Summary
In vitro induction of human primordial germ cell-like cells (hPGCLCs) provides an ideal platform to recapitulate hPGC development. However, the detailed molecular mechanisms regulating the induction of hPGCLCs remain largely uncharacterized. Here, we profiled the chromatin accessibility and transcriptome dynamics throughout the process of hPGCLC induction. Genetic ablation of SOX15 indicated the crucial roles of SOX15 in the maintenance of hPGCLCs. Mechanistically, SOX15 exerted its roles via suppressing somatic gene expression and sustaining latent pluripotency. Notably, ETV5, a downstream regulator of SOX15, was also uncovered to be essential for hPGCLC maintenance. Finally, a stepwise switch of OCT4/SOX2, OCT4/SOX17, and OCT4/SOX15 binding motifs were found to be enriched in closed-to-open regions of human embryonic stem cells, and early- and late-stage hPGCLCs, respectively. Collectively, our data characterized the chromatin accessibility and transcriptome landscapes throughout hPGCLC induction and defined the SOX15-mediated regulatory networks underlying this process.
Keywords: human primordial germ cell-like cells, chromatin accessibility, SOX15, germ cell, ETV5
Graphical abstract
Highlights
-
•
Chromatin accessibility landscape is revealed throughout hPGCLC induction
-
•
SOX15 is involved in hPGCLC maintenance via dual effects
-
•
ETV5, a downstream regulator of SOX15, is essential for hPGCLC maintenance
-
•
A stepwise OCT4:SOX motifs switch is uncovered throughout hPGCLC induction
Zhao and colleagues reveals the chromatin accessibility and transcriptome dynamics throughout hPGCLC induction. They show the essential roles of SOX15-mediated regulatory network in maintaining the hPGCLC identity by suppressing somatic gene expression and sustaining latent pluripotency. Moreover, they uncover a stepwise OCT4:SOX motifs switch throughout hPGCLC induction.
Introduction
The formation of human primordial germ cells (hPGCs) is critical for establishing the human germline and transmission of genetic information (Leitch et al., 2013). The recent development of in vitro differentiation protocols for human primordial germ cell-like cells (hPGCLCs) from human pluripotent stem cells (hPSCs) has minimized technical and ethical limitations inherent in using human tissues. This system has facilitated our understanding of hPGC biology, and might eventually provide a source of haploid germ cells for infertility treatments (Saitou and Miyauchi, 2016). However, the regulatory networks for germ cell and somatic lineage bifurcation are still unclear and the establishment of stable hPGCLCs and their further maturation remain challenging.
In mammals, primordial germ cells (PGCs) are specified from early embryonic cells through the sophisticated interactions between WNT and BMP pathways, which is highly conserved in humans, monkeys, pigs, and mice (Kobayashi et al., 2017). It has been reported that the transcription factors (TFs) BLIMP1 (PRDM1), TFAP2C, and PRDM14 are general regulators of PGC specification in both mice and humans (Irie et al., 2015; Sasaki et al., 2015; Sybirna et al., 2020). However, accumulating evidence indicated that the germ line specifications are actually quite different between humans and mice (Irie et al., 2015; Kobayashi et al., 2017; Kojima et al., 2017; Tang et al., 2015). For instance, the pluripotency factor SOX2 is essential for mouse PGC (mPGC) induction, but it is not expressed in human PGCs (Campolo et al., 2013; Perrett et al., 2008). Vice versa, SOX17 acts as a key regulator of initial induction of hPGCLCs, but is dispensable for that in mPGC specification (Irie et al., 2015; Kanai-Azuma et al., 2002).
The SOX family member SOX15, which shares a very similar HMG domain with SOX2 (Kamachi and Kondoh, 2013), is highly expressed in both hPGCs and mPGCs (Guo et al., 2015; Sarraj et al., 2003). Interestingly, the developmental defects due to Sox2 deficiency in mESCs can be rescued by overexpression of Sox15 (Niwa et al., 2016). Notably, a recent study uncovered the role of SOX15 in maintaining hPGCLC identity, but how SOX15 regulates hPGCLC induction is still unclear (Pierson Smela et al., 2019). Most SOX factors including SOX2, SOX17, and SOX15 bind to similar CATTGT-like DNA motifs (Hou et al., 2017; Maruyama et al., 2005). SOX2 and SOX15 also possess the ability to heterodimerize with OCT4 and bind a canonical SOXOCT motif composed of SOX and OCT half-sites (CATTGTCATGCAAAT-like) (Chang et al., 2017). The canonical SOXOCT motif is critical for the induction and maintenance of pluripotency in mice and humans (Aksoy et al., 2013a, 2013b; Jauch et al., 2011; Veerapandian et al., 2018). In addition, a recent study in seminoma cell lines revealed that the canonical SOXOCT motifs are bound by SOX17 to regulate pluripotency-related genes (Jostes et al., 2020). Therefore, it is speculated that OCT4/SOX17 or OCT4/SOX15 complexes exert overlapping regulatory roles in hPGCs or hPGCLCs.
In this study, we investigated the genome-wide chromatin changes and transcriptome dynamics in the process of hPGCLC induction via time course ATAC-seq (assay for transposase-accessible chromatin using sequencing) and RNA-seq (RNA sequencing) analyses. We obtained distinct patterns of CO/OC (closed-to-open/open-to-closed) loci that underlie the bifurcation of germline and non-germline lineage. The combined genetic ablation assay and integrated analysis of RNA-seq, ATAC-seq, and CUT&Tag-seq (cleavage under targets and tagmentation sequencing) demonstrated that SOX15 was crucial for the maintenance of hPGCLC identity by simultaneous somatic gene expression suppression and latent pluripotency preservation. ETV5, a downstream regulator of SOX15, was validated to be essential for hPGCLC maintenance. Moreover, in late-stage hPGCLCs, there was a switch toward utilization of an OCT4/SOX15, which was distinct from that in human embryonic stem cells (hESCs) (OCT4/SOX2) and early-stage hPGCLCs (OCT4/SOX17).
Results
Chromatin accessibility and gene regulation dynamics during hPGCLC induction
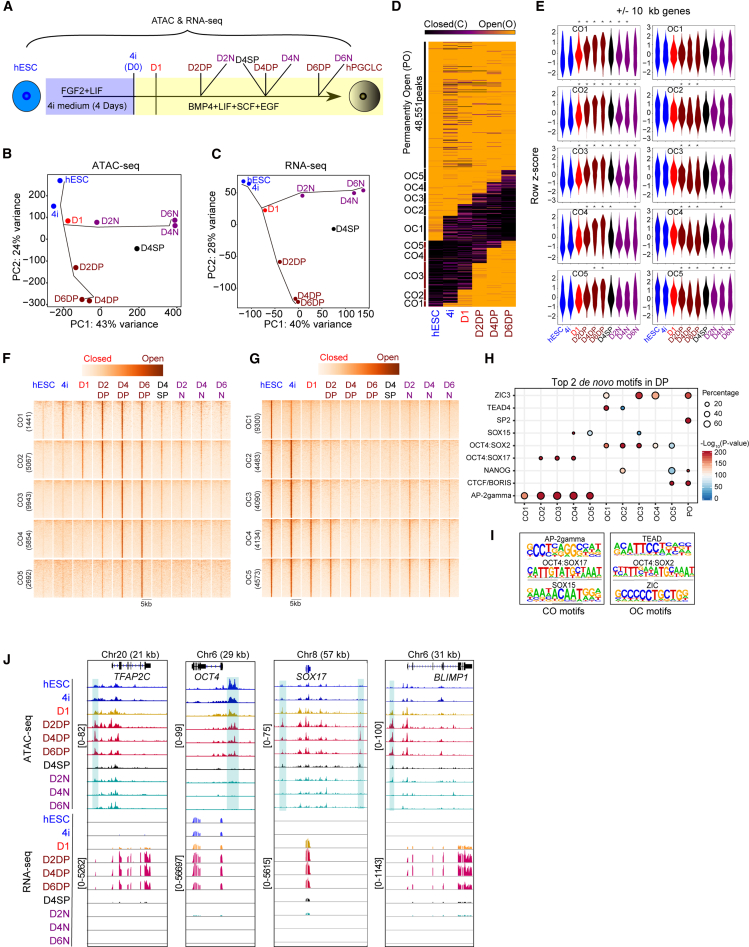
To investigate the dynamic genome regulation during the induction of hPGCLCs from hESCs, we used a modified protocol based on a previous study (Mitsunaga et al., 2017) to obtain EpCAM+/INTEGRINα6+ (DP) and EpCAM−/INTEGRINα6− (N) cells (Figure S1A). The PGC marker genes such as TFAP2C and SOX17 were upregulated in EpCAM+/INTEGRINα6+ cells, while somatic genes such as HOXA1 were upregulated in EpCAM−/INTEGRINα6− cells (Figure S1B). We further confirmed the protein expression of OCT4, SOX17, and TFAP2C in embryoid bodies (EBs) at day 2, 4, and 6 via immunostaining (Figure S1C). We then performed a time course ATAC-seq and RNA-seq analysis throughout hPGCLC induction (Figure 1A). Principal-component analysis (PCA) revealed a cell-fate bifurcation between EpCAM+/INTEGRINα6+ and EpCAM−/INTEGRINα6− cells along the trajectory of hPGCLC induction from day 1 (D1) onward (Figures 1B, 1C, S1D, and S1E).
