Abstract
Parkinson’s disease is the second most common neurodegenerative disease, and its prevalence has been projected to double over the next generation. Nonetheless, an accurate diagnosis of Parkinson’s disease remains challenging, and identifying the earliest stages of the disease is a major unmet need. Recent developments include the validation of modified clinical diagnostic criteria, the introduction and testing of research criteria for prodromal Parkinson’s disease, and the identification of genetic subtypes and a growing number of genetic variants associated with Parkinson’s disease risk. There has also been significant progress in the development of diagnostic biomarkers, of which genetic and imaging tests are already part of routine work-up protocols in clinical practice, while novel tissue and fluid markers are under investigation. Parkinson’s disease is evolving from a clinical to a biomarker-supported diagnostic entity, in which an earlier identification is possible, different subtypes with diverse prognosis are recognized and novel disease-modifying treatments are in development.
1. Introduction
Parkinson’s disease is the second most common neurodegenerative disease with a global prevalence of more than 6 million individuals. This number corresponds to a 2.5-fold increase in prevalence over the past generation, making Parkinson’s disease one of the leading causes of neurological disability.1,2 The pathological hallmark of Parkinson’s disease consists of neural inclusions in the form of Lewy bodies and Lewy neurites with cell loss in the substantia nigra and other brain areas. Given that aggregated and misfolded α-synuclein species are the major constituents of Lewy bodies, Parkinson’s disease is classified as a synucleinopathy. Braak and others have proposed a pattern of spread of Lewy pathology, starting in the caudal brainstem and progressing rostrally through the upper brainstem, limbic regions, and finally the neocortex, but such spread probably does not occur in all cases.3 Recent research strongly suggests that prion-like cell-to-cell transmission and permissive templating of synuclein are key mechanisms of disease progression.4
Age is the most significant risk factor for developing Parkinson’s disease, and men are more susceptible than women with a prevalence ratio of approximately 3:2. There is a strong genetic component to disease risk, with over 90 genetic risk loci currently identified.5 Additionally, several possibly modifiable environmental (e.g., pesticides, water pollutants) and behavioural factors (e.g., use of tobacco, coffee, exercise, or head trauma)1 have been found to have a role in the pathogenesis of Parkinson’s disease in different populations. While these advances in our understanding of pathogenesis and epidemiology have been impressive,6,7 the cause of Parkinson’s disease remains enigmatic, and no cure or preventive therapy has yet been found.
Modified clinical diagnostic criteria, designed to enhance the diagnostic accuracy of Parkinson’s disease, have been recently validated. However, diagnosis remains a challenge since clinical features of the disease overlap with other neurodegenerative conditions, and diagnostic tests or biomarkers still do not allow for a definitive diagnosis from the earliest stages. As a result, clinical diagnostic accuracy remains suboptimal, even when the condition is clinically fully manifest.8 Identification of prodromal disease is an even greater unmet need given that future disease-modifying therapies will have their greatest chance for success at this stage.9,10 Finally, there is a need to better define Parkinson’s disease subtypes,11–13 which not only have different clinical presentation and prognosis, but also differ in underlying disease mechanisms, calling for personalised treatment approaches. The most obvious example is monogenic Parkinson’s disease, where subtype-specific therapies are already being tested in clinical trials.14,15
This Review, directed towards the general neurologist and movement disorder specialists involved in the diagnosis and care of Parkinson’s disease as well as toward clinical and basic neuroscientists, describes the motor and non-motor features of Parkinson’s disease, and delineates the issues involved in identifying the currently recognized subtypes, and the increasing role of genetics in the diagnosis. It also lists the challenges encountered when diagnosing the manifest and ‘premotor’ stages of the disease and critically reviews those imaging, fluid and tissue biomarkers that best support the diagnosis of Parkinson’s disease.
2. Parkinson’s disease – more than a movement disorder
The clinical hallmark of Parkinson’s disease is a motor syndrome characterised by bradykinesia, rest tremor, and rigidity as well as changes in posture and gait. The motor disturbances cause progressive disability with impairment in activities of daily living and reduced quality of life. While the classic motor symptoms occur early and are the pillars of current diagnostic criteria, the development of postural instability and increasing gait difficulties, as well as dysphagia and dysarthria, drive the progression of motor disability.16
Although Parkinson’s disease is defined as a movement disorder, it is associated with a variety of non-motor symptoms (NMS) in virtually all patients, including hyposmia, constipation, urinary dysfunction, orthostatic hypotension, memory loss, depression, pain, and sleep disturbances (Panel 1). While the classic motor signs of Parkinson’s disease are linked to nigral degeneration and striatal dopamine depletion, NMS are likely related to neurodegeneration of other structures, including the peripheral autonomic nervous system.17,18 NMS are frequent at early stages and, although intense and disturbing for some patients, observational studies indicate that they are mild in most cases,19,20 increasing in severity with disease duration.19 NMS in the evolution of Parkinson’s disease cause an important burden, reduce quality of life, and are a driver of the overall cost of care.21 Particularly, cognitive decline and hallucinations are a common cause of hospitalisation and institutionalisation in advanced Parkinson’s disease.22
Panel 1 -.
