Abstract
Objective
To evaluate the effect of inserting epidemiological information into lumbar spine imaging reports on subsequent nonsurgical and surgical procedures involving the thoracolumbosacral spine and sacroiliac joints.
Design
Analysis of secondary outcomes from the Lumbar Imaging with Reporting of Epidemiology (LIRE) pragmatic stepped-wedge randomized trial.
Setting
Primary care clinics within four integrated health care systems in the United States.
Subjects
238,886 patients ≥18 years of age who received lumbar diagnostic imaging between 2013 and 2016.
Methods
Clinics were randomized to receive text containing age- and modality-specific epidemiological benchmarks indicating the prevalence of common spine imaging findings in people without low back pain, inserted into lumbar spine imaging reports (the “LIRE intervention”). The study outcomes were receiving 1) any nonsurgical lumbosacral or sacroiliac spine procedure (lumbosacral epidural steroid injection, facet joint injection, or facet joint radiofrequency ablation; or sacroiliac joint injection) or 2) any surgical procedure involving the lumbar, sacral, or thoracic spine (decompression surgery or spinal fusion or other spine surgery).
Results
The LIRE intervention was not significantly associated with subsequent utilization of nonsurgical lumbosacral or sacroiliac spine procedures (odds ratio [OR] = 1.01, 95% confidence interval [CI] 0.93–1.09; P = 0.79) or any surgical procedure (OR = 0.99, 95 CI 0.91–1.07; P = 0.74) involving the lumbar, sacral, or thoracic spine. The intervention was also not significantly associated with any individual spine procedure.
Conclusions
Inserting epidemiological text into spine imaging reports had no effect on nonsurgical or surgical procedure utilization among patients receiving lumbar diagnostic imaging.
Keywords: Epidural Injections, Zygapophyseal Joint, Injection, Injections, Radiofrequency Ablation, Radiology, Spine, Lumbar
Introduction
Low back pain (LBP) has been the leading contributor to years lived with disability in the United States over the past 25 years [1]. LBP is also a major contributor to health-related spending in the United States [2]. Despite considerable increases in expenditures for LBP over time [2], there is a lack of evidence of a corresponding improvement in health status for U.S. adults [3]. Indeed, there is concern that more treatment increases costs without leading to better health outcomes [3, 4]. Procedural treatments for LBP or conditions associated with LBP, such as lumbosacral radiculopathy and symptomatic lumbar spinal stenosis, include both surgical (e.g., spinal fusion or laminectomy) and nonsurgical (e.g., epidural corticosteroid injections or other nonsurgical percutaneous procedures) invasive treatments, often performed on an elective basis [5, 6]. For the purposes of the present study, we define “procedural treatments for LBP” as including both surgical and nonsurgical percutaneous procedures, either for LBP itself or for the treatment of specific spine-related pain syndromes (e.g., lumbosacral radiculopathy or symptomatic lumbar spinal stenosis) that may be associated with LBP. Surgical procedures for conditions associated with LBP are commonly used in the United States and comprise a substantial component of LBP-related health spending [7]. Nonsurgical percutaneous procedures for conditions associated with LBP are also commonly used [8–12]. Decreasing procedural treatments for LBP may be one way to decrease LBP-related health care spending in the United States.
Procedural treatments for LBP are typically directed at correcting an underlying structural or anatomic problem or eliminating pain attributed to such a problem. A fundamental issue in spine care is that many of the commonly noted anatomic or structural “findings” described on lumbar spinal imaging reports (e.g., intervertebral disc height loss or facet degeneration) are highly prevalent even among those without LBP and therefore lack specificity, making it problematic to attribute individual cases of LBP to these findings [13, 14]. However, certain spine imaging findings of lower prevalence (e.g., nerve root displacement/compression or disc extrusion) are less commonly found in asymptomatic individuals and may be more strongly associated with LBP or specific spine syndromes such as lumbosacral radiculopathy [15–17].
