Abstract
Purpose.
There is no consensus for the treatment of melanoma metastatic to the liver. Percutaneous hepatic perfusion with melphalan (PHP-Mel) is a method of delivering regional chemotherapy selectively to the liver. In this study, we report the results of a multicenter, randomized controlled trial comparing PHP-Mel with best alternative care (BAC) for patients with ocular or cutaneous melanoma metastatic to the liver.
Patients and Methods.
A total of 93 patients were randomized to PHP-Mel (n = 44) or BAC (n = 49). On the PHP-Mel arm, melphalan was delivered via the hepatic artery, and the hepatic effluent captured and filtered extracorporeally prior to return to the systemic circulation via a venovenous bypass circuit. PHP-Mel was repeatable every 4–8 weeks. The primary endpoint was hepatic progression-free survival (hPFS), and secondary endpoints included overall PFS (oPFS), overall survival (OS), hepatic objective response (hOR), and safety.
Results.
hPFS was 7.0 months for PHP-Mel and 1.6 months for BAC (p < 0.0001), while oPFS was 5.4 months for PHP-Mel and 1.6 months for BAC (p < 0.0001). Median OS was not significantly different (PHP-Mel 10.6 months vs. BAC 10.0 months), likely due to crossover to PHP-Mel treatment (57.1 %) from the BAC arm, and the hOR was 36.4 % for PHP-Mel and 2.0 % for BAC (p < 0.001). The majority of adverse events were related to bone marrow suppression. Four deaths were attributed to PHP-Mel, three in the primary PHP-Mel group, and one post-crossover to PHP-Mel from BAC.
Conclusion.
This randomized, phase III study demonstrated the efficacy of the PHP-Mel procedure. hPFS, oPFS, and hOR were significantly improved with PHP-Mel. PHP with melphalan should provide a new treatment option for unresectable metastatic melanoma in the liver.
Isolated hepatic metastases from melanoma represent a significant treatment dilemma in many patients with primary cutaneous and ocular melanoma. For patients with cutaneous melanoma, the development of hepatic disease is often associated with synchronous extrahepatic disease, but in approximately 3–5 % of patients, liver disease is present in isolation.1 The problem is much more significant for patients with ocular melanoma, where 50 % of patients will eventually develop metastatic disease, and the majority of those will have liver metastases as the sole or life-limiting component of distant disease.2 For such patients, effective therapies are limited and life expectancy is characterized by a median survival of 2–12 months and a 1-year survival rate of 10–25 %.3,6–8 Multiple reports have demonstrated the efficacy of liver-directed therapy for patients with isolated hepatic disease. Liver resection is associated with prolonged survival in highly selected patients where resection addresses all metastatic disease.9,10 Arterially delivered therapeutic strategies utilizing chemoembolization, immunoembolization, and radioembolization with yttrium 90 have demonstrated antitumor activity in selected patients reported in case series and phase I and II clinical trials.7,11–15 No comprehensive examination of novel systemic therapies has been performed in patients with metastatic ocular melanoma.
Melphalan has long been established as an effective regional therapeutic agent for patients with regionally confined melanoma to the extremity.8,16 We and others have demonstrated the antitumor efficacy of isolated hepatic perfusion (IHP) with melphalan for patients with multiple tumor histologic subtypes, including metastatic ocular melanoma.5,17,18 For patients with metastatic ocular melanoma, response rates have ranged from 60 to 80 %.7,15 In a subset of these patients, durable responses have been observed but, overwhelmingly, the recurrence and progression of intrahepatic disease has been the life-limiting component of disease. A phase I trial utilizing a minimally invasive approach to administration of hepatic directed, intra-arterial melphalan (PHP-Mel) was undertaken, yielding clinically relevant response rates in patients with metastatic melanoma confined to the liver, and formed the basis for a phase III trial examining the value of this therapy.19 This report details the results of a phase III, random assignment, multicenter trial, and represents the first such trial comparing regional therapy with standard systemic therapies for this disease.
PATIENTS AND METHODS
Between February 2006 and July of 2009, a total of 93 patients with liver-predominant ocular or cutaneous melanoma were accrued to this trial. The initial 33 patients were enrolled within the Surgery Branch of the National Cancer Institute (NCI), prior to the pre-planned study expansion to multiple extramural sites. The trial schema is presented in Fig. 1. All patients were required to have biopsy proven, unresectable melanoma metastatic to the liver. Eligibility criteria included Eastern Cooperative Oncology Group performance status of ≤2, a serum bilirubin <2.0 mg/dl, a platelet count >100,000, serum creatinine <1.5 mg/dl, and liver function tests <10 times the upper limit of normal. Patients were evaluated with computed tomography (CT) of the chest, abdomen, and pelvis, as well as magnetic resonance imaging (MRI) of the liver and brain. Patients were excluded for brain metastases, conditions precluding anticoagulation, latex allergy, cirrhosis, or significant portal hypertension. Patients with limited extrahepatic disease in the presence of clearly progressive advanced liver metastases that were the life-limiting component of their disease were deemed eligible.
FIG. 1.
