Abstract
Summary
Adrenoleukodystrophy is a peroxisomal X-linked recessive disease caused by mutations in the ABCD1 gene, located on the X-chromosome (Xq28). Gene mutations in patient with adrenoleukodystrophy induce metabolic alterations characterized by impaired peroxisomal beta-oxidation and accumulation of very long chain fatty acid (VLCFA) in plasma and in all tissues. Although nutritional intervention associated with a various mixture of oil prevents the accumulation of VLCFA, to date no causal treatment is available. Therefore, haematopoietic stem cell transplantation (HSCT) and gene therapy are allowed only for very early stages of cerebral forms diagnosed during childhood.We reported a case series describing five family members affected by X-linked adrenoleukodystrophy caused by a novel mutation of the ABCD1 gene. Particularly, three brothers were affected while the sister and mother carried the mutation of the ABCD1 gene. In this family, the disease was diagnosed at different ages and with different clinical pictures highlighting the wide range of phenotypes related to this novel mutation. In addition, these characteristics stress the relevant role of early diagnosis to properly set a patient-based follow-up.
Learning points
We report a novel mutation in the ABCD1 gene documented in a family group associated to an X-ALD possible Addison only phenotype.
All patients present just Addison disease but with different phenotypes despite the presence of the same mutations. Further follow-up is necessary to complete discuss the clinical development.
The diagnosis of ALD needs to be included in the differential diagnosis in all patients with idiopathic PAI through accurate evaluation of VLCFA concentrations and genetic confirmation testing.
Early diagnosis of neurological manifestation is important in order to refer timely to HSCT.
Further follow-up of these family members is necessary to characterize the final phenotype associated with this new mutation.
Patient Demographics: Adolescent/young adult, Paediatric, Adult, Male, Female, White, Italy
Clinical Overview: Adrenal, Adrenal
Related Disciplines: Paediatrics, Neurology, Genetics
Publication Details: Unique/unexpected symptoms or presentations of a disease, May, 2021
Background
X-linked adrenoleukodystrophy (X-ALD) is a peroxisomal metabolic disorder caused by mutations in the ABCD1 gene located on the Xq28 chromosome, coding for an adrenoleukodystrophy protein (ALDP) (1). ALDP is an ATP-binding-cassette-transporter that is involved in the active transport of very long chain fatty acid (VLCFA)≥ C22:0 across the peroxisomal membrane for β-oxidation (1, 2). Therefore, a dysfunction of ALDP induces accumulation of VLCFAs in tissues and body fluids, including the cerebral white matter, the spinal cord and the adrenal cortex. Accordingly, this leads to progressive CNS demyelination and primary adrenal insufficiency (PAI) as predominant manifestations, in typically X-ALD patients (3).
The incidence rate for X-ALD in males is between 1:20 000 and 1:30 000 individuals worldwide (2). In addition, Horn et al. for the first time have also included the affected females demonstrating an incidence rate of 1:61 000 for males and females within the Norwegian population (4). The prevalence rates were established of 1:200 000 for males and 1:100 000 for females (2, 4). Moreover, this condition occurs with a similar frequency in all populations and ethnicity, without any evidence of different incidence rates between countries across the world (2).