Motor and non-motor symptoms of Parkinson’s disease
| Motor features occurring at early stages. Considered the “classical or cardinal” motor features of Parkinson disease | |
| Bradykinesia | General slowness and paucity of spontaneous movement; decreased arm swing,reduced facial expresión, reduced gesticulation, micrographia, turning in bed, hipophonia. Progressive reduction in speed and amplitude of voluntary repetitive movement (finger taps, hand grips, pronation–supination movements, toe taps and heel stamps) |
| Rigidity | Increased muscular tone by a resistance of passive movements of equal degree in opposing muscle groups (“lead-pipe” type). If interrupted by tremor a cog-wheel phenomenon results |
| Tremor | Rest tremor 4–6 Hz common in limbs (“pill-rolling” in hands), lips, chin or jaw, rarer in head. Amplitude diminishes or is abolished during goal-directed voluntary movements; exam hand rest tremors with hands in a relaxed position and arms supported, e.g. hands folded into the lap while sitting, and forearms in pronation (not supination). Low amplitud hand action tremor also common at presentation. |
| Gait alterations | Decreased arm swing; dragging one leg; slightly bent posture while walking |
| Motor features present at later stages. These motor features generally occur in addition of earlier ones and respond poorly to dopaminergic treatment | |
| Posture alterations | Trunk bent forward when standing. Lateral (“Pisa syndrome”) or anterior (camptocormia) deviation of trunk, or head flexion (“dropped head”). Arms abducted, flexed at elbow. Flexed wrist and metacarpophalangeal joints, and extended hand fingers and thumb. |
| Freezing of gait | Freezing of gait: sudden and brief episode of inability to produce effective forward stepping: at initiation of gait (“start hesitation”), during gait (motor block), when turning or approaching narrow spaces. Festination: patients are compelled to accelerate the gait forward. |
| Balance alterations | Unsteadiness when standing and walking. Altered postural reflexes (tested with the “pull test”); falls |
| Other | Dysarthria, dysphagia |
| Non motor features present at early stages. Not uncommon at the time of diagnosis. May precede the onset of motor features | |
| Hyposmia | Smell loss reported by up to 70% of patients and when formally tested present in almost 90%. Frequent smell tests used: UPSIT and the Sniffin’sticks test. |
| Sleep disorders | REM sleep behavior disorder: parasomnia characterized clinically by vivid, generally unpleasant dreams (eg, being attacked or robbed) and vigorous behaviors in which the patients seem to be enacting their dreams (eg, punching, shouting, laughing). Also insomnia, periodic limb movements, restless leg syndrome, akathisia, excessive daytime sleepiness. |
| Neuropsychiatric features | Prominent apathy. Anxiety: generalized anxiety, panic attacks and social phobias. Depression, usually mild, associated to anhedonia and apathy |
| Autonomic dysfunction | Constipation. Delayed gastric emptying. Urinary urgency or incontinence. Erectile dysfunction.Orthostatic hypotension.Heat intolerance |
| Mild cognitive impairment | Mild cognitive decline in executive and attention domains |
| Pain and somatosensory disturbances | Pain, paresthesias, burning sensations |
| Non motor features present at later stages. Early non motor features frequently persist and worsen at this stage | |
| Dementia | About 30% of PD patients develop dementia, affecting visual spatial recognition and construction, and semantic and episodic memory. Prevalence increases with disease duration. Fequently associated to hallucinations and psychosis |
The prodrome of Parkinson’s disease
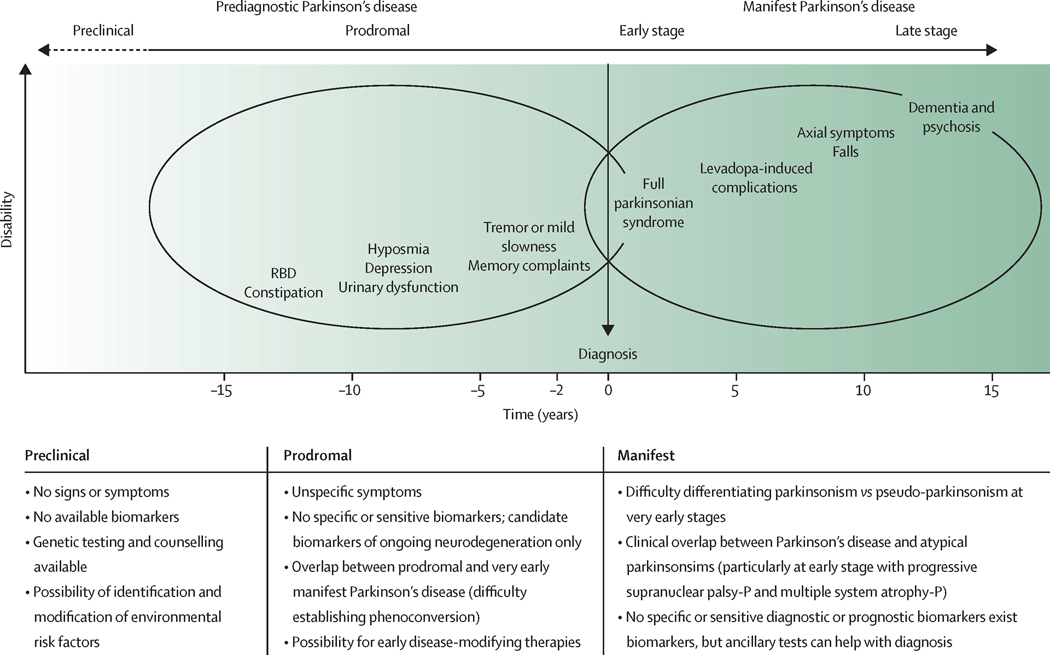