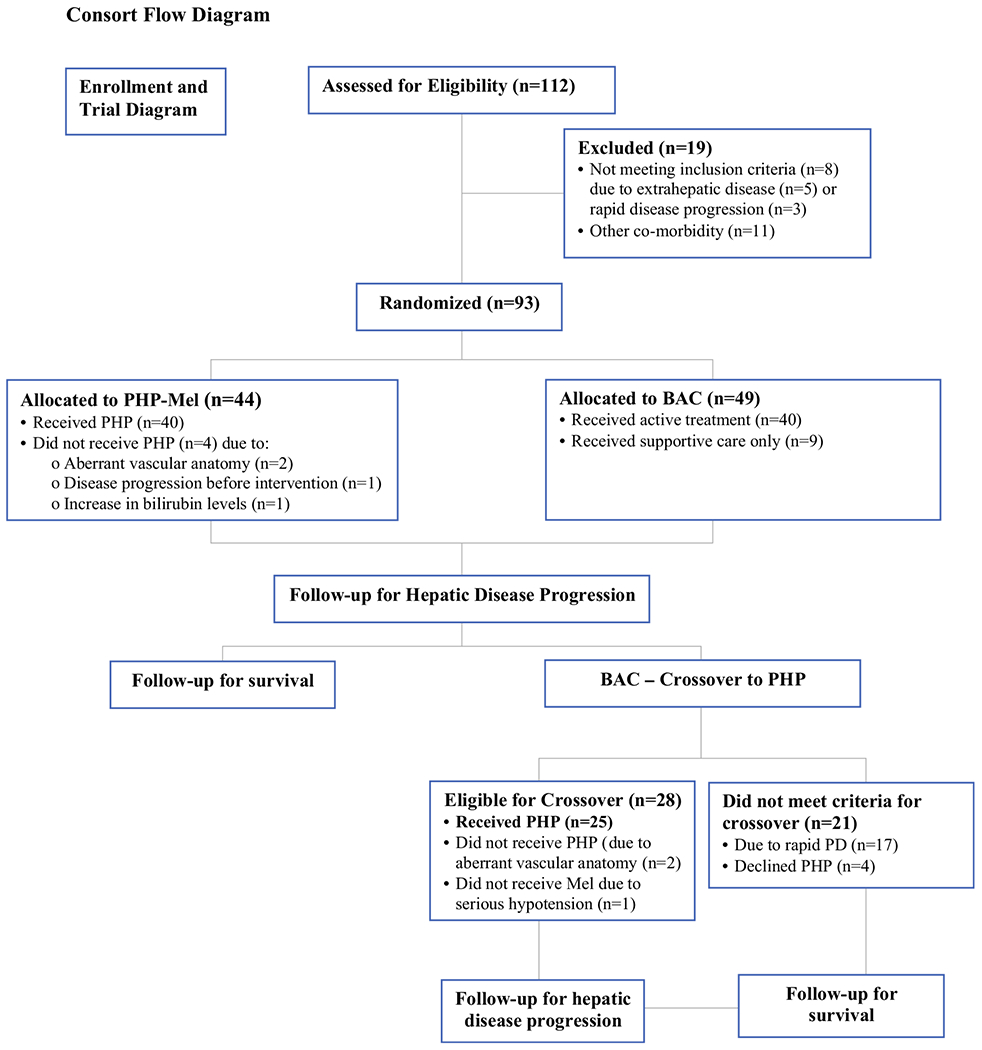
Consort flow diagram of phase III trial of PHP (melphalan) compared with BAC. PHP percutaneous hepatic perfusion, BAC best alternative care, Mel melphalan
Treatment Arms
Forty-four patients were randomly assigned to receive PHP-Mel (47.3 %) and 49 (52.7 %) assigned to receive best alternative care (BAC). Primary BAC treatment strategies included systemic chemotherapy, embolization, and supportive care. Crossover to PHP-Mel was permitted at hepatic progression provided all entry and/or retreatment criteria were met.
Procedure
Percutaneous hepatic perfusion is a percutaneous technique that allows delivery of high-dose melphalan directly to the liver via the hepatic artery over 30 min. A unique double balloon inferior vena cava catheter system (Delcath Systems, Inc., Queensbury, NY, USA)19 was used to catch the hepatic venous outflow and funnel the blood extracorporeally through melphalan-extracting charcoal filters, before returning the blood to the systemic vasculature via the internal jugular vein. All PHP-Mel procedures were carried out under general anesthesia and systemic anticoagulation with heparin.
Melphalan was administered at a dose of 3 mg/kg based on ideal body weight. The melphalan dose on subsequent PHPs was reduced to 2.5 mg/kg if a dose-limiting toxicity (DLT) was encountered, defined as any of the following: grade 4 neutropenia >5 days in duration, with growth factor support or associated with neutropenic fever; grade 4 thrombocytopenia >5 days in duration or associated with bleeding requiring transfusion; grade 4 anemia >48 h in duration; grade 3 or 4 major non-hematologic organ toxicity not correctable within 24 h of the procedure (excluding fever, nausea, and weight gain). Subjects randomized to PHP-Mel received treatment approximately every 4–8 weeks when hematologic toxicity resolved to grade 2 or less. Up to six PHP procedures could be performed in any given patient in the absence of progressive disease.
Follow-Up
While receiving active treatment, all patients were followed and imaged at 6 week ± 2-week intervals. When off active treatment, patients entered the ‘follow-up phase’ during which they were evaluated for disease progression every 8 weeks for the remainder of the first year, every 3 months the second year, every 4 months for the third year, every 6 months for the fourth year, and yearly thereafter. Survival was assessed every 6 months for the first 2 years and then yearly thereafter.
Study Objectives
In accordance with pre-study meetings with the US FDA, hepatic progression-free survival (hPFS) was the primary endpoint. Secondary endpoints included hPFS, xPFS (defined as the time from the date of randomization to the first observation of extrahepatic disease progression or death due to any cause), hepatic objective response (hOR), objective response rate (ORR), overall PFS (oPFS), overall survival (OS), and safety. Although all treatment decisions were based on investigator (INV) assessment of response, survival and response calculations were based on a blinded, outside independent image review (IRC).
Statistics
For analysis of the primary endpoint of PFS, 46 patients per treatment arm had 80 % power to detect an approximate median difference of 4 months, under the assumption that the median time of hPFS was 4 months for those patients randomized to BAC and 7.7 months for those randomized to PHP-Mel. Survival endpoints were calculated from the time of treatment randomization to event. All treatment decisions were based on INV assessment of response, but response and survival calculations were based on a blinded, outside IRC.
