Abstract
BACKGROUND
Increases in cardiac troponin (cTn) in coronavirus disease 2019 (COVID-19) have been associated with worse prognosis. Nonetheless, data about the significance of cTn in elderly subjects with COVID-19 are lacking.
METHODS
From a registry of consecutive patients with COVID-19 admitted to a hub hospital in Italy from 25/02/2020 to 03/07/2020, we selected those ≥ 60 year-old and with cTnI measured within three days from the molecular diagnosis of SARS-CoV-2 infection. When available, a second cTnI value within 48 h was also extracted. The relationship between increased cTnI and all-cause in-hospital mortality was evaluated by a Cox regression model and restricted cubic spline functions with three knots.
RESULTS
Of 343 included patients (median age: 75.0 (68.0−83.0) years, 34.7% men), 88 (25.7%) had cTnI above the upper-reference limit (0.046 µg/L). Patients with increased cTnI had more comorbidities, greater impaired respiratory exchange and higher inflammatory markers on admission than those with normal cTnI. Furthermore, they died more (73.9%vs. 37.3%, P < 0.001) over 15 (6−25) days of hospitalization. The association of elevated cTnI with mortality was confirmed by the adjusted Cox regression model (HR = 1.61, 95%CI: 1.06−2.52, P = 0.039) and was linear until 0.3 µg/L, with a subsequent plateau. Of 191 (55.7%) patients with a second cTnI measurement, 49 (25.7%) had an increasing trend, which was not associated with mortality (univariate HR = 1.39, 95%CI: 0.87−2.22, P = 0.265).
CONCLUSIONS
In elderly COVID-19 patients, an initial increase in cTn is common and predicts a higher risk of death. Serial cTn testing may not confer additional prognostic information.
Coronavirus disease 2019 (COVID-19) is the clinical manifestation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The most common symptoms are fever, dry cough, fatigue, myalgia, anosmia, and dysgeusia.[1] About 80% of patients with COVID-19 have mild pneumonia, whilst around 15% suffer from acute respiratory failure, and about 5% are critically ill with rapid progression towards acute respiratory distress syndrome (ARDS), cytokine storm, and multisystem failure.[1,2]
Within this clinical picture, cardiovascular (CV) alterations are common and have been associated with worse prognosis.[3–6] The spectrum includes elevation of biomarkers of cardiac injury, arterial and venous thromboembolism, and arrhythmias. In severe cases, cardiogenic shock and fatal cardiac arrest may occur.[4,7]
Among these CV abnormalities, particular emphasis has been given to an increase in cardiac troponin (cTn) concentrations. During COVID-19, cardiac stress and damage may arise because of a variety of mechanisms, including type 2 ischemia, hypoxia, sepsis and systemic inflammation, pulmonary thrombosis and embolism, cardiac adrenergic hyperstimulation during cytokine storm syndrome, and myocarditis.[2,8] A rise in cTn may be also due to pre-existing cardiac disease and concomitant comorbidities.[9]
Irrespective of the underlying causes, evidence of cardiac injury at the time of admission for COVID-19 has been associated with a more severe clinical course and higher mortality.[2,6,8,10] Limited data also indicate that a rising trend of cTn levels during the hospitalization identifies a subset of COVID-19 patients with worse outcome.[10,11]
Nonetheless, the specific impact of cTn measurement has not been investigated yet in the elderly. This lack of evidence has potential practical implications, since COVID-19 patients older than 60 years of age need hospitalization more often than younger ones,[12–14] and thereby, are more likely to be tested for cTn.
The aims of this study were to evaluate the relationship between cardiac injury, as demonstrated by cTn elevation either at baseline or on a second measurement within 48 h, and all-cause in-hospital mortality in ≥ 60 year-old patients hospitalized for COVID-19.
METHODS
Study Population
This is a retrospective analysis of a prospective registry enrolling all consecutive patients diagnosed with COVID-19 in a hub hospital in Genova, Italy, from February 25th to July 3rd, 2020. Genova is the main city of an Italian region with an overall old population (around 1,500,000 inhabitants, with 35.6% being ≥ 60-year-old).
The registry was developed by modifying an established registry of patients with infectious diseases,[15,16] was approved by the local Ethics Committee (study number 163/2020) and contained anonymized data; all capable subjects gave written informed consent to the use of such data for research purposes. SARS-CoV-2 infection was confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) of pharyngeal swabs or bronchoalveolar aspirates. Laboratory exams and diagnostic procedures were performed as per standard clinical practice.