Providing patients and providers with epidemiological information to educate them about the high prevalence of common imaging findings even in those without LBP may increase awareness that certain imaging findings are unlikely to pinpoint the cause of pain for a given patient [18]. This may reduce potentially unnecessary subsequent procedural treatments for LBP. The Lumbar Imaging with Reporting of Epidemiology (LIRE) randomized controlled trial examined the effect of inserting epidemiological “benchmark” information about the prevalence of common imaging findings among individuals without LBP into lumbar spine imaging reports, as compared with the usual practice of not providing such information [19]. Importantly, the LIRE benchmark epidemiological information does not include less common imaging findings that may have stronger links to spine-related symptoms (e.g., nerve root displacement/compression, disc extrusions). The LIRE trial found that providing epidemiological information did not reduce overall subsequent spine-related costs as reflected by relative value units [19]. However, providing epidemiological information in lumbar spine imaging reports resulted in a slightly lower likelihood of patients receiving a subsequent opioid prescription (odds ratio [OR] = 0.95, 95% confidence interval [CI] 0.91–1.00), as compared with not providing such information [20]. A prespecified analysis of procedural treatments (nonsurgical percutaneous spine procedures and spine surgeries) for LBP and conditions associated with LBP was originally planned as an examination of secondary outcomes in the LIRE trial protocol [21]. The aims of the present study were to examine the effects of inserting epidemiological text into lumbar spine imaging reports on 1) the likelihood of subsequent nonsurgical lumbosacral or sacroiliac spine procedures and 2) the likelihood of subsequent spine surgery involving the lumbar, sacral, or thoracic spine. We hypothesized that inserting epidemiological text into spine imaging reports would decrease the likelihood of nonsurgical lumbosacral or sacroiliac spine procedures and decrease the likelihood of subsequent spine surgery involving the lumbar, sacral, or thoracic spine.
Methods
Study Design
The LIRE trial was a pragmatic, multicenter, stepped-wedge, cluster-randomized trial conducted within four large integrated health care systems. Primary care clinics were randomly assigned to different start dates for receiving imaging reports containing several additional lines of text describing age- and imaging modality–appropriate epidemiological benchmarks for the prevalence of common degenerative imaging findings in adults without LBP, such that all primary care providers (PCPs) within a clinic would begin receiving the intervention at approximately the same time. Clinics received standard imaging reports (without the addition of epidemiological benchmarks) before their assigned intervention date. We used clinic-level cluster randomization to minimize potential contamination that might result from having some providers receiving epidemiological benchmark information and others not receiving epidemiological benchmark information within the same clinic. We used a stepped-wedge randomization scheme to facilitate implementation of the intervention within all clinics by the end of the study; the design permitted both within-cluster and between-cluster comparisons. We reported previously a detailed description of the LIRE trial protocol [21].
Study Participants
We enrolled clinics and their patients from four integrated health care systems: Kaiser Permanente, Northern California; Henry Ford Health System, Detroit, Michigan; Kaiser Permanente Washington (formerly Group Health Cooperative), Seattle, Washington; and Mayo Clinic Health System, Rochester, Minnesota. These health care systems have comprehensive electronic health record (EHR) systems allowing capture of health care utilization data, including procedural care. Within each health care system, we identified adult primary care clinics and associated PCPs. We defined “LIRE providers” as PCPs who were based primarily at a single primary care clinic and who ordered at least one lumbar imaging examination during the study period [19]. When a provider ordered a lumbar imaging examination, an automated screening process determined whether the PCP, patient, and clinic were eligible for our study. PCPs in the participating health care systems were able to order x-rays, magnetic resonance imaging, and computed tomography (i.e., magnetic resonance imaging/computed tomography did not have to be ordered by a specialist). We enrolled participants from the population of eligible patients ≥18 years of age whose PCP had ordered a diagnostic imaging study of the lumbar spine between October 1, 2013, and September 30, 2016. Exclusion criteria included patients who had received spine imaging within the 12 months before the lumbar diagnostic imaging study and those who had opted out of research study participation. The institutional review boards for the participating health systems determined that the study was minimal risk and granted waivers of consent and Health Insurance Portability and Accountability Act (HIPAA) authorizations.
Randomization
We used a stepped-wedge cluster randomization study design, randomly assigning clinics from each health care system to begin receiving the intervention at one of five calendar times, separated by 6-month intervals, between April 2014 and April 2016. Clinics were classified into tertiles of clinic size by the number of PCPs within each clinic. The randomization was stratified by clinic size tertile and health care system so that health care systems and clinics of similar size were represented similarly in each randomization wave. Because of the stepped-wedge temporal randomization scheme, we labeled clinics as “control” clinics if insertion of the intervention text into spine imaging reports had not yet started and as “intervention” clinics after insertion of the intervention text had begun. Because the intervention text was visible to providers, blinding of the participating clinics was infeasible. The study investigators at the data coordinating center were blinded to clinic and participant randomization status, except for the biostatistician (ENM) who received and cleaned the data.
Intervention
The “intervention text” consisted of age- and modality-specific epidemiological benchmark information on the prevalence of common findings in adults without LBP (Supplementary Data File 1) [12–14]. Using a fully automated approach through the radiology information system or EHR, we inserted the intervention text into thoracic or lumbar spine imaging reports at intervention clinics. PCPs in control clinics continued to receive their usual imaging reports without the intervention text.