RESULTS
Baseline Patient Characteristics
Ninety-three patients were randomized at nine institutions across the US—44 patients to the PHP-Mel arm (47.3 %) and 49 to the BAC arm (52.7 %). Patient and tumor clinicopathologic characteristics were similar between the two groups (Table 1). In general, this group of patients had extensive liver disease, characterized by 51 % of patients having five or more liver lesions at baseline and a mean hepatic replacement with tumor of 31.6 %. Nineteen patients (20.5 %) in the PHP-Mel arm had >50 % hepatic replacement with tumor. After a single patient death secondary to hepatic failure, the protocol was amended to mandate biopsy of non-tumor liver for all patients with >50 % hepatic replacement with tumor in order to ensure adequate hepatic reserve (see the “Discussion” section). Patients with surgically resectable disease were excluded from the trial. The mean baseline lactate dehydrogenase (LDH) was 524 IU/L (range 106–2716). The majority of patients had not received prior regional therapy for their metastatic melanoma (90.9 % for PHP patients and 93.9 % for BAC patients).
TABLE 1.
Baseline characteristics (intention-to-treat population)
| Category | PHP-Mel (N = 44) |
BAC (N = 49) |
Treatment group comparison p value |
|---|---|---|---|
| Age [years; median (range)] | 55.0 (33–74) | 56.0 (31–77) | 0.9534 |
| Gender [n (%)] | 0.4797 | ||
| Male | 23 (52.3) | 22 (44.9) | |
| Female | 21 (47.7) | 27 (55.1) | |
| ECOG performance status [n (%)] | 0.0284 | ||
| 0 | 28 (63.6) | 42 (85.7) | |
| 1 | 13 (29.5) | 6 (12.2) | |
| Site of primary tumor [n (%)] | 0.8577 | ||
| Ocular | 39 (88.6) | 44 (89.8) | |
| Cutaneous | 5 (11.4) | 5 (10.2) | |
| Duration of hepatic metastasis [months; mean ± SD] | 4.6 ± 7.7 | 4.6 ± 5.5 | |
| Hepatic tumor burden [%; median (range)] | 32.5 (5–85) | 25.0 (5–90) | 0.5342 |
| Site of metastases [n (%)] | 0.9305 | ||
| Hepatic only | 27 (61.4) | 28 (57.1) | |
| Hepatic and extrahepatic | 17 (38.6) | 21 (42.9) | |
| Previous treatment for liver metastases [n (%)] | |||
| Chemotherapy/immunotherapy | 8 (18.2) | 10 (20.4) | 0.9008 |
| Regional therapya | 4 (9.1) | 3 (6.1) | 0.7038 |
PHP-Mel Percutaneous hepatic perfusion with melphalan, BAC best alternative care, ECOG Eastern Cooperative Oncology Group
Included chemoembolization, radioembolization, or ablation
Forty of the 44 (91 %) patients randomized to PHP-Mel were treated, receiving a median of three treatments. Twenty-eight patients (64 % of randomized) received at least three treatments, and eight patients completed four treatments (18.1 %).
Forty-nine patients were randomized to the BAC arm. Primary treatment regimens included systemic chemotherapy with dacarbazine/temozolomide (42.9 %), carboplatin/taxol (6.1 %), chemoembolization (22.4 %), radioembolization (6.1 %), or supportive care (18.4 %) (Table 2). On progression of disease, crossover to PHP-Mel treatment occurred in 28 of 49 patients (57.1 %) at a mean time from randomization of 3.8 months (range 1.1–23.7); however, only 25 of the 28 crossover patients received PHP-Mel. Crossover did not occur in 21 patients (42.9 %) secondary to death (n = 4), patient decision (n = 1), progression of hepatic and extrahepatic disease (n = 15), and an unspecified reason (n = 1).
TABLE 2.
Treatments administered to patients in the BAC arm
| Category and regimen | Overall (N = 49) [n (%)] |
|---|---|
| Systemic chemotherapy | 24 (49.0) |
| Carboplatin/paclitaxel | 3 (6.1) |
| Dacarbazine | 1 (2.0) |
| Temozolomide | 20 (40.8) |
| Chemoembolization | 11 (22.4) |
| Carmustinea | 3 (6.1) |
| Cisplatin | 2 (4.1) |
| Doxorubicin/cisplatin/mitomycin | 3 (6.1) |
| Doxorubicin | 1 (2.0) |
| Doxorubicin/cisplatin | 2 (4.1) |
| Radioembolization (with Yttrium Y-90 SirSpheres) | 3 (6.1) |
| Combination systemic chemotherapy/embolizationb | 1 (2.0) |
| Surgery | 1 (2.0) |
| Supportive care | 9 (18.4) |
BAC Best alternative care
Treatments administered after randomization and before disease progression
Patient 012–354 received two cycles of inter-arterial carmustine followed by one cycle of intra-arterial paclitaxel
Patient 03–259 received seven cycles of inter-arterial gemcitabine combined with intravenous paclitaxel
Hepatic and Overall Progression-Free Survival
The median hPFS (by IRC assessment) in the PHP-Mel group was 7.0 months (95 % CI 5.2–9.7 months) compared with 1.6 months (95 % CI 1.5–2.9 months) in the BAC group (p < 0.0001) (Fig. 2a). The median oPFS (by INV assessment) in the PHP-Mel group was 5.4 months (95 % CI 3.4—8.1 months), and 1.6 months (95 % CI 1.5–2.3 months) in the BAC group (p = 0.0001) (Fig. 2b).
FIG. 2.