The study sample consisted of patients aged ≥ 60 years with cTnI measured within three days from the molecular confirmation of SARS-CoV-2 infection. A second cTnI measurement within 48 h from the first one was available for a subset. The 60-year age cut-off was chosen according to the general agreement that ≥ 60 year-old COVID-19 patients represent a distinctive population with specific features.[12–14]
Demographic and Clinical Data
For every patient, the following information was retrieved: age, gender, Charlson comorbidities index (CCI), prior myocardial infarction (MI), history of chronic heart failure (CHF), and presence of hypertension, atrial fibrillation (AF), neurological disorder, chronic obstructive pulmonary disease (COPD), diabetes, cancer or chronic kidney disease (CKD), need of non-invasive or invasive ventilation, and admission to the intensive care unit (ICU), as reported in the medical records.
We also assessed the clinical features on admission, including laboratory exams. Plasma cTnI concentration was measured using a sandwich chemiluminescent immunoassay based on LOCI® technology on Dimension Vista® 1500 System. The limit of quantitation (functional sensitivity), which corresponds to the cTnI concentration at which the coefficient of variation is 10%, was < 0.04 µg/L. [17] The upper-reference limit (URL), as defined at the 99th percentile of the reference interval, was 0.046 µg/L.
All-cause in-hospital mortality was ascertained by review of the medical records.
Statistical Analysis
Categorical variables are presented as frequencies and percentages and were compared by chi-square test or Fisher’s exact test. Continuous variables are reported as mean ± SD or median and interquartile range according to their distribution. Normally distributed variables were compared by means of unpaired Student’s t test and non-normally distributed ones with the Mann-Whitney U non-parametric test.
For those patients for whom a second cTnI determination was available, the trend between the second and the first measurement was categorized as increase or non-increase, depending on whether the difference between the two values was > 0 or ≤ 0.
Time to all-cause in-hospital death was graphically depicted using the Kaplan-Meier method and compared by log-rank test. Patients were right-censored if they were discharged from the hospital alive or were still hospitalized at the time of data extraction (July 3, 2020).
A Cox regression model was used to estimate the hazard ratios (HRs) with 95% confidence interval (CI) of all-cause in-hospital mortality according to cTnI values below or above the URL. The model was adjusted for clinically meaningful covariates that were different between dead and alive patients with P < 0.05.
A potentially non-linear relationship between admission cTnI and all-cause in-hospital mortality was tested by using restricted cubic spline functions with three knots; data were then displayed graphically. As a sensitivity analysis, fitting a proportional sub-distribution hazards regression to the same variables included in the Cox regression model, we performed a competing risk analysis in which discharge from the hospital was treated as a competing risk for all-cause in-hospital mortality.
All analyses were performed with R environment 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) and packages finalfit, survival, ggplot2, survminer, rms, and cmprsk.
RESULTS
A total of 1275 consecutive patients admitted during the study period were included in the registry. Of them, 468 had cTnI measured within three days from the RT-PCR for SARS-CoV-2. One-hundred twenty-five subjects were excluded from the analysis because they had < 60 years of age, leaving a final sample of 343 patients. Their baseline characteristics are shown in Table 1. Median age was 75.0 (68.0−83.0) years and 119 (34.7%) were men. One-hundred ninety-eight (58.1%) patients had hypertension and 17.6% diabetes. Based on medical history, the prevalence of cardiovascular comorbidities was around 10%. Most patients presented with fever and around half had dyspnoea (Table 1). Median oxygen saturation (SpO2) and arterial oxygen partial pressure (pO2) were 94.0% (90.0%−97.0%) and 67.1 (55.0−84.3) mmHg, respectively. Inflammatory biomarkers were elevated (Table 1).
Table 1. Characteristics of the study patients according to baseline cardiac troponin I.