Baseline Measures
As a key design feature of our pragmatic approach, we collected all measures passively through the EHR. EHR data were obtained for patients beginning 12 months before their index imaging and continuing up to 24 months after the index imaging. These data included International Classification of Diseases (ICD), Ninth Revision, Clinical Modification (ICD-9-CM) and Tenth Revision [ICD-10-CM] diagnostic and procedure codes; Current Procedural Terminology (CPT) procedure codes; and site-specific procedure codes. At study baseline, we collected data on patient age (categorized as 18–39, 40–60, and ≥61 years); sex; insurance type (Medicare, Medicaid/state-subsidized, commercial, Veterans Affairs [VA], self-pay, and unknown/not reported); study site; and clinic size. The Charlson comorbidity index (categorized as 0, 1, 2, and ≥3 conditions) [22–24] was calculated from diagnostic codes present in the 12 months before the index imaging. The index imaging modality (x-ray, computed tomography, or magnetic resonance imaging) was determined from CPT and site-specific codes.
Outcome Measures
Outcomes were evaluated over an 18-month follow-up because of some missing EHR data between 18 and 24 months of follow-up. The primary outcome for aim 1 of the present study was the occurrence of any nonsurgical lumbosacral or sacroiliac spine procedure from among the four types of nonsurgical procedures considered in this analysis (epidural steroid injections [ESI], facet joint injections [including both intra-articular joint injections and medial branch blocks], facet joint radiofrequency ablation [RFA], and sacroiliac joint injections) during the 18-month follow-up. In other words, this was a single primary outcome reflecting the occurrence of any one of the four major types of nonsurgical lumbosacral or sacroiliac spine procedures. The CPT codes and site-specific codes used to identify these nonsurgical lumbosacral or sacroiliac spine procedures are provided in Supplementary Data File 2. Other nonsurgical lumbosacral or sacroiliac spine procedures were not considered because of their lower frequency of utilization. In exploratory secondary analyses, we examined the same four nonsurgical procedures separately. These four analyses examined the outcomes of 1) any lumbosacral ESI, 2) any lumbosacral facet joint injection, 3) any lumbosacral facet joint RFA, and 4) any sacroiliac joint injections during the 18-month follow-up. These four exploratory secondary analyses were conducted because changes relevant to specific procedures might be obscured in the primary analysis grouping various procedures together. To account for the fact that nonsurgical lumbosacral and sacroiliac procedures are often repeated, secondary analyses were also conducted for each outcome, examining the number of procedures (as a count) conducted during the 18-month follow-up.
The primary outcome for aim 2 of the present study was the occurrence of any lumbar, sacral, or thoracic spine surgery (spinal fusion or proxies for fusion, such as disc arthroplasty, decompression surgery, or other spine surgeries) during the 18-month follow-up. We used ICD-9, ICD-10, CPT, and site-specific codes to identify surgical spine procedures, including algorithms with ≥98% sensitivity and specificity for identifying decompression surgery and spinal fusion [25]; these codes are provided in Supplementary Data File 2. There were two exploratory secondary spine surgery outcomes for aim 2: the occurrence of 1) any spinal fusion or proxy for fusion and 2) any decompression surgery.
Statistical Analysis
We used descriptive statistics to compare baseline characteristics of the intervention and control groups. To evaluate the effect of the LIRE epidemiological benchmark intervention on binary outcomes, we used generalized linear models that clustered on clinic and then provider within the clinic, using robust standard errors. Models included fixed effects (site, clinic size by tertile, computed tomography [vs. magnetic resonance imaging], Charlson Comorbidity category [0, 1, 2, 3+], site-specific time [linear], sex, age range [<40, 40–60, >60)], and random effects for clinic [intercept and treatment] and provider [intercept only]). Models for nonsurgical lumbosacral or sacroiliac spine procedures also included prior nonsurgical procedural utilization in the 1 year preceding the index image as a fixed effect. Because there were very few patients with surgical procedures involving the lumbar, sacral, or thoracic spine in the 1 year preceding the index image (n = 90 [0.1%] in controls not receiving the LIRE intervention; n = 89 [0.1%] in those receiving the LIRE intervention) relative to the total sample size, prior surgical procedure utilization was not included in the models for surgical spine procedures. Secondary analyses examined the number of nonsurgical spinal procedures via negative binomial regression where possible and Poisson regression where negative binomial regression models did not converge. Statistical significance was determined by a P value <0.05 for each of the two primary outcomes (any nonsurgical procedure and any spine surgery). A Bonferroni correction was used to determine the threshold for statistical significance for exploratory secondary outcomes examining individual nonsurgical lumbosacral or sacroiliac spine procedures (0.05/4 individual procedures = 0.0125) and surgical procedures involving the lumbar, sacral, or thoracic spine (0.05/2 individual procedures = 0.025). Analyses used the intention-to-treat method. SAS software version 9.4 (Cary, NC, USA) was used for all analyses.