X-ALD is now assessed as a disease with distinct phenotypes (5), which differ according to the development of damages on the adrenal gland or CNS. These alterations might occur as isolated, concurrent or following tissue involvement (2). These specific characteristics have allowed to adopt a classification system according to two main components, and particularly to the age at onset and the appearance of neurological involvement (3). Thus, X-ALD now includes six major groups: childhood cerebral ALD (characterized by cerebral demyelination during childhood, CCALD), adolescent cerebral ALD (AdolCALD), adult cerebral ALD (ACALD), adrenomyeloneuropathy (AMN) with or without cerebral disease, Addison only and women with X-ALD (6). During childhood and adulthood, the CCALD and AMN have a frequency of 31–35% and 20–60%, respectively, while the AdolCALD and ACALD represent rare conditions with a frequency less than 7% (3). Interestingly, the Addison only form is supposed to be rare and its frequency characteristically decreases with age. Furthermore, the frequency of women with X-ALD is unknown as most are asymptomatic although subtle signs and symptoms of the disease might appear usually between the second and the fourth decade of life (7). Engelen M et al, in a recent prospective cross-sectional cohort study, demonstrated that only 18% of women with heterozygous X-ALD under the age of 40 years had symptoms. Interestingly, in women older than 60 years old, the percentage of symptomatic carriers increase significantly, reaching 88% (3). Analogously, Horn et al reported that no women carriers older than 50 years were free of neurological symptoms (2, 4). Available data clearly show that there is no correlation between phenotype and genotype of this disease (8). Consequently, it is impossible to predict the course of the disease, even within the same family.
To date, the precise mechanism by which the accumulation of VLCFA determines neurotoxicity and PAI is not completely known. Therefore, it has been supposed that cell membrane instability, oxidative stress and inflammatory processes are directly activated by fatty acids accumulation (8). Only a minority of VLCFAs derive from the diet while the highest amount is synthesized through elongation of long-chain fatty acids by the ELOVL enzymes. In particular, ELOVL6 is responsible for the synthesis of C18:0–C22:0, and ELOV1 of C24:0–C22:0 (9). A deficiency in ALDP reduces VLCFA-CoA penetration into peroxisomes, thus resulting in an increase of cytosolic VLCFA-CoA concentration and incorporation into complex lipids through the ELOV1 elongation activity (10). However, the complete characterization of the pathophysiology of VLCFA metabolism is needed in order to offer relevant information on the perspectives on novel treatments.
Although during childhood the CCALD is the most common phenotype, the Addison only variant might have some specific peculiarities especially in relation to the age of onset. In fact, most of X-ALD with PAI have an early onset of the disease, although few cases have been described in young adults reaching to adolescent or post-pubertal subjects (11). Nonetheless, patients with Addison only can develop neurological symptoms subsequently (3). The growing knowledge of novel genetic mutations might enhance the complete characterization of the pathophysiology of this complex disease with the aim of new treatments and follow-up. We describe a family with five members carrying a novel mutation in the ABCD1 gene (c.853C> G (p.R285G)). The case series is characterized by some relevant peculiarities: the wide range of phenotype in clinical manifestations, the different mode and age at onset of ALD. Thus, these aspects emphasize the importance of early diagnosis of this condition.
Cases series presentation
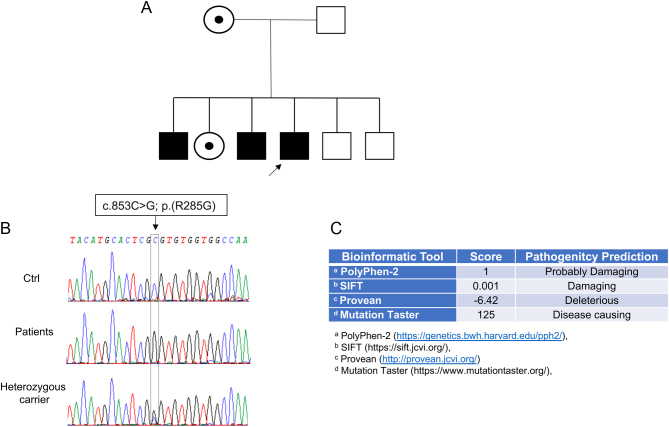
The family tree is illustrated in Fig. 1.
Figure 1.
Pedigree of the family with a novel mutation in the ABCD1 gene. (A) Family tree; (B) electropherogram analysis of the mutation; (C) results from bioinformatic programs: Polyphen, Sift, Provean, Mutation Taster.