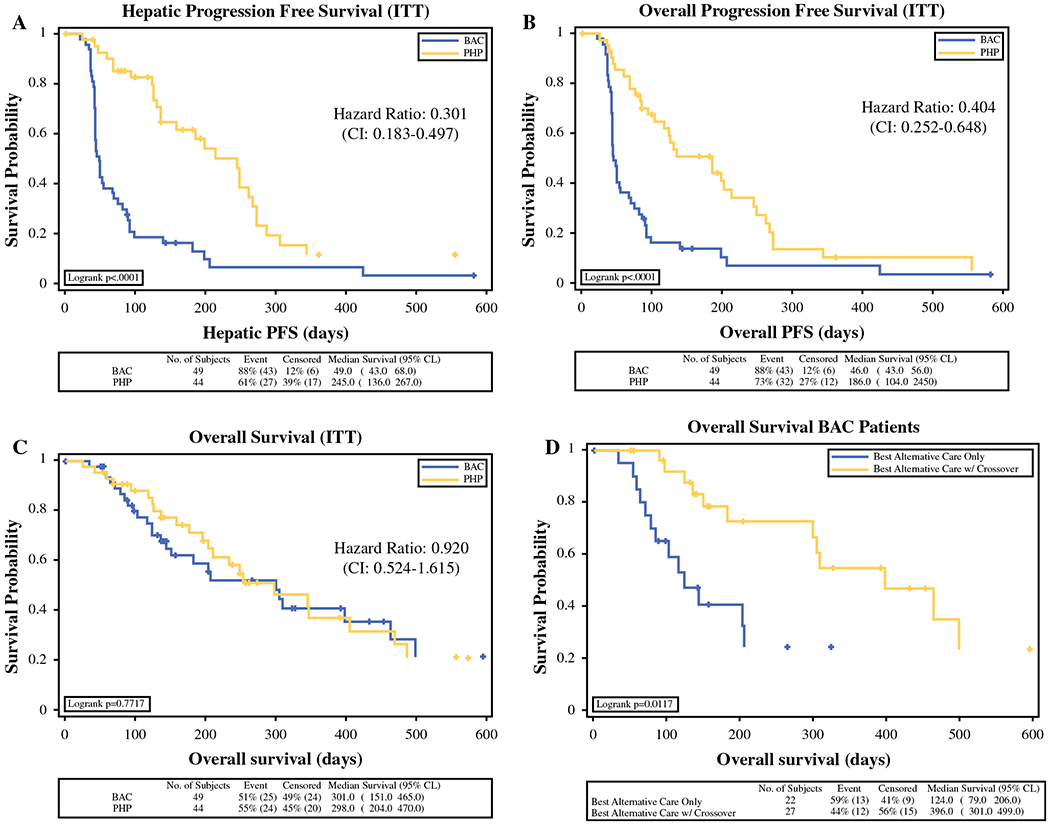
ITT intention-to-treat, CI confidence interval, PFS progression-free survival, BAC best alternative care, PHP percutaneous hepatic perfusion
Objective Response
The hOR (by IRC assessment) for PHP-Mel was 36.4 % (n = 16, all partial response (PRs)), with an additional stable disease (SD) rate of 52.3 % (n = 23), and an overall hepatic disease control rate (the proportion of patients with a response or SD) of 75.0 % (Fig. 3). On the BAC arm, a single patient (2.0 %) achieved a PR and 20 additional patients (40.8 %) achieved SD, for an overall disease control rate of 42.9 %. When comparing the hOR between the two treatment groups, there was a significant improvement in response favoring PHP-Mel patients (p < 0.001).
FIG. 3.
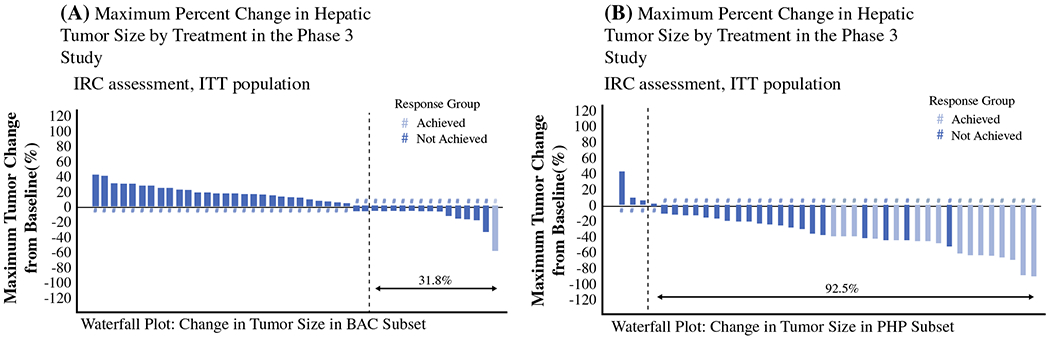
Maximum percent change in hepatic tumor size by treatment in the phase III study. ITT intention-to-treat, BAC best alternative care, PHP percutaneous hepatic perfusion
The ORR rate (by INV assessment) for the PHP-Mel group (27.3 %) was significantly higher (p = 0.003) than that seen in the BAC group (4.1 %). All of the ORRs were PRs. The median duration of objective response was 6.3 months for PHP-Mel and 3.7 months in the BAC group (Fig. 4).
FIG. 4.