Results
Study Sample
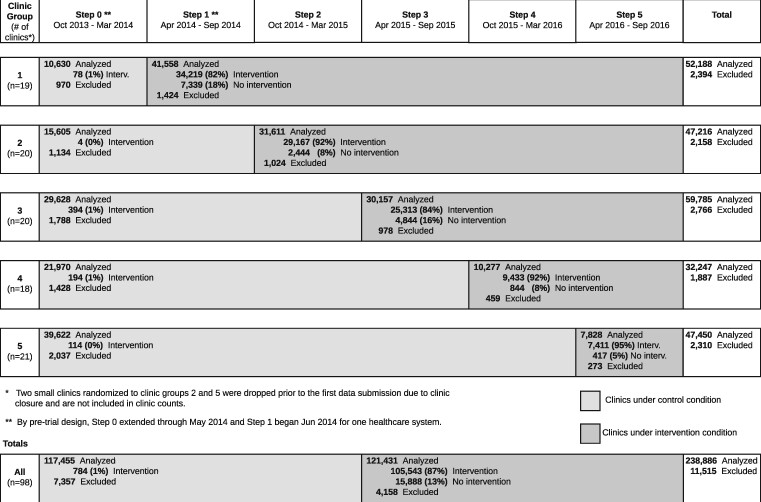
The study sample included 238,886 patients (Figure 1). Baseline characteristics were generally comparable between patients in the intervention and control groups (Table 1).
Figure 1.
Stepped-wedge allocation of trial subjects. Patients were excluded for any of the following reasons: a prior lumbar spine image within 12 months (n = 11,149; 97% of exclusions), an imaging report finalization date more than 4 days after image completion date (n = 354; 3%), an image completion date before report finalization date (n = 3), and not having a link to utilization data (n = 9). For clinics under the control condition, “Intervention” indicates that the intervention text was mistakenly included in the image report. For clinics under the intervention condition, “Intervention” indicates that the intervention text was successfully included in the image report, and “No intervention” indicates that the intervention text was not included.
Table 1.
Baseline characteristics
| Characteristic | Control (N = 117,455) | Intervention (N = 121,431) |
|---|---|---|
| Site, n (%) | ||
| A | 6,950 (6) | 7,388 (6) |
| B | 96,275 (82) | 100,729 (83) |
| C | 7,846 (7) | 7,726 (6) |
| D | 6,384 (5) | 5,588 (5) |
| Age in years, n (%) | ||
| 18–39 | 21,237 (18) | 22,105 (18) |
| 40–60 | 45,032 (38) | 44,995 (37) |
| >60 | 51,186 (44) | 54,331 (45) |
| Sex,* n (%) | ||
| Female | 67,915 (58) | 69,458 (57) |
| Male | 49,534 (42) | 51,965 (43) |
| Charlson Comorbidity Index, n (%) | ||
| 0 | 75,106 (64) | 77,973 (64) |
| 1 | 20,675 (18) | 21,193 (17) |
| 2 | 11,451 (10) | 11,760 (10) |
| 3+ | 10,223 (9) | 10,505 (9) |
| Primary insurance at index, n (%) | ||
| Medicare | 44,362 (38) | 46,479 (38) |
| Medicaid/state-subsidized | 5,546 (5) | 6,510 (5) |
| Commercial | 65,375 (56) | 66,368 (55) |
| VA | 117 (0) | 131 (0) |
| Self-pay | 731 (1) | 570 (0) |
| Unknown or not reported | 1,324 (1) | 1,373 (1) |
| Socioeconomic index,† mean±SD | 57±6 | 57±7 |
| Prior injection, n (%) | 1,399 (1) | 966 (1) |
| Prior surgery, n (%) | 90 (0.1) | 89 (0.1) |
Does not include 14 patients with other or unknown sex.
Does not include 6,810 (3%) patients with unknown socioeconomic index.
Table 2 shows the frequencies and proportions of patients who received nonsurgical lumbosacral or sacroiliac spine procedures and surgical procedures involving the lumbar, sacral, or thoracic spine over the 18-month follow-up. Nearly 12% of patients received at least one nonsurgical lumbosacral or sacroiliac spine procedure. ESIs were by far the most common nonsurgical procedure (received by 10% of patients), with much lower proportions of patients receiving facet joint injections (2%), sacroiliac joint injections (1%), and facet joint RFA (1%). A minority of those receiving nonsurgical procedures had repeat procedures, with 3% receiving two procedures and 2% receiving three or more procedures over 18 months. Repeat procedures consisted mainly of repeat ESIs (Table 2).