| Overall population(n = 343) | cTnI ≤ 0.046 µg/L(n = 255) | cTnI > 0.046 µg/L( n = 88) | P-value | |
| Data are presented as mean ± SD or median (range). AF: atrial fibrillation; ALT: alanine-transaminase; AST: aspartate transaminase; BP: blood pressure; CHF: chronic heart failure; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CPK: creatine-phosphokinase; CRP: C-reactive protein; ICU: intensive care unit; INR: international normalized ratio; IL-6: interleuchin-6; MI: myocardial infarction; MV: mechanical ventilation; NIV: non-invasive ventilation; pO2: partial oxygen pressure; SpO2: oxygen saturation. | ||||
| Baseline and clinical characteristics | ||||
| Age, yrs | 75.0 (68.0−83.0) | 73.0 (66.0−81.0) | 81.0 (74.0−89.3) | < 0.001 |
| Male sex | 119 (34.7%) | 86 (33.7%) | 33 (37.5%) | 0.609 |
| CCI | 3.0 (2.0−4.0) | 3.0 (2.0−4.0) | 4.0 (3.0−4.0) | < 0.001 |
| Hypertension | 198 (58.1%) | 145 (57.3%) | 53 (60.2%) | 0.725 |
| Diabetes | 60 (17.6%) | 46 (18.2%) | 14 (16.1%) | 0.781 |
| Prior MI | 41 (12.1%) | 26 (10.4%) | 15 (17.0%) | 0.143 |
| CHF | 26 (8.2%) | 17 (7.1%) | 9 (11.5%) | 0.308 |
| AF | 40 (11.9%) | 24 (9.6%) | 16 (18.6%) | 0.041 |
| CKD | 29 (8.6%) | 14 (5.6%) | 15 (17.2%) | 0.002 |
| Neurological disorder | 36 (10.7%) | 18 (7.1%) | 18 (20.9%) | 0.001 |
| COPD | 41 (12.2%) | 32 (12.8%) | 9 (10.3%) | 0.680 |
| Cancer | 38 (11.3%) | 24 (9.6%) | 14 (16.3%) | 0.136 |
| Symptoms and signs | ||||
| Fever, °C | 37.7 (36.8−38.3) | 37.7 (36.8−38.3) | 37.8 (36.6−38.3) | 0.721 |
| Dyspnoea | 179 (52.8%) | 131 (52.0%) | 48 (55.2%) | 0.697 |
| Cough | 138 (40.7%) | 109 (43.3%) | 29 (33.3%) | 0.134 |
| Heart rate, per min | 88.0 (77.0−100.0) | 87.0 (77.5−99.5) | 90.0 (76.8−102.5) | 0.142 |
| Respiratory rate, per min | 20.0 (18.0−28.0) | 20.0 (18.0−28.0) | 22.0 (18.0−28.0) | 0.481 |
| Systolic BP, mmHg | 130.0 (120.0−150.0) | 130.0 (120.0−150.0) | 130.0 (116.5−145.0) | 0.179 |
| Diastolic BP, mmHg | 75.0 (70.0−85.0) | 75.0 (70.0−85.0) | 75.5 (67.3−80.0) | 0.651 |
| Laboratory findings | ||||
| SpO2 | 94.0% (90.0%−97.0%) | 94.0% (91.0%−97.0%) | 90.5% (85.0%−96.0%) | < 0.001 |
| Arterial pO2, mmHg | 67.1 (55.0−84.3) | 68.5 (58.5−86.0) | 57.0 (48.0−73.0) | 0.001 |
| Creatinine, mg/dL | 1.0 (0.8−1.3) | 1.0 (0.8−1.2) | 1.1 (0.9−1.8) | < 0.001 |
| Haemoglobin, g/L | 134.0 (120.0−147.0) | 135.0 (123.5−148.0) | 127.5 (114.0−143.0) | 0.013 |
| Platelets, × 109/L | 177.0 (134.0−240.5) | 174.0 (135.5−234.5) | 184.0 (130.8−268.5) | 0.196 |
| White blood cells, × 109 /L | 6.4 (4.8−9.4) | 6.2 (4.7−8.2) | 8.1 (5.3−13.1) | < 0.001 |
| Lymphocytes, × 109 /L | 0.8 (0.6−1.1) | 0.8 (0.6−1.1) | 0.8 (0.5−1.0) | 0.163 |
| ALT, IU/L | 30.0 (22.0−47.0) | 30.0 (21.5−47.0) | 30.0 (22.5−46.5) | 0.803 |
| AST, IU/L | 38.5 (26.3−58.0) | 37.5 (26.0−54.0) | 47.0 (29.8−78.3) | 0.021 |