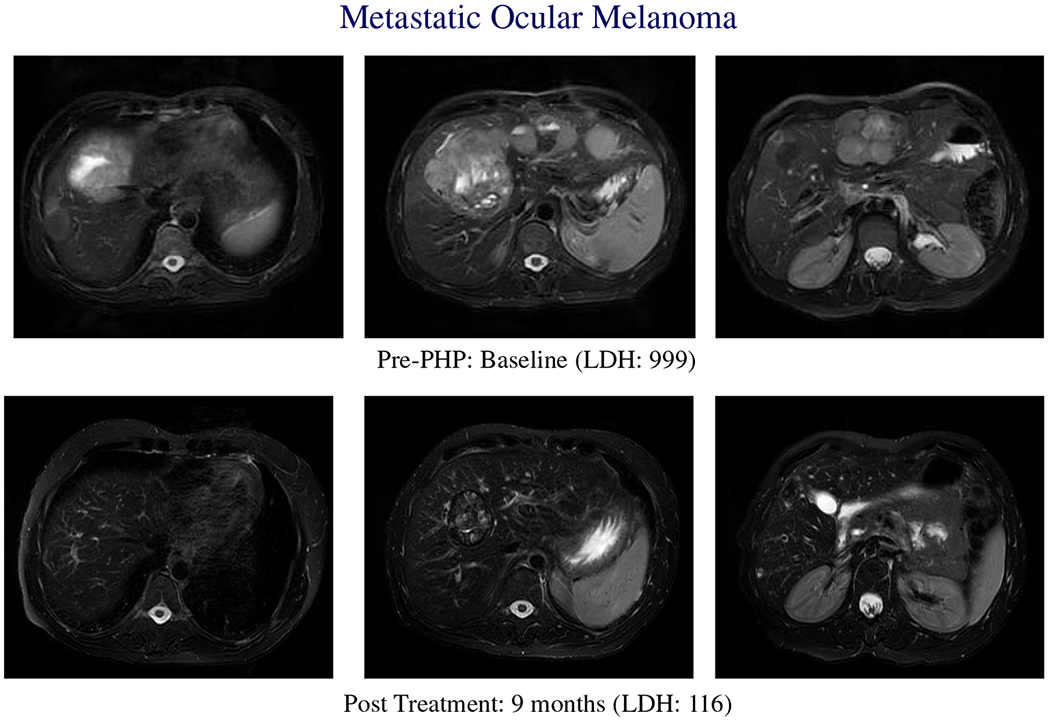
Metastatic ocular melanoma. Baseline and post-treament scans on a patient with ocular melanoma with bilobar liver metastases treated with PHP-mel. Treatment response correlates with decrease in LDH. Patient was successfully retreated with melphalan via IHP at disease recurrence within the liver.
Overall Survival
Median OS was 10.6 months (95 % CI 6.9–13.6 months) in the PHP-Mel group compared with 10.0 months (95 % CI 6.0–13.1 months) in the BAC group (Fig. 2c). While there was no significant difference in OS between the two randomized groups, a subgroup analysis revealed median OS to be 13.1 months (95 % CI 10.0–20.3 months) in BAC patients (n = 28, 57.1 %) who crossed over and received treatment with PHP-Mel (Fig. 2d).
Follow-Up and Survival
Four patients (8.6 %) were still alive as of September 2014—two PHP-Mel patients (2.2 %) and two BAC patients (2.2 %), both of whom crossed over from BAC to PHP-Mel.
SAFETY
A detailed toxicity assessment was performed on all patients who received PHP-Mel (randomized and crossover) for two distinct study periods (peri- and post-procedural) in order to separate procedure-related events from events related to systemic melphalan exposure. The most common events occurring during these time frames are shown in Table 3.
TABLE 3.
Overview of adverse events by time period (grade 3/4 events)
| Peri-procedural (N = 70) [n (%)] |
Post-procedural (N = 70) [n (%)] |
|
|---|---|---|
| Any TEAE | 63 (90.0) | 64 (91.4) |
| Hemoglobin | 42 (60.0) | 44 (62.9) |
| decreased | ||
| Platelet count | 52 (74.3) | 56 (80.0) |
| decreased | ||
| aPTT prolonged | 18 (25.7) | NA |
| AST increased | 14 (20.0) | 7 (10.0) |
| Blood albumin | 26 (37.1) | 4 (5.7) |
| decreased | ||
| Blood bilirubin | 7 (10.0) | 10 (14.3) |
| increased | ||
| Blood calcium | 16 (22.9) | NA |
| decreased | ||
| INR increased | 14 (20.0) | 1 (1.4) |
| Febrile | NA | 12 (17.1) |
| neutropenia | ||
| Neutrophil count | 3 (4.3) | 60 (85.7) |
| decreased | ||
aPTT Activated partial thromboplastin time, INR international normalized ratio, TEAE treatment-emergent adverse event, AST aspartate transaminase, NA not available
Peri-Procedural Events
Peri-procedural events were defined as occurring from the date of the planned procedure until the earlier of 72 h post-procedure or patient discharge from the hospital. Events occurring during this time period were more likely to be related to the device and/or the PHP-Mel procedure. Peri-procedural adverse events (AEs) were observed in nearly all PHP-Mel-treated patients (n = 63/70, 90.0 %), and were primarily grade 3/4. The most common events included thrombocytopenia (74.3 %) and anemia (60.0 %); AEs are listed in Table 3. These events were primarily related to platelet sequestration and/or hemodilution, and frequently required transfusion for correction. During this period, patients received a mean of 8.5 units of cryoprecipitate, 3.5 units of fresh frozen plasma, 2.3 units of packed red blood cells, and 7.6 units of platelets.
Procedure-associated hypotension was routinely noted on initiation of venous hemofiltration, necessitating transient use of vasopressors. Hepatic artery spasm was observed and treated with intra-arterial administration of nitroglycerin in 67 % of patients. End organ toxicity that occurred during this period, and that was potentially attributable to hypotension, occurred in several patients. Cardiac toxicity was manifested by elevated troponin (n = 6), sinus tachycardia (n = 2), myocardial infarction (n = 1), atrial fibrillation (n = 1), pericardial effusion (n = 1), and ventricular tachycardia (n = 1). Central nervous system-associated events included cerebral ischemia (n = 1) and facial paresis (n = 1). No patients experienced renal toxicity during the peri-procedural period.
Post-Procedural Events
Post-procedural events were defined as occurring between the end of the peri-procedural period until 30 days after dosing, or until the start of the next treatment cycle. Events occurring during this time period were more likely to be melphalan-related. Post procedural AEs were observed in nearly all PHP-Mel-treated patients (n = 64/70, 91.4 %), and were primarily grade 3/4. Despite the routine utilization of stem cell support, the most common post-procedural AEs were related to the effects of bone marrow suppression, and included neutropenia (85.7 %), thrombocytopenia (80.0 %), and anemia (62.9 %). Hepatic dysfunction, as manifested by grade III/IV bilirubin elevation, was observed in ten (14.3 %) patients and was universally self-limited (Table 3). Rare complications included venous thrombosis, acute cholecystitis, and gastroduodenal ulcer.
