Abstract
Background: Few studies have reported the association of Guillain-Barre syndrome (GBS) and coronavirus disease-2019 (COVID-19) infection. In this study, we reported GBS in six patients infected with COVID-19 and reviewed all existing literature about GBS in association with COVID-19.
Methods: This study was performed in three referral centers of COVID-19 in Iran, and six patients with the diagnosis of GBS were enrolled. Patients enrolled in the study with acute progressive weakness according to the demyelinating or axonal variant of GBS, according to Uncini's criteria.
Results: Four of our patients had axonal polyneuropathy, two patients had demyelinating polyneuropathy, and one patient required mechanical ventilation. All our patients had a favorable response to treatment. In one patient, the GBS symptoms recurred four months after the first episode.
Conclusion: Limited case reports suggest a possible association between GBS and COVID-19. Such associations may be an incidental concurrence or a real cause-and-effect linkage; however, more patients with epidemiological studies are necessary to support a causal relationship.
Key Words: Guillain-Barre Syndrome, COVID-19, Severe Acute Respiratory Syndrome Coronavirus 2, Polyneuropathies
Introduction
After a cluster of pneumonia cases in Wuhan, China, the severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), named coronavirus disease 2019 (COVID-19), has been under intense investigation and research. COVID-19 typically affects the respiratory system, ranging from mild flu-like symptoms to severe pneumonia. Nevertheless, involvement of extrapulmonary organs has also been reported. 1 A broad spectrum of neurological manifestations including febrile seizures, headache, myalgia, encephalopathy, encephalitis, stroke, and acute polyneuropathy has been published in association with COVID-19. 2 Guillain-Barre syndrome (GBS) is an acute-onset, immune-mediated polyradiculoneuropathy with diverse clinical manifestations including ascending quadriparesis, facial paresis, dysautonomia, and respiratory failure resulting to mechanical ventilation and admission in intensive care unit (ICU). 3 It is estimated that around 60% of GBS cases are related to a recent infection, often gastrointestinal (GI) or respiratory infections. 3 In addition to the evidence of molecular mimicry between epitopes of Campylobacter jejuni and gangliosides of nerves, many microorganisms have been considered in its pathogenesis, e.g., the recent reports of Zika virus (ZIKV)-related GBS. 4 Noticeably, through the current pandemic, some adult cases of GBS have been reported with COVID-19.5-9
To add to the existing data, we hereby describe six patients with GBS, with a possible association with COVID-19 infection.
Materials and Methods
This study was performed in three referral centers of COVID-19 in Iran: Shariati Hospital affiliated to Tehran University of Medical Sciences, Tehran, Firoozgar Hospital affiliated to Iran University of Medical Sciences, Tehran, and Al-Zahra Hospital affiliated to Isfahan University of Medical Sciences, Isfahan.
Patients enrolled in the study with acute progressive weakness according to the demyelinating or axonal variant of GBS, according to Uncini's criteria. 10 Also, COVID-19 infection was diagnosed with consistent clinical symptoms with positive nasopharyngeal polymerase chain reaction (PCR) test or typical lung involvement in computed tomography (CT) scan, including ground-glass opacities (GGOs) reported by radiologists.
Results
The clinical manifestations, cerebrospinal fluid (CSF) findings, electrodiagnostic findings, and outcome of six patients with GBS and COVID-19 infection are summarized in table 1.
Table 1.
