Abstract
Coronavirus disease 2019 (COVID‐19) can cause severe lymphopenia and respiratory failure requiring prolonged invasive mechanical ventilation (MV). COVID‐19 patients with severe lymphopenia or respiratory failure are at risk of developing secondary infections. Here, we present the needle autopsy findings of a critically ill patient with COVID‐19 who required reintubation and prolonged MV, and eventually died of secondary cytomegalovirus (CMV) pneumonia. This case highlights the potential risk of long‐term steroid use and the need for routine monitoring for CMV infection in critically ill patients with COVID‐19.
Keywords: Co‐infection, corticosteroid therapy, COVID‐19, cytomegalovirus pneumonia, lymphopenia
Here, we present the needle autopsy findings of a critically ill patient with coronavirus disease 2019 (COVID‐19) who required reintubation and prolonged mechanical ventilation, and eventually died of secondary cytomegalovirus (CMV) pneumonia. This case highlights the potential risk of long‐term steroid use and the need for routine monitoring for CMV in critically ill patients with COVID‐19.
Introduction
Cytomegalovirus (CMV) is a common herpesvirus that has infected the majority of people worldwide [1]. CMV reactivation may lead to CMV end‐organ disease in immunocompromised patients and immunocompetent patients who are critically ill. The incidence of CMV reactivation in immunocompetent patients with acute respiratory distress syndrome has been reported to be approximately 30%, and reactivation is associated with a significant increase in intensive care unit (ICU) mortality [2]. CMV reactivation and CMV pneumonia due to reactivation are clinically relevant issues also in patients with coronavirus disease 2019 (COVID‐19). However, there is still a significant lack of information regarding CMV infection or CMV end‐organ disease in COVID‐19 patients. Here, we report a needle autopsy case of fatal CMV pneumonia in a critically ill patient with COVID‐19.
Case Report
An 80‐year‐old woman non‐smoker presented to the emergency department with a one‐week history of dry cough and high fever. She had a medical history of hypertension and diabetes mellitus. Chest computed tomography showed bilateral, peripheral ground‐glass opacities. A real‐time reverse transcriptase‐polymerase chain reaction test for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) using a nasopharyngeal swab showed a positive result. She was admitted and started on supplemental oxygen therapy, favipiravir on a compassionate use basis, and dexamethasone (6 mg daily). However, three days later, she was admitted to the ICU and mechanically ventilated because of respiratory failure. She continued to receive dexamethasone and additionally received antibiotics and remdesivir. Her respiratory condition and chest infiltration improved, and she was extubated after seven days of mechanical ventilation (MV). Dexamethasone was discontinued after 10 days.
However, she developed a fever and rapidly progressive hypoxaemia in the next two days, and she was reintubated. Chest X‐ray revealed a recurrence of the bilateral chest infiltrations. Cultures and laboratory tests were performed, but there was no evidence of co‐infection. Because there was a possibility that the COVID‐19‐related inflammation had relapsed, she again received dexamethasone (6 mg daily) with subsequent, gradual improvement of the chest shadows. Thereafter, the steroid was tapered on a weekly basis.
On the ninth day after reintubation, the chest shadows worsened again. Her serum β‐d‐glucan was found to be elevated (53.7 pg/mL; upper limit of normal, 11 pg/mL). Empirical treatment with micafungin and trimethoprim‐sulfamethoxazole was initiated. Candida parapsilosis was found on blood culture, and so the micafungin was changed to fosfluconazole. Trimethoprim‐sulfamethoxazole was discontinued after she tested negative for Pneumocystis jirovecii on polymerase chain reaction. Her serum β‐d‐glucan decreased, and a follow‐up blood culture six days after the initial culture was negative for Candida. However, her respiratory condition and the chest shadows continued to worsen. A repeat screening test for co‐infection was performed. The CMV antigenemia assay for detecting pp65 antigen in peripheral blood leucocytes was elevated (674 antigen‐positive cells per 50,000 leucocytes). This result suggested that CMV pneumonia was the cause of her respiratory failure and bilateral chest infiltrations (Fig. 1A), and ganciclovir was immediately initiated. However, she died four days after the positive CMV antigenemia test result. With the consent of the family, a needle autopsy from the lung was performed approximately 3 h after her death, which demonstrated CMV‐infected cells (Fig. 1B). This confirmed the diagnosis of CMV pneumonia.