Discontinuation of Study Therapy
The reasons provided for discontinuation of PHP-Mel treatment in both randomized and crossover patients are shown in Table 4. The primary cause for discontinuation was an AE. Of the 70 patients who underwent PHP-Mel treatment, 24 (34.3 %) discontinued treatment due to AEs, the most common of which were attributable to bone marrow suppression. Other AEs influencing study withdrawal included hepatic toxicity, as evidenced by hyperbilirubinemia (5.7 %) and increases in alanine transaminase (ALT) and aspartate transaminase (AST; 2.9 % each). The second most common reason for the discontinuation of PHP-Mel was disease progression (n = 20, 28.6 %). Fifteen of the 20 patients withdrew due to extrahepatic progression, and five withdrew due to hepatic progression.
TABLE 4.
Reasons for discontinuation of PHP-Mel treatment
| Reason for discontinuation | Randomized to PHP | After crossover to PHP | Total PHP patients |
|---|---|---|---|
| (N = 42) [n (%)] | (N = 28) [n (%)] | (N = 70) [n (%)] | |
| Death | 3 (4.8) | 1 (3.6) | 4 (4.3) |
| Disease progression | 12 (28.6) | 8 (28.6) | 20 (28.6) |
| Hepatic progression | 3 (9.5) | 1 (3.6) | 4 (7.1) |
| Extrahepatic progression | 8 (19.0) | 7 (25.0) | 15 (21.4) |
| Adverse events | 15 (35.7) | 9 (32.1) | 24 (34.3) |
| Platelet count decreased | 6 (14.3) | 6 (21.4) | 12 (17.1) |
| Neutrophil count decreased | 3 (7.1) | 2 (7.1) | 5 (7.1) |
| Blood bilirubin increased | 3 (7.1) | 1 (3.6) | 4 (5.7) |
| Patient decision | 1 (2.4) | 0 | 1 (1.4) |
| Investigator’s opinion | 7 (16.7) | 2 (7.1) | 9 (12.9) |
| Lost to follow-up | 0 | 0 | 0 |
| Completed four cycles of therapy and no clinical indication to continue | 2 (4.8) | 3 (10.7) | 5 (7.1) |
| Completed six cycles of PHP therapy or equivalent of BAC (twelve 3-week cycles) | 1 (2.4) | 1 (3.6) | 2 (2.9) |
| Other | 2 (4.8) | 4 (14.3) | 6 (8.6) |
PHP Percutaneous hepatic perfusion, BAC best alternative care
Deaths
Three deaths were attributed to treatment in patients randomized to PHP-Mel. Two were associated with bone marrow suppression; one from complications of neutropenia and one from streptococcal sepsis. A single death was associated with progressive hepatic failure in a patient with near-complete hepatic replacement with tumor. An additional death occurred in the crossover patient population, resulting from a gastric perforation.
DISCUSSION
This report demonstrates the efficacy of this novel approach to the regional, intra-arterial delivery of high-dose melphalan with venous hemofiltration in patients with isolated or liver-predominant hepatic metastases from primary ocular or cutaneous melanoma. As patients randomized to receive PHP-Mel experienced a significant improvement in hPFS compared with patients treated with BAC, the primary endpoint of the study was met. The treatment effect resulted in a similarly significant impact on oPFS, despite the fact that therapy was only delivered into the liver. No impact on OS was noted. However, since the majority of subjects in the BAC arm (57.1 %) were able to crossover with similar efficacy, the trial was not able to address the question of OS. A hepatic response to therapy was observed in 16 patients (36.3 %) randomized to PHP-Mel and one patient (2.0 %) on the BAC arm. Ocular melanoma has an unusual predilection for liver metastases, and the vast majority of patients present with unresectable, bilobar disease. The group of patients in this trial represents this biology, with 20 % of the treated patients enrolled with >50 % liver replaced with tumor. Differences in hPFS (PHP-Mel vs. BAC) were observed in patients with high (>50 % hepatic replacement) and low (<50 % hepatic replacement) hepatic tumor burden. There was a trend towards improved survival within the PHP-Mel group for patients with low hepatic tumor burden but this did not reach statistical significance nor was the study powered to address this analysis. A single patient death was attributable to hepatic failure, but subsequent pathologic assessment of the non-tumor liver via core needle biopsy in patients with >50 % hepatic tumor burden helped to exclude the rare patient with normal bilirubin who has liver disease that is too extensive to safely undergo therapy. Crossover to the experimental treatment was permitted at disease progression as long as the patient met all study entry criteria. The median time to liver progression on the BAC arm was 1.6 months, while post-crossover liver progression occurred at a median of 8.4 months after PHP therapy, demonstrating the efficacy of the therapy to salvage patients at an advanced stage. Additionally, median OS for crossover patients (N = 28) was 13.1 months compared with 10.6 months for patients randomized to PHP-Mel. The 57 % of patients who were allowed to crossover confounded the ability to analyze any survival advantage associated with PHP-Mel.
Toxicity associated with therapy was significant but not resistant to effective management in the majority of patients. Toxicity was primarily hematologic and was observed during one of two prospectively defined periods—peri-procedural (during which AEs were more likely to be related to the PHP-Mel procedure) or post-procedural (during which AEs were more likely to be attributable to systemic exposure to melphalan. Immediate post-procedure toxicity is centered around the use of paired, activated charcoal filters used to extract melphalan from the hepatic venous effluent. Patients underwent standard anticoagulation with heparin during the procedure. Despite the routine use of protamine to reverse the heparin, grade 3 or 4 peri-procedural toxicity in the form of coagulopathy was observed in 31.0 %, and is thought to be primarily related to consumption of clotting factors by the filters. The routine use of protamine and cryoprecipitate in the immediate post-procedure period facilitated the correction of these clotting defects. Additionally, the initiation of blood flow through the filters led to thrombocytopenia (grade IV; n = 20, 47.6 %), requiring transfusion in 33.8 %. Careful attention to flow rates and pressures within the circuit minimized clotting derangements, and clinically relevant bleeding complications associated with this coagulopathy were infrequent. The impact of the coagulation and platelet toxicity warranted attentive management as the treatment catheters and sheaths need to be removed promptly under good hemostatic conditions. An additional effect of the initiation of the filters was associated with an expected but profound decrease in systemic blood pressure, observed in 5.7 % of patients. These events were effectively treated with vasopressors but mandated careful monitoring and assessment for secondary hepatic artery spasm. Clinically relevant cardiac and vascular complications were uncommon.
