; Vallier et al., 2005), and also for formation of the primary body axes and specification of mesendoderm cell fates through reciprocal interactions between embryonic and extra-embryonic tissues. Recently, it has been suggested that Nodal signaling is also involved in embryo elongation via cooperation with the planar cell polarity signaling pathway (Schauer et al., 2020; Williams and Solnica-Krezel, 2020). In post-implantation embryos, Nodal is expressed in EPI and PrE cells, and sustains Bmp4 expression in the adjacent extra-embryonic ectoderm (ExE). In turn, BMP4 from the ExE proximal to the EPI induces Wnt3 expression in the juxtaposed EPI and PrE, and Wnt, in turn, further activates Nodal (Ben-Haim et al., 2006). Elevated Nodal and Wnt activities define the posterior side where the primitive streak forms (Figure 1A). Alongside EPI development, PrE cells in contact with EPI become VE. A group of distal VE cells, known as the distal VE (DVE), express the Nodal and Wnt antagonists, Lefty1, Cerberus-like 1 (Cer1), and DKK1. Before gastrulation takes place, DVE cells migrate anteriorly and ultimately form the anterior VE (AVE) (Yamamoto et al., 2004). Nodal and Wnt antagonists secreted by the AVE inhibit Nodal and Wnt signaling in the anterior EPI and restrict their activities, which induce the primitive streak, to the posterior side.
During gastrulation, EPI cells differentiate into three germ layers, definitive endoderm, mesoderm, and ectoderm. It is believed that graded Nodal, Wnt, and FGF signaling levels along the proximal-posterior to distal-anterior axis dictate cell decisions during gastrulation (Arnold and Robertson, 2009), although the relationships between signaling and fate are incompletely understood. For example, multiple lines of evidence suggest that the highest Nodal levels are required for the cell fates defined in the anterior primitive streak, definitive endoderm, and axial mesoderm (Dougan et al., 2003; Gritsman et al., 2000; Thisse et al., 2000; Vincent et al., 2003), while the expression pattern of Nodal suggests greater activity in the posterior primitive streak (Brennan et al., 2001; Collignon et al., 1996). One possibility is that cell fates are determined according to the timing and speed by which cells migrate through the signaling gradients around the primitive streak (Heemskerk and Warmflash, 2016). However, evaluating this and other hypotheses requires precise measurements of signaling dynamics and cell fate specification, which has proven difficult due to the inaccessibility of the embryo implanted in utero and the technical challenges involved in imaging.
Measuring morphogen signaling dynamics
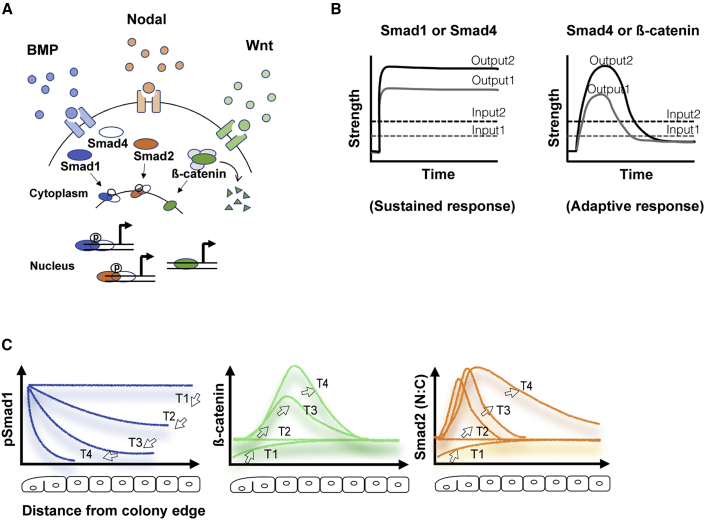
Approaches using fluorescent proteins have enabled monitoring of signaling dynamics in individual living cells. For TGF-β superfamily signaling, binding of ligands, such as BMP4 or Nodal, to their receptor complexes leads to phosphorylation and activation of pathway-specific receptor-regulated Smads (R-Smads), which then complex with a common co-Smad (Smad4) and translocate to the nucleus where they regulate gene expression (Figure 2A). Thus, fusions of fluorescent proteins with Smads can be used to monitor the activity of these pathways, an approach which has been used in a variety of model systems (Bourillot et al., 2002; Heemskerk et al., 2019; Nemashkalo et al., 2017; Nicolás et al., 2004; Sorre et al., 2014; Warmflash et al., 2012; Yoney et al., 2018). These studies have revealed that, in human ESCs (hESCs), SMAD4 enters the nucleus stably in response to BMP stimulation (Heemskerk et al., 2019; Nemashkalo et al., 2017; Yoney et al., 2018), while the response to Activin/Nodal is adaptive, returning to baseline even in the continued presence of ligand and activated receptors (Figure 2B). This adaptation renders cells sensitive to the rate of change of ligand in addition to its absolute concentration (Heemskerk et al., 2019; Sorre et al., 2014; Yoney et al., 2018), with corresponding effects on the differentiation of hESCs (Heemskerk et al., 2019).
Figure 2.
Signaling dynamics of the BMP-Wnt-Nodal pathways
(A) Schematic of BMP, Nodal, and canonical Wnt pathways. Upon ligand binding, the corresponding transducers (i.e., Smad1 and Smad4 for BMP4, Smad2 and Smad3 for Nodal, β-catenin for canonical Wnt) translocate to the nucleus.
(B) In the continued presence of BMP4, the response of transducers is sustained. In contrast, the response of SMAD4 to Nodal/Activin signaling and nuclear β-catenin to WNT3A is adaptive (Heemskerk et al., 2019; Massey et al., 2019).
(C) Signaling cascades in micropatterned hESC colonies. In response to uniformly added BMP4, SMAD1 activity is initially high across the entire colony, and is then restricted to the colony edge (Etoc et al., 2016). BMP4 signaling at the colony edge leads to elevated canonical Wnt signaling (β-catenin), which subsequently expands inward. Upregulated by Wnt, Nodal signaling expands inward, eventually surpassing Wnt signaling (Chhabra et al., 2019).
For the canonical Wnt pathway, ligand engagement with receptors inhibits a destruction complex, allowing β-catenin to accumulate and translocate to the cell nucleus where it binds TCF/LEF transcription factors and regulates target genes (Figure 2A). Fusions of β-catenin with GFP therefore allow monitoring of the status of the Wnt pathway (Kafri et al., 2016; Massey et al., 2019). In hESCs, Wnt signaling is adaptive in response to ligand stimulation but not in response to inhibition of glycogen-synthase kinase-3β (GSK-3β), a key component of the destruction complex (Massey et al., 2019). In contrast to Nodal signaling, which is nearly fully adaptive at all ligand doses, the degree of adaptation is more complete at lower doses, but some adaptation is seen even at saturating doses. Together these results reveal that the BMP, Wnt, and Nodal pathways each have a unique dynamical profile during differentiation which must be accounted for when trying to understand events in the embryo. Thus, live imaging approaches have enabled insights into these pathways which were not easily captured by traditional analysis of fixed samples.