Figure 1.
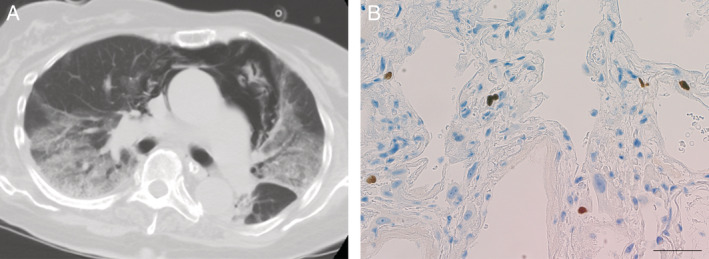
(A) Chest computed tomography scan at the time of positive cytomegalovirus (CMV) antigenemia showing bilateral ground‐glass opacity with consolidation. Pneumomediastinum is also present. (B) Immunohistochemical stain of a lung biopsy specimen showing a positive stain for CMV. Scale bar: 50 μm.
Discussion
This report describes a critically ill patient with COVID‐19 who required reintubation and eventually died of secondary infection with CMV pneumonia. There are a limited number of published reports regarding co‐infection with SARS‐CoV‐2 and CMV, and these report have focused on gastrointestinal CMV disease [3, 4, 5]. To our knowledge, this is the first case of CMV pneumonia confirmed by a needle autopsy in a patient with COVID‐19.
COVID‐19 can cause severe lymphopenia [6] and present respiratory failure requiring prolonged invasive MV [7]. Lymphopenia and prolonged MV have both been identified as risk factors for secondary infections in COVID‐19 patients [8]. A preliminary report suggests that reactivation of Herpesviridae such as herpes simplex virus and CMV might be common in patients with COVID‐19 pneumonia than in critically ill patients other than COVID‐19 [9]. Currently, the use of dexamethasone is recommended in COVID‐19 patients who require invasive MV [10]. Although the dose of dexamethasone used in the RECOVERY trial (6 mg daily for up to 10 days) may not act as immunosuppressive, it may contribute to the reactivation of CMV when combined in the presence of factors such as severe lymphopenia or prolonged MV [2, 11]. In fact, previous studies have reported that corticosteroid use is associated with increased risk of CMV reactivation [12, 13, 14]. The cause of the recurrent respiratory distress requiring reintubation in the present case was probably due to remnant inflammation caused by COVID‐19 pneumonia. It may also have been as a result of the discontinuation of steroids. Considering the good response to initial steroid treatment and the possibility of a relapse after discontinuation, we administered steroids for a total of more than 10 days. However, long‐term steroid administration might have contributed to an increased risk of co‐infection, such as candidemia or CMV pneumonia. Therefore, surrogate markers for identifying COVID‐19‐related inflammation are required to avoid unnecessary long‐term administration of immunosuppressive agents. A recent preliminary study suggests that the combination of lymphocyte counts and IgG antibody levels against spike and receptor‐binding domain may help detect remnant COVID‐19‐related inflammation [15].
It is impossible to differentiate COVID‐19 pneumonia from other respiratory infections based solely on clinical and imaging findings, which may lead to underestimating the incidence of co‐infection in clinical practice. In particular, late co‐infections in patients requiring prolonged MV may be more likely to be underestimated [16]. Therefore, clinicians should consider CMV pneumonia in the differential diagnosis when they encounter unexplained worsening or relapse of respiratory failure in critically ill patients with COVID‐19, especially in those with prolonged lymphopenia and MV. Considering that the screening test for CMV infection in the present case showed a negative result two weeks before it was found to be positive, the routine monitoring to detect CMV infection, similar to the monitoring of transplant recipients, may be justified in critically ill patients with COVID‐19.
Disclosure Statement
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Author Contribution Statement
All authors meet the following ICMJE authorship criteria: All authors contributed to the intellectual content of this manuscript. Saori Amiya and Takayuki Shiroyama wrote the first draft of this manuscript. Haruhiko Hirata, Takayuki Shiroyama, and Akinori Uchiyama designed this study. Saori Amiya, Haruhiko Hirata, Yuichi Adachi, Takayuki Niitsu, Yoshimi Noda, Takatoshi Enomoto, Reina Hara, Moe Koide, and Akinori Uchiyama participated in the treatment of the patient and collected the clinical data. Takayuki Shiroyama, Kiyoharu Fukushima, Yasuhiko Suga, Kotaro Miyake, Yoshito Takeda, and Atsushi Kumanogoh made intellectual contributions and helped in patient management and writing the manuscript. All authors critically reviewed the manuscript and approved the final version.