Index case: case 1
The index case is a prepubertal 9-year-old boy, born to a non-consanguineous family. He was admitted for progressive and increasing anorexia, fatigue, weight loss, salt craving and abdominal pain. Perinatal history and past medical history were unremarkable. Development milestones in areas of motor, language and cognitive skills were normal. He attended grammar school with good performance.
On physical examination, he was alert, cooperative and well-nourished. He had no skin rash or pigmentation. His speech was fluent and he did not present ataxia or difficulties in balancing. Cranial nerve examination was normal. He had a normal sensation to pinprick, light touch, and proprioception. Cardiovascular examination (blood pressure, heart rate), pulmonary and abdominal examination were normal.
The laboratory investigations revealed hyponatremia (123 mmol/L), normal kalemia levels (4.5 mmol/L) hypochloremia (87 mmol/L), severely elevated ACTH level (>1500 pg/mL, normal range 4.7–48.8 pg/mL) with inappropriate borderline serum cortisol (8.3 µg/dL, normal range 8.0–25.0 µg/dL), and increased renin levels (>500 mu/mL, normal range 4.4–46.1 mu/mL), thus confirming the diagnosis of PAI. He started adrenal corticosteroid replacement therapy (dose 25 mg/ die; 15–10 mg) with rapid resolution of symptoms. Treatment was continued and follow-up was started after discharge.
Case 2
A 15-year-old boy, brother of the index case, 1 month later, came to our observation with a similar clinical picture. He was admitted for progressive and increasing fatigue and dehydration. He presented a longstanding history of weakness, anorexia, weight loss and increased thirst.
Perinatal history and past medical history were unremarkable. Milestones of neurodevelopment in areas of motor, language and cognitive skills were normal. His growth and development were normal such as a normal pubertal development. He attended high school with very good performance. On physical examination, his mental status and neurological evaluation were normal. He was alert and cooperative, and his speech was fluent. No apparent cortical deficit was detected. He did not have difficulties in balancing or ataxia and the Romberg test was negative. Cranial nerve evaluation was normal. He had normal sensation to pinprick, light touch, and proprioception. Osteotendinous reflexes were reduced in the upper limbs, present but reduced in the lower limbs. Cardiovascular examination, pulmonary and abdominal examination were normal. In addition, he presented dehydrated skin and mucous. Areas of hyperpigmentation especially on the tongue were documented. He had a normal growth with normal height. He presented normal pubertal development.
The laboratory examination revealed normal serum electrolytes, plasma glucose, kidney and hepatic function. Endocrine evaluation demonstrated elevated plasma ACTH level (>1500 pg/mL, normal range 4.7–48.8 pg/mL) with low serum cortisol (2.5 µg/dL, normal range 8.0–25.0 mcg/dL). Mineralocorticoid function (renin and aldosterone levels) was normal. Abdominal CT revealed normal adrenal glands. Therefore, the diagnosis of PAI was made and glucocorticoid replacement treatment was started (dose 35 mg/die; 20–15 mg).
The presence of two cases of PAI disease within the same family suggested the possible recurrence of a familiar or genetic form of PAI. Autoimmune hypoadrenalism was excluded by lack of anti-21-hydroxylase antibodies. In addition, the diagnosis of Triple A disease was excluded for the absence of achalasia and alacrimia evaluated with digestive X-ray first tract and Schirmer test. To further explore the presence of cerebral manifestations of ALD, a neurological examination and a brain MRI were performed in the two brothers. Results in both subjects showed no significant neurological alterations and white matter lesions. Loes score was negative in both subjects. In addition, patients underwent neuropsychological assessments. No abnormal findings were documented on electrophysiologic studies including ECG, nerve conduction studies, brainstem auditory evoked potentials and somatosensory evoked potential studies. In contrast, visual evoked potentials showed slowing of nerve conduction along the optical pathway of the right visual nerve after foveal stimulation for the case index and case 2. Lastly, ALD Addison only was suggested and genetic analysis was performed confirming that the two patients were positive for genetic diagnosis of adrenoleukodystrophy. Particularly, the genetic test shared a novel mutation in the ABCD1 gene not previously described in the literature. In addition, blood of the two brothers was collected to determine the levels of VLCFAs, showing elevated concentrations (values of case index: C26:0: 2.090 μmol/L, C26:0/C22:0: 0.036 μmol/L, C24:0/C22:0: 1.149 μmol/L; values of case 2: C26:0: 1.150 μmol/L, C26:0/C22:0: 0.033 μmol/L, C24:0/C22:0: 1.161 μmol/L, normal range: C26:0: 0.01–0.9 μmol/L, C26:0/C22:0: 0.006–0.025 μmol/L, C24:0/C22:0: 0.46–1.27 μmol/L).