Characteristics of six cases of Guillain-Barre syndrome (GBS) after coronavirus disease 2019 (COVID-19)
|
Patient
number |
Age
(year)/sex |
Neurological symptoms and signs |
Need for
intubation |
Electrodiagnostic findings | Treatent | Outcome |
|---|---|---|---|---|---|---|
| 1 | 64/F | Progressive symmetric ascending quadriparesis, areflexia, loss of ambulation |
No | Day 25 of symptom onset: axonal motor polyradiculoneuropathy |
0.4 g/kg/day IVIg for 5 days |
Discharged with significant improvement |
| 2 | 38/M | Paresthesia and progressive muscle weakness involving upper and lower limbs, bifacial weakness, areflexia, loss of ambulation |
Yes | Day 21 of symptom onset: demyelinating sensorimotor polyneuropathy |
Plasma exchange for five sessions |
Discharged with significant improvement |
| 3 | 48/F | Asymmetric (right > left) quadriparesis, areflexia, loss of position, and light touch sensation in distal lower limbs |
No | Day 32 of symptom onset, day 2 of admission: asymmetric axonal sensorimotor polyradiculoneuropathy |
0.4 g/kg/day IVIg for 5 days |
Discharged with significant improvement |
| 4 | 85/F | Progressive symmetric ascending quadriparesis and paresthesia, areflexia, loss of ambulation |
No | Day 30 of symptom onset, day 7 of admission: axonal sensorimotor polyradiculoneuropathy |
Plasma exchange for five sessions |
Discharged with some improvement |
| 5 | 58/M | Ataxia and distal lower limbs paresthesia, mild foot dorsiflexion weakness, areflexia, glove and stocking sensory loss, abnormal position, and vibration senses |
No | Day 11 of symptom onset, day 4 of admission: demyelinating sensorimotor polyneuropathy |
0.4 g/kg/day IVIg for 5 days |
Discharged with significant improvement |
| 6 | 43/F | Asymmetric weakness in both legs and then left upper limbs, areflexia in lower limbs, glove and stocking sensory loss, abnormal position, and vibration senses |
No | Day 8 of symptom onset, day 5 of admission: axonal motor polyradiculoneuropathy |
Plasma exchange for five sessions |
Discharged with partial improvement |
IVIg: Intravenous immunoglobulin
The mean age of patients was 56.0 ± 17.0 years. Four patients were female. History, physical examination, and paraclinical evaluations for each patient are explained in detail.
Patient 1: A 64-year-old woman was admitted to the emergency department with clinical manifestation of acute progressive symmetric ascending quadriparesis. Her symptoms started with acute progressive weakness of distal lower extremities three days before admission. The weakness gradually increased in severity, leading to the inability to walk at the time of admission. Moreover, the patient had dyspnea, fever, and a non-productive cough of 20-day duration. Vital signs on admision were remarkable for a respiratory rate of 18 breaths per minute with an oxygen saturation of 85% without supplemental oxygen, but she seemed otherwise stable. The initial neurological examination was notable for Medical Research Council scale (MRC scale) of muscle strength 3/5 at proximal, 2/5 at distal of the upper extremities and 2/5 at proximal, 1/5 at distal of the lower extremities, but no apparent sensory deficits were detected. Deep tendon reflexes (DTRs) were globally absent. No facial or bulbar weakness was seen.
Lung CT scan revealed typical changes of COVID-19 pneumonia, including bilateral GGOs, but the first pharyngeal swab test for SARS-COV-2 PCR was negative. Initial routine laboratory investigations were unremarkable. Also, brain and spinal magnetic resonance imaging (MRI) were normal. Her laboratory data were as follows: white blood cell (WBC) count: 11800 cells/µl (eosinophil: 20%). A complete metabolic panel was within normal limits. Patient received 0.4 g/kg/day intravenous immunoglobulin (IVIg) for five days in accordance with clinical presentations related to GBS. On day 13, her CSF analysis result was normal. A nerve conduction study was performed on day 25 of symptom onset that revealed reduced right median and left peroneal compound muscle action potential (CMAP) amplitudes with normal sensory nerve action potentials (SNAPs). Electromyography (EMG) showed diffuse fibrillation and positive sharp waves (PSWs) potentials. She was thus suggested as a pattern of acute motor axonal neuropathy (AMAN) variant of GBS. After 20 days of admission, the patient stabilized and was discharged to a rehabilitation facility. Two weeks after discharge, she was able to walk without any assistance.