| Total bilirubin, mg/dL | 0.5 (0.4−0.7) | 0.5 (0.4−0.7) | 0.6 (0.4−0.9) | 0.034 |
| INR | 1.2 (1.1−1.3) | 1.2 (1.1−1.3) | 1.3 (1.2−1.4) | 0.001 |
| CRP, mg/L | 93.4 (43.1−136.0) | 87.0 (42.3−134.0) | 106.0 (52.2−160.0) | 0.076 |
| IL-6, pg/mL | 50.7 (23.1−95.9) | 46.0 (21.1−82.3) | 75.6 (37.4−146.3) | < 0.001 |
| Fibrinogen, mg/dL | 5.7 (4.5−7.1) | 5.7 (4.6−7.3) | 5.3 (4.1−7.0) | 0.128 |
| D-dimer, ng/mL | 1081.0 (677.0−1704.3) | 970.0 (622.1−1459.0) | 1627.5 (1070.2−4333.3) | < 0.001 |
| CPK, µg/L | 126.5 (70.8−293.3) | 114.0 (69.0−203.0) | 186.00 (93.0−516.5) | < 0.001 |
| Critically ill | ||||
| No ventilation support | 183 (53.7%) | 128 (50.6%) | 55 (62.5%) | 0.152 |
| NIV | 98 (28.7%) | 77 (30.4%) | 21 (23.9%) | |
| MV | 60 (17.6%) | 48 (19.0%) | 12 (13.6%) | |
| MV duration, days | 10.0 (5.5−18.0) | 12.0 (5.0−19.0) | 8.0 (6.0−10.5) | 0.329 |
| Admitted to ICU | 64 (18.8%) | 51 (20.2%) | 13 (14.8%) | 0.339 |
Median cTnI was 0.02 (0.02−0.05) µg/L; 88 (25.7%) patients had a cTnI value above the URL. These latter were older and had more often AF, CKD and a neurological disorders than the subjects with normal cTnI (Table 1). Although the frequency of dyspnoea was not different between the two groups, patients with cTnI above the URL presented with lower SpO2 and arterial pO2. Concentrations of creatinine, aspartate transaminase (AST), bilirubin, inflammatory parameters, D-dimer, creatine phosphokinase (CPK), and international normalized ratio (INR) were higher in subjects with baseline cTnI above the URL, whilst haemoglobin levels were lower (Table 1).
Overall, 28.7% and 17.6% patients received non-invasive and mechanical ventilation, respectively; 18.8% were admitted to the ICU. The frequencies of non-invasive and invasive ventilation support, as well as of ICU admission, were non-significantly lower in the group with elevated cTnI (Table 1).
During a median hospital stay of 15 (6−25) days, 160 (46.6%) patients died (Supplementary Table 1). All-cause mortality was higher in patients with increased admission cTnI (65 deaths/88 patients, 73.9%vs. 95 deaths/255 patients, 37.3%, P < 0.001).
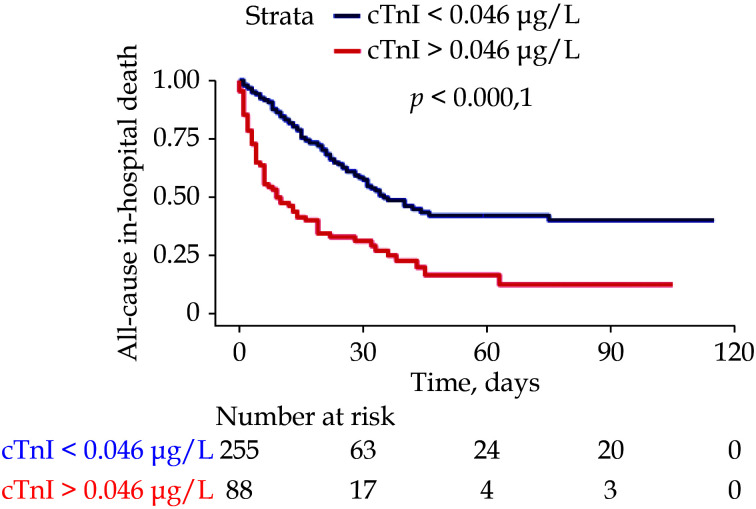
Kaplan-Meyer curve showed that patients with increased cTnI survived less throughout the hospitalization than those with normal cTnI (Figure 1). The association between baseline cTnI and all-cause in-hospital mortality was confirmed by the adjusted Cox regression model (HR = 1.61, 95%CI: 1.06−2.52,P = 0.039) (Table 2). The sensitivity competing risk analysis yielded results consistent with the Cox regression model (HR for admission cTnI: 1.46, 95%CI: 1.11−2.93,P = 0.043) (Supplementary Table 2).
Figure 1.
Kaplan-Meyer curves for all-cause in-hospital mortality according to baseline cardiac troponin I above or below the limit of quantification.
cTnI: cardiac troponin I.