Figure 1.
Chromatin accessibility and gene regulation dynamics during hPGCLC induction
(A) Schematic representation of time course ATAC-seq and RNA-seq library induction during the hPGCLC induction from hESCs. Day is represented as “D,” EpCAM+/INTEGRINα6+ cells are represented as DP, EpCAM−/INTEGRINα6− cells are represented as N, and EpCAM+/INTEGRINα6− cells are represented as SP.
(B and C) PCA of ATAC-seq (B) and RNA-seq data (C). Cell types are labeled as described in (A) and two independent replicates are merged.
(D) Dynamically closed-to-open (CO), open-to-closed (OC), and permanently open (PO) chromatin regions are clustered and shown as a heatmap. CO, OC, and PO refer to closed in hESCs but open in D6DP, open in hESCs but closed in D6DP, and PO in both hESCs and D6DP, respectively.
(E) Violin plots showing the expression levels of all genes with a TSS within 10 kb of an ATAC-seq peak for each CO or OC group. The Wilcoxon rank-sum test was performed. ∗p < 0.01.
(F and G) Heatmap showing the genome coverage of ATAC-seq signals on each CO (F) and OC (G) group.
(H) Bubble plot showing the top 2 de novo motifs in COs/OCs.
(I) Selected top ranked de novo motifs from CO (left) and OC (right).
(J) Representative genome coverage plots for ATAC-seq and RNA-seq signals for key germ cell genes.
We next used our ATAC-seq data to define the chromatin accessibility dynamics (Figures 1D and S1F) (Li et al., 2017). We defined the open chromatin peaks in each ATAC library using macs2 (Zhang et al., 2008) (Figures S1F and S1G) and grouped the open/closed regions as reported in previous studies (Li et al., 2017) (Figure 1D; Table S1). We could identify dynamically CO, OC, and permanently open (PO) regions. Many PO regions were enriched in the proximal promoters (Figure S1H). We then evaluated the gene expression patterns associated with the dynamic chromatin changes throughout hPGCLC induction, and observed a significant difference in gene expression patterns from D1 onward when compared with hESCs or 4i stage (Figure 1E). We then evaluated the OC1-5 and CO1-5 genomic regions in the N cells and found that N cells failed to close (FC) in OC3-5 and failed to robustly open in CO2-5 (Figures 1F and 1G).
To understand the mechanisms underlying the global chromatin dynamics, we measured the enrichment of TF binding motifs. AP-2gamma, OCT4:SOX17 (compressed SOXOCT motif) and single SOX15 motifs were enriched in the CO regions. In contrast, TEAD, OCT4:SOX2 (canonical SOXOCT motif), and ZIC motifs were enriched in the OC regions (Figure 1H). Interestingly, the compressed OCT4:SOX17 motif emerged in the chromatin regions that open early, while the single SOX15 motif was specially enriched at the sites that open later throughout PGCLC induction (CO4 and CO5; Figures 1H, 1I, S1I, and S1J). In addition, the DP cells exhibited expected stage-specific open chromatin signals at the TFAP2C, OCT4, SOX17, and BLIMP1 loci, and the corresponding transcripts were upregulated (Figure 1J). In summary, we comprehensively profiled the chromatin accessibility and transcriptome dynamics throughout hPGCLC induction, obtaining the specific CO/OC patterns and the enriched TF binding motifs.
Determination of the regulatory elements underlying cell-fate bifurcation of germline and non-germline lineages
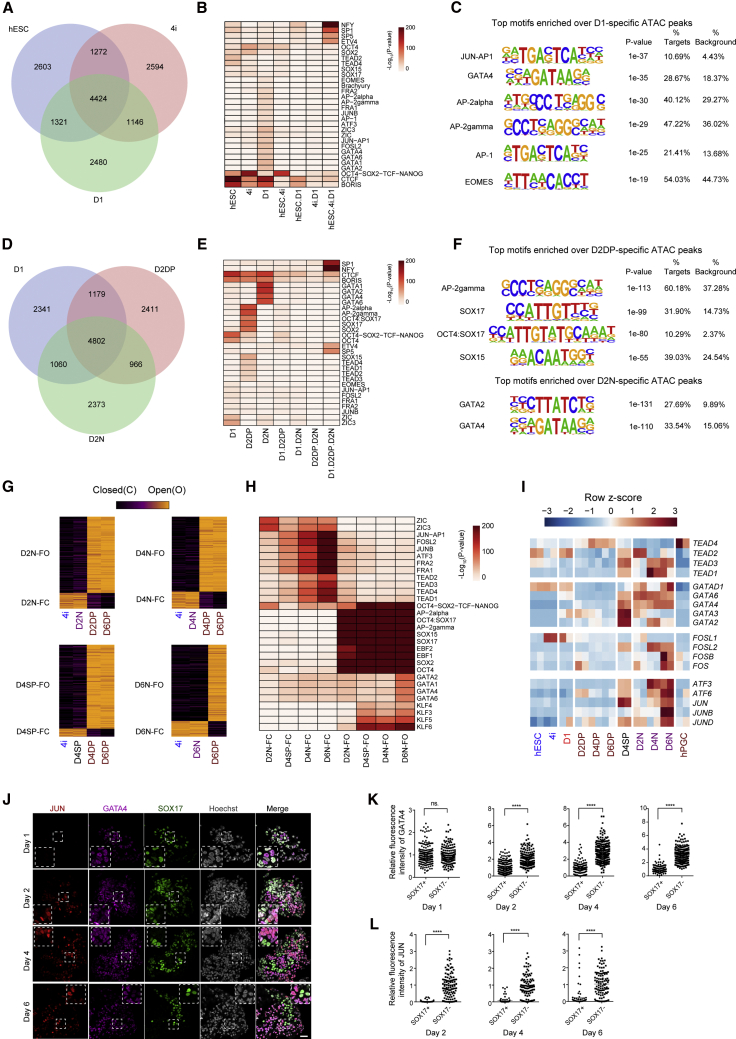
Compared with EpCAM+/INTEGRINα6+ cells (DP cells committing to the germline lineage), EpCAM−/INTEGRINα6− cells (N cells uncommitted to the germline lineage) were enriched with the binding motifs of representative somatic TFs, such as JUN-AP1, JUNB, and GATA motifs in the CO regions and pluripotency-associated TFs in the OC regions (Figures S2A–S2D). To elucidate the regulators accounting for the cell-fate bifurcation of germline and non-germline lineage, we first focused on the top 10k peaks from hESC, 4i, and D1 libraries and intersected the peaks from each library to obtain the specific and common peaks (Figure 2A). Interestingly, the top significantly enriched motifs over D1-specific peaks (2,480 peaks) are JUN-AP1/AP1, EOMES, AP-2alpha/AP-2gamma, and GATA binding motifs (Figures 2B, 2C, and S2E). It is well known that EOMES and TFAP2C (AP-2gamma) play critical roles in hPGCLC induction (Kojima et al., 2017); however, it is still unknown that, if the AP1 and GATA family TFs are essential for this process. We next compared the top 10 kb peaks from D1, D2DP, and D2N libraries and found that the D2DP-specific peaks were enriched with AP2, OCT4:SOX17, SOX17, and SOX15 motifs, while the D2N-specific peaks were enriched with GATA motifs (Figures 2D–2F and S2F).
Figure 2.
Determination of the regulatory elements underlying cell-fate bifurcation of germline and non-germline lineages
(A) Venn diagram showing the top 10k common and specific peaks in hESCs, 4i cells, and day 1 cells.
(B) Heatmap showing TF motifs significantly enriched in the common and specific peaks in hESCs, 4i cells, and day 1 cells defined in (A).
(C) The top binding motifs enriched in day 1-specific peaks.
(D) Venn diagram showing the top 10k common and specific peaks in day 1, D2DP, and D2N cells.
(E) Heatmap showing TF motifs significantly enriched for common and specific peaks in day 1, D2DP, and D2N cells defined in (D).
(F) The top binding motifs enriched in D2DP-specific and D2N-specific peaks.
(G) Failed-to-open (FO) and failed-to-close (FC) peaks for D2N, D4N, D4SP, and D6N cells compared with D6DP cells are shown. These peaks are derived from peaks open in 4i cells yet closed in D6DP cells or peaks closed in 4i cells yet open in D6DP cells. (H) Heatmap showing TF motifs significantly enriched in FO and FC peaks in D2N, D4N, D4SP, and D6N cells.
(I) Gene expression of TEAD, GATA family, and AP1 family TFs among DP and N cells as well as 7-week hPGCs (Irie et al., 2015).
(J) Immunofluorescence of JUN, GATA4, and SOX17 in EBs from day 1 to day 6, Scale bar, 50 μm. The dotted boxes enclose representative zoomed images.
(K and L) Quantification of relative fluorescence intensity of GATA4 (K) or JUN (L) for SOX17-positive and -negative cells measured by the ImageJ software. Eight slides of immunostaining from three independent experiments were used. Two-tailed Student's t test was performed, ∗∗∗∗p < 0.0001.