Patient 2: A 38-year-old man was admitted to the emergency room with paresthesia of his hands and feet and progressive muscle weakness. Neurological manifestations of the patient had begun three days before admission with acute progressive weakness of distal lower extremities. The weakness progressed to the point that he could not get off bed, and this led to seeking immediate medical attention. Fourteen days before the onset of these symptoms, he had upper respiratory syndrome with fever and cough that had a spontaneous resolution after a few days. An initial nasopharyngeal swab was negative for SARS-COV infection, but lung CT showed typical lung involvement of COVID-19. On examination, the patient did not have fever with a respiratory rate of 15/minute, and oxygen saturation of 95% on room air. Initial neurological examination revealed neck flexion and upper limb muscles MRC strength of 4-/5, lower limb muscles MRC strength of 2/5, generalized areflexia, and impaired vibratory and proprioceptive sensory modalities at the toes. Four days after admission, he developed bilateral facial weakness, progressive arm weakness, and neuromuscular respiratory failure requiring mechanical ventilation. CSF analysis was not performed due to the unstable patient condition. The laboratory examination results were as follows: WBC count: 10500 cells/µl. The complete metabolic panel was within normal limits. He received five sessions of plasma exchange (PLEX). His respiratory status improved with weaning from mechanical ventilation 19 days after GBS symptom onset. Three weeks after onset, a nerve conduction study disclosed the absence of F-waves along with a diffuse prolonged distal motor latency and reduced distal CMAP amplitudes with reduction of conduction velocities and conduction block, suggesting a demyelination pattern; moreover, no SNAP was registered. He was referred to a rehabilitation center to get physical therapy. Two months after discharge, he was walking without assistance.
Patient 3: A 48-year-old woman presented with muscle weakness (right > left) for two weeks before admission. She had concurrent dyspnea and fever diagnosed with pneumonia due to coronavirus with a positive nasopharyngeal PCR assay for COVID-19. She received hydroxychloroquine with mild improvement. On neurological examination, she could not walk on the heels and toes, Romberg sign was positive, the MRC muscle strenght in right upper and lower limbs was 3/5 and in the left side was 4/5, DTR was absent in lower limbs and 2+ in the upper limbs, position sense was abnormal. She had decreased pinprick and light touch sensation to the ankle. The electrodiagnostic studies revealed absent CMAP in both peroneal and left tibial nerve and reduced CMAP amplitude in right tibial, both median and both ulnar nerves in an asymmetric pattern. There was an absent SNAP in both sural and left median nerves and reduced SNAP amplitude in the right median and both ulnar nerves. EMG showed fibrillation and PSW in the distal muscles of the upper and lower extremities. These findings were consistent with severe subacute asymmetric axonal sensorimotor polyradiculoneuropathy. CSF analysis was done after two days and was abnormal due to albuminocytologic dissociation (Table 1).
The patient received 0.4 g/kg/day IVIg for five days, and after 20 days of admission, her symptoms stabilized, and she was discharged to a rehabilitation facility. Two weeks after discharge, she was walking without assistance with MRC muscle strenght of 4+ in lower limbs.
Patient 4: An 85-year-old woman was admitted to the neurology ward with a three-week history of paresthesia and progressive quadriparesis. Her symptoms started with paresthesia and weakness of distal lower limbs that had an ascending progression to involve proximal of lower limbs and distal and proximal of upper limbs after one week. Two weeks into the onset, the weakness of the patient stabilized. The patient had no autonomic, bulbar, or sphincteric features or backpain. There was no history of recent fever, diarrhea, or upper respiratory symptoms. Her past medical history was notable for hypertension (HTN), ischemic heart disease (IHD), and right femur fracture. On examination, her vital signs were normal, and her oxygen saturation was 87% without supplemental oxygen. The neurologic exam showed intact cranial nerves, limb and neck weakness (MRC score 3/5 at neck flexors, 3/5 at upper limbs, 3-/5 at distal lower limbs, and 3/5 at proximal lower limbs), absent DTRs, glove and stocking hypesthesia, and impaired position sense in distal lower limbs.