In-vitro-established ESCs mirror in vivo pluripotency
Studies with cells that can be stably propagated in culture and correspond to different embryonic populations enable detailed studies of signaling dynamics. In this section, we review the different cell types available to researchers studying embryonic development in vitro.
Naive and primed pluripotent cells
The remarkable abilities of ESCs to indefinitely self-renew and to give rise to all the cell types found in the embryo make them a unique model to understand mammalian development. While all pluripotent states are transient in vivo, ESC cultures have enabled researchers to stabilize cells in a spectrum of temporally ordered states, which are difficult to capture in rapidly developing embryos.
ESCs were firstly isolated from mouse pre-implantation blastocysts, by seeding ICM cells on embryonic feeder cells in the presence of serum (Evans and Kaufman, 1981; Martin, 1981). Subsequently, a minimal culture medium supplemented with the cytokine leukemia inhibitory factor (LIF) and inhibitors of mitogen-activated protein kinase andGSK-3, termed LIF/2i, was shown to be sufficient to support mESC self-renewal without compromising their pluripotency (Ying et al., 2008). This formulation leads to activated Wnt signaling (through GSK3 inhibition) thereby promoting pluripotency, and inhibited FGF signaling, which prevents differentiation to a more mature EPI state. The roles of these pathways in maintaining mESCs mimic their roles in pluripotency progression in vivo (Neagu et al., 2020). mESCs possess the potential to give rise to all embryonic lineages and to contribute to chimeras when reintroduced into blastocysts. Furthermore, mESC studies paved the road for the derivation of pluripotent cells from other species, including the successful derivation of hESCs after nearly two more decades (Thomson et al., 1998).
The maintenance of hESCs requires completely different signaling conditions compared with mESCs, in particular, pluripotency is maintained by the combined effects of FGF and Activin/Nodal signaling activation (James et al., 2005; Vallier et al., 2005). Although hESCs can differentiate into all three embryonic germ layers and form teratomas in immune-compromised mice, they exhibit distinct transcriptomic and epigenetic features when compared with mESCs (De Los Angeles et al., 2015).
Differences between mESCs and hESCs raised questions about the nature of pluripotency and inspired isolation of pluripotent cells from post-implantation mouse embryos termed EPI stem cells (EpiSCs). Derivation of distinct pluripotent stem cells from mouse embryos helped reveal the existence of different pluripotent states during EPI development. Traditional mESCs correspond to the pre-implantation ICM and their state is referred to as “naive” due to their inability to respond to differentiating signals without transitioning through additional pluripotent states. EpiSCs correspond to the post-implantation EPI and are referred to as “primed” to reflect their ability to directly respond to differentiation-inducing signals. hESCs show more similarity to mEpiSCs than mESCs, and are similarly believed to be primed pluripotent stem cells. Naive hESCs, which display similar features to mESCs and contribute to interspecies chimeras, have also been derived from the human embryo. Of note, mouse induced pluripotent stem cells (iPSCs) exhibit similar developmental potential as naive pluripotent cells while human iPSCs are similar to primed pluripotent cells (De Los Angeles et al., 2015). Recently, it was shown that both mouse and human ESCs can be stabilized in a state between the naive and primed states known as “formative” pluripotency (Kinoshita et al., 2020). Readers are directed to reviews for more extensive summaries of the history of ESC derivation and their developmental relevance (De Los Angeles et al., 2015; Wu and Izpisua Belmonte, 2015).
Expanded potential stem cells
When reintroduced into a blastocyst, naive ESCs give rise to all embryonic lineages, however, rarely contribute to extra-embryonic tissues. Recently, so-called extended potential stem cells (EPSCs) were established and shown to be capable of contributing to both embryonic and extra-embryonic lineages in chimeric embryos. EPSCs have been derived from in-vitro-cultured ESCs, iPSCs, or blastocysts (Gao et al., 2019; Yang et al., 2017a, 2017b). The developmental identity of the EPSCs is not clear. Do EPSCs represents a natural embryonic state (totipotency) or an artificial product? Further study is required to answer this question. Nonetheless, the ability of EPSCs to give rise to both embryonic and extra-embryonic lineages opens up possibilities for in vitro modeling of embryogenesis (Li et al., 2019).
Signaling dynamics in micropatterned 2D gastruloids
While conventional pluripotent stem cell culture conditions lack reproducible spatial patterning, and 3D models lack precise reproducibility in size and shape, 2D micropatterned models have emerged as a method of choice for studying signaling dynamics in self-organization due to their quantitative reproducibility and ideal imaging conditions.
Control of hESC colony size affects differentiation trajectories and changes the proportion of cell types within a colony (Bauwens et al., 2008). Further studies have shown that hESCs confined to circular micropatterns and differentiated with morphogens involved in gastrulation self-organize into an ordered array of ExE-like cells and three germ layers along the radial axis of the colony (Warmflash et al., 2014). High-resolution single-cell transcriptomic data have identified the existence of TE or amnion-like cell types as well as primordial germ cell-like cells, in addition to cells of the EPI and the three germ layers in these 2D human gastruloids. These data also reveal the presence of mesoderm at varying stages of maturation, indicative of ongoing differentiation. Cross-species comparisons revealed that these human micropatterned colonies possess a similar composition of cell types as the embryonic day 7.0 (E7.0) mouse embryo and the cynomolgus monkey 16 days post-fertilization gastrulae (Minn et al., 2020).
BMP4 activity induces extra-embryonic fates in the peripheral cells and stimulates Wnt and Nodal activities in adjacent cells, which then differentiate to mesendoderm (Etoc et al., 2016; Warmflash et al., 2014). The role of these extra-embryonic cells is reminiscent of the role of extra-embryonic tissue-derived BMP4 in upregulating Wnt and Nodal levels in the adjacent EPI at the onset of gastrulation (see above). Consistent with this hypothesis, treating the micropatterned hESCs with only WNT3A results in primitive streak fates at the colony edge while extra-embryonic fates are lacking. Adjusting Nodal activities in the presence of WNT3A leads to fate shifting between mesoderm and endoderm (Martyn et al., 2018), in agreement with genetic evidence that Nodal acts as a morphogen (Robertson, 2014).
This micropattern approach also has been demonstrated using mouse EPI-like cells (EpiLCs). Direct comparison of the micropattern model with relevant stages of mouse embryo allows quantitative assessment of timing and levels of signaling required for specific regions of mouse gastrulating embryo (Morgani et al., 2018). This system allows for more refined evaluation of the requirement for signals than is possible in vivo. For example, assessment of the role of Bmp4 in cell fate allocation during gastrulation is difficult in the mouse embryo because disruption of signaling leads to developmental arrest at early gastrulation stage (Winnier et al., 1995). By removing the BMP4 from the cocktail of gastrulation-promoting ligands supplied to micropatterned mouse EpiLCs, Morgani et al. (2018) confirmed the requirement for BMP4 for induction of posterior mesodermal fates, while anterior fates emerged in the absence of BMP4.