As previously noted, hemofiltration does not remove 100 % of melphalan administered into the hepatic artery, even when imaging demonstrates complete isolation of the retrohepatic vena cava. During the dose escalation portion of the phase I trial, DLT related to the administration of melphalan was defined as grade 3 and 4 hepatic or hematologic (bone marrow suppression) toxicity which did not return to baseline by the eighth post-procedure week. The phase I trial defined bone marrow suppression, not hepatic toxicity, as DLT, manifesting as prolonged grade 4 neutropenia and/or thrombocytopenia, and led to 3.0 mg/kg being established as the maximum tolerated dose (MTD).19 At the MTD, manageable grade 3 and 4 neutropenia and thrombocytopenia were observed in the majority of patients.19 Subsequent trials and treatment guidelines were developed with planned mitigation and treatment of these expected marrow-related toxicities in mind, including the use of prophylactic bone marrow growth factors in all patients, and the close monitoring of laboratories between treatments. Dose reductions were used in 18 (25.7 %) of all patients receiving PHP-Mel secondary to the development of grade 3 or 4 bone marrow suppression. Completion of all four planned treatments was accomplished in 19.0 % of patients receiving PHP-Mel, with bone marrow suppression resulting in treatment cessation in eight patients. Ongoing research has led to the development of better filtration systems in combination with prospective strategies to limit hemodynamic perturbations that have demonstrated early evidence of reduced intraoperative and postoperative toxicity profiles. Such changes will be a vital and important factor in facilitating the more widespread adoption of this technology.
Despite significant increases in efficacy observed with new systemic chemotherapy and immunotherapy directed against metastatic melanoma, the significant majority of patients with unresectable metastatic disease will succumb to their disease. Even the newest agents, which have such durability and promise in cutaneous melanoma, are limited in patients with uveal melanoma.6 Considerable debate surrounds the efficacy and value of regional therapy for patients with advanced cancer, but there are numerous reports indicating that regional therapies in selected patients are associated with high response rates and durable responses. There are examples in the literature to support the therapeutic benefit of regional therapy for patients with metastatic melanoma.6–8,16,20 Because regional therapies can often result in prompt control of advanced burden of disease confined to the limb or liver, its role as a component of an integrated treatment plan that includes molecular targeting agents, chemotherapy, or immunotherapy should be developed. For patients with isolated limb metastases from melanoma, long-term disease control, defined as disease-free and with OS >5 years, has been demonstrated with both amputation and isolated limb perfusion.8,16,21–24 Similarly, multiple INVs have demonstrated the efficacy of surgical resection and IHP with melphalan for properly selected patients with liver-confined metastatic melanoma.15 For patients with low-volume disease, complete resection of disease allows for a median survival of 24–50 months.18 IHP with melphalan is associated with an ORR of 70 % and median survival of 14 months, with significantly higher rates in patients with low baseline LDH.18,25
CONCLUSIONS
The present study demonstrates improved control of liver disease in patients treated with intra-arterial melphalan compared with standard available therapy. Although treatment was limited to the liver, this benefit extended to oPFS. Despite the significant morbidity associated with this therapy, there was a clear-cut impact on hepatic and overall PFS, indicating the aggressive nature of the underlying malignancy. The ability to improve the rate of perioperative and longer-term toxicity will allow for more widespread adoption of this technology, and is an ongoing research focus. Clearly, the landscape of available treatments for patients with metastatic melanoma has changed since the initiation of this study. Immunotherapies such as anti-CTLA-4 and anti-PD-1 have shown great promise in those patients with metastatic cutaneous melanoma,26–29 but results for patients with ocular melanoma are far less encouraging.
ACKNOWLEDGMENT
The authors would like to acknowledge all of the patients and their families for participation in this clinical trial. In addition, we would like to thank all of the interventional radiologists, nurses, and physicians who helped provide excellent care to our patients. This study was funded by the Intramural Program of the National Cancer Institute, National Institutes of Health. Additional funding was supplied via a Cooperative Research and Development Agreement (CRADA) between Delcath Systems, Inc., and the Surgery Branch of the National Cancer Institute.