The genetic diagnosis was confirmed by direct sequencing of ABCD1 gene that showed hemizygous mutation c.853C>G(p.R285G) not reported in the database of gene mutations for ALD disease (https://adrenoleukodystrophy.info/). The use of specific bioinformatics software (Polyphen, Sift, Provean, Mutation Taster) confirmed its probable pathogenicity (Fig. 1). Therefore, genetic counselling was performed in all members and thus even asymptomatic siblings or relatives were studied with the aim of evaluating the presence of the genetic mutation.
In Index case and case 2 besides the steroid replacement treatment, diet therapy was also started, including a reduction of the intake of foods with VLCFA and a mixture of erucic acid, glycerolotrioleate acid and conjugate linoleic acid (EA/GTO/CLA) was started (12).
At the age of 12 years and 18 years old, respectively, they continued steroid replacement therapy and diet with a good control of VLCFA metabolism. No neurological symptoms neither any matter lesions on brain MRI have been developed during the follow-up. To date, however, it is difficult to predict what form of X-ALD they would develop due to the novel mutation detected.
Case 3
This asymptomatic 12-year-old brother demonstrated the same ABCD1 gene mutation and high levels of VLCFA.
Perinatal history and past medical history were unremarkable. Neurodevelopmental milestones in areas of motor, language and cognitive skills were normal. No abnormalities in CNS were detected in clinical evaluation and in brain MRI, normal cognitive abilities as well as visuospatial and visuomotor function or attention. The school performance was good and he practiced basketball without difficulties.
ACTH test showed reduced cortisol response (ACTH 65.1 pg/mL; cortisol 11.2 µg/dL). Therefore, steroid replacement therapy and diet were started. In addition, no abnormal findings on electrophysiologic studies were detected (ECG, nerve conduction studies, brainstem auditory evoked potentials and somatosensory evoked potentials studies, and visual evoked potentials). MRI study was normal and the Loes score was 0. Neuropsychological assessments were performed and were normal. He started a diet therapy, with a reduction of the intake of foods with VLCFA and the same oil mixture mentioned above.
To date, the patient is in good general condition, his development is similar to his peers and he tolerates all effort well.
Case 4
The 14-year-old sister was studied and she carried the same new mutation described in heterozygosity. She was asymptomatic but presented high VLCFA levels so she promptly started diet and follow-up. No abnormal findings on electrophysiologic studies were detected (ECG, nerve conduction studies, brainstem auditory evoked potentials and somatosensory evoked potentials studies, and visual evoked potentials). Brain MRI was normal and the Loes score was 0.
Case 5
The 41-year-old woman, mother of the described cases, carries the new gene mutation (c.853C>G(p.R285G)) in heterozygosity.
In the past medical history, she had a Guillian–Barrè episode at the age of 39 years old which resolved in 10 days but it required 18 months of rehabilitation.