Micropatterning has allowed the dissection of signaling dynamics associated with self-organized fate patterning (Figure 2C). Based on the expression patterns of Nodal and Wnt ligands, it is believed that these pathways are activated in a gradient from proximal-posterior to distal-anterior in the peri-gastrulation embryo (Arnold and Robertson, 2009); however, how the activity changes in space and time has not been measured in vivo. Several studies have together quantified BMP-Wnt-Nodal signaling dynamics in the micropatterned hESC-based gastrulation model. Soon after BMP application, signaling through this pathway is elevated throughout the circular colony, but activity is later restricted to the peripheral region due to expression of Noggin and polarization of receptors to the basal side of the cells (Etoc et al., 2016; Heemskerk et al., 2019). Extended BMP activity in the peripheral region triggers Wnt and Nodal activity in the adjacent EPI-like domain. Rather than stable signaling gradients, Wnt and Nodal signaling both exhibit spreading dynamics during the patterning of mesendodermal and ectodermal layers within the EPI-like domain (Chhabra et al., 2019). That is, activity starts at a point near the colony edge and then the domain of active signaling expands inward to cover nearly the entire colony. An important unanswered question is that given that both the Wnt and Nodal territories spread further than the region of mesoderm, precisely what features of the dynamics of these signals are required for differentiation?
Interestingly, waves of Wnt activity are required for self-organized patterning in both BMP4-treated (Chhabra et al., 2019) and WNT3A-treated (Martyn et al., 2018) micropatterns; however, they propagate by different mechanisms. In the case of BMP4 treatment, endogenous Wnt is activated by BMP and appears to diffuse long-range while simultaneously being strengthened by autoactivation of additional Wnt ligand (Chhabra et al., 2019). In contrast, when colonies are treated with exogenous WNT3A, only the edge is initially sensitized to respond by differentiating; however, edge cells then undergo EMT, which in turn sensitizes the next layer of cells to the exogenous WNT3A. The wave of Wnt signaling, therefore, is tightly coupled to a wave of differentiation which permits cells to respond. This wave does not require endogenous Wnt, as it is not blocked by inhibition of Wnt secretion. Both of these studies reveal elaborate mechanisms of signaling propagation in tissue which do not rely on establishing gradients of signaling molecules. These studies demonstrate the power of the 2D micropatterning approach. The relative ease of manipulating the cultures allows for dissection of which pathways are involved and at what time intervals, and the accessibility for imaging allows direct observation of signaling activities. Imaging and manipulation can be applied for multiple pathways in concert to understand how combinatorial interactions govern fate patterning.
Signaling dynamics in 3D stem cell models
In addition to the pluripotent cells discussed above, in vitro equivalents of TE (trophoblast stem cells [TSCs]) (Tanaka, 1998) and extra-embryonic endoderm (XEN cells) (Kunath et al., 2005) have been established. Various combinations of these cell types are enabling reconstruction of 3D embryo-like structures and study of inductive signaling interactions and morphogenesis, which is largely missing in 2D models (Figure 3).
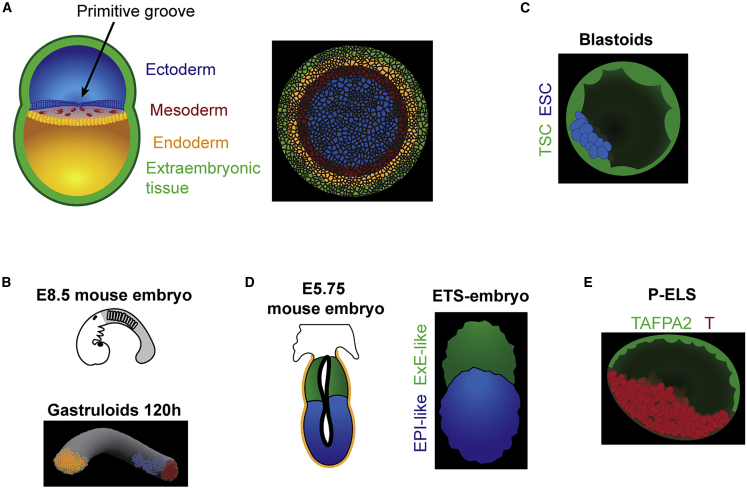
Figure 3.
Examples of stem cell-based embryo models
(A) Schematic of a human gastrulation-staged embryo, showing the correspondence between the embryo and a micropatterned 2D colony of hESCs that differentiates into extra-embryonic (CDX2-positive, green), endodermal (SOX17-positive, yellow), mesodermal (Brachyury [BRA]-positive, red), and ectodermal (SOX2-positive, blue) (Warmflash et al., 2014) fate layers.
(B) 3D rendering of elongated gastruloids at 120 h, expressing Bra (red), Sox2 (blue), and Gata6 (yellow), resembling the post-occipital portion of the E8.5 mouse embryo (Beccari et al., 2018).
(C) Schematic of blastoids formed with ESCs (blue) and TSCs (green) (Rivron et al., 2018).
(D) A 3D rendered of an ETS-embryo comprising an epiblast-like compartment (OCT4-positive, blue) and trophoblast/ExE-like compartment (Eomes-positive, green), resembling an egg cylinder-staged mouse embryo (Harrison et al., 2017).
(E) An example of posteriorized embryonic-like sac (P-ELS) modeling human epiblast and amnion development. TFAP2A (green), a putative amniotic ectoderm marker. T (red), a primitive streak marker (Zheng et al., 2019).
Blastoids
It was recently demonstrated that mouse ESCs and TSCs mixed at a particular ratio will self-organize into a structure with a trophoblastic cyst surrounding an internal mass of ESCs positioned to one side of the cavity, resembling the E3.5 mouse blastocyst. Dissection of signaling activities within the blastoids revealed communication between the embryonic compartment and extra-embryonic compartment through the TGF-β ligands Bmp4 and Nodal (Rivron et al., 2018). Blastoids do not progress further in development, in part due to the PrE compartment being smaller than in the blastocyst in vivo or absent altogether. Substituting ESCs with EPSCs resulted in specification and maturation of PrE-like cells and development of the TSC-derived cells into more mature TE lineages. These advances allow the further progression of the blastocyst-like structure into a post-implantation cylindrical embryo-like structure, and facilitated attachment to maternal tissues when transplanted in foster mothers, albeit without complete implantation (Sozen et al., 2019). Strikingly, Belmonte and colleagues recently demonstrated that blastoids can be derived from a single mouse EPSC (EPS-blastoids) (Li et al., 2019). These EPS-blastoids not only developed from pre- to post-implantation stages, but also induced decidualization (in approximately 7% of cases), and grew inside the uterus upon transfer into pseudopregnant mice. Of note, embryo-like structures derived from EPS-blastoids were disorganized when compared with their in vivo counterparts, indicating that there are still gaps in our understanding of recreating early development that prevent these models from developing further (Li et al., 2019).