To examine the failure to commit to the germline lineage, we determined the loci that failed to open (FO) and FC in the mid and late stages, in which all EpCAM−/INTEGRINα6− and EpCAM+/INTEGRINα6− (SP) cells were compared with the EpCAM+/INTEGRINα6+ cells (Figure 2G). Of note, the FO loci were significantly enriched with AP2, OCT4, OCT4:SOX17, SOX17, SOX15, and EBF motifs, while the FC loci were enriched with AP1 and TEAD motifs (Figure 2H). Consistently, somatic lineage genes such as GATA, AP1, and TEAD family members were upregulated in N cells (Figure 2I). Next, we found that the day 1 EBs exhibited a higher proportion of GATA4-positive cells than that of SOX17-positive cells by immunostaining (Figures 2J, S2G, and S2H). Notably, there was no difference for GATA4 expression between SOX17-positive and -negative cells in day 1 EBs, while the SOX17-positive cells exhibited significantly lower GATA4 expression than that of SOX17-negative cells from day 2 (Figures 2J and 2K). Although the AP1-JUN motifs were enriched in D1-specific open regions, JUN protein cannot be detected until day 2 (Figure 2J). Interestingly, a mutually exclusive expression pattern between JUN and SOX17 was identified (Figures 2J and 2L), suggesting the potential antagonism between JUN and SOX17.
Transcriptional determinants during the induction of hPGCLCs from hESCs
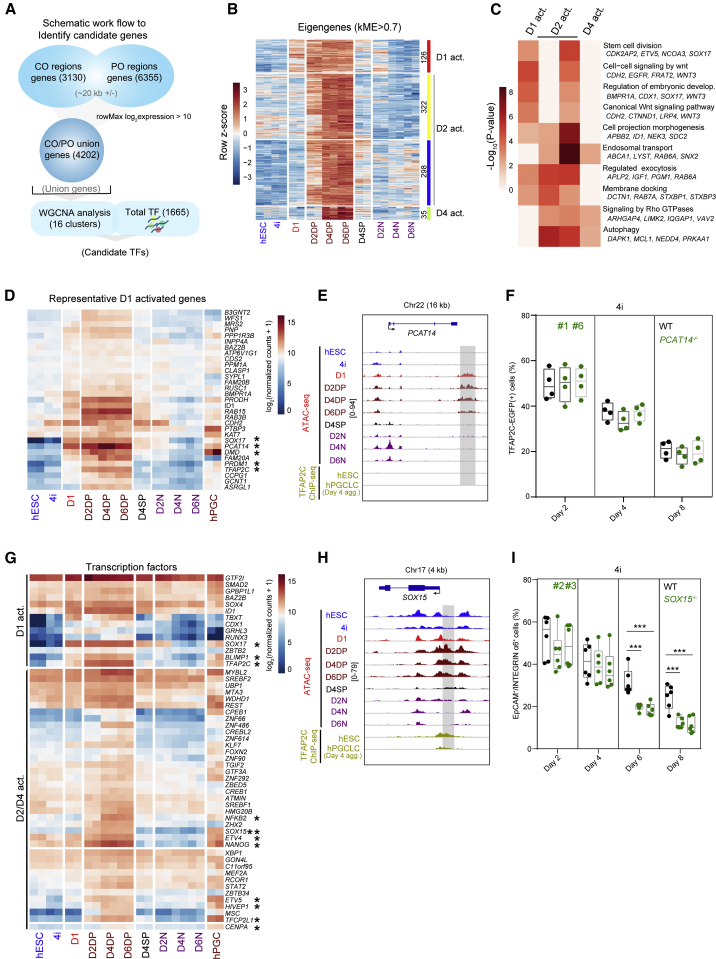
In contrast to EpCAM−/INTEGRINα6− (N) cells, the EpCAM+/INTEGRINα6+ (DP) cells were on a trajectory toward gonadal hPGCs (Figure S3A). To evaluate the key transcriptional regulators that control hPGCLCs, we annotated all genes with a transcription start site (TSS) within 20 kb of the CO1-5 regions (3,130 genes) and constituently opened PO regions (6,355 genes) from Figure 1D. From this, we obtained 4,202 union genes from the CO and PO regions after removing lowly expressed genes (Figure 3A). We clustered the genes using weighted gene correlation network analysis (WGCNA) (Langfelder and Horvath, 2008) and obtained 16 modules across hPGCLC induction (Figure S3B; Table S2). These modules showed distinct patterns of gene expression and gene ontology (GO) (Figures 3B, 3C, and S3C–S3F). Based on the expression patterns, we assigned the WGCNA-identified modules: red as day 1 activated (D1 act.), yellow/blue as day 2 activated (D2 act.), and green as day 4 activated (D4 act.) (Figure 3B). GO analysis showed that nucleic acid metabolism-related terms were enriched in genes highly expressed from hESCs to D1 (Figures S3C and S3D), while genes in D1, D2, and D4 act. modules were related to terms such as “stem cells division” and “WNT signaling related” (Figure 3C; Table S2). Conversely, genes highly expressed in N cells were enriched in GO terms associated with somatic differentiation (Figures S3E and S3F; Table S2).
Figure 3.
Transcriptional determinants during the induction of hPGCLCs from hESCs
(A) Schematic representation of candidate genes identification.
(B) Heatmap showing the expression of selected modules in which genes are specifically expressed in DP cells. Module eigengenes score (kME score > 0.7) was used to set the threshold to obtain candidate genes. The red, yellow/blue, and green modules were assigned to day 1-activated (D1 act.), day 2-activated (D2 act.), and day 4-activated (D4 act.) groups.
(C) Gene ontology (GO) analysis of the genes in the D1 act., D2 act., and D4 act. groups as defined in (B).
(D) Heatmap showing the expression pattern of representative D1 act. genes.
(E) Selected genomic views showing the ATAC-seq signals and TFAP2C chromatin immunoprecipitation sequencing (ChIP) signals (Chen et al., 2019) for PCAT14 in the indicated samples. The specific open regions from day 1 are marked with a gray box.
(F) The percentages of TFAP2C-EGFP(+) cells of floating embryoids of WT (black) and PCAT14 knockout (KO) lines (green) upon hPGCLC induction at the indicated days via the 4i method. Results of four independent experiments were shown (n = 4).
(G) Heatmap showing the overall expression of all TFs from the D1/D2/D4 act. modules. Key genes with relatively high expression in hPGCLCs and hPGCs are highlighted.
(H) Selected genomic views showing the ATAC-seq signals and TFAP2C ChIP signals (Chen et al., 2019) for SOX15 in the indicated samples. The specific open regions with TFAP2C binding are marked with a gray box.
(I) The percentages of EpCAM+/INTEGRINα6+ cells of floating embryoids from WT (black) and SOX15 KO lines (green) upon hPGCLC induction at the indicated days via the 4i method. Results of six independent experiments were shown (n = 6). Two-tailed Student's t test was performed, ∗∗∗p < 0.001.
To find the critical genes involved in the induction of hPGCLCs, we first focused on the D1 act. genes that showed the similar expression patterns to SOX17. PCAT14, a long non-coding RNA, was activated from day 1 and exhibited high expression and specific open regions in hPGCLCs (Figures 3D, 3E, and S3G). But PCAT14 knockout (KO) exhibited no obvious effect on the induction of hPGCLCs (Figures 3F and S3H–S3J). Then we intersected genes in D1, D2, and D4 act. groups with a database of TFs (Hu et al., 2019) and 53 unique TFs were identified. In detail, SOX17, BLIMP1, and TFAP2C were activated at day 1, while NFKB2, SOX15, ETV4, NANOG, ETV5, HIVEP1, and TFCP2L1 were activated from day 2 or day 4 (Figure 3G). All the TFs highlighted in Figure 3G showed significant expression levels in DP cells (hPGCLCs) like that in hPGCs, but were downregulated in N cells. Notably, there were specific open regions at the loci of SOX15 in DP cells from day 2 (Figure 3H), which were consistent with the gene expression pattern. In addition, the specific open regions of SOX15 genome loci showed the enrichment of TFAP2C peaks (Figure 3H). To confirm if SOX15 was essential for the induction of hPGCLCs from hESCs, we generated SOX15 KO hESC clones and confirmed the absence of the SOX15 protein (Figures S4A and S4B). The resulting SOX15 KOs were karyotypically normal and expressed pluripotency marker genes (Figures S4C and S4D). Interestingly, we found that the proportion of hPGCLCs was dramatically decreased on D6 and D8 in the SOX15 KO lines (Figures 3I, S4E, and S4F), indicating that SOX15 might be crucial for the maintenance of hPGCLC identity. In addition, genetic ablation of SOX15 also led to a decrease of EpCAM+/INTEGRINα6+ cells from D4 in the iMeLC system (Figures S4G–S4I). And SOX15 KO had no obvious effect on the cell-proliferation and apoptosis status of hPGCLCs (Figures S4J–S4M).