Neurological evaluation was normal. Particularly, she showed normal walking without ataxia but reported pain in the legs and mild tiredness as prominent symptoms. In addition, she had a peculiar phenotype characterized by a fragile and thin hair. Laboratory evaluation showed a normal adrenal function (cortisol, ACTH aldosterone and renin levels). However, levels of VLCFA were elevated, thus she started a diet and used the same mixture of oil utilized for the sons with follow-up. No abnormal findings on electrophysiologic studies (ECG, nerve conduction studies, brainstem auditory evoked potentials and somatosensory evoked potentials studies, and visual evoked potentials). At brain MRI multifocal leukoencephalopathy was described. Particularly, point-like signals on the supratentorial white matter were detected. The MRI lesions are not related to an X- ALD demyelination.
The main clinical features and approaches to our patients are summarized in Table 1.
Table 1.
Main clinical and biochemical features, brain MRI characteristics and treatments of the family members with X-ALD.
| Cases | |||||
|---|---|---|---|---|---|
| 1† | 2 | 3 | 4 | 5 | |
| Sex | M | M | M | F | F |
| Age at diagnosis, years | 9 | 15 | 12 | 14 | 41 |
| Symptoms | Anorexia, asthenia, abdominal pain, salt craving | Anorexia, fatigue, hyperpigmentation | None | None | Asthenia, tiring easily on walking, sensitive alteration |
| Adrenal function | |||||
| Sodium, mmol/L | 123 | ||||
| Cloremia, mmol/L | 87 | ||||
| ACTH, pg/mL | >1500 | >1500 | 65.1 | Normal | Normal |
| Cortisol, µg/dL | 8.3 | 2.5 | 11.2* | ||
| Renin, µ/mL | >500 | ||||
| VLCFA, µmol/L | |||||
| C26:0 | 2.090 | 1.150 | 1.290 | 2.390 | 2.390 |
| C26:0/C22:0 | 0.036 | 0.033 | 0.034 | 0.025 | 0.036 |
| C24:0/C22 | 1.149 | 1.161 | 1.243 | 1.089 | 1.089 |
| Brain MRI | Normal | Normal | Normal | Normal | MLE |
| Therapy | HC, DR, oil mix | HC, DR, oil mix | HC, DR, oil mix | DR | HC, DR, oil mix |
†Index case; *Reduced cortisol response to ACTH.
DR, dietary restrictions; HC, hydrocortisone; MLE, multifocal leuco-encephalopathy; Oil mix: erucic acid/ glycerolotrioleate acid/ conjugate linolenic acid; VLCFA, very long chain fatty acid.
Other family members
The gene analysis was also performed further in two brothers (4 and 6 years old, respectively) reporting no mutation (wild type) in ABCD1 gene. However, the youngest one presented epilepsy and the oldest was affected by a complex neuropsychological picture of unknown nature.
Discussion
For the first time, we report a novel mutation in the ABCD1 gene documented in a family group associated to an X-ALD possible Addison only phenotype. The diagnostic confirmation was obtained with direct sequencing of ABCD1 gene that showed hemizygous mutation c.853C>G(p.R285G) not previously reported. More importantly, the use of specific bioinformatics software (Polyphen, Sift, Provean, Mutation Taster) confirmed its probable pathogenicity. Clinical and instrumental investigations of all the affected male family members showed the presence of PAI requiring steroid replacement therapy. In addition, due to the characteristic increase of VLCFA levels detected in male subjects and carriers of X-ALD (sister and mother) diet therapy was started and associate in all patients with a reduction of VLCFA acid concentrations. In all subjects, no neurological involvement or MRI alterations were documented, besides the mother who showed multifocal leukoencephalopathy associated to a positive history of a Guillian-Barrè episode requiring a long rehabilitation period. In addition, she complained a mild pain in the legs and tiredness, partially reduced with diet treatment. Therefore, the follow-up of all the family members might add relevant information on the effects of this mutation on neurological alterations. This is particularly relevant in order to add further pieces to the pathophysiology of the disease. In fact, it is well known that to date there is a lack of knowledge on why neurological or adrenal involvement is not detected in all subjects and seems to be not related directly to VLCFA concentrations. Therefore, further studies and case reports are needed in order to clarify these key elements.