ETS/X embryoids
By adjusting culture conditions, mixed embryonic and extra-embryonic stem cells can also model a more advanced developmental stage. Zernicka-Goetz and colleagues, by juxtaposing mouse ESCs and TSCs in a 3D extracellular matrix (ECM) scaffold, demonstrated the formation of a cylindrical structure consisting of EPI-like and ExE-like compartments and resembling the egg-cylinder-staged mouse embryo, termed ETS-embryos or ETS-embryoids (Harrison et al., 2017). Polarization and lumenogenesis of the EPI-like compartment, was followed by the formation of a cavity in the ExE-like compartment. Lumenogenesis has been suggested to be an intrinsic capacity of primed ESCs (Taniguchi et al., 2015), while these studies with ETS-embryoids demonstrated that, in the ExE-like compartment, lumen formation requires external signals. Nodal signaling emanated from the EPI-like compartment is required for the formation of the extra-embryonic cavity. Simultaneously, the extra-embryonic compartment may provide signaling cues, such as BMP4, to induce polarization of the adjacent EPI-like compartment. The two cavities ultimately fuse into one mirroring the pro-amniotic cavity. Notably, ETS-embryos develop embryo-like architecture and form regionalized mesoderm in the absence of PrE, highlighting the intrinsic self-organizing capability of ESCs. As in the embryo, this mesoderm differentiation requires endogenous Wnt signaling. Adding XEN cells to the system resulted in anterior-posterior (A-P) polarization and specification of mesoderm and definitive endoderm lineages (Harrison et al., 2017; Sozen et al., 2018). While, as noted above, requirements for several different signals have been determined, the dynamics of these signals and their relationship to fates remains largely unexplored. These models represent an exciting opportunity to tackle these challenging questions in vitro.
3D gastruloid/somitogenesis models
Aggregates of ESCs grown in a non-adhesive environment in the absence of pluripotency supporting factors, undergo spontaneous differentiation and give rise to masses of various cell types, called embryoid bodies (EBs) (Desbaillets et al., 2000; Wobus et al., 1984). EBs to some extent reflect post-implantation development; however, they lack reproducible spatial patterning. Recently, Martinez Arias and colleagues showed that treating mESCs aggregates composed of a defined number of cells on day 3 (48–72 h) with a small-molecule Wnt agonist (CHIR) or ligand (Wnt3A) enhanced polarization of the primitive streak marker Brachyury (BRA also called T) at one end, forming a posterior-like pole with high Wnt and Nodal activities (Beccari et al., 2018). Concomitantly, those aggregates elongated along this presumptive A-P axis, reaching up to 500–1,000 μm in size, with continued patterning resulting in cell lineages of all three germ layers. Because of their ability to form a body axis and undergo germ layer differentiation, key features of gastrulation, these structures were termed gastruloids (Baillie-Johnson et al., 2015; Beccari et al., 2018; Turner et al., 2017; Van Den Brink et al., 2014). Nonetheless, transcriptomic experiments have revealed a close resemblance to the E8.5, somitogenesis-stage, embryo. Extended growth of elongated gastruloids displays features reminiscent of axial morphogenesis marked by sequential activation of Hox genes (Beccari et al., 2018). Embedding of the elongated gastruloids in a low percentage of Matrigel facilitated the formation of structures that morphologically and molecularly resemble somites (van den Brink et al., 2020; Veenvliet et al., 2020). These findings are indicative of a role for ECM in somite formation, but it remains unclear whether ECM provides biochemical or mechanical cues. A similar model has been developed using hESCs (Moris et al., 2020), with a key difference that the pulse of Wnt activation is added on the first day rather than the third, likely reflecting the later developmental stage of human ESCs compared with mouse ESCs. Whether the human model is capable of sequentially activating Hox genes and forming morphological somites remains to be determined.
Gastruloids also provide insights into signaling activities that drive spatial organization. Uniform application of CHIR or Wnt3A enhances the polarization and elongation, suggesting that Wnt signaling plays a role in the process, but an external Wnt signaling gradient is not required. Experiments with Wnt inhibitors show that blocking receptors or Wnt ligands has a more pronounced effect than inhibiting β-catenin activity, indicating the likely involvement of non-canonical Wnt signaling (Turner et al., 2017). Moreover, while gastruloids made from Nodal knockout cells failed to elongate, the addition of Nodal to the medium rescued this phenotype, indicating that polarized Nodal activity might not be required either. BMP signaling is dispensable for the A-P polarization of gastruloids. Given the fact that extra-embryonic cells are missing in gastruloids (as shown by the absence of Bmp4 and Gata6 expression, markers for ExE and PrE, respectively), the authors argue that extra-embryonic cells are dispensable for polarized expression of Bra, and the function of extra-embryonic tissues might be to bias instead of to induce polarization of Bra expression (Turner et al., 2017; Van Den Brink et al., 2014). In contrast, in ETS/X embryoids, interactions between embryonic and extra-embryonic cells appear to be essential for polarized Bra expression. Understanding this discrepancy could provide insight into the mechanisms of symmetry breaking at gastrulation stages.
A recently developed 3D human EPI model demonstrates that homogeneously applied BMP4 in the medium is capable of driving symmetry breaking of a spherical epithelial cyst of hESCs with mesoderm differentiation occurring only in half of the cyst (Simunovic et al., 2019). This symmetry breaking involves a WNT/DKK circuit, while the CHIR applied in the gastruloids models discussed above likely bypasses this circuit. Therefore, it seems likely that different mechanisms are involved in different models for symmetry breaking. The relationship between these mechanisms and what occurs in vivo is an important topic for future study.
Human post-implantation amniotic sac embryoids
During human embryogenesis, the amnion differentiates from the EPI before gastrulation. Recently, it has been suggested that amniotic cells may serve as sources for gastrulation-inducing signals in primate embryos (Sasaki et al., 2016; Yang et al., 2020) (Figure 1B); however, until recently, systems to study human amniotic development were lacking. hESCs grown on a gel bed with gel supplemented into the media will differentiate into 3D sacs consisting of amniotic tissue in a manner requiring BMP activity (Shao et al., 2017a). A small portion of these hESC cysts spontaneously self-organized into asymmetric structures composed of an amniotic ectoderm-like domain and an EPI-like domain, resembling the post-implantation human amniotic sac, a model termed a post-implantation amniotic sac embryoid (PASE) (Shao et al., 2017b). Utilization of microfluidic technology to provide polarized BMP signaling to one side of hESC clusters, thus providing an exogenous cue for symmetry breaking, results in robust formation of PASEs (Zheng et al., 2019). These sacs then further develop with features of the primitive streak arising on the EPI side, albeit in a disorganized fashion that results in the dissolution of the PASE. These gastrulation-like events depend on signals from the amniotic cells. These results are in line with the idea that, given its physical proximity to the EPI, the amnion may play a role similar to the one that ExE plays in mouse development, serving as a source of gastrulation-inducing signals, such as BMP4.