Absence of SOX15 in hPGCLCs derails the germline fate and initiates a somatic lineage program
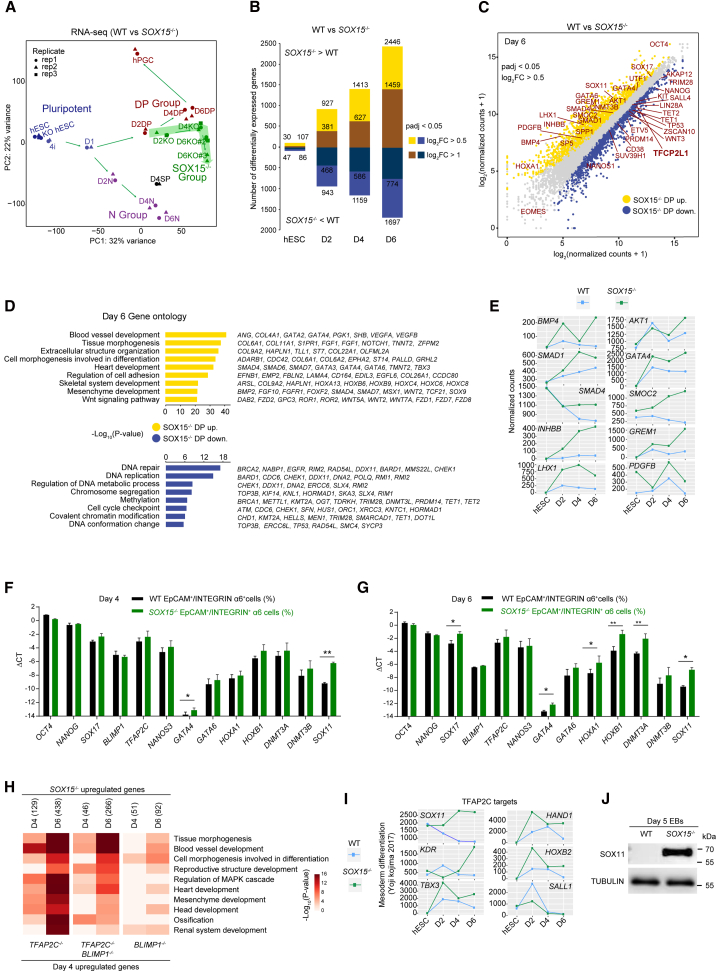
To obtain a comprehensive insight into the roles of SOX15 throughout hPGCLC induction, we evaluated the impact of the SOX15 KO on the transcriptome via time course RNA-seq. Intriguingly, PCA showed that the divergence between the SOX15 wild type (WT) and KO started at D2 (Figure 4A). In support of this, the number of differentially expressed genes (DEGs) increased from day 2 onward (Figure 4B; Table S3). Among the late-stage (day 6) DEGs, we noticed that pluripotency genes were downregulated; however, a range of somatic genes were upregulated in SOX15−/− hPGCLCs relative to the control (Figures 4C–4E; Table S3). qPCR further validated the aberrant upregulation of somatic genes (GATA4, GATA6, HOXA1, and HOXB1) and the de novo DNA methyltransferases (DNMT3A) (Figures 4F and 4G). These data support the notion that SOX15 is essential for maintaining the germ cell identity of late-stage hPGCLCs.
Figure 4.
Absence of SOX15 in hPGCLCs derails the germline fate and initiates a somatic lineage program
(A) PCA of the RNA-seq data of WT and SOX15 KO samples. Cell types are indicated by different colors. The green color shows the diverted pathway of SOX15 KO cells. Results of three independent experiments were shown and the replicates are represented by triangles, squares, and circles.
(B) Bar plot showing the number of differentially expressed genes (DEGs) during the induction of hPGCLCs from SOX15−/− hESCs (padj < 0.05, log2 fold change [FC] > 0.5 or > 1).
(C) Scatterplot showing the DEGs in SOX15−/− DP cells at day 6. The SOX15−/− upregulated and downregulated genes are color coded (log2 fold change > 0.5).
(D) GO terms enriched in DEGs in SOX15−/− DP cells (log2 fold change > 0.5).
(E) Line plots showing gene expression dynamics of the indicated genes.
(F and G) qPCR of the indicated genes in EpCAM+/INTEGRINα6+ cells of day 4 embryoids (F) and day 6 embryoids (G) derived from WT and SOX15−/− hESCs, respectively. Relative expression levels are shown normalized to GAPDH. Error bars indicate mean ± SD from three independent replicates. Two-tailed Student's t test was performed, ∗p < 0.05, ∗∗p < 0.01.
(H) Heatmap showing the GO terms enriched in the upregulated genes (day 4 and day 6) in SOX15−/− cells shared with BLIMP1−/− or TFAP2C−/− cells (day 4). The gene numbers here are from Figure S6.
(I) Line plots showing the gene expression of the downstream genes regulated by TFAP2C.
(J) Western blot analysis of SOX11 protein in day 5 SOX15−/− embryoids relative to the control. Tubulin was used as an inner control.
Among the affected genes in SOX15−/− hPGCLCs, we found that several pluripotency-associated genes (such as TFCP2L1) were downregulated (Figure 4C). Thus, we next attempted to see if the naive stem cell-specific gene TFCP2L1 (Wang et al., 2019) was also involved in hPGCLC induction; however, TFCP2L1 KO did not impact the induction of hPGCLCs (Figures S5A–S5H).
SOX15 might act as a downstream regulator of TFAP2C
To identify the upstream regulator of SOX15, we first compared the expression of hPGC and pluripotency-associated marker genes in the SOX15−/−, SOX17−/−, TFAP2C−/−, and BLIMP1−/− cells throughout hPGCLC induction (Kojima et al., 2017) (Figures S6A and S6B). We found that, at day 2, SOX17−/− cells exhibited the complete loss of expression of early hPGC and pluripotency-associated marker genes, while TFAP2C−/− cells maintained lower levels of SOX17 and BLIMP1 until day 2. Compared with SOX17−/− and TFAP2C−/− cells, the BLIMP1−/− cells expressed SOX17 at similar levels to WT until day 2 and maintained higher levels of pluripotency and hPGC-associated markers until day 4 (Figure S6B). Notably, SOX15 expression was not activated in both SOX17−/− or TFAP2C−/− cells, while it was indistinguishable in BLIMP1−/− cells. This implies that SOX15 might be the downstream regulator of SOX17 and TFAP2C but not BLIMP1. To further test whether SOX15 expression is dependent on SOX17 or TFAP2C, we analyzed the up- and downregulated genes in hPGCLCs using the RNA-seq data: SOX15−/− (day 2, day 4, or day 6), SOX17−/− (day 2), and TFAP2C−/− and BLIMP1−/− (day 4), compared with their controls (Kojima et al., 2017) (Figures S6C–S6H; Table S4). Interestingly, we observed that several canonical pathways such as ATF2 and AP1 were significantly enriched in commonly upregulated genes in SOX15−/− and TFAP2C−/− at day 4 and day 6, but not genes in SOX15−/− and BLIMP1−/− (Figure S6I). Furthermore, SOX15−/− cells and TFAP2C−/− cells shared many somatic lineage-related GO terms in the upregulated genes (Figure 4H). In addition, the downregulated genes shared with TFAP2C−/− or BLIMP1−/− had only a few significant associated GO terms (Figure S6J).
To establish the direct relationship between SOX15 and TFAP2C as well as BLIMP1, we examined the target genes of BLIMP1, BLIMP1/TFAP2C, and TFAP2C, respectively (Kojima et al., 2017). We found that the targets of BLIMP1 and BLIMP1/TFAP2C were not affected in SOX15−/− cells (Figure S6K); however, TFAP2C target genes associated with mesoderm differentiation were significantly upregulated in SOX15−/− hPGCLCs (Figure 4I). Notably, chromatin immunoprecipitation sequencing analysis showed that TFAP2C can bind to several proximal elements at the SOX15 locus, supporting the notion that TFAP2C might be an upstream regulator of SOX15 (Figure 3H) (Chen et al., 2019). Western blot results further demonstrated that SOX11, a shared marker gene of mesoderm and ectoderm lineages, was upregulated in SOX15−/− cells (Figure 4J). Together, these results suggest that SOX15 might act as a downstream regulator of TFAP2C to exert its functions.
The suppression of somatic gene expression mediated by SOX15 is associated with chromatin accessibility
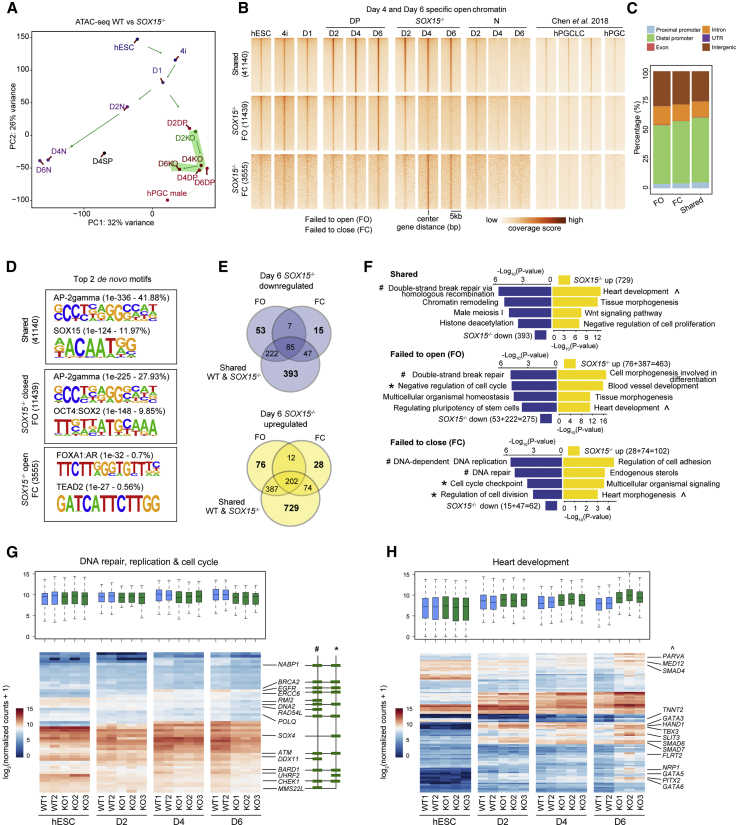
To determine how SOX15 exerts its roles in somatic gene expression suppression during hPGCLC induction, we performed ATAC-seq in WT and SOX15−/− hPGCLCs. PCA analysis demonstrated that SOX15−/− hPGCLCs cells diverged from the hPGCLC trajectory from day 4 onward (Figure 5A). We also combined the D4 and D6 ATAC-seq data and clustered them into three categories, shared-open, FO, and FC (Figure 5B). The open chromatin regions of shared, FC, and FO groups were highly enriched around the distal and intergenic regions (Figure 5C). Of note, we discovered the top TF binding motifs of each category (shared-open, AP2 and SOX15; FC, FOXA1:AR and TEAD2; FO, OCT4:SOX2) (Figure 5D).