X-ALD is a complex disease with a still unknown physiopathology and very few therapeutic options available. Patients with X-ALD do not show genotype/phenotype correlation, and other factors such as genetic, epigenetic, or environmental features should contribute to determine the possibility of progression towards cerebral ALD feature. In a recent international multicentre retrospective review, the authors showed data on 159 male patients with ALD, PAI disease was present in 71.1%, while spinal cord disease was present in 41.7% and cerebral disease in 53.3% (13). By performing survival analyses aiming to define the development of PAI over time, authors showed that the cumulative probability was high until the age of 10 years and increased afterwards. Therefore, PAI should be considered as a main clinical symptom both in adults and in children (13). Accordingly, recent studies reported that neurological manifestations due to demyelination can develop after the diagnosis of PAI. Moreover, spinal cord or cerebral complication should occur at the 2nd–3rd decades (13). Engelen M et al, in a recent prospective cross-sectional cohort study, demonstrated that only 18% of women heterozygous X-ALD under the age of 40 years had symptoms. Interestingly, in women older than 60 years old, the percentage of subjects with symptoms increases significantly, reaching 88% in heterozygous X-ALD (3). Accordingly, it is more difficult to predict their final phenotype and, therefore, a close monitoring is needed. These effects can be clearly shown in our case series. Particularly, we identified a novel mutation in the ABCD1 gene, all the patients present only Addison disease but within the family, there could be several different phenotypes despite the presence of the same mutations they might develop other manifestations, not known.
Finally, most importantly in patients with idiopathic PAI, diagnosis of ALD should be considered and accurate evaluation through detection of VLCFA concentrations and genetic confirmation test needs to be performed.
Early diagnosis allows to detect neurological manifestation and refer properly to HSCT in early phase of cerebral phenotype. Moreover, the early diagnosis of X-ALD also in Addison only phenotype is more important to avoid adrenal crisis and to start corticosteroid replacement therapy. Nutrition therapy based on restriction of VLCFA intake and use of the new oil mixture, that inhibits elongation of saturated fatty acids in the body needs to be started. An important option to improve the prognosis of X-ALD is the prenatal tests or screening for newborns in order to intercept CCALD cases before neurological manifestation develop (5, 14, 15). This will allow for appropriate definitive cures such as HSCT or gene therapy, but to date, neonatal screening for X-ALD is not yet available in Italy.
In conclusion, long-term prospective and observational studies of cases are needed to elucidate the natural history of adrenal insufficiency in ALD. Particularly, the follow-up of these family members is useful to predict the final phenotype associated with this new mutation and might clarify if it remains Addison only or whether progress in further phenotypes adding novel knowledge to this complex disease.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent has been obtained from the patients and patients’ guardians for publication of the case series.