Conclusions
Stem cell-based embryo models provide insights into development and open up opportunities to address questions regarding signaling dynamics that have proven to be difficult to address in mammalian animal models. These novel approaches pave new ways to disentangle the interplay between the signaling pathways involved in embryonic development. The models are valuable both for the insights they afford into development and for a more general understanding of how to build self-organizing biological systems, in a bottom-up manner. While 2D micropattern systems show remarkable reproducibility and are ideal for live-cell imaging, they undergo very limited morphogenesis. On the other hand, although 3D models can recapitulate some morphogenetic events, reproducibility sufficient to perform quantitative studies of signaling remains a challenge that may be addressed by future advances in bioengineering techniques. Live-cell imaging of fluorescently tagged signaling pathway components has already yielded new insights into how signals propagate during self-organization. A current challenge is to quantitatively relate these dynamics to cell fate decisions. Ultimately, direct comparisons of in vitro models with natural embryos will be required to validate any new findings, which is currently feasible for rodents but unrealistic for humans in the foreseeable future. Limited data from the ex vivo culture of pre-implantation human embryos or from non-human primates may provide alternative ways forward for this purpose.
Author contributions
L.L. and A.W. wrote and revised the manuscript.
Acknowledgments
This work was funded by Rice University and grants from the Welch Foundation (C-2021), NSF (MCB-1553228), NIH (R01GM126122), and Simons Foundation (511079). We thank members of the Warmflash lab for helpful discussions and Elena Camacho Aguilar for creating Figure 3.
References
- Arnold S.J., Robertson E.J. Making a commitment: cell lineage allocation and axis patterning in the early mouse embryo. Nat. Rev. Mol. Cell Biol. 2009;10:91–103. doi: 10.1038/nrm2618. [DOI] [PubMed] [Google Scholar]
- Baillie-Johnson P., van den Brink S.C., Balayo T., Turner D.A., Martinez Arias A. Generation of aggregates of mouse embryonic stem cells that show symmetry breaking, polarization and emergent collective behaviour in vitro. JoVE. 2015;24:e53252. doi: 10.3791/53252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauwens C.L., Peerani R., Niebruegge S., Woodhouse K.A., Kumacheva E., Husain M., Zandstra P.W. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 2008;26:2300–2310. doi: 10.1634/stemcells.2008-0183. [DOI] [PubMed] [Google Scholar]
- Beccari L., Moris N., Girgin M., Turner D.A., Baillie-Johnson P., Cossy A.-C., Lutolf M.P., Duboule D., Arias A.M. Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature. 2018;562:272–276. doi: 10.1038/s41586-018-0578-0. [DOI] [PubMed] [Google Scholar]
- Bedzhov I., Bialecka M., Zielinska A., Kosalka J., Antonica F., Thompson A.J., Franze K., Zernicka-Goetz M. Development of the anterior-posterior axis is a self-organizing process in the absence of maternal cues in the mouse embryo. Cell Res. 2015;25:1368–1371. doi: 10.1038/cr.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Haim N., Lu C., Guzman-Ayala M., Pescatore L., Mesnard D., Bischofberger M., Naef F., Robertson E.J., Constam D.B. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev. Cell. 2006;11:313–323. doi: 10.1016/j.devcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Beppu H., Kawabata M., Hamamoto T., Chytil A., Minowa O., Noda T., Miyazono K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev. Biol. 2000;221:249–258. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- Biechele S., Cockburn K., Lanner F., Cox B.J., Rossant J. Porcn-dependent Wnt signaling is not required prior to mouse gastrulation. Dev. 2013;140:2961–2971. doi: 10.1242/dev.094458. [DOI] [PubMed] [Google Scholar]
- Bourillot P.-Y., Garrett N., Gurdon J.B. A changing morphogen gradient is interpreted by continuous transduction flow. Development. 2002;129:2167–2180. doi: 10.1242/dev.129.9.2167. [DOI] [PubMed] [Google Scholar]
- van Boxtel A.L., Chesebro J.E., Heliot C., Ramel M., Stone R.K., Hill C.S. A temporal window for signal activation dictates the dimensions of a nodal signaling domain. Dev. Cell. 2015;35:175–185. doi: 10.1016/j.devcel.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J., Lu C.C., Norris D.P., Rodriguez T.A., Beddington R.S.P., Robertson E.J. Nodal signalling in the epiblast patterns the early mouse embryo. Nature. 2001;411:965–969. doi: 10.1038/35082103. [DOI] [PubMed] [Google Scholar]
- van den Brink S.C., Alemany A., van Batenburg V., Moris N., Blotenburg M., Vivié J., Baillie-Johnson P., Nichols J., Sonnen K.F., Martinez Arias A. Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Nature. 2020;582:405–409. doi: 10.1038/s41586-020-2024-3. [DOI] [PubMed] [Google Scholar]
- Chen Q., Shi J., Tao Y., Zernicka-Goetz M. Tracing the origin of heterogeneity and symmetry breaking in the early mammalian embryo. Nat. Commun. 2018;9:1819. doi: 10.1038/s41467-018-04155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhabra S., Liu L., Goh R., Kong X., Warmflash A. Dissecting the dynamics of signaling events in the BMP, WNT, and NODAL cascade during self-organized fate patterning in human gastruloids. Plos Biol. 2019;17:e3000498. doi: 10.1371/journal.pbio.3000498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G.C., Dunn N.R., Anderson D.C., Oxburgh L., Robertson E.J. Differential requirements for Smad4 in TGFβ-dependent patterning of the early mouse embryo. Development. 2004;131:3501–3512. doi: 10.1242/dev.01248. [DOI] [PubMed] [Google Scholar]
- Collignon J., Varlet I., Robertson E.J. Relationship between asymmetric nodal expression and the direction of embryonic turning. Nature. 1996;381:155–158. doi: 10.1038/381155a0. [DOI] [PubMed] [Google Scholar]
- Deglincerti A., Croft G.F., Pietila L.N., Zernicka-Goetz M., Siggia E.D., Brivanlou ali H. Self-organization of the in vitro attached human embryo. Nature. 2016;533:251–254. doi: 10.1038/nature17948. [DOI] [PubMed] [Google Scholar]
- Van Den Brink S.C., Baillie-Johnson P., Balayo T., Hadjantonakis A.K., Nowotschin S., Turner D.A., Arias A.M. Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Dev. 2014;141:4231–4242. doi: 10.1242/dev.113001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbaillets I., Ziegler U., Groscurth P., Gassmann M. Embryoid bodies: an in vitro model of mouse embryogenesis. Exp. Physiol. 2000;85:645–651. [PubMed] [Google Scholar]