Figure 5.
The suppression of somatic gene expression mediated by SOX15 is associated with chromatin accessibility
(A) PCA plot showing ATAC-seq analysis of the hPGCLC induction under the normal and SOX15 KO states. Two independent replicates are merged.
(B) Heatmap of ATAC-seq signals in the indicated samples over shared-open chromatin regions constituting 41,140 peaks, SOX15−/− FO regions constituting 11,439 peaks, and SOX15−/− FC regions constituting 3,555 peaks.
(C) Bar plot showing the percentage of genomic features from FO, FC, and shared regions.
D) Top 2 de novo motifs from shared, FO, and FC genomic regions. The name of the motifs with respective p value and percentage are shown on each motif.
(E) Venn diagram showing the intersection of nearby genes from FO, FC, or shared regions that shared with the downregulated and upregulated genes in day 6 SOX15−/− cells, respectively. Log2 fold change > 0.5.
(F) GO analysis for upregulated and downregulated genes nearby shared, FO, and FO regions, respectively, as shown in (E).
(G and H) Boxplots (with the median and 25th and 75th percentiles) and heatmap showing the expression patterns of specific genes representing the GO terms of DNA repair, DNA replication, and cell cycle (G), or heart development (H). The symbols #, ∗, and ˆ represent the GO terms shown in (F) and key genes are indicated.
We next extracted the day 6 DEGs around the shared, FO, and FC open chromatin regions, respectively. There were 53 downregulated genes and 76 upregulated genes for FO, 15 downregulated genes and 28 upregulated genes for FC, and 393 downregulated genes and 729 upregulated genes for shared-open regions (Figure 5E). Among the unique genes for shared-open regions, we observed that the heart development (i.e., somatic mesoderm)-related genes were upregulated, while double-strand break repair via homologous recombination-related genes were downregulated in SOX15−/− hPGCLCs (Figure 5F; Table S5). This result indicates that the genes nearby were still affected by the absence of SOX15, albeit no change of these shared-open chromatin regions. Due to the limited number of DEGs, we combined the common genes near shared regions of FO or FC regions for further analysis. GO analysis of genes near the FO and FC regions revealed that the downregulated genes were enriched in DNA replication and pluripotency-related GO terms, while the upregulated genes were enriched in heart development-related GO terms (Figure 5F; Table S5). In addition, we combined the genes in similar GO terms of shared, FC, as well as FO groups and found that the downregulated or upregulated genes of these GO terms in SOX15−/− cells exhibited differential expression patterns throughout hPGCLC induction (Figures 5G and 5H). Overall, loss of SOX15 in hPGCLCs disturbed the genes near the unchanged chromatin open regions or resulted in aberrant chromatin changes, both of which might further induce the observed cell-fate bifurcation to somatic lineages.
SOX15 exerts its function in hPGCLC maintenance by directly suppressing somatic gene expression and sustaining latent pluripotency
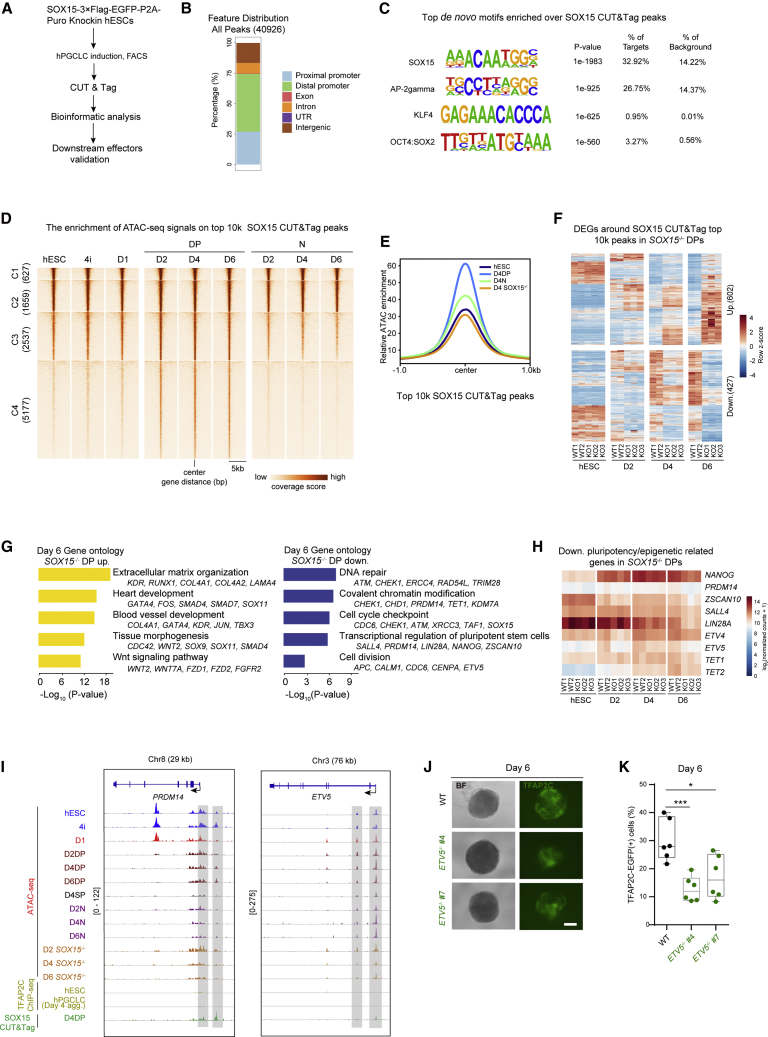
To find the target genes bound by SOX15, we first established SOX15-3×Flag-P2A-EGFP-Puro knockin cell lines and obtained day 4 EpCAM+/INTEGRINα6+ (DP) cells to perform CUT&Tag assays (Figures 6A, S7A, and S7B) (Kaya-Okur et al., 2020). SOX15 peaks were mainly enriched around the proximal and distal promoter regions (Figure 6B). Next, we performed de novo motif search on SOX15 peaks. Interestingly, we found AP2-gamma, KLF4, and OCT4:SOX2 on the top enriched motif list, indicating that the AP2-gamma (TFAP2C) and KLF4 might also bind near the SOX15-bound regions (Figure 6C). For the extinguishment of SOX2 in hPGCLCs, the OCT4:SOX2 motifs regions should be bound by OCT4/SOX17 or/and OCT4/SOX15. By overlaying the enrichment of ATAC-seq signals on top 10k SOX15 peaks, we found four different signals clusters. Interestingly, cluster 4 (5,177 regions) showed a stronger signal specific to DP cells (Figure 6D). Moreover, the chromatin accessibility status near SOX15 peaks were dramatically decreased in D4 SOX15 KO DP cells (Figure 6E). Surprisingly, the relative ATAC signals on D2 for SOX15 peaks were highly enriched in both WT and SOX15 KO DP cells; however, the ATAC signal for SOX15 peaks were only remained enriched in WT DP cells on D4 and D6 (Figure S7C). These results indicate that SOX15 might exert its function by regulating chromatin accessibility and thereby target gene expression.
Figure 6.
SOX15 exerts its function in hPGCLC maintenance by directly suppressing somatic gene expression and sustaining latent pluripotency
(A) Schematic representation of the SOX15 CUT&Tag analysis workflow in hPGCLCs.
(B) Bar plot showing the percentage of genomic feature distribution of SOX15 peaks.
(C) The top binding motifs enriched in SOX15 peaks.
(D) Heatmap of ATAC-seq signals in the indicated samples over the top 10k SOX15 peaks.
(E) Pileup of the ATAC-seq signals over the top 10k SOX15 peaks regions in the indicated cells.
(F) Heatmap showing the expression patterns of upregulated or downregulated genes around the top 10k SOX15 peaks in day 6 SOX15 KO DP cells.
(G) GO analysis for the upregulated or downregulated genes as described in (F).
(H) Heatmap showing the expression patterns of downregulated pluripotency-related genes in SOX15 KO DP cells.
(I) Selected genomic views showing the ATAC-seq signals, TFAP2C ChIP signals (Chen et al., 2019), and SOX15 signals at the PRDM14 and ETV5 genome loci in the indicated samples. The specific open regions with SOX15 signals and decreased ATAC-seq signals from day 4 KO DP cells compared with those in DP cells are marked with a gray box.