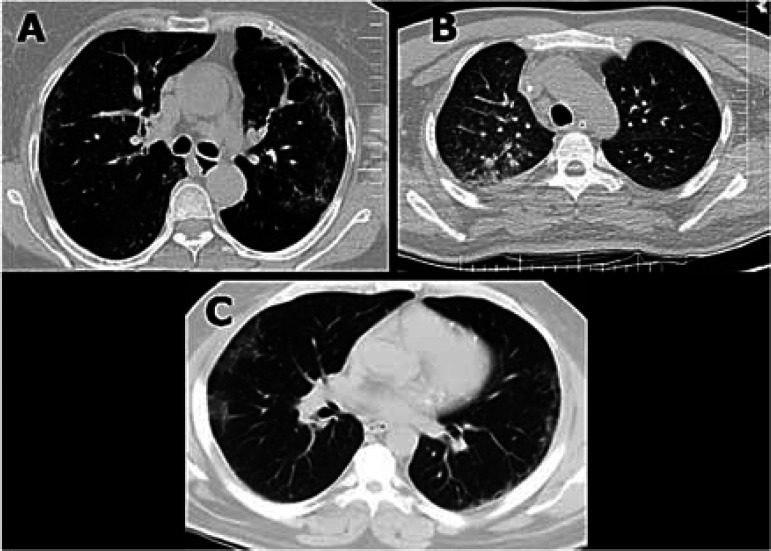
Nasopharyngeal swab test for SARS-COV-2 PCR was negative, but a chest CT scan revealed typical changes of COVID-19 pneumonia (Figure 1).
Figure 1.
Lung computed tomography (CT) scan of patients 1, 2, and 4; CT scan showed ground-glass opacities (GGOs)
Electrodiagnostic studies were performed seven days after admission that showed the decreased amplitude of CMAPs in both median and ulnar nerves and absent CMAP in both tibial and peroneal nerves. SNAPs were globally absent. EMG revealed fibrillations and PSWs in both tibialis anterior (TA) and first dorsal interosseous (FDI) muscles with decreased recruitment in all studied muscles indicating a subacute axonal sensorimotor polyradiculoneuropathy. CSF analysis was as follows: WBC count: 0 per mm3, protein: 46 mg/dl, glucose: 67 mg/dl. Other laboratory tests were unremarkable. The patient was treated with five sessions of PLEX that resulted in significant improvement. At the time of discharge, she was able to walk with bilateral assistance and had minimal symptoms in upper limbs.
Patient 5: A 58-year-old man was admitted to the emergency department with progressive gait abnormality, ataxia, and distal paresthesia from several days ago. He had a history of GBS that presented with ataxia and lower limb weakness four months ago. He was treated with PLEX and recovered completely. One week before hospitalization, the patient suffered from dyspnea and malaise, and he was referred to an infectious disease specialist. An oropharyngeal swab test for SARS-COV-2 PCR was sent that returned negative. His past medical history was notable for type 2 diabetes mellitus (DM) and renal failure. Vital signs on presentation were remarkable for oxygen saturation of 87% on room air, but the patient was otherwise stable. On neurological examination, the patient was conscious, the muscle strength examination with MRC score was 4/5 in dorsiflexion and 5/5 in other tested muscles, and DTRs were absent generally. He had glove and stocking sensory loss and also abnormal position and vibration senses. Romberg's sign was positive, and he had ataxic gait. The laboratory examination results were as follows: serum glucose: 250 mg/dl, blood urea nitrogen (BUN): 19 mg/dl, creatinine: 2.4 mg/dl, WBC count: 6500 cells per µl, and erythrocyte sedimentation rate (ESR): 63 mm/hour. Brain MRI was normal. A lung CT scan showed GGOs in both lungs. Given the epidemiologic scenario, abnormal lung CT, and decreased oxygen saturation, COVID-19 was suspected, and second oropharyngeal PCR was sent that was positive. On day 4, an electrodiagnostic study was performed and demonstrated decreased CMAP amplitude in both peroneal nerves and absent response in all SNAPs in upper and lower limbs. EMG showed decreased recruitment in the distal muscles of lower limbs. There was clinical suspicion for the Miller Fisher syndrome (MFS) variant of GBS. The ganglioside antibody panel was negative. Considering the clinical manifestations related to GBS relapse, we excluded GBS mimicking conditions in this patient based on his clinical history, brain MRI, and response to treatment. The patient was immediately started on 0.4 g/kg/day of IVIg for a planned five days course after a nephrology consult. His clinical course improved, and he was discharged.