Acknowledgments
We thank all the colleagues and staff who were involved in the treatment of patients with COVID‐19 at our hospital. This research was supported by “Research and Development Grant Program for COVID‐19, 2020‐2021” from the Strategic Global Partnership & the X (cross)‐Innovation Initiative, Graduate School of Medicine, Faculty of Medicine, and Osaka University Hospital. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Amiya, S , Hirata, H , Shiroyama, T , et al. (2021) Fatal cytomegalovirus pneumonia in a critically ill patient with COVID‐19. Respirology Case Reports, 9(7), e00801. 10.1002/rcr2.801
Associate Editor: Tow Keang Lim
References
- 1. Griffiths P, Baraniak I, and Reeves M. 2015. The pathogenesis of human cytomegalovirus. J. Pathol. 235:288–297. [DOI] [PubMed] [Google Scholar]
- 2. Al‐Omari A, Aljamaan F, Alhazzani W, et al. 2016. Cytomegalovirus infection in immunocompetent critically ill adults: literature review. Ann. Intensive Care 6:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Geisen WR, Berger J, Schwartz C, et al. 2020. Cytomegalovirus enterocolitis secondary to experimental COVID‐19 therapy. IDCases 22:e00962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amaral PH, Ferreira BM, Roll S, et al. 2020. COVID‐19 and cytomegalovirus co‐infection: a challenging case of a critically ill patient with gastrointestinal symptoms. Eur. J. Case Rep. Intern. Med. 7:001911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marchi G, Vianello A, Crisafulli E, et al. 2020. Cytomegalovirus‐induced gastrointestinal bleeding and pancreatitis complicating severe Covid‐19 pneumonia: a paradigmatic case. Mediterr. J. Hematol. Infect. Dis. 12:2020060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang Y, Zhang L, Sang L, et al. 2020. Kinetics of viral load and antibody response in relation to COVID‐19 severity. J. Clin. Invest. 130:5235–5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Botta M, Tsonas AM, Pillay J, et al. 2020. Ventilation management and clinical outcomes in invasively ventilated patients with COVID‐19 (PRoVENT‐COVID): a national, multicentre, observational cohort study. Lancet Respir. Med. 9:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ripa M, Galli L, Poli A, et al. 2021. Secondary infections in patients hospitalized with COVID‐19: incidence and predictive factors. Clin. Microbiol. Infect. 27:451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Le Balc'h P, Pinceaux K, Pronier C, et al. 2020. Herpes simplex virus and cytomegalovirus reactivations among severe COVID‐19 patients. Crit. Care 24:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Institutes of Health . COVID‐19 Treatment Guidelines Therapeutic Management of Patients with COVID‐19. https://www.covid19treatmentguidelines.nih.gov/therapeutic-management/ (accessed 9 December 2020).
- 11. Honore PM, Barreto Gutierrez L, Kugener L, et al. 2020. SARS‐CoV‐2 infection as a risk factor for herpesviridae reactivation: consider the potential influence of corticosteroid therapy. Crit. Care 24:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cook CH, Martin LC, Yenchar JK, et al. 2003. Occult herpes family viral infections are endemic in critically ill surgical patients. Crit. Care Med. 31:1923–1929. [DOI] [PubMed] [Google Scholar]
- 13. Ong DSY, Spitoni C, Klein Klouwenberg PMC, et al. 2016. Cytomegalovirus reactivation and mortality in patients with acute respiratory distress syndrome. Intensive Care Med. 42:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li L, Hsu SH, Gu X, et al. 2020. Aetiology and prognostic risk factors of mortality in patients with pneumonia receiving glucocorticoids alone or glucocorticoids and other immunosuppressants: a retrospective cohort study. BMJ Open 10(10):e037419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adachi Y, Shiroyama T, Yamaguchi Y, et al. 2021. Predicting recurrence of respiratory failure in critically ill patients with COVID‐19: a preliminary study. J. Infect. 82:e33–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rawson TM, Moore LSP, Zhu N, et al. 2020. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID‐19 antimicrobial prescribing. Clin. Infect. Dis. 71:2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]