Table 2. Univariate and multivariate Cox regression models for all-cause in-hospital mortality with baseline cardiac troponin I.
| Predictor | Univariate | Multivariate | |||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| AF: atrial fibrillation; BP: blood pressure; CHF: chronic heart failure; CKD: chronic kidney disease; CRP: C-reactive protein; SpO2: oxygen saturation. | |||||||
| Age | 1.06 | 1.04−1.08 | < 0.001 | 1.07 | 1.04−1.09 | < 0.001 | |
| Increased baseline troponin | 2.73 | 1.99−3.74 | < 0.001 | 1.61 | 1.06−2.52 | 0.039 | |
| CKD | 2.66 | 1.71−4.15 | < 0.001 | 1.01 | 0.45−1.93 | 0.854 | |
| CHF | 1.86 | 1.16−2.99 | 0.010 | 1.00 | 0.47−2.29 | 0.928 | |
| AF | 1.83 | 1.20−2.79 | 0.005 | 1.55 | 0.86−2.77 | 0.143 | |
| Dyspnoea | 1.74 | 1.26−2.41 | 0.001 | 2.29 | 1.48−3.54 | < 0.001 | |
| Systolic BP | 0.99 | 0.98−1.00 | 0.094 | 1.00 | 0.99−1.01 | 0.475 | |
| SpO2 | 0.96 | 0.94−0.97 | < 0.001 | 0.98 | 0.95−1.01 | 0.202 | |
| Haemoglobin | 0.99 | 0.99−1.00 | 0.111 | 1.00 | 0.99−1.01 | 0.870 | |
| White blood cells | 1.02 | 1.00−1.05 | 0.051 | 0.96 | 0.91−1.02 | 0.215 | |
| Creatinine | 1.30 | 1.17−1.45 | < 0.001 | 1.25 | 1.00−1.58 | 0.052 | |
| D-dimer | 1.01 | 1.01−1.02 | 0.003 | 1.00 | 1.00−1.00 | 0.183 | |
| CRP | 1.01 | 1.00−1.01 | < 0.001 | 1.00 | 1.00−1.01 | 0.001 | |
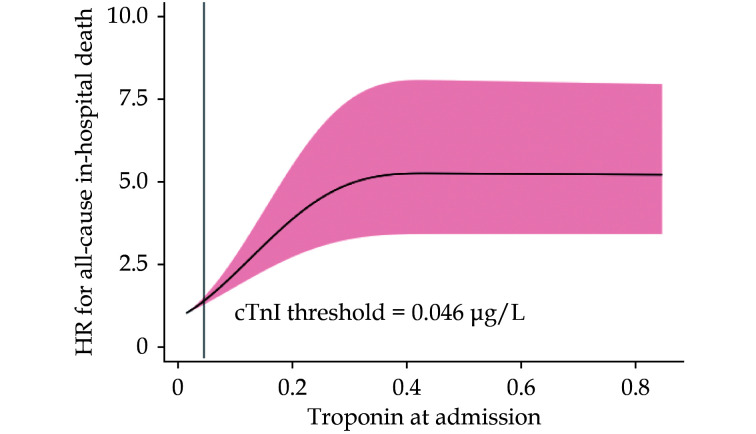
As shown in Figure 2, the association of admission cTnI with all-cause in-hospital mortality began with concentrations within the range of normality as per manufacturer’s indications and was linear until the threshold of 0.3 µg/L, after which a plateau was observed with no further increase in mortality.
Figure 2.
Spline curve for the hazard ratios of all-cause mortality according to baseline cardiac troponin I.
cTnI: cardiac troponin I.