Patient 6: A 43-year-old woman presented with asymmetric weakness in both legs and then left upper limbs from 3 days before admission. She had a history of Crohn's disease and was treated with mesalazine. Two weeks before hospitalization, the patient suffered from fever, headache, and intermittent dyspnea. She was diagnosed with COVID-19 and was treated with hydroxychloroquine and azithromycin. The patient recovered from COVID-19, and the nasopharyngeal swab test and chest CT scan were negative before the onset of neuropathic symptoms.
At the time of admission, her vital signs were unremarkable, and oxygen saturation of 94% on room air was detected. On neurological examination, the cranial nerves were intact, and the muscle strength examination showed weakness in left arm and hand (MRC: 4/5), right leg and foot (MRC: 4/5), and left leg and foot (MRC: 4-/5). DTRs were reduced in the ankle. There was a glove and stocking light touch sensory loss and distal loss of vibration and position senses. All laboratory examination results, such as vasculitis tests, were normal. Cervical and brain MRI were normal. Nerve conduction studies (day 5) showed reduced amplitude in right peroneal nerve, and EMG showed reduced recruitment in distal muscles in lower limbs supporting AMAN. She was diagnosed with GBS, and treatment started with PLEX. Her clinical condition improved gradually, and she was discharged with partial improvement.
Discussion
In this study, we reported GBS in six patients infected with COVID-19 and reviewed all existing literature about GBS in association with COVID-19.
GBS is triggered by an abnormal immune cell response to an earlier infection which evokes a cross-reaction against ganglioside components of the peripheral nerves. 3 The most commonly identified infections are Campylobacter jejuni, cytomegalovirus (CMV), Epstein-Barr virus (EBV), influenza-A virus, mycoplasma pneumonia, Haemophilus influenza, earlier coronavirus-types [severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS)], and ZIKV. 3,4,11
The pathomechanism of GBS in patients infected with COVID-19 has not yet been scrutinzied. The neuropathological effect of COVID-19 infections results from the immune-mediated process, either directly by viral invasion or via molecular changes. Development of GBS in patients might support the immunological mechanisms, as described in MERS coronavirus (MERS-COV) infection, 11 but further studies should be organized.
After the new coronavirus pneumonia caused by SARS-COV-2, several studies showed that this virus was capable of causing a disproportionate immune reaction with an increased level of cytokines as interleukin-6 (IL-6), which are produced by activated leukocytes and excite the inflammatory cascade leading to widespread tissue injury.2,12
IL-6 plays a central role in multiple organ damage, which is often lethal for patients with COVID-19 infection. These dysimmunity processes are likely responsible for the main part of the different organ manifestations, including neurological complications. According to the literature, it is likely that patients with severe symptoms of COVID-19 and rapid clinical decline have additional risk to develop serious neurological complications. 1,2,9,12,13
Surprisingly, one of our patients (patient 5) had a history of GBS four months ago and now developed recurrence of the illness in association with COVID-19 infection. As we know, GBS is usually a monophasic illness and recurrence is rare; however, an estimated 5% of patients have recurrent attacks.3 Most patients with recurrent GBS respond favorably to treatment with plasmapheresis or IVIg as our patient.
In three of our patients (patients 1, 3, and 4), concurrency of GBS and COVID-19 infection suggested a parainfectious profile because COVID-19 and neuropathic symptoms were present simultaneously. Still, in patients 2, 5, and 6 due to delayed neuropathic presentation after complete improvement of COVID-19, the postinfectious pattern may be considered.
Interestingly, in six patients in previous studies,7,9,14,15 antiganglioside antibodies panel was checked and was negative as three of our patients (patients 3, 5, and 6). Direct infection may be the mechanism of neuropathy instead of the immune process, but more cases are necessary to support a causal relationship.