REFERENCES
- 1.Karlen AI, Clark JJ, Wong LL. Two cases of partial hepatectomy for malignant melanoma. Hawaii J Med Public Health. 2012;71:92–6 [PMC free article] [PubMed] [Google Scholar]
- 2.Damato B Progress in the management of patients with uveal melanoma. The 2012 Ashton Lecture. Eye (Lond). 2012;26:1157–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldman ED, Pingpank JF, Alexander HR Jr. Regional treatment options for patients with ocular melanoma metastatic to the liver. Ann Surg Oncol. 2004;11:290–7 [DOI] [PubMed] [Google Scholar]
- 4.Jovanovic P, Mihajlovic M, Djordjevic-Jocic J, Vlajkovic S, Cekic S, Stefanovic V. Ocular melanoma: an overview of the current status. Int J Clin Exp Pathol. 2013;6:1230–44 [PMC free article] [PubMed] [Google Scholar]
- 5.Alexander HR Jr, Libutti SK, Pingpank JF, Steinberg SM, Bartlett DL, Helsabeck C. Hyperthermic isolated hepatic perfusion using melphalan for patients with ocular melanoma metastatic to liver. Clin Cancer Res. 2003;9:6343–9 [PubMed] [Google Scholar]
- 6.Pereira PR, Odashiro AN, Lim LA, Miyamoto C, Blanco PL, Odashiro M, et al. Current and emerging treatment options for uveal melanoma. Clin Ophthalmol. 2013;7:1669–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forster MR, Rashid OM, Perez MC, Choi J, Chaudhry T, Zager J, et al. Chemosaturation with percutaneous hepatic perfusion for unresectable metastatic melanoma or sarcoma to the liver: a single institution experience. J Surg Oncol. 2014;109:434–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rashid OM, Sloot S, Zager JS. Regional therapy in metastatic melanoma: an update on minimally invasive intraarterial isolated limb infusion and percutaneous hepatic perfusion. Expert Opin Drug Metab Toxicol. 2014;10:1355–64 [DOI] [PubMed] [Google Scholar]
- 9.Gomez D, Wetherill C, Cheong J, Jones L, Marshall E, Damato B, et al. The liverpool uveal melanoma liver metastases pathway: outcome following liver resection. J Surg Oncol. 2014;109:542–7 [DOI] [PubMed] [Google Scholar]
- 10.Frenkel S, Nir I, Hendler K, Lotem M, Eid A, Jurim O, et al. Long-term survival of uveal melanoma patients after surgery for liver metastases. Br J Ophthalmol. 2009;93:1042–6 [DOI] [PubMed] [Google Scholar]
- 11.Klingenstein A, Haug AR, Zech CJ, Schaller UC. Radioembolization as locoregional therapy of hepatic metastases in uveal melanoma patients. Cardiovasc Interv Radiol. 2013;36:158–65 [DOI] [PubMed] [Google Scholar]
- 12.Gonsalves CF, Eschelman DJ, Sullivan KL, Anne PR, Doyle L, Sato T. Radioembolization as salvage therapy for hepatic metastasis of uveal melanoma: a single-institution experience. AJR Am J Roentgenol. 2011;196:468–73 [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto A, Chervoneva I, Sullivan KL, Eschelman DJ, Gonsalves CF, Mastrangelo MJ, et al. High-dose immunoembolization: survival benefit in patients with hepatic metastases from uveal melanoma. Radiology. 2009;252:290–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato T, Eschelman DJ, Gonsalves CF, Terai M, Chervoneva I, McCue PA, et al. Immunoembolization of malignant liver tumors, including uveal melanoma, using granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2008;26:5436–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwala SS, Eggermont AM, O’Day S, Zager JS. Metastatic melanoma to the liver: a contemporary and comprehensive review of surgical, systemic, and regional therapeutic options. Cancer. 2014;120:781–9 [DOI] [PubMed] [Google Scholar]
- 16.Han D, Beasley GM, Tyler DS, Zager JS. Minimally invasive intra-arterial regional therapy for metastatic melanoma: isolated limb infusion and percutaneous hepatic perfusion. Expert Opin Drug Metab Toxicol. 2011;7:1383–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander HR, Libutti SK, Bartlett DL, Puhlmann M, Fraker DL, Bachenheimer LC. A phase I–II study of isolated hepatic perfusion using melphalan with or without tumor necrosis factor for patients with ocular melanoma metastatic to liver. Clin Cancer Res. 2000;6:3062–70 [PubMed] [Google Scholar]
- 18.Olofsson R, Cahlin C, All-Ericsson C, Hashimi F, Mattsson J, Rizell M, et al. Isolated hepatic perfusion for ocular melanoma metastasis: registry data suggests a survival benefit. Ann Surg Oncol. 2014;21:466–72 [DOI] [PubMed] [Google Scholar]
- 19.Pingpank JF, Libutti SK, Chang R, Wood BJ, Neeman Z, Kam AW, et al. Phase I study of hepatic arterial melphalan infusion and hepatic venous hemofiltration using percutaneously placed catheters in patients with unresectable hepatic malignancies. J Clin Oncol. 2005;23:3465–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto M, Zager JS. Isolated hepatic perfusion for metastatic melanoma. J Surg Oncol. 2014;109:383–8 [DOI] [PubMed] [Google Scholar]
- 21.Paulsen IF, Chakera AH, Drejoe JB, Klyver H, Dahlstrøm K, Oturai P, et al. Tumour response after hyperthermic isolated limb perfusion for locally advanced melanoma. Dan Med J. 2014;61:A4741. [PubMed] [Google Scholar]
- 22.Steinman J, Ariyan C, Rafferty B, Brady MS. Factors associated with response, survival, and limb salvage in patients undergoing isolated limb infusion. J Surg Oncol. 2014;109:405–9 [DOI] [PubMed] [Google Scholar]
- 23.Wong J, Chen YA, Fisher KJ, Beasley GM, Tyler DS, Zager JS. Resection of residual disease after isolated limb infusion (ILI) is equivalent to a complete response after ILI-alone in advanced extremity melanoma. Ann Surg Oncol. 2014;21:650–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beasley GM, Speicher P, Augustine CK, Dolber PC, Peterson BL, Sharma K, et al. A multicenter phase I dose escalation trial to evaluate safety and tolerability of intra-arterial temozolomide for patients with advanced extremity melanoma using normothermic isolated limb infusion. Ann Surg Oncol. 2015;22:287–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leyvraz S, Spataro V, Bauer J, Pampallona S, Salmon R, Dorval T, et al. Treatment of ocular melanoma metastatic to the liver by hepatic arterial chemotherapy. J Clin Oncol. 1997;15:2589–95 [DOI] [PubMed] [Google Scholar]
- 26.Foletto MC, Haas SE. Cutaneous melanoma: new advances in treatment. An Bras Dermatol. 2014;89:301–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danielli R, Ridolfi R, Chiarion-Sileni V, Queirolo P, Testori A, Plummer R. Ipilimumab in pretreated patients with metastatic uveal melanoma: safety and clinical efficacy. Cancer Immunol Immunother. 2012;61:41–8 [DOI] [PMC free article] [PubMed] [Google Scholar]