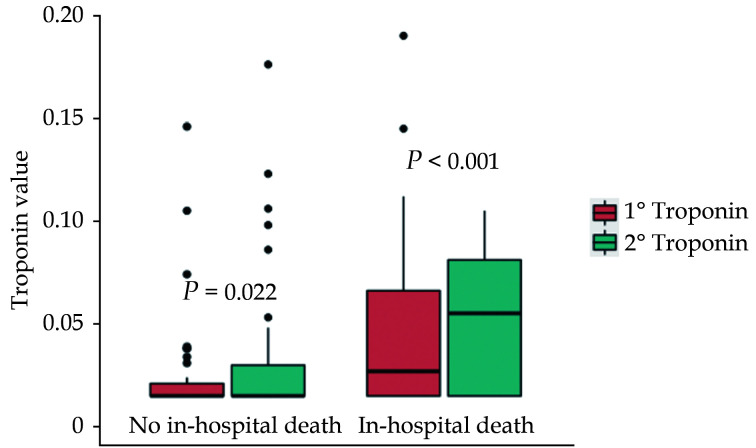
A second cTnI measurement within 48 h was available for 191 (55.7%) patients, the median value being 0.02 (0.02−0.05) µg/L; an increasing trend was found in 49 (25.7%) of them. The concentrations of cTnI were higher at both the first (0.03 (0.02−0.06) vs. 0.02 (0.02−0.02) µg/L, P < 0.001) and the second determination (0.05 (0.02−0.08) µg/L vs. 0.02 (0.02−0.03) µg/L, P < 0.001) in patients who died than in those who did not ( Figure 3). However, an increase in cTnI within 48 h was not associated with higher all-cause in-hospital mortality (univariate HR = 1.39, 95%CI: 0.87−2.22,P = 0.265).
Figure 3.
Baseline and subsequent cardiac troponin I levels according to all-cause in-hospital mortality.
DISCUSSION
In this study, we show that an initially increased cTn value portends a higher risk of in-hospital mortality in subjects older than 60 years with COVID-19. In the population examined, an increased cTnI concentration within three days from the molecular diagnosis of SARS-CoV-2 infection conferred a 60% higher risk of all-cause in-hospital death. By contrast, a further elevation in cTn over the following 48 h did not provide additional prognostic information.
CV involvement is common in COVID-19 and has been pointed out as one of the factors contributing to the dismal prognosis that many patients face.[1] The elevation of markers of myocardial injury, especially cTn, has drawn much attention, since it can be readily assessed and is clinically relevant.[2,4,7,8] Several authors have reported that an increase in cTn concentrations portends a higher risk of in-hospital death.[3–6,10,11,18–20] This evidence has been gathered by analyzing various cohorts with important differences, for instance in the severity of COVID-19 and, thus, the intensity of treatments, in ethnicity and in the burden of comorbidities.[9] Therefore, it is assumed that the results of these studies can be generalized to all patients hospitalized for COVID-19.[8] However, data about the value of cTn specifically in elderly subjects admitted for COVID-19 are scarce. This lack of information is remarkable, considering the epidemiology of the SARS-CoV-2 pandemic, in which old individuals are the most affected and often need hospitalization.[13,21,22]
Our results confirm that cTnI elevation is an independent predictor of in-hospital mortality in elderly patients hospitalized for COVID-19, like it is in younger ones. The cohort we investigated was particularly old. In fact, the median age was 75 years, whilst the mean or median age in the other investigations of cTn in COVID-19 was ≤ 70 years (Supplementary Table 3). Furthermore, almost 1 in 2 patients died during the hospitalization. A similar proportion of deaths has been described for critically ill COVID-19 Italian patients with a median age of 63 (56−69) years.[23] We believe that the comparable mortality of our patients, who were admitted to the ICU only in about 20% of cases, is explained by the substantially older age. It is notable that, even within this vulnerable population, an initial increase in cTn identified a frailer group with worse prognosis. The mechanisms leading to cTn elevation during COVID-19 are manifold and likely often concomitant.[24] Autopsy studies indicate that myocardial ischemia due to plaque rupture, coronary artery spasm or direct injury, or microthrombi are rare in COVID-19,[25] and the pathogenesis of cardiac damage has been primarily ascribed to other events.[4,9,10,19] First, the heart may suffer from severe hypoxia in the contest of acute respiratory insufficiency. Second, hyperinflammation and sepsis with cytokine storm may directly affect cardiomyocytes, up to causing stress cardiomyopathy or myocarditis. Third, cardiac injury may develop following pulmonary thromboembolism. Finally, SARS-CoV-2 can localize to the myocardium, although an ensuing inflammatory infiltrate has not been demonstrated.[18] The elderly may be more prone to all these events because of the reduced resistance of the aging heart to stressors and of concomitant asymptomatic or overt cardiac disease. Other authors found a continuous relationship between cTn concentrations and mortality.[6] Moreover, in subjects admitted for COVID-19 younger than those evaluated by us, the trend between two measurements of cTn obtained at the beginning of the hospitalization may help better stratifying the risk of death.[21] By contrast, in our cohort, the troponin-mortality risk curve plateaued after a relatively low level and the changes in cTn did not refine prognostication. Thus, our analysis suggests that the presence, but not the entity of cardiac injury corresponds to worse outcomes in elderly patients hospitalized for COVID-19, and that multiple determinations of cTn may be of value only if clinically motivated.