Table 2.
Frequencies of spine procedures performed over 18-month follow-up
| Number of Procedures, n (%) |
||||
|---|---|---|---|---|
| Any | 1 | 2 | 3 or more | |
| Nonsurgical lumbosacral or sacroiliac spine procedures | ||||
| Primary outcome (Aim 1) | ||||
| Any type of nonsurgical procedure* | 28,339 (12) | 15,422 (6) | 7,921 (3) | 4,996 (2) |
| Secondary outcomes (Aim 1) | ||||
| Any ESI | 24,450 (10) | 14,060 (6) | 6,850 (3) | 3,540 (1) |
| Any facet joint injection | 3,905 (2) | 2,842 (1) | 837 (0) | 226 (0) |
| Any facet joint RFA | 1,420 (1) | 1,074 (0) | 290 (0) | 56 (0) |
| Any sacroiliac joint injection | 1,722 (1) | 1,309 (1) | 327 (0) | 86 (0) |
| Surgical procedures involving the lumbar, sacral, or thoracic spine | ||||
| Primary outcome (Aim 2) | ||||
| Any spine surgery† | 7,538 (3) | 4,719 (2) | 2,186 (1) | 633 (0) |
| Secondary outcomes (Aim 2) | ||||
| Fusion‡ | 2,629 (1) | 2,490 (1) | 129 (0) | 10 (0) |
| Decompression | 6,734 (3) | 6,223 (3) | 458 (0) | 53 (0) |
Including lumbosacral ESI, facet joint injection (medial branch blocks or intra-articular injections), or facet joint RFA; or sacroiliac joint injection (the occurrence of any one of these types of procedures).
Any spine surgery includes decompression surgery, spinal fusion or proxies for spine fusion, or other surgeries involving the lumbar, sacral, or thoracic spine (the occurrence of any one of these types of surgeries).
Spinal fusion or proxies for spinal fusion (e.g., disc arthroplasty). Fusion may or may not have also involved decompression.
The results of generalized linear models to evaluate the effect of the LIRE intervention on nonsurgical lumbosacral or sacroiliac spine procedures are presented in Table 3. Inserting epidemiological text into lumbar spine imaging reports did not have a significant effect on the occurrence of any nonsurgical lumbosacral or sacroiliac spine procedure over the subsequent 18 months (OR = 1.01, 95% CI 0.93–1.09; P = 0.79), the primary outcome for aim 1. Similarly, there were no significant effects on the four exploratory secondary outcomes for aim 1 (the four specific procedure types: ESIs, facet joint injections, facet joint RFA, and sacroiliac joint injections), after accounting for multiple statistical comparisons (Table 3). Results were also similar when outcomes were treated as counts of procedures, showing no significant effects on nonsurgical lumbosacral or sacroiliac spine procedure utilization (Table 3).
Table 3.
Effects of the LIRE intervention on utilization of nonsurgical lumbosacral or sacroiliac spine procedures over 18-month follow-up
| Any |
Count |
|||||||
|---|---|---|---|---|---|---|---|---|
| Adjusted Proportions |
Adjusted Rate |
|||||||
| Control (n = 117,455) | Intervention (n = 121,431) | OR‖ (95% CI) | P | Control (n = 117,455) | Intervention (n = 121,431) | IRR‖ (95% CI) | P | |
| Nonsurgical lumbosacral or sacroiliac spine procedures | ||||||||
| Primary outcome (Aim 1) | ||||||||
| Any type of nonsurgical procedure* | 11.8% | 11.9% | 1.01 (0.93–1.09) | 0.79† | 0.205 | 0.207 | 1.01 (0.93–1.10) | 0.86‡ |
| Secondary outcomes (Aim 1) | ||||||||
| Any ESI | 10.1% | 10.4% | 1.03 (0.95–1.12) | 0.46§ | 0.166 | 0.169 | 1.02 (0.93–1.11) | 0.67‡ |
| Any facet joint injection | 1.7% | 1.5% | 0.89 (0.78–1.02) | 0.09§ | 0.023 | 0.021 | 0.94 (0.80–1.10) | 0.44‡ |
| Any facet joint RFA | 0.6% | 0.6% | 0.99 (0.81–1.20) | 0.89§ | 0.008 | 0.008 | 1.03 (0.80–1.34) | 0.81‡ |
| Any sacroiliac joint injection | 0.7% | 0.8% | 1.13 (0.93–1.37) | 0.22§ | 0.009 | 0.010 | 1.08 (0.87–1.34) | 0.50‡, ¶ |
IRR=incidence rate ratio.