- Dessaud E., Yang L.L., Hill K., Cox B., Ulloa F., Ribeiro A., Mynett A., Novitch B.G., Briscoe J. Interpretation of the sonic hedgehog morphogen gradient by a temporal adaptation mechanism. Nature. 2007;450:717–720. doi: 10.1038/nature06347. [DOI] [PubMed] [Google Scholar]
- Dougan S.T., Warga R.M., Kane D.A., Schier A.F., Talbot W.S. The role of the zebrafish nodal-related genes squint and cyclops in patterning of mesendoderm. Development. 2003;130:1837–1851. doi: 10.1242/dev.00400. [DOI] [PubMed] [Google Scholar]
- Dubrulle J., Jordan B.M., Akhmetova L., Farrell J.A., Kim S.H., Solnica-Krezel L., Schier A.F. Response to Nodal morphogen gradient is determined by the kinetics of target gene induction. eLife. 2015;4:e05042. doi: 10.7554/eLife.05042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eling N., Morgan M.D., Marioni J.C. Challenges in measuring and understanding biological noise. Nat. Rev. Genet. 2019;20:536–548. doi: 10.1038/s41576-019-0130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etoc F., Metzger J., Ruzo A., Kirst C., Yoney A., Ozair M.Z., Brivanlou A.H., Siggia E.D. A balance between secreted inhibitors and edge sensing controls gastruloid self-organization. Dev. Cell. 2016;39:302–315. doi: 10.1016/j.devcel.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Gao X., Nowak-Imialek M., Chen X., Chen D., Herrmann D., Ruan D., Chen A.C.H., Eckersley-Maslin M.A., Ahmad S., Lee Y.L. Establishment of porcine and human expanded potential stem cells. Nat. Cell Biol. 2019;21:687–699. doi: 10.1038/s41556-019-0333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham S.J.L., Wicher K.B., Jedrusik A., Guo G., Herath W., Robson P., Zernicka-Goetz M. BMP signalling regulates the pre-implantation development of extra-embryonic cell lineages in the mouse embryo. Nat. Commun. 2014;5:5667. doi: 10.1038/ncomms6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier C., Gurchenkov V., Perea-Gomez A., Camus A., Ott S., Papanayotou C., Iranzo J., Moreau A., Reid J., Koentges G. Nodal cis-regulatory elements reveal epiblast and primitive endoderm heterogeneity in the peri-implantation mouse embryo. Dev. Biol. 2011;349:350–362. doi: 10.1016/j.ydbio.2010.10.036. [DOI] [PubMed] [Google Scholar]
- Gritsman K., Talbot W.S., Schier A.F. Nodal signaling patterns the organizer. Development. 2000;127:921–932. doi: 10.1242/dev.127.5.921. [DOI] [PubMed] [Google Scholar]
- Harrison S.E., Sozen B., Christodoulou N., Kyprianou C., Zernicka-Goetz M. Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science. 2017;356:eaal1810. doi: 10.1126/science.aal1810. [DOI] [PubMed] [Google Scholar]
- Heemskerk I., Warmflash A. Pluripotent stem cells as a model for embryonic patterning: from signaling dynamics to spatial organization in a dish. Dev. Dyn. 2016;245:976–990. doi: 10.1002/dvdy.24432. [DOI] [PubMed] [Google Scholar]
- Heemskerk I., Burt K., Miller M., Chhabra S., Guerra M.C., Liu L., Warmflash A. Rapid changes in morphogen concentration control self-organized patterning in human embryonic stem cells. eLife. 2019;8:e40526. doi: 10.7554/eLife.40526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirate Y., Hirahara S., Inoue K.I., Suzuki A., Alarcon V.B., Akimoto K., Hirai T., Hara T., Adachi M., Chida K. Polarity-dependent distribution of angiomotin localizes hippo signaling in preimplantation embryos. Curr. Biol. 2013;23:1181–1194. doi: 10.1016/j.cub.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J., Vogel R., Brinkmann V., Erdmann B., Birchmeier C., Birchmeier W. Requirement for β-catenin in anterior-posterior axis formation in mice. J. Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D., Levine A.J., Besser D., Hemmati-Brivanlou A. TGFβ/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132:1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- Johnson M.H., Ziomek C.A. The foundation of two distinct cell lineages within the mouse morula. Cell. 1981;24:71–80. doi: 10.1016/0092-8674(81)90502-x. [DOI] [PubMed] [Google Scholar]
- Kafri P., Hasenson S.E., Kanter I., Sheinberger J., Kinor N., Yunger S., Shav-Tal Y. Quantifying β-catenin subcellular dynamics and cyclin D1 mRNA transcription during Wnt signaling in single living cells. eLife. 2016;5:e16748. doi: 10.7554/eLife.16748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M., Piliszek A., Artus J., Hadjantonakis A.K. FGF4 is required for lineage restriction and salt-and-pepper distribution of primitive endoderm factors but not their initial expression in the mouse. Development. 2013;140:267–279. doi: 10.1242/dev.084996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M., Garg V., Hadjantonakis A.K. Lineage establishment and progression within the inner cell mass of the mouse blastocyst requires FGFR1 and FGFR2. Dev. Cell. 2017;41:496–510.e5. doi: 10.1016/j.devcel.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M., Barber M., Mansfield W., Cui Y., Spindlow D., Stirparo G.G., Dietmann S., Nichols J., Smith A. Capture of mouse and human stem cells with features of formative pluripotency. Cell Stem Cell. 2020;28:453–471.e8. doi: 10.1016/j.stem.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkevich E., Niwayama R., Courtois A., Friese S., Berger N., Buchholz F., Hiiragi T. The apical domain is required and sufficient for the first lineage segregation in the mouse embryo. Dev. Cell. 2017;40:235–247.e7. doi: 10.1016/j.devcel.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawchuk D., Honma-Yamanaka N., Anani S., Yamanaka Y. FGF4 is a limiting factor controlling the proportions of primitive endoderm and epiblast in the ICM of the mouse blastocyst. Dev. Biol. 2013;384:65–71. doi: 10.1016/j.ydbio.2013.09.023. [DOI] [PubMed] [Google Scholar]
- Kunath T., Arnaud D., Uy G.D., Okamoto I., Chureau C., Yamanaka Y., Heard E., Gardner R.L., Avner P., Rossant J. Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts. Development. 2005;132:1649–1661. doi: 10.1242/dev.01715. [DOI] [PubMed] [Google Scholar]
- Kurowski A., Molotkov A., Soriano P. FGFR1 regulates trophectoderm development and facilitates blastocyst implantation. Dev. Biol. 2019;446:94–101. doi: 10.1016/j.ydbio.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Zhong C., Yu Y., Liu H., Sakurai M., Yu L., Min Z., Shi L., Wei Y., Takahashi Y. Generation of blastocyst-like structures from mouse embryonic and adult cell cultures. Cell. 2019;179:687–702.e18. doi: 10.1016/j.cell.2019.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H.Y.G., Alvarez Y.D., Gasnier M., Wang Y., Tetlak P., Bissiere S., Wang H., Biro M., Plachta N. Keratins are asymmetrically inherited fate determinants in the mammalian embryo. Nature. 2020;585:404–409. doi: 10.1038/s41586-020-2647-4. [DOI] [PubMed] [Google Scholar]