The retrospective design is the main limitation of this work. However, most of the literature about the CV complications in COVID-19 is based on data collected retrospectively. Moreover, in Italy the most intense phase of the SARS-CoV-2 epidemic, when the majority of patients was hospitalized, had a relatively brief course, making the conduct of prospective studies very challenging. The possibility of a selection bias must be also acknowledged, since only part of the subjects included in the registry we analysed had cTnI measured.
In conclusion, an initial elevation in cTnI is associated with all-cause in-hospital mortality in elderly patients admitted for COVID-19. Beyond the relatively low threshold of 0.3 µg/L, additional increases in the cTnI concentrations do not confer a higher risk of mortality, indicating that the rise in cTnI in itself, rather than its entity, is marker of worse outcome. An increasing trend in cTnI levels on a second assessment does not give further information about prognosis.
Acknowledgements
GECOVID-19 Study group: Anna Alessandrini; Marco Camera; Emanuele Delfino; Andrea De Maria; Chiara Dentone; Antonio Di Biagio; Ferdinando Dodi; Antonio Ferrazin; Giovanni Mazzarello; Malgorzata Mikulska; Laura Nicolini; Federica Toscanini; Daniele Roberto Giacobbe; Antonio Vena; Lucia Taramasso; Elisa Balletto; Federica Portunato; Eva Schenone; Nirmala Rosseti; Federico Baldi; Marco Berruti; Federica Briano; Silvia Dettori; Laura Labate; Laura Magnasco; Michele Mirabella; Rachele Pincino; Chiara Russo; Giovanni Sarteschi; Chiara sepulcri; Stefania Tutino (Clinica di Malattie Infettive); Roberto Pontremoli; Valentina Beccati; Salvatore Casciaro; Massimo Casu; Francesco Gavaudan; Maria Ghinatti; Elisa Gualco; Giovanna Leoncini; Paola Pitto; Kassem salam (Clinica di Medicina interna 2); Angelo Gratarola; Mattia Bixio; Annalisa Amelia; Andrea Balestra; Paola Ballarino; Nicholas Bardi; Roberto Boccafogli; Francesca Caserza; Elisa Calzolari; Marta Castelli; Elisabetta Cenni; Paolo Cortese; Giuseppe Cuttone; Sara Feltrin; Stefano Giovinazzo; Patrizia Giuntini; Letizia Natale; Davide Orsi; Matteo Pastorino; Tommaso Perazzo; Fabio Pescetelli; Federico Schenone; Maria Grazia Serra; Marco Sottano (Anestesia e Rianimazione; Emergenza Covid padiglione 64); Iole Brunetti; Maurizio Loconte; Lorenzo Ball; Denise Battaglini; Chiara Robba; Nicolò Patroniti (Anestesiologia e Terapia Intensiva); Roberto Tallone; Massimo Amelotti; Marie Jeanne Majabò; Massimo Merlini; Federica Perazzo (Cure Intermedie); Nidal Ahamd; Paolo Barbera; Marta Bovio; Paola Campodonico; Andrea Collidà; Ombretta Cutuli; Agnese Lomeo; Francesca Fezza; Nicola Gentilucci; Nadia Hussein; Emanuele Malvezzi; Laura Massobrio; Giulia Motta; Laura Pastorino; Nicoletta Pollicardo; Stefano Sartini; Paola Vacca; Valentina Virga (Dipartimento di Emergenza ed Accettazione); Italo Porto; Gian Paolo Bezante; Roberta Della Bona; Giovanni La Malfa; Alberto Valbusa; Vered Gil Ad (Clinica Malattie Cardiovascolari); Emanuela Barisione; Michele Bellotti; Aloe’ Teresita; Alessandro Blanco; Marco Grosso; Maria Grazia Piroddi (Pneumologia ad Indirizzo Interventistico); Paolo Moscatelli; Paola Ballarino; Matteo Caiti; Elisabetta Cenni; Patrizia Giuntini; Ottavia Magnani (Medicine d’Urgenza); Samir Sukkar; Ludovica Cogorno; Raffaella Gradaschi; Erica Guiddo; Eleonora Martino; Livia Pisciotta (Dietetica e Nutrizione clinica); Bruno Cavagliere; Rossi Cristina; Farina Francesca (Direzione delle Professioni Sanitarie); Giacomo Garibotto; Pasquale Esposito (Clinica nefrologica, Dialisi e Trapianto); Giovanni Passalacqua; Diego Bagnasco; Fulvio Braido; Annamaria Riccio; Elena Tagliabue (Clinica Malattie Respiratorie ed Allergologia); Claudio Gustavino; Antonella Ferraiolo (Ostetricia e Ginecologia); Salvatore Giuffrida; Nicola Rosso (Direzione Amministrativa); Alessandra Morando; Riccardo Papalia; Donata Passerini; Gabriella Tiberio (Direzione di Presidio); Giovanni Orengo; Alberto Battaglini (Gestione del Rischio Clinico); Silvano Ruffoni; Sergio Caglieris.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
References