Lumbosacral ESI, facet joint injection, or facet joint RFA; or sacroiliac joint injection (the occurrence of any one of these types of procedures).
Statistical significance defined as P < 0.05.
Poisson regression.
Statistical significance defined as P < 0.0125 (with Bonferroni correction accounting for four individual nonsurgical spine procedures).
Because of lack of model convergence, the clinic-level treatment random effects term was dropped from this model.
Models adjusting for fixed effects (site, clinic size tertile, computed tomography vs. magnetic resonance imaging, Charlson Comorbidity category [0, 1, 2, 3+], site-specific time [linear], gender, age range [<40, 40–60, >60], and prior injection) and random effects (clinic [intercept and treatment] and provider [intercept only]).
More than 3% of patients received spine surgery, with 3% receiving decompression surgery and 1% receiving spinal fusion or a proxy for fusion, such as disc arthroplasty, over 18 months of follow-up (Table 2). Inserting epidemiological text into lumbar spine imaging reports did not have a significant effect on the occurrence of any spine surgery over 18 months of follow-up (OR = 0.99, 95 CI 0.91–1.07; P = 0.74) (Table 4), the primary outcome for aim 1. Similarly, there were no significant effects on the occurrence of decompression surgery or the occurrence of spinal fusion over 18 months of follow-up (Table 4), the two exploratory secondary outcomes for aim 2.
Table 4.
Effects of the LIRE intervention on utilization of lumbar, sacral, or thoracic spine surgery over 18-month follow-up
| Any |
||||
|---|---|---|---|---|
| Adjusted Proportions |
||||
| Control (n = 117,455) | Intervention (n = 121,431) | OR‖ (95% CI) | P | |
| Primary outcome (Aim 2) | ||||
| Any spine surgery* | 3.2% | 3.1% | 0.99 (0.91–1.07) | 0.74† |
| Secondary outcomes (Aim 2) | ||||
| Fusion‡ | 1.1% | 1.1% | 1.01 (0.89–1.14) | 0.85§ |
| Decompression | 2.9% | 2.8% | 0.97 (0.88–1.06) | 0.47§ |
Any spine surgery includes decompression surgery, spinal fusion or proxies for spine fusion, or other surgeries involving the lumbar, sacral, or thoracic spine.
Statistical significance defined as P < 0.05.
Spinal fusion or proxies for spinal fusion (e.g., disc arthroplasty). Fusion may or may not have also involved decompression.
Statistical significance defined as P < 0.025 (with Bonferroni correction accounting for two individual surgical spine procedures).
Models adjusting for fixed effects (site, clinic size tertile, computed tomography vs. magnetic resonance imaging, Charlson Comorbidity category [0, 1, 2, 3+], site-specific time [linear], gender, age range [<40, 40–60, >60]) and random effects (clinic [intercept and treatment] and provider [intercept only]).
Discussion
In this prespecified secondary analysis of the LIRE stepped-wedge, cluster-randomized trial, 12% of study patients who received lumbar spine imaging also received one or more nonsurgical lumbosacral or sacroiliac spine procedures over the 18 months after the index image report. Inserting epidemiological text into spine imaging reports had no significant effect on the subsequent occurrence or frequency of nonsurgical lumbosacral or sacroiliac spine procedures. Similarly, although 3% of patients who received lumbar spine imaging also received thoracolumbar spine surgery over 18 months of follow-up, there was no significant effect of inserting epidemiological text into spine imaging reports on subsequent spine surgery involving the lumbar, sacral, or thoracic spine.
The findings of our study are consistent with the main results of the LIRE trial, in which there was no statistically significant impact of the insertion of epidemiological text into spine imaging reports on spine-related relative value units [19]. Because spine procedures are associated with substantial direct health care costs [3, 8, 12], it is perhaps unsurprising that we found no effect of the intervention on such procedures. The decision of whether or not to offer spine procedures is made largely by clinical spine specialists, such as spine surgeons, pain medicine physicians, or other nonsurgical specialists.
Spine specialists are likely already aware of the high prevalence of incidental and non–clinically meaningful findings on spine imaging, and such knowledge may already be incorporated into their clinical decision-making on the suitability of spine procedures for a given patient. Thus, although providing benchmark epidemiological information in spine imaging reports to nonspecialists (such as PCPs) may offer new information or a useful reminder of information previously learned, this is likely not the case for spine specialists. This may explain the null effect of the LIRE intervention on nonsurgical lumbosacral or sacroiliac spine procedures or spine surgery involving the lumbar, sacral, or thoracic spine in the present study.