Several NMS associated with Parkinson’s disease, such as smell loss or constipation, are commonly experienced by patients prior to the onset of classic motor symptoms – sometimes preceding the occurrence of motor features by years or even decades.23 The period when these symptoms arise have been conceptualized as the prodromal phase of Parkinson’s disease, corresponding to a stage of disease where neurodegenerative changes involve extranigral sites, such as the lower brainstem, the olfactory bulb and tracts, and the peripheral autonomic nervous system (Braak stages 1–3).3 Similar to Alzheimer’s disease, an even earlier period when future patients are still free of any symptoms, but disease-specific pathology is assumed to be present, and there is biomarker evidence of disease, has also been postulated for Parkinson’s disease ( termed pre-clinical Parkinson’s disease).24 (Figure)
Figure.
The natural history of Parkinson’s disease and diagnostic challenges by disease stage RBD=REM sleep behavior disorder. Progressive supranuclear palsy-P=progressive supranuclear palsy with predominant parkinsonism. Multiple system atrophy-P=multiple system atrophy with predominant parkinsonism. The time of diagnosis is represented in the axis as time “0”. The timepoints on the left side of diagnosis represent the number of years before diagnosis, and the timepoints on the right represent the years after diagnosis. These periods of time are orientative. The dotted arrow indicates that the duration of the preclinical phase is unknown, unlike the prodromal phase, which can extend between 10 and 15 years.
The evidence that NMS are markers of a prodromal phase of Parkinson’s disease is based on retrospective assessments as well as prospective epidemiological and observational studies,23,25 and is most compelling for constipation, smell loss, and REM-sleep behaviour disorder (RBD). Additionally, urinary urgency, sexual dysfunction, hypotension, anxiety, depression, colour vision impairments, and dysexecutive syndrome have also been described to antedate the onset of motor symptoms in Parkinson’s disease (Figure). 19,23,25 Important issues about the prodromal phase of Parkinson’s disease remain to be clarified, such as the sequence in which prodromal symptoms develop and the speed of disease progression.26 Prodromal features can also vary depending on etiology (e.g., idiopathic vs. monogenic Parkinson’s disease).27 In addition to NMS, subtle motor signs such as decreased facial mobility, voice changes, loss of finger dexterity, a mildly stooped posture, or decreased arm swing when walking may also antedate the evolution of definitive motor symptoms. But such mild parkinsonian signs may be difficult to distinguish from unspecific mobility changes associated with normal aging.28
3. Parkinson’s disease subtypes
Parkinson’s disease is strikingly heterogeneous regarding the age of onset, clinical presentation, rate of progression, and treatment response. Several clinical subtypes of Parkinson’s disease have been proposed. Additionally, the discovery of genetically defined forms of the disease, which may differ from classic Parkinson’s disease in a number of clinical variables, has challenged the unitarian view of Parkinson’s disease and opened the door for a biological definition of sub-entities within the Parkinson’s disease spectrum.
Approaches towards subtyping Parkinson’s disease have either used empirical assessments of individual clinical features or the more objective and hypothesis-free methodology of hierarchical cluster analysis and other forms of machine learning.12,13,29 Clinical features that have been used for subtyping with either approach included age at onset (early-onset versus late-onset), prevailing motor phenotype (tremor-dominant versus non-tremor cases), motor complications in response to chronic levodopa, non-motor features (particularly autonomic dysfunction, cognitive dysfunction, and RBD), as well as the rate of progression.
Empirically defined subtypes include young-onset Parkinson’s disease or early-onset Parkinson’s disease, usually defined by age at onset cut-offs below ages of 40 or 50 years and characterised by slower progression, preserved cognition, and increased risk to develop motor complications in response to levodopa.30–31 Benign-tremulous Parkinson’s disease or tremor-dominant Parkinson’s disease are two terms that