(J) Bright-field (BF) and fluorescence (TFAP2C-EGFP) images of floating embryoids from WT and ETV5−/− lines at day 6. Scale bar, 200 μm.
(K) The percentages of TFAP2C-EGFP(+) cells of floating embryoids from day 6 WT (black) and ETV5 KO lines (green) upon hPGCLC induction via the 4i method. Results of six independent experiments are shown (n = 6). Two-tailed Student's t test was performed, ∗p < 0.05, ∗∗∗p < 0.001.
Then, we searched for D6 DEGs around the top 10k SOX15 peaks. About 602 upregulated genes and 427 downregulated genes were obtained (Figure 6F). GO analysis of these genes revealed that the upregulated genes were enriched in the terms associated with somatic lineage differentiation, while the downregulated genes were enriched for the DNA repair and pluripotency-related terms (Figure 6G; Table S6). Moreover, SOX15 peaks were detected at the proximal regulatory elements of several pluripotency-related genes, such as PRDM14, NANOG, ETV4, and ETV5 (Figures 6G and S7D) (Kalkan et al., 2019; Murakami et al., 2016; Sybirna et al., 2020). These results suggest that SOX15 might be involved in maintaining the latent pluripotency of hPGCLCs (Leitch and Smith, 2013). In support of this, the regulatory elements bound by SOX15 of these genes showed decreased ATAC signals in day 4/6 SOX15 KO DP cells compared with that in WT DP cells, which were consistent with the downregulated expression of these genes (Figures 6G–6I and S7D). These results indicated that SOX15 exerted its functions in maintaining the identity of hPGCLCs through dual effect-simultaneous suppression of somatic gene expression and the retention of latent pluripotency.
Given the fact that PRDM14 and NANOG are implicated in the induction of PGCLCs (Murakami et al., 2016; Sybirna et al., 2020), we then investigated whether ETV5 was also involved in the maintenance of hPGCLCs by acting as a direct target of SOX15. To this end, the expression pattern of ETV5 was first evaluated, and we found that ETV5 was downregulated in D4 SOX15 KO DP cells (Figure S7E). Then, ETV5 hESC KO clones (TFAP2C-EGFP knockin) were generated and the absence of the ETV5 protein was confirmed (Figures S7F and S7G). The resulting ETV5 KOs can be induced into hPGCLCs with decreased ratio of hPGCLCs compared with WT control (Figures 6J, 6K, S7G, and S7H). These data proved that ETV5, which acted as a downstream regulator of SOX15, was essential for hPGCLC maintenance.
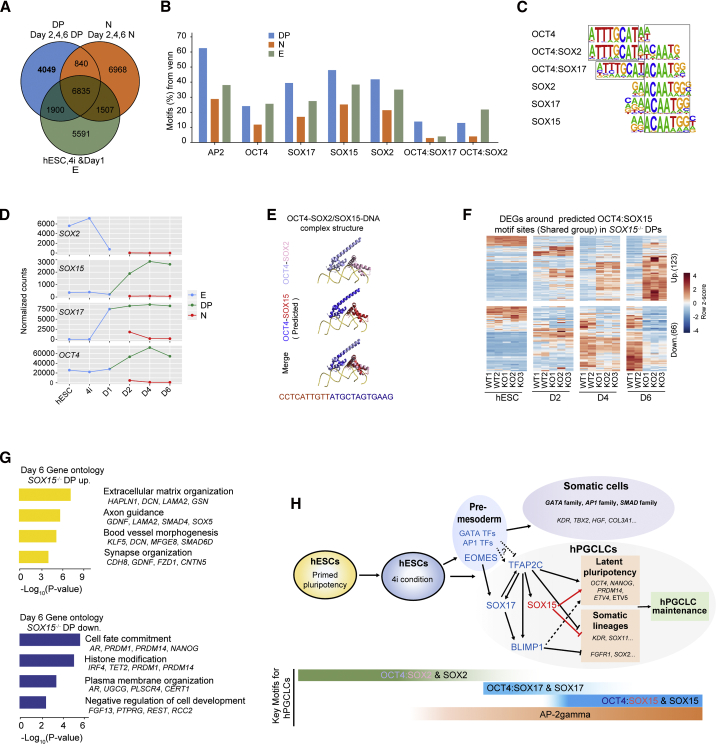
A stepwise OCT4:SOX motifs switch throughout hPGCLC induction
To further study the stage-specific role of SOXs and OCT4/SOXs in the induction of hPGCLCs, we performed a focused analysis of SOX motifs in open chromatin. First, we defined peaks in DP (top 10k peaks from day 2/4/6 DP libraries), N (peaks from day 2/4/6 N libraries), and E (peaks from early stage: hESCs, 4i, and day 1 libraries) groups and intersected the peaks to obtain specific peaks in each group (Figure 7A). The DP-specific ATAC signals (4,049 peaks) were enriched in hPGCLCs and gonadal hPGCs (Chen et al., 2018), while the N-specific ATAC signals (6,968 peaks) were not found in hPGCLCs and hPGCs (Figure S7I). These DP-specific regions showed an enrichment of known single SOX or OCTSOX motifs (Figures 7B and 7C). Notably, SOX2 and OCT4:SOX2 motifs (canonical SOXOCT motifs) were enriched in DP-specific regions (Figures 7B and 7C), which was not consistent with the absence of SOX2 in hPGCLCs (Figure 7D). This prompted us to ask if the SOX2 and OCT4:SOX2 motifs sites in DP group were engaged by SOX17 or SOX15 to form an OCT4/SOX17 or OCT4/SOX15 heterodimer. Notably, co-immunoprecipitation results in HEK293 cells showed that there was an interaction between OCT4 and SOX15 or SOX17 (Figures S7J and S7K). In addition, overexpression of Sox15 can rescue the defects that result from the absence of Sox2 in mESCs (Niwa et al., 2016). It is known that SOX17 heterodimerize with OCT4 to bind a compressed motif (OCT4:SOX17), which lacks a single base pair between the SOX and OCT half-sites compared with the canonical motifs (OCT4:SOX2) (Figure 7C), while SOX15 can heterodimerize with OCT4 on canonical elements (Chang et al., 2017), albeit there is no direct evidence to demonstrate the presence of OCT4:SOX15 motifs so far. Molecular modeling results further showed that human OCT4-SOX15-DNA complex shared a similar overall fold with mouse OCT4-SOX2-DNA complex (Figure 7E). Based on this evidence, the OCT4:SOX2 motifs enriched in the DP-specific group and SOX15 CUT&Tag peaks (Figure 6C) were most likely to be OCT4:SOX15 motifs.
Figure 7.
A stepwise OCT4:SOX motifs switch throughout hPGCLC induction
(A) Venn diagram showing the common and different peaks in DP, N, and E groups.
(B) Bar plot representing the percentage of known OCTSOX motifs enriched in DP/N/E-specific open chromatin regions.
(C) Known SOX and OCT motifs with respective TF binding sequences.
(D) Line plot showing the gene expression of SOX2, SOX15, SOX17, and OCT4 in E (hESC, 4i, day 1), DP, and N cells.
(E) Ribbon diagrams showing the similarity between the structure of known mouse OCT4-SOX2-DNA complex (PDB: 6HT5) and the predicted modeled structure of human OCT4-SOX15-DNA complex.
(F) Heatmap showing the expression pattern of upregulated or downregulated genes around putative OCT4:SOX15 binding motif sites, which belong to the “Shared” group as described in Figure 5B, in day 6 SOX15 KO DP cells.
(G) GO analysis for the upregulated or downregulated genes as described in (F).
(H) Schematic showing the roles of SOX15, ETV5, and the key motifs during the induction of hPGCLCs.
To determine if the predicted OCT4:SOX15 motifs was functionally relevant, we first extracted 1,595, 68, and 3 ATAC-seq peaks including OCT4:SOX15 motifs sites in the shared, FO, and FC groups, respectively (Figure S7L). Next, we searched the DEGs around the predicted OCT4:SOX15 binding motif sites in SOX15−/− DP cells. GO analysis of genes around the shared regions showed that the 123 upregulated genes in SOX15−/− DP cells were enriched in terms such as “extracellular matrix organization,” while the 66 downregulated genes were enriched in terms such as “cell fate commitment” (Figures 7F and 7G; Table S6). Notably, the downregulated genes included PRDM14 and NANOG, which are critical for the latent pluripotency of germline.
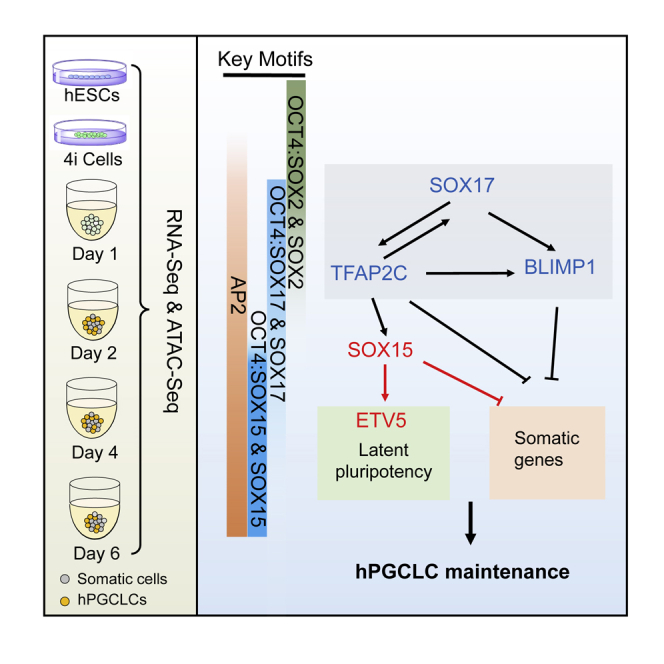
Based on these results, we established a model that supports a stepwise switch of OCT/SOX heterodimerization preferences, from OCT4/SOX2 in pluripotent cells, to OCT4/SOX17 in early-stage cells, and then to a putative OCT4/SOX15 binding module in the late stage (Figure 7H). This model describes the critical roles of SOX15 in the maintenance of hPGCLC identity via suppressing somatic gene expression and sustaining latent pluripotency.