The LIRE intervention consisted of short segments of text that could easily be inserted into electronic lumbar spine radiology report templates, making it an intervention that is very low cost and relatively simple to implement in many health care contexts. Given the LIRE trial’s finding of significantly lower opioid prescription rates in those randomized to receive the LIRE intervention text, a case could be made for widespread adoption of the LIRE intervention text. A potential limitation of such an approach might be if the intervention text led treating clinicians to devalue the importance of all spine conditions and “undertreat” conditions that might otherwise have benefited from treatment. In this sense, the lack of any significant effects of the LIRE intervention on spine procedure utilization seen in the present study is reassuring. In particular, decompressive spine surgery may be performed nonelectively for progressive neurological deficits or cauda equina syndrome, and rates of decompression did not appear to be affected by the LIRE intervention in our study. Therefore, our findings add to the strength of the case for broader adoption of the LIRE intervention and routine incorporation into lumbar imaging reports, beyond the evidence we have reported previously [19].
This study has some limitations. First, our study examined the effects of the LIRE intervention in a broad sample of participants receiving lumbar spine imaging. This imaging may have been for patients with LBP or for specific spine diagnoses that are often (but not necessarily) associated with LBP, such as lumbosacral radiculopathy or symptomatic lumbar spinal stenosis. Our approach did not restrict analyses according to specific diagnostic subgroups that may be more appropriate clinical candidates for a given procedure (such as analyses of surgical decompression performed only in those with lumbosacral radiculopathy). Second, although the LIRE trial was intentionally designed such that all study outcomes could be evaluated through the EHR, patient-reported outcomes such as pain and back pain–related functional limitations were not collected. Patient-reported outcomes may have added an element of depth to the present findings. On the other hand, the present study’s outcomes are high-cost spine procedures, which are generally accepted to be accurate in EHR documentation because of their high costs, and the EHR-based algorithms used for identifying spine surgeries in the present study have been validated [25]. Another potential limitation of the study is that we did not have access to data that would have enabled us to examine whether the LIRE intervention affected PCPs’ recommendations for spine procedures or referrals to spine specialists, which might have been impacted without manifesting as an overall change in procedural outcomes, given that final procedural eligibility is ultimately determined by the treating specialist. Nevertheless, our results are likely to represent the overall effect that could be expected from applying the LIRE intervention in usual clinical practice.
In summary, in this secondary analysis of a stepped-wedge randomized controlled trial, inserting epidemiological text into spine imaging reports did not affect use of nonsurgical lumbosacral or sacroiliac spine procedures or surgical procedures involving the lumbar, sacral, or thoracic spine.
Authors’ Contributions
PS, ENM, KT, JAT, RAD, ALA, PJH, JGJ, and JLF contributed to the study concept and design. ENM, KT, DFK, KJS, PHL, ALA, BG, KTJ, SDR, and JGJ contributed to the acquisition of data. ENM, PS, LSG, ZAM, SKJ, BWB, MOR, JAT, DFK, RAD, KJS, PHL, ALA, BG, PJH, SDR, JGH, and JLF contributed to the analysis and interpretation of data. All authors contributed to the drafting and revision the manuscript and approval of final version.
Supplementary Data
Supplementary Data may be found online at http://painmedicine.oxfordjournals.org.
Supplementary Material
Funding sources: This work was supported within the National Institutes of Health (NIH) Health Care Systems Research Collaboratory by the NIH Common Fund through cooperative agreement U24AT009676 from the Office of Strategic Coordination within the Office of the NIH Director and cooperative agreements UH2AT007766 and UH3AR066795 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). It was also supported by the UW Clinical Learning, Evidence and Research (CLEAR) Center for Musculoskeletal Disorders funded by NIH/NIAMS P30AR072572. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Suri is a Staff Physician at the VA Puget Sound Health Care System and was funded by the VA Puget Sound Health Care System. The contents of this work do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Conflict of interest: Dr. Jarvik reported receiving royalties from Springer Publishing and Wolters Kluwer/UpToDate and receiving travel reimbursement from GE–Association of University Radiologists outside the submitted work. Dr. Deyo reported having an endowed professorship funded by Kaiser Permanente and receiving personal fees from UpToDate outside the submitted work. Dr. Friedly reported receiving grants from the Department of Defense and salary support from the American Academy of Physical Medicine and Rehabilitation for serving as editor-in-chief outside the submitted work. None of the other authors has potential conflicts of interest to report.
Trial registration: Clinicaltrials.gov NCT02015455.
Prior presentation: None.