Sixteen cases of GBS in patients with COVID-19 have been newly reported after the recent pandemic (Table 2).3, 5-9,14-20 The electrodiagnostic findings were in accordance with an axonal variant of GBS in five out of sixteenth patients. In 10 other patients, a demyelinating subtype was found, and in one patient, the electrodiagnostic study was not performed. In our study, four patients had axonal polyneuropathy, and two patients had demyelinating polyneuropathy.
Table 2.
Neurological manifestations and paraclinical data of sixteen patients with Guillain-Barre syndrome (GBS) and coronavirus disease 2019 (COVID-19) infection reported in the literature
| Study |
Neurological signs and
symptoms |
CSF findings | EMG-NCV result | Treatment and outcome at 4 weeks |
|---|---|---|---|---|
| Toscano et al. 9 | Flaccid areflexic tetraplegia evolving to facial weakness, upper-limb paresthesia (36 hours), and respiratory failure (day 6) |
Day 2: normal protein level, no cells, negative PCR assay for SARS- COV-2; Day 10: protein level: 101 mg/dl, WBC: 4 per mm3, negative PCR assay for SARS-COV-2 |
* | Receiving 2 cycles of IVIg, having poor outcomes including the persistence of severe upper-limb weakness, dysphagia, and lower-limb paraplegia |
| Toscano et al. 9 | Facial diplegia and generalized areflexia evolving to lower limb paresthesia with ataxia (day 2) |
Day 3: protein level: 123 mg/dl, no cells, negative PCR assay for SARS- COV-2 |
* | Receiving IVIg, having improvements including a decrease in ataxia and a mild decrease in facial weakness |
| Toscano et al. 9 | Flaccid tetraparesis and facial weakness evolving to areflexia (day 2) and respiratory failure (day 5) |
Day 3: protein level: 193 mg/dl, no cells, negative PCR assay for SARS-COV-2 |
* | Receiving 2 cycles of IVIg, having poor outcomes including ICU admission owing to neuromuscular respiratory failure and flaccid tetraplegia |
| Toscano et al. 9 | Flaccid areflexic tetraparesis and ataxia (day 4) |
Day 5: normal protein level, no cells, negative PCR assay for SARS-COV-2 |
* | Receiving IVIg, having mild improvement but being unable to stand 1 month after the onset |
| Toscano et al. 9 | Facial weakness, flaccid areflexic paraplegia (days 2-3), and respiratory failure (day 4) |
Day 3: protein level: 40 mg/dl, WBC: 3 per mm3, CSF/serum albumin ratio: 1.2%, negative PCR assay for SARS-COV-2 |
* | Receiving IVIg and plasma exchange, having bacterial pneumonia during IVIg treatment, which delayed plasma exchange |
| Virani et al. 17 | Difficulty breathing before admission, flaccid areflexic paraparesis, areflexia, evolving upper limb weakness, fever, diarrhea, urinary retention |
Not tested | Not tested | Receiving IVIg, his respiratory status improved with liberation from mechanical ventilation. His upper extremity weakness resolved after completion of the course of IVIg. Lower extremity weakness persisted. |
| Zhao et al.19 | Flaccid paraparesis, severe fatigue (day 2), areflexia (day 3), evolving upper limb weakness (day 4), cough and fever (day 8) |
Day 4: normal cell counts and increased protein level: 124 mg/dl |
AIDP | IVIg, 0.4 g/kg × 5; she had normal muscle strength in both arms and legs and the return of tendon reflexes in both legs and feet. Her respiratory symptoms resolved as well. |
| Sedaghat and Karimi 5 |
Flaccid quadriparesis and areflexia |
Not tested | Axonal neuropathy | IVIg, 0.4 g/kg × 5; they did not mention about the outcome |
| Ottaviani et al. 7 |
Rapidly progressive paraparesis, areflexia, initial distal weakness in the upper limbs |
Albumin-cytological dissociation (0 cells/ul, 108 mg/dl proteins) |
A mixed pattern of demyelination and axonal | IVIg, 0.4 g/kg × 5, it did not benefit and the patient required intubation |
| Alberti et al. 6 | Subacute onset of paresthesia and distal weakness at the limb, flaccid tetraparesis |
Mild increase in the protein content (54 mg/dl) and mild leukocytosis (9 cells/μl) |