- Liu P., Wakamiya M., Shea M.J., Albrecht U., Behringer R.R., Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat. Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- De Los Angeles A., Ferrari F., Xi R., Fujiwara Y., Benvenisty N., Deng H., Hochedlinger K., Jaenisch R., Lee S., Leitch H.G. Hallmarks of pluripotency. Nature. 2015;525:469–478. doi: 10.1038/nature15515. [DOI] [PubMed] [Google Scholar]
- Ma H., Zhai J., Wan H., Jiang X., Wang X., Wang L., Xiang Y., He X., Zhao Z.-A., Zhao B. In vitro culture of cynomolgus monkey embryos beyond early gastrulation. Science. 2019;366:eaax7890. doi: 10.1126/science.aax7890. [DOI] [PubMed] [Google Scholar]
- Maître J.-L., Turlier H., Illukkumbura R., Eismann B., Niwayama R., Nédélec F., Hiiragi T. Asymmetric division of contractile domains couples cell positioning and fate specification. Nature. 2016;536:344–348. doi: 10.1038/nature18958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn I., Kanno T.Y., Ruzo A., Siggia E.D., Brivanlou A.H. Self-organization of a human organizer by combined Wnt and Nodal signalling. Nature. 2018;558:132–135. doi: 10.1038/s41586-018-0150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey J., Liu Y., Alvarenga O., Saez T., Schmerer M., Warmflash A. Synergy with TGFβ ligands switches WNT pathway dynamics from transient to sustained during human pluripotent cell differentiation. Proc. Natl. Acad. Sci. U S A. 2019;116:4989–4998. doi: 10.1073/pnas.1815363116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minn K.T., Fu Y.C., He S., Dietmann S., George S.C., Anastasio M.A., Morris S.A., Solnica-Krezel L. High-resolution transcriptional and morphogenetic profiling of cells from micropatterned human ESC gastruloid cultures. eLife. 2020;9:e59445. doi: 10.7554/eLife.59445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgani S.M., Metzger J.J., Nichols J., Siggia E.D., Hadjantonakis A.-K. Micropattern differentiation of mouse pluripotent stem cells recapitulates embryo regionalized cell fate patterning. eLife. 2018;7:e32839. doi: 10.7554/eLife.32839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moris N., Anlas K., van den Brink S.C., Alemany A., Schröder J., Ghimire S., Balayo T., van Oudenaarden A., Martinez Arias A. An in vitro model of early anteroposterior organization during human development. Nature. 2020;582:410–415. doi: 10.1038/s41586-020-2383-9. [DOI] [PubMed] [Google Scholar]
- Neagu A., van Genderen E., Escudero I., Verwegen L., Kurek D., Lehmann J., Stel J., Dirks R.A.M., van Mierlo G., Maas A. In vitro capture and characterization of embryonic rosette-stage pluripotency between naive and primed states. Nat. Cell Biol. 2020;22:534–545. doi: 10.1038/s41556-020-0508-x. [DOI] [PubMed] [Google Scholar]
- Nemashkalo A., Ruzo A., Heemskerk I., Warmflash A. Morphogen and community effects determine cell fates in response to BMP4 signaling in human embryonic stem cells. Development. 2017;144:3042–3053. doi: 10.1242/dev.153239. [DOI] [PubMed] [Google Scholar]
- Nicolás F.J., De Bosscher K., Schmierer B., Hill C.S. Analysis of Smad nucleocytoplasmic shuttling in living cells. J. Cell Sci. 2004;117:4113–4125. doi: 10.1242/jcs.01289. [DOI] [PubMed] [Google Scholar]
- Poh Y.-C., Chen J., Hong Y., Yi H., Zhang S., Chen J., Wu D.C., Wang L., Jia Q., Singh R. Generation of organized germ layers from a single mouse embryonic stem cell. Nat. Commun. 2014;5:4000. doi: 10.1038/ncomms5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivron N.C., Frias-Aldeguer J., Vrij E.J., Boisset J.-C., Korving J., Vivié J., Truckenmüller R.K., van Oudenaarden A., van Blitterswijk C.A., Geijsen N. Blastocyst-like structures generated solely from stem cells. Nature. 2018;557:106–111. doi: 10.1038/s41586-018-0051-0. [DOI] [PubMed] [Google Scholar]
- Robertson E.J. Dose-dependent nodal/smad signals pattern the early mouse embryo. Semin. Cell Dev. Biol. 2014;32:73–79. doi: 10.1016/j.semcdb.2014.03.028. [DOI] [PubMed] [Google Scholar]
- Rossant J. Genetic control of early cell lineages in the mammalian embryo. Annu. Rev. Genet. 2018;52:185–201. doi: 10.1146/annurev-genet-120116-024544. [DOI] [PubMed] [Google Scholar]
- Saiz N., Mora-Bitria L., Rahman S., George H., Herder J.P., Garcia-Ojalvo J., Hadjantonakis A.-K. Growth-factor-mediated coupling between lineage size and cell fate choice underlies robustness of mammalian development. eLife. 2020;9:e56079. doi: 10.7554/eLife.56079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Nakamura T., Okamoto I., Yabuta Y., Iwatani C., Tsuchiya H., Seita Y., Nakamura S., Shiraki N., Takakuwa T. The germ cell fate of cynomolgus monkeys is specified in the nascent amnion. Dev. Cell. 2016;39:169–185. doi: 10.1016/j.devcel.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Schauer A., Pinheiro D., Hauschild R., Heisenberg C.-P. Zebrafish embryonic explants undergo genetically encoded self-assembly. eLife. 2020;9:e55190. doi: 10.7554/eLife.55190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazi M.N., Jedrusik A., Vuoristo S., Recher G., Hupalowska A., Bolton V., Fogarty N.M.E., Campbell A., Devito L.G., Ilic D. Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol. 2016;18:700–708. doi: 10.1038/ncb3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Taniguchi K., Gurdziel K., Townshend R.F., Xue X., Yong K.M.A., Sang J., Spence J.R., Gumucio D.L., Fu J. Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nat. Mater. 2017;16:419–427. doi: 10.1038/nmat4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y., Taniguchi K., Townshend R.F., Miki T., Gumucio D.L., Fu J. A pluripotent stem cell-based model for post-implantation human amniotic sac development. Nat. Commun. 2017;8:1–15. doi: 10.1038/s41467-017-00236-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C.S., Rahman S., Raina D., Schröter C., Hadjantonakis A.-K. Live visualization of ERK activity in the mouse blastocyst reveals lineage-specific signaling dynamics. Dev. Cell. 2020;55:341–353.e5. doi: 10.1016/j.devcel.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simunovic M., Metzger J.J., Etoc F., Yoney A., Ruzo A., Martyn I., Croft G., You D.S., Brivanlou A.H., Siggia E.D. A 3D model of a human epiblast reveals BMP4-driven symmetry breaking. Nat. Cell Biol. 2019;21:900–910. doi: 10.1038/s41556-019-0349-7. [DOI] [PubMed] [Google Scholar]