Discussion
Here, time course ATAC-seq and RNA-seq analyses were performed to resolve the dynamics of genome regulation in both hPGCLCs and non-hPGCLCs. In addition, we identified the involvement of SOX15 in maintaining the identity of hPGCLCs. Further studies showed that SOX15 exerted its functions in hPGCLCs by suppression of somatic gene expression and retainment of latent pluripotency. Among the SOX15-mediated regulatory networks underlying latent pluripotency preservation, ETV5 was revealed to be critical for hPGCLC maintenance by acting as a downstream regulator of SOX15. Finally, a stepwise OCT4:SOX motifs switch was uncovered to have potential functions throughout hPGCLC induction. Based on our data and the accumulated evidence, we propose a model that SOX15 is involved in facilitating the establishment of hPGCLC regulatory networks (Figure 7H).
The analysis for chromatin dynamics of both hPGCLCs and non-hPGCLCs from hESCs revealed that several TF motifs as “accelerators” (AP2, OCT4:SOX17, and SOX15) or potential “suppressors” (GATA, AP1, and TEAD) of hPGCLC induction. However, it is noteworthy that GATA and AP1 motifs are not only enriched in non-hPGCLCs (Figures S2B and S2C), but also in the regions over D1-specific peaks, in which the EOMES motif is also enriched (Figure 2C). Therefore, it would be appealing to validate the functions of GATA and AP1 in the induction of hPGCLCs, which might provide new insights into the cell-fate bifurcation of germline and somatic lineage.
SOX17 and TFAP2C exert their functions in hPGCLC induction in an interdependent manner, and TFAP2C has a decisive role in the somatic lineage suppression to maintain the hPGCLC identity (Kobayashi et al., 2017; Kojima et al., 2017); growing evidence shows that TFAP2C is involved in the activation of OCT4 naive enhancers and the prevention of hPGCLCs from somatic lineages (Chen et al., 2018, 2019; Pastor et al., 2018). Consistent with these findings, our genome-wide analysis revealed that the hPGCLCs were enriched with TFAP2C motif elements as well as SOX17, SOX15, and OCT4/SOX motif elements, coinciding with the suppression of the somatic transcriptome. Moreover, we found that removal of SOX15 destabilizes hPGCLCs after day 4. A recent study demonstrates that the absence of SOX15 derails the germline fate of hPGCLCs and reactivation of SOX15 could rescue the hPGCLC identity in the SOX15−/− cell line (Pierson Smela et al., 2019); however, the detailed mechanisms of SOX15 in hPGCLCs are still unclear. Combined with ATAC-seq and CUT&Tag-seq analysis, we discovered that SOX15 played critical roles in the maintenance of hPGCLC identity by suppression of somatic gene expression and retainment of latent pluripotency.
In this study, a stepwise switch of the OCT4:SOX motif is uncovered throughout hPGCLC induction, in which OCT4/SOX2, OCT4/SOX17, and predicted OCT4/SOX15 motifs are enriched in open regions of hESCs, and early- and late-stage hPGCLCs, respectively. Further analysis demonstrated that the predicted OCT4/SOX15 binding motif is most likely to be functionally relevant, as exemplified by the involvement in the suppression of somatic gene expression. Previous studies reveal that the proper downregulation of SOX2 in the initial induction of hPGCLCs is possibly dependent on EOMES, but not SOX17 (Kojima et al., 2017). Coincident with the suppression of SOX2, the emergence of SOX17 expression from the early stage is mainly controlled by EOMES (Kojima et al., 2017). In this regard, it would be interesting to know the mechanisms regulating the shift from OCT4/SOX2 (pluripotent cells) to OCT4/SOX17 (early-stage hPGCLCs) and then to OCT4/SOX15 (mid- to late-stage hPGCLCs).
Collectively, this work characterizes the chromatin accessibility and transcriptome dynamics from hESCs to hPGCLCs or to non-hPGCLCs, providing novel insights into in vitro human germ cell induction, as exemplified by the critical role of SOX15 in the maintenance of hPGCLC identity by suppressing somatic gene expression and retaining latent pluripotency.
Experimental procedures
Induction of 4i hESCs and hPGCLCs
hPGCLCs were generated from hESCs based on a previously reported protocol (Mitsunaga et al., 2017) with slight modifications. Further information is provided in the supplemental experimental procedures.
Statistical analysis
Statistical analyses were performed using GraphPrism 6.0 software. All values are depicted as the mean ± SD. The statistical parameters, such as statistical analysis, n values, and statistical significance, are shown in the figure legends. Statistical significance is presented in the figures as ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, and not significant (ns, p > 0.05) (Student's t test) unless stated otherwise. The other statistical tests for DEG analysis, GO analysis, and motif discovery are implemented as part of the respective computational framework of the above websites and tools.
Data and code availability
The accession number for the ATAC-seq, RNA-seq and CUT&Tag-seq data reported in this paper is Gene Expression Omnibus (GEO): GSE143345.
Author contributions
X.-Y.Z., G.C., and Z.T.L. conceived and designed the experiments. X.M.W., Z.T.L., K.S., X.Y.X., M.M.C., X.H.X., C.W., W.Y.Y., Z.K.Y., X.R.W., and Y.Z. conducted the experiments. V.V., Y.X.Y., X.L.S., and F.L. performed all bioinformatics analysis. V.V., X.M.W., Z.T.L., G.C., and X.-Y.Z. wrote the manuscript. A.P.H., R.J., M.Y.L., and C.H.W. helped with data interpretation and manuscript reviewing. X.-Y.Z. supervised the project.
Acknowledgments
We are grateful to Dr. Yong Fan for providing us with the human ESC line Fy-hES-3. This work was supported by the National Key R&D Program of China (2017YFA0105001 to X.-Y.Z., 2016YFC1000606 to X.-Y.Z), the National Natural Science Foundation of China (31671544 to X.-Y.Z., 31601208 to Z.T.L., 31970787 to G.C.., 31700676 to F.L., 32000579 to X.M.W.), the Key Research & Development Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (2018GZR110104002 to X.-Y.Z.), Guangzhou Science And Technology project key project topic (201904020031 to X.-Y.Z.), the Natural Science Foundation of Guangdong Province (2019A1515010446 to G.C., 2017A030313098 to Z.T.L., 2016A030313604 to F.L.), the Clinical Innovation Research Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (2018GZR0201003 to F.-F.H.), the Outstanding Scholar Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory (2018GZR110102004 to F.-F.H.), Guangdong Provincial Science and Technology Program (2019B030301009), the Natural Science Foundation of Shenzhen (JCYJ20180305163311448 to G.C.), and the China Postdoctoral Science Foundation (2020M672707 to X.M.W.).
Published: April 29, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2021.03.032.
Contributor Information
Zhaoting Liu, Email: liuzhaoting@i.smu.edu.cn.
Gang Chang, Email: changgang@szu.edu.cn.
Xiao-Yang Zhao, Email: zhaoxiaoyang@smu.edu.cn.