AIDP | IVIg, 0.4 g/kg × 5, the patient died a few hours later because of progressive respiratory failure |
| El Otmani et al. 20 |
Weakness and tingling sensation in all four extremities |
Increased protein level at 1 g per liter (normal range: 0.2-0.4) with normal WBC count |
AMSAN | IVIg, 0.4 g/kg × 5, no significant improvement |
| Coen et al. 14 | Paraparesis, distal allodynia, difficulties in voiding and constipation |
Albuminocytologic dissociation without intrathecal IgG synthesis |
AIDP | IVIg, 0.4 g/kg × 5, improvement was rapid |
| Camdessanche et al. 15 |
Flaccid severe tetraparesis, swallowing disturbance |
Protein level: 1.66 g per liter, cell count: normal |
AIDP | IVIg, 0.4 g/kg × 5, they did not mention about outcome |
| Padroni et al. 16 |
Asthenia, hands and feet paresthesia, and gait difficulties progressing |
CSF proteins = 48 mg/dl (normal = 0-40 mg/dl), WBC = 1 × 106/l (normal = 0-8 × 106/l) |
Acute polyradiculoneuropathy | IVIg, 0.4 g/kg × 5, the patient was intubated |
| Scheidl et al. 8 | Acute, proximally pronounced, moderate, symmetric paraparesis |
An albuminocytologic dissociation with increased protein level (140 g/l) and normal cell count |
AIDP | IVIg, 0.4 g/kg × 5, which was followed by an almost complete recovery |
| Su et al. 18 | Symmetric paresthesias and ascending appendicular weakness |
WBC: 1 cell/µl, protein: 313 mg/dl on day 8 |
AIDP | IVIg, 0.4 g/kg × 5, without response, severe dysautonomia and remaining in the ICU with severe weakness after 3 weeks |
*Toscano et al.9 mentioned that three patients presented with axonal neuropathy and two patients presented with demyelinating neuropathy but it was not clear which patients had axonal or demyelinating neuropathy.
CSF: Cerebrospinal fluid; EMG: Electromyography; NCV: Nerve conduction velocity; IVIg: Intravenous immunoglobulin; AIDP: Acute inflammatory demyelinating polyradiculoneuropathy; AMSAN: Acute motor and sensory axonal neuropathy; PCR: Polymerase chain reaction; SARS-COV-2: Severe acute respiratory syndrome coronavirus 2; IgG: Immunoglobulin G; WBC: White blood cell; ICU: Intensive care unit
In contrast to the previous reports,9,17,20 although four of our patients' electrophysiological studies demonstrated axonal patterns, their responses to treatment were acceptable without any severe deficits and disability.
Toscano et al. reported the largest series of GBS in patients with COVID-19. 9 They analyzed the data of five GBS patients with COVID-19, hospitalized in three northern Italian medical centers. Three of them had earlier anosmia or ageusia. Four patients had a facial weakness, and three patients established respiratory failure in the course of the disease, leading to a poor outcome. 9 Four weeks after treatment, two patients continued in the ICU getting mechanical ventilation, two were undertaking physical therapy for severe quadriparesis, and only one could be discharged and was able to walk independently. Four patients had a positive PCR test for COVID-19 at the onset of the neurological symptoms, but in all the cases, PCR was negative for COVID-19 in CSF.
In sixteen reported patients in the literature, 9 had respiratory failure and required mechanical ventilation. 7-9,15,17,18 It was not evident provided that the cause of the respiratory failure was the neuromuscular dysfunction owing to GBS or the earlier severe COVID-19 pneumonia. In our patients, one patient required mechanical ventilation. Seemingly, more serious respiratory symptoms in the acute phase of COVID-19 are related to a more severe form of GBS, as an indicator of the extent of the pathological immune response. Instead, the preceding respiratory syndrome might worsen the GBS symptoms leading to a worse outcome.8,9
Conclusion
Limited case reports suggest a possible association between GBS and COVID-19. Such associations may be an incidental concurrence or a real cause-and-effect linkage; however, more patients with epidemiological studies are necessary to support a causal relationship.