- Sirard C., De La Pompa J.L., Elia A., Itie A., Mirtsos C., Cheung A., Hahn S., Wakeham A., Schwartz L., Kern S.E. The tumor suppressor gene Smad4/Dpc4 is required for gastrulation and later for anterior development of the mouse embryo. Genes Dev. 1998;12:107–119. doi: 10.1101/gad.12.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorre B., Warmflash A., Brivanlou A.H., Siggia E.D. Encoding of temporal signals by the TGF-β pathway and implications for embryonic patterning. Dev. Cell. 2014;30:334–342. doi: 10.1016/j.devcel.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozen B., Amadei G., Cox A., Wang R., Na E., Czukiewska S., Chappell L., Voet T., Michel G., Jing N. Self-assembly of embryonic and two extra-embryonic stem cell types into gastrulating embryo-like structures. Nat. Cell Biol. 2018;20:979–989. doi: 10.1038/s41556-018-0147-7. [DOI] [PubMed] [Google Scholar]
- Sozen B., Cox A.L., De Jonghe J., Bao M., Hollfelder F., Glover D.M., Zernicka-Goetz M. Self-organization of mouse stem cells into an extended potential blastoid. Dev. Cell. 2019;51:698–712.e8. doi: 10.1016/j.devcel.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sozen B., Demir N., Zernicka-Goetz M. BMP signalling is required for extra-embryonic ectoderm development during pre-to-post-implantation transition of the mouse embryo. Dev. Biol. 2021;470:84–94. doi: 10.1016/j.ydbio.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tanaka S. Promotion of trophoblast stem cell proliferation by FGF4. Science (80-. ) 1998;282:2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Shao Y., Townshend R.F., Tsai Y.H., Delong C.J., Lopez S.A., Gayen S., Freddo A.M., Chue D.J., Thomas D.J. Lumen formation is an intrinsic property of isolated human pluripotent stem cells. Stem Cell Reports. 2015;5:954–962. doi: 10.1016/j.stemcr.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski A.K. Experiments on the development of isolated blastomeres of mouse eggs. Nature. 1959;184:1286–1287. doi: 10.1038/1841286a0. [DOI] [PubMed] [Google Scholar]
- Thisse B., Wright C.V.E., Thisse C. Activin- and Nodal-related factors control antero–posterior patterning of the zebrafish embryo. Nature. 2000;403:425–428. doi: 10.1038/35000200. [DOI] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Turner D.A., Girgin M., Alonso-Crisostomo L., Trivedi V., Baillie-Johnson P., Glodowski C.R., Hayward P.C., Collignon J., Gustavsen C., Serup P. Anteroposterior polarity and elongation in the absence of extra-embryonic tissues and of spatially localised signalling in gastruloids: mammalian embryonic organoids. Development. 2017;144:3894–3906. doi: 10.1242/dev.150391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallier L., Alexander M., Pedersen R.A. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J. Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- Veenvliet J.V., Bolondi A., Kretzmer H., Haut L., Scholze-Wittler M., Schifferl D., Koch F., Guignard L., Kumar A.S., Pustet M. Mouse embryonic stem cells self-organize into trunk-like structures with neural tube and somites. Science (80-. ) 2020;370:eaba4937. doi: 10.1126/science.aba4937. [DOI] [PubMed] [Google Scholar]
- Vincent S.D., Dunn N.R., Hayashi S., Norris D.P., Robertson E.J. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev. 2003;17:1646–1662. doi: 10.1101/gad.1100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmflash A., Zhang Q., Sorre B., Vonica A., Siggia E.D., Brivanlou A.H. Dynamics of TGF-signaling reveal adaptive and pulsatile behaviors reflected in the nuclear localization of transcription factor Smad4. Proc. Natl. Acad. Sci. U S A. 2012;109:E1947–E1956. doi: 10.1073/pnas.1207607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmflash A., Sorre B., Etoc F., Siggia E.D., Brivanlou A.H. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat. Methods. 2014;11:847–854. doi: 10.1038/nmeth.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.L.K., Solnica-Krezel L. Nodal and planar cell polarity signaling cooperate to regulate zebrafish convergence and extension gastrulation movements. eLife. 2020;9:e54445. doi: 10.7554/eLife.54445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnier G., Blessing M., Labosky P.A., Hogan B.L.M. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Wobus A.M., Holzhausen H., Jäkel P., Schöneich J. Characterization of a pluripotent stem cell line derived from a mouse embryo. Exp. Cell Res. 1984;152:212–219. doi: 10.1016/0014-4827(84)90246-5. [DOI] [PubMed] [Google Scholar]
- Wu J., Izpisua Belmonte J.C. Dynamic pluripotent stem cell states and their applications. Cell Stem Cell. 2015;17:509–525. doi: 10.1016/j.stem.2015.10.009. [DOI] [PubMed] [Google Scholar]
- Xiang L., Yin Y., Zheng Y., Ma Y., Li Y., Zhao Z., Guo J., Ai Z., Niu Y., Duan K. A developmental landscape of 3D-cultured human pre-gastrulation embryos. Nature. 2020;577:537–542. doi: 10.1038/s41586-019-1875-y. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Saijoh Y., Perea-Gomez A., Shawlot W., Behringer R.R., Ang S.-L., Hamada H., Meno C. Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature. 2004;428:387–392. doi: 10.1038/nature02418. [DOI] [PubMed] [Google Scholar]
- Yang X., Li C., Xu X., Deng C. The tumor suppressor SMAD4/DPC4 is essential for epiblast proliferation and mesoderm induction in mice. Proc. Natl. Acad. Sci. U S A. 1998;95:3667–3672. doi: 10.1073/pnas.95.7.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Ryan D.J., Wang W., Tsang J.C.H., Lan G., Masaki H., Gao X., Antunes L., Yu Y., Zhu Z. Establishment of mouse expanded potential stem cells. Nature. 2017;550:393–397. doi: 10.1038/nature24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Liu B., Xu J., Wang J., Wu J., Shi C., Xu Y., Dong J., Wang C., Lai W. Derivation of pluripotent stem cells with in vivo embryonic and extraembryonic potency. Cell. 2017;169:243–257.e5. doi: 10.1016/j.cell.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Goedel A., Kang Y., Si C., Chu C., Zheng Y., Chen Z., Gruber P., Xiao Y., Zhou C. Essential amnion signals for primate primitive streak formation resolved by scRNA map. bioRxiv. 2020 doi: 10.1101/2020.05.28.118703. 2020.05.28.118703. [DOI] [Google Scholar]
- Ying Q.L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoney A., Etoc F., Ruzo A., Carroll T., Metzger J.J., Martyn I., Li S., Kirst C., Siggia E.D., Brivanlou A.H. WNT signaling memory is required for ACTIVIN to function as a morphogen in human gastruloids. eLife. 2018;7:e38279. doi: 10.7554/eLife.38279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.T., Hiiragi T. Symmetry breaking in the mammalian embryo. Annu. Rev. Cell Dev. Biol. 2018;34:405–426. doi: 10.1146/annurev-cellbio-100617-062616. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Xue X., Shao Y., Wang S., Esfahani S.N., Li Z., Muncie J.M., Lakins J.N., Weaver V.M., Gumucio D.L. Controlled modelling of human epiblast and amnion development using stem cells. Nature. 2019;573:421–425. doi: 10.1038/s41586-019-1535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]