Supplemental information
References
- Aksoy I., Jauch R., Chen J., Dyla M., Divakar U., Bogu G.K., Teo R., Leng Ng C.K., Herath W., Lili S. Oct4 switches partnering from Sox2 to Sox17 to reinterpret the enhancer code and specify endoderm. EMBO J. 2013;32:938–953. doi: 10.1038/emboj.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy I., Jauch R., Eras V., Chng W.B., Chen J., Divakar U., Ng C.K., Kolatkar P.R., Stanton L.W. Sox transcription factors require selective interactions with Oct4 and specific transactivation functions to mediate reprogramming. Stem Cells. 2013;31:2632–2646. doi: 10.1002/stem.1522. [DOI] [PubMed] [Google Scholar]
- Campolo F., Gori M., Favaro R., Nicolis S., Pellegrini M., Botti F., Rossi P., Jannini E.A., Dolci S. Essential role of Sox2 for the establishment and maintenance of the germ cell line. Stem Cells. 2013;31:1408–1421. doi: 10.1002/stem.1392. [DOI] [PubMed] [Google Scholar]
- Chang Y.K., Srivastava Y., Hu C., Joyce A., Yang X., Zuo Z., Havranek J.J., Stormo G.D., Jauch R. Quantitative profiling of selective Sox/POU pairing on hundreds of sequences in parallel by Coop-seq. Nucleic Acids Res. 2017;45:832–845. doi: 10.1093/nar/gkw1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Liu W., Zimmerman J., Pastor W.A., Kim R., Hosohama L., Ho J., Aslanyan M., Gell J.J., Jacobsen S.E. The TFAP2C-regulated OCT4 naive enhancer is involved in human germline formation. Cell Rep. 2018;25:3591–3602.e5. doi: 10.1016/j.celrep.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D., Sun N., Hou L., Kim R., Faith J., Aslanyan M., Tao Y., Zheng Y., Fu J., Liu W. Human primordial germ cells are specified from lineage-primed progenitors. Cell Rep. 2019;29:4568–4582.e5. doi: 10.1016/j.celrep.2019.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F., Yan L., Guo H., Li L., Hu B., Zhao Y., Yong J., Hu Y., Wang X., Wei Y. The transcriptome and DNA methylome landscapes of human primordial germ cells. Cell. 2015;161:1437–1452. doi: 10.1016/j.cell.2015.05.015. [DOI] [PubMed] [Google Scholar]
- Hou L., Srivastava Y., Jauch R. Molecular basis for the genome engagement by Sox proteins. Semin. Cell Dev Biol. 2017;63:2–12. doi: 10.1016/j.semcdb.2016.08.005. [DOI] [PubMed] [Google Scholar]
- Hu H., Miao Y.R., Jia L.H., Yu Q.Y., Zhang Q., Guo A.Y. AnimalTFDB 3.0: a comprehensive resource for annotation and prediction of animal transcription factors. Nucleic Acids Res. 2019;47:D33–D38. doi: 10.1093/nar/gky822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie N., Weinberger L., Tang W.W., Kobayashi T., Viukov S., Manor Y.S., Dietmann S., Hanna J.H., Surani M.A. SOX17 is a critical specifier of human primordial germ cell fate. Cell. 2015;160:253–268. doi: 10.1016/j.cell.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauch R., Aksoy I., Hutchins A.P., Ng C.K., Tian X.F., Chen J., Palasingam P., Robson P., Stanton L.W., Kolatkar P.R. Conversion of Sox17 into a pluripotency reprogramming factor by reengineering its association with Oct4 on DNA. Stem Cells. 2011;29:940–951. doi: 10.1002/stem.639. [DOI] [PubMed] [Google Scholar]
- Jostes S.V., Fellermeyer M., Arevalo L., Merges G.E., Kristiansen G., Nettersheim D., Schorle H. Unique and redundant roles of SOX2 and SOX17 in regulating the germ cell tumor fate. Int. J. Cancer. 2020;146:1592–1605. doi: 10.1002/ijc.32714. [DOI] [PubMed] [Google Scholar]
- Kalkan T., Bornelov S., Mulas C., Diamanti E., Lohoff T., Ralser M., Middelkamp S., Lombard P., Nichols J., Smith A. Complementary activity of ETV5, RBPJ, and TCF3 drives formative transition from naive pluripotency. Cell Stem Cell. 2019;24:785–801.e7. doi: 10.1016/j.stem.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi Y., Kondoh H. Sox proteins: regulators of cell fate specification and differentiation. Development. 2013;140:4129–4144. doi: 10.1242/dev.091793. [DOI] [PubMed] [Google Scholar]
- Kanai-Azuma M., Kanai Y., Gad J.M., Tajima Y., Taya C., Kurohmaru M., Sanai Y., Yonekawa H., Yazaki K., Tam P.P. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- Kaya-Okur H.S., Janssens D.H., Henikoff J.G., Ahmad K., Henikoff S. Efficient low-cost chromatin profiling with CUT&Tag. Nat. Protoc. 2020;15:3264–3283. doi: 10.1038/s41596-020-0373-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T., Zhang H., Tang W.W.C., Irie N., Withey S., Klisch D., Sybirna A., Dietmann S., Contreras D.A., Webb R. Principles of early human development and germ cell program from conserved model systems. Nature. 2017;546:416–420. doi: 10.1038/nature22812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima Y., Sasaki K., Yokobayashi S., Sakai Y., Nakamura T., Yabuta Y., Nakaki F., Nagaoka S., Woltjen K., Hotta A. Evolutionarily distinctive transcriptional and signaling programs drive human germ cell lineage specification from pluripotent stem cells. Cell Stem Cell. 2017;21:517–532.e5. doi: 10.1016/j.stem.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Langfelder P., Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch H.G., Smith A. The mammalian germline as a pluripotency cycle. Development. 2013;140:2495–2501. doi: 10.1242/dev.091603. [DOI] [PubMed] [Google Scholar]
- Leitch H.G., Tang W.W., Surani M.A. Primordial germ-cell development and epigenetic reprogramming in mammals. Curr. Top Dev. Biol. 2013;104:149–187. doi: 10.1016/B978-0-12-416027-9.00005-X. [DOI] [PubMed] [Google Scholar]
- Li D., Liu J., Yang X., Zhou C., Guo J., Wu C., Qin Y., Guo L., He J., Yu S. Chromatin accessibility dynamics during iPSC reprogramming. Cell Stem Cell. 2017;21:819–833.e6. doi: 10.1016/j.stem.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Maruyama M., Ichisaka T., Nakagawa M., Yamanaka S. Differential roles for Sox15 and Sox2 in transcriptional control in mouse embryonic stem cells. J. Biol. Chem. 2005;280:24371–24379. doi: 10.1074/jbc.M501423200. [DOI] [PubMed] [Google Scholar]
- Mitsunaga S., Odajima J., Yawata S., Shioda K., Owa C., Isselbacher K.J., Hanna J.H., Shioda T. Relevance of iPSC-derived human PGC-like cells at the surface of embryoid bodies to prechemotaxis migrating PGCs. Proc. Natl. Acad. Sci. U S A. 2017;114:E9913–E9922. doi: 10.1073/pnas.1707779114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Gunesdogan U., Zylicz J.J., Tang W.W.C., Sengupta R., Kobayashi T., Kim S., Butler R., Dietmann S., Surani M.A. NANOG alone induces germ cells in primed epiblast in vitro by activation of enhancers. Nature. 2016;529:403–407. doi: 10.1038/nature16480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H., Nakamura A., Urata M., Shirae-Kurabayashi M., Kuraku S., Russell S., Ohtsuka S. The evolutionally-conserved function of group B1 Sox family members confers the unique role of Sox2 in mouse ES cells. BMC Evol. Biol. 2016;16:173. doi: 10.1186/s12862-016-0755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor W.A., Liu W., Chen D., Ho J., Kim R., Hunt T.J., Lukianchikov A., Liu X., Polo J.M., Jacobsen S.E. TFAP2C regulates transcription in human naive pluripotency by opening enhancers. Nat. Cell Biol. 2018;20:553–564. doi: 10.1038/s41556-018-0089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett R.M., Turnpenny L., Eckert J.J., O'Shea M., Sonne S.B., Cameron I.T., Wilson D.I., Rajpert-De Meyts E., Hanley N.A. The early human germ cell lineage does not express SOX2 during in vivo development or upon in vitro culture. Biol. Reprod. 2008;78:852–858. doi: 10.1095/biolreprod.107.066175. [DOI] [PubMed] [Google Scholar]
- Pierson Smela M., Sybirna A., Wong F.C.K., Surani M.A. Testing the role of SOX15 in human primordial germ cell fate. Wellcome Open Res. 2019;4:122. doi: 10.12688/wellcomeopenres.15381.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M., Miyauchi H. Gametogenesis from pluripotent stem cells. Cell Stem Cell. 2016;18:721–735. doi: 10.1016/j.stem.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Sarraj M.A., Wilmore H.P., McClive P.J., Sinclair A.H. Sox15 is up regulated in the embryonic mouse testis. Gene Expr. Patterns. 2003;3:413–417. doi: 10.1016/s1567-133x(03)00085-1. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Yokobayashi S., Nakamura T., Okamoto I., Yabuta Y., Kurimoto K., Ohta H., Moritoki Y., Iwatani C., Tsuchiya H. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell. 2015;17:178–194. doi: 10.1016/j.stem.2015.06.014. [DOI] [PubMed] [Google Scholar]
- Sybirna A., Tang W.W.C., Pierson Smela M., Dietmann S., Gruhn W.H., Brosh R., Surani M.A. A critical role of PRDM14 in human primordial germ cell fate revealed by inducible degrons. Nat. Commun. 2020;11:1282. doi: 10.1038/s41467-020-15042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W.W., Dietmann S., Irie N., Leitch H.G., Floros V.I., Bradshaw C.R., Hackett J.A., Chinnery P.F., Surani M.A. A unique gene regulatory network resets the human germline epigenome for development. Cell. 2015;161:1453–1467. doi: 10.1016/j.cell.2015.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerapandian V., Ackermann J.O., Srivastava Y., Malik V., Weng M., Yang X., Jauch R. Directed evolution of reprogramming factors by cell selection and sequencing. Stem Cell Reports. 2018;11:593–606. doi: 10.1016/j.stemcr.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Wang X., Zhang S., Sun H., Li S., Ding H., You Y., Zhang X., Ye S.D. The transcription factor TFCP2L1 induces expression of distinct target genes and promotes self-renewal of mouse and human embryonic stem cells. J. Biol. Chem. 2019;294:6007–6016. doi: 10.1074/jbc.RA118.006341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu T., Meyer C.A., Eeckhoute J., Johnson D.S., Bernstein B.E., Nusbaum C., Myers R.M., Brown M., Li W. Model-based analysis of ChIP-seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number for the ATAC-seq, RNA-seq and CUT&Tag-seq data reported in this paper is Gene Expression Omnibus (GEO): GSE143345.