- 1.Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 2.Imazio M, Klingel K, Kindermann I, et al COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart. 2020;106:1127–1131. doi: 10.1136/heartjnl-2020-317186. [DOI] [PubMed] [Google Scholar]
- 3.Kang Y, Chen T, Mui D, et al Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020;106:1132–1141. doi: 10.1136/heartjnl-2020-317056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 5.Ruan Q, Yang K, Wang W, Jiang L, Song J Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi S, Qin M, Shen B, et al Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long B, Brady WJ, Koyfman A, Gottlieb M Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38:1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaze DC Clinical utility of cardiac troponin measurement in COVID-19 infection. Ann Clin Biochem. 2020;57:202–205. doi: 10.1177/0004563220921888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandoval Y, Januzzi JL, Jr., Jaffe AS Cardiac Troponin for the Diagnosis and Risk-Stratification of Myocardial Injury in COVID-19: JACC Review Topic of the Week. J Am Coll Cardiol. 2020;76:1244–1258. doi: 10.1016/j.jacc.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lala A, Johnson KW, Januzzi JL, et al Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C, Jiang J, Wang F, et al Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J Mol Cell Cardiol. 2020;147:74–87. doi: 10.1016/j.yjmcc.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller AL, McNamara MS, Sinclair DA Why does COVID-19 disproportionately affect older people? Aging (Albany NY) 2020;12:9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nanda A, Vura N, Gravenstein S COVID-19 in older adults. Aging Clin Exp Res. 2020;32:1199–1202. doi: 10.1007/s40520-020-01581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shahid Z, Kalayanamitra R, McClafferty B, et al COVID-19 and Older Adults: What We Know. J Am Geriatr Soc. 2020;68:926–929. doi: 10.1111/jgs.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannini B, Riccardi N, Cenderello G, et al From Liguria HIV Web to Liguria Infectious Diseases Network: How a Digital Platform Improved Doctors' Work and Patients' Care. AIDS Res Hum Retroviruses. 2018;34:239–240. doi: 10.1089/aid.2017.0064. [DOI] [PubMed] [Google Scholar]
- 16.Vena A, Giacobbe DR, Di Biagio A, et al Clinical characteristics, management and in-hospital mortality of patients with COVID-19 In Genoa, Italy. Clin Microbiol Infect. 2020;26:1537–1544. doi: 10.1016/j.cmi.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apple FS, Sandoval Y, Jaffe AS, et al Cardiac Troponin Assays: Guide to Understanding Analytical Characteristics and Their Impact on Clinical Care. Clin Chem. 2017;63:73–81. doi: 10.1373/clinchem.2016.255109. [DOI] [PubMed] [Google Scholar]
- 18.Guo T, Fan Y, Chen M, et al Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lippi G, Lavie CJ, Sanchis-Gomar F Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63:390–391. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lombardi CM, Carubelli V, Iorio A, et al Association of Troponin Levels With Mortality in Italian Patients Hospitalized With Coronavirus Disease 2019: Results of a Multicenter Study. JAMA Cardiol. 2020;5:1274–1280. doi: 10.1001/jamacardio.2020.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lithander FE, Neumann S, Tenison E, et al COVID-19 in older people: a rapid clinical review. Age Ageing. 2020;49:501–515. doi: 10.1093/ageing/afaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolich-Zugich J, Knox KS, Rios CT, et al SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience. 2020;42:505–514. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grasselli G, Greco M, Zanella A, et al Risk Factors Associated With Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tersalvi G, Vicenzi M, Calabretta D, et al Elevated Troponin in Patients With Coronavirus Disease 2019: Possible Mechanisms. J Card Fail. 2020;26:470–475. doi: 10.1016/j.cardfail.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradley BT, Maioli H, Johnston R, et al Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.