References
- 1. Mokdad AH, Ballestros K, Echko M, et al. ; The US Burden of Disease Collaborators. The State of US Health, 1990-2016: Burden of diseases, injuries, and risk factors among US States. JAMA 2018;319(14):1444–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dieleman JL, Baral R, Birger M, et al. US spending on personal health care and public health, 1996–2013. JAMA 2016;316(24):2627–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martin BI, Deyo RA, Mirza SK, et al. Expenditures and health status among adults with back and neck problems. JAMA 2008;299(6):656–64. [DOI] [PubMed] [Google Scholar]
- 4. Deyo RA, Mirza SK, Turner JA, Martin BI.. Overtreating chronic back pain: Time to back off? J Am Board Fam Med 2009;22(1):62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chou R, Loeser JD, Owens DK, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: An evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976) 2009;34(10):1066–77. [DOI] [PubMed] [Google Scholar]
- 6. Bernstein DN, Brodell D, Li Y, Rubery PT, Mesfin A.. Impact of the economic downturn on elective lumbar spine surgery in the United States: A national trend analysis, 2003 to 2013. Global Spine J 2017;7(3):213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim LH, Vail D, Azad TD, et al. Expenditures and health care utilization among adults with newly diagnosed low back and lower extremity pain. JAMA Netw Open 2019;2(5):e193676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Starr JB, Gold L, McCormick Z, Suri P, Friedly J.. Trends in lumbar radiofrequency ablation utilization from 2007 to 2016. Spine J 2019;19(6):1019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Starr JB, Gold LS, McCormick Z, Suri P, Friedly J.. Repeat procedures and prescription opioid use after lumbar medial branch nerve radiofrequency ablation in commercially insured patients. Spine J 2020;20(3):344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abbott ZI, Nair KV, Allen RR, Akuthota VR.. Utilization characteristics of spinal interventions. Spine J 2012;12(1):35–43. [DOI] [PubMed] [Google Scholar]
- 11. Friedly J, Chan L, Deyo R.. Geographic variation in epidural steroid injection use in medicare patients. J Bone Joint Surg Am 2008;90(8):1730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Friedly J, Chan L, Deyo R.. Increases in lumbosacral injections in the Medicare population: 1994 to 2001. Spine 2007;32(16):1754–60. [DOI] [PubMed] [Google Scholar]
- 13. Brinjikji W, Luetmer PH, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol 2015;36(4):811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Endean A, Palmer KT, Coggon D.. Potential of magnetic resonance imaging findings to refine case definition for mechanical low back pain in epidemiological studies: A systematic review. Spine (Phila Pa 1976) 2011;36(2):160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suri P, Boyko EJ, Goldberg J, Forsberg CW, Jarvik JG.. Longitudinal associations between incident lumbar spine MRI findings and chronic low back pain or radicular symptoms: Retrospective analysis of data from the longitudinal assessment of imaging and disability of the back (LAIDBACK). BMC Musculoskel Disord 2014;15(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jarvik JJ, Hollingworth W, Heagerty P, Haynor DR, Deyo RA.. The Longitudinal Assessment of Imaging and Disability of the Back (LAIDBack) study: Baseline data. Spine 2001;26(10):1158–66. [DOI] [PubMed] [Google Scholar]
- 17. Herlin C, Kjaer P, Espeland A, et al. Modic changes—their associations with low back pain and activity limitation: A systematic literature review and meta-analysis. PLoS One 2018;13(8):e0200677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roland M, van Tulder M.. Should radiologists change the way they report plain radiography of the spine? Lancet 1998;352(9123):229–30. [DOI] [PubMed] [Google Scholar]
- 19. Jarvik JG, Meier EN, James KT, et al. The effect of including benchmark prevalence data of common imaging findings in spine image reports: A stepped-wedge, pragmatic randomized trial. JAMA Network Open 2020;3(9):e2015713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jarvik JG, Meier EN, James KT, et al. The effect of including benchmark prevalence data of common imaging findings in spine image reports on health care utilization among adults undergoing spine imaging: A stepped-wedge randomized clinical trial. JAMA Netw Open 2020;3(9):e2015713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jarvik JG, Comstock BA, James KT, et al. Lumbar Imaging with Reporting of Epidemiology (LIRE)—Protocol for a pragmatic cluster randomized trial. Contempor Clin Trials 2015;45(Pt B):157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Charlson ME, Pompei P, Ales KL, MacKenzie CR.. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 23. Charlson M, Szatrowski TP, Peterson J, Gold J.. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47(11):1245–51. [DOI] [PubMed] [Google Scholar]
- 24. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011;173(6):676–82. [DOI] [PubMed] [Google Scholar]
- 25. Kazberouk A, Martin BI, Stevens JP, McGuire KJ.. Validation of an administrative coding algorithm for classifying surgical indication and operative features of spine surgery. Spine (Phila Pa 1976) 2015;40(2):114–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.