Author contribution statement
All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
References
- 1.Hartley MD, Kirkemo LL, Banerji T, Scanlan TS.A thyroid hormone-based strategy for correcting the biochemical abnormality in X-linked adrenoleukodystrophy. Endocrinology 2017. 158 1328–1338. ( 10.1210/en.2016-1842) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiesinger C, Eichler FS, Berger J.The genetic landscape of X-linked adrenoleukodystrophy: inheritance, mutations, modifier genes, and diagnosis. Application of Clinical Genetics 2015. 8 109–121. ( 10.2147/TACG.S49590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engelen M, Kemp S, de Visser M, van Geel BM, Wanders RJ, Aubourg P, Tien Poll-The BT.X-linked adrenoleukodystrophy (X-ALD): clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet Journal of Rare Diseases 2012. 7 51. ( 10.1186/1750-1172-7-51) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horn MA, Retterstol L, Abdelnoor M, Skjeldal OH, Tallaksen CM.Adrenoleukodystrophy in Norway: high rate of de novo mutations and age-dependent penetrance. Pediatric Neurology 2013. 48 212–219. ( 10.1016/j.pediatrneurol.2012.12.007) [DOI] [PubMed] [Google Scholar]
- 5.Turk BR, Theda C, Fatemi A, Moser AB.X-linked adrenoleukodystrophy: pathology, pathophysiology, diagnostic testing, newborn screening and therapies. International Journal of Developmental Neuroscience 2020. 80 52–72. ( 10.1002/jdn.10003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemp S, Huffnagel IC, Linthorst GE, Wanders RJ, Engelen M.Adrenoleukodystrophy—neuroendocrine pathogenesis and redefinition of natural history. Nature Reviews: Endocrinology 2016. 12 606–615. ( 10.1038/nrendo.2016.90) [DOI] [PubMed] [Google Scholar]
- 7.Schirinzi T, Vasco G, Aiello C, Rizzo C, Sancesario A, Romano A, Favetta M, Petrarca M, Paone L, Castelli E. et al. Natural history of cohort of ABCD1 variants female carriers. European Journal of Neurology 2019. 26 326–332. ( 10.1111/ene.13816) [DOI] [PubMed] [Google Scholar]
- 8.Kemp S, Berger J, Aubourg P.X-linked adrenoleukodystrophy: clinical, metabolic, genetic and pathophysiological aspects. Biochimica et Biophysica Acta 2012. 1822 1465–1474. ( 10.1016/j.bbadis.2012.03.012) [DOI] [PubMed] [Google Scholar]
- 9.Ofman R, Dijkstra IM, van Roermund CW, Burger N, Turkenburg M, van Cruchten A, van Engen CE, Wanders RJ, Kemp S.The role of ELOVL1 in very long-chain fatty acid homeostasis and X-linked adrenoleukodystrophy. EMBO Molecular Medicine 2010. 2 90–97. ( 10.1002/emmm.201000061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kemp S, Wanders R.Biochemical aspects of X-linked adrenoleukodystrophy. Brain Pathology 2010. 20 831–837. ( 10.1111/j.1750-3639.2010.00391.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alsaleem M, Saadeh L.Adrenoleukodystrophy. In StatPearls (Internet). Treasure Island (FL): StatPearls Publishing, 2020. PMID 32965999. [PubMed] [Google Scholar]
- 12.Cappa M, Bizzarri C, Petroni A, Carta G, Cordeddu L, Valeriani M, Vollono C, De Pasquale L, Blasevich M, Banni S.A mixture of oleic, erucic and conjugated linoleic acids modulates cerebrospinal fluid inflammatory markers and improve somatosensorial evoked potential in X-linked adrenoleukodystrophy female carriers. Journal of Inherited Metabolic Disease 2012. 35 899–907. ( 10.1007/s10545-011-9432-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huffnagel IC, Laheji FK, Aziz-Bose R, Tritos NA, Marino R, Linthorst GE, Kemp S, Engelen M, Eichler F.The natural history of adrenal insufficiency in X-linked adrenoleukodystrophy: an international collaboration. Journal of Clinical Endocrinology and Metabolism 2019. 104 118–126. ( 10.1210/jc.2018-01307) [DOI] [PubMed] [Google Scholar]
- 14.Kemper AR, Brosco J, Comeau AM, Green NS, Grosse SD, Jones E, Kwon JM, Lam WK, Ojodu J, Prosser LA. et al. Newborn screening for X-linked adrenoleukodystrophy: evidence summary and advisory committee recommendation. Genetics in Medicine 2017. 19 121–126. ( 10.1038/gim.2016.68) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu J, Eichler F, Biffi A, Duncan CN, Williams DA, Majzoub JA.The changing face of adrenoleukodystrophy. Endocrine Reviews 2020. 41 577–5. ( 10.1210/endrev/bnaa013) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a