Acknowledgments
None.
Notes:
How to cite this article: Okhovat AA, Ansari B, Hemasian H, Haghi-Ashtiani B, Advani S, Ziaadini B, et al. Guillain-Barre syndrome in patients with coronavirus disease-2019: Report of six cases and review of literature. Curr J Neurol 2020; 19(3): 122-30.
Conflict of Interests
The authors declare no conflict of interest in this study.
References
- 1.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–90. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382(23):2268–70. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barre syndrome. Lancet. 2016;388(10045):717–27. doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 4.Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, et al. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet. 2016;387(10027):1531–9. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: A case report. J Clin Neurosci. 2020;76:233–5. doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alberti P, Beretta S, Piatti M, Karantzoulis A, Piatti ML, Santoro P, et al. Guillain-Barre syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e741. doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ottaviani D, Boso F, Tranquillini E, Gapeni I, Pedrotti G, Cozzio S, et al. Early Guillain-Barre syndrome in coronavirus disease 2019 (COVID-19): A case report from an Italian COVID-hospital. Neurol Sci. 2020;41(6):1351–4. doi: 10.1007/s10072-020-04449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheidl E, Canseco DD, Hadji-Naumov A, Bereznai B. Guillain-Barre syndrome during SARS-CoV-2 pandemic: A case report and review of recent literature. J Peripher Nerv Syst. 2020;25(2):204–7. doi: 10.1111/jns.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain-Barre syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574–6. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uncini A, Kuwabara S. The electrodiagnosis of Guillain-Barre syndrome subtypes: Where do we stand? Clin Neurophysiol. 2018;129(12):2586–93. doi: 10.1016/j.clinph.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Kim JE, Heo JH, Kim HO, Song SH, Park SS, Park TH, et al. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol. 2017;13(3):227–33. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carod-Artal FJ. Neurological complications of coronavirus and COVID-19. Rev Neurol. 2020;70(9):311–22. doi: 10.33588/rn.7009.2020179. [DOI] [PubMed] [Google Scholar]
- 13.Whittaker A, Anson M, Harky A. Neurological Manifestations of COVID-19: A systematic review and current update. Acta Neurol Scand. 2020;142(1):14–22. doi: 10.1111/ane.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coen M, Jeanson G, Culebras Almeida LA, Hubers A, Stierlin F, Najjar I, et al. Guillain-Barre syndrome as a complication of SARS-CoV-2 infection. Brain Behav Immun. 2020;87:111–2. doi: 10.1016/j.bbi.2020.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camdessanche JP, Morel J, Pozzetto B, Paul S, Tholance Y, Botelho-Nevers E. COVID-19 may induce Guillain-Barre syndrome. Rev Neurol (Paris) 2020;176(6):516–8. doi: 10.1016/j.neurol.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padroni M, Mastrangelo V, Asioli GM, Pavolucci L, Abu-Rumeileh S, Piscaglia MG, et al. Guillain-Barre syndrome following COVID-19: New infection, old complication? J Neurol. 2020;267(7):1877–9. doi: 10.1007/s00415-020-09849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Virani A, Rabold E, Hanson T, Haag A, Elrufay R, Cheema T, et al. Guillain-Barre syndrome associated with SARS-CoV-2 infection. IDCases. 2020;20:e00771. doi: 10.1016/j.idcr.2020.e00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su XW, Palka SV, Rao RR, Chen FS, Brackney CR, Cambi F. SARS-CoV-2-associated Guillain-Barre syndrome with dysautonomia. Muscle Nerve. 2020;62(2):E48–9. doi: 10.1002/mus.26988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barre syndrome associated with SARS-CoV-2 infection: Causality or coincidence? Lancet Neurol. 2020;19(5):383–4. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Otmani H, El Moutawakil B, Rafai MA, El Benna N, El Kettani C, Soussi M, et al. Covid-19 and Guillain-Barre syndrome: More than a coincidence! Rev Neurol (Paris) 2020;176(6):518–9. doi: 10.1016/j.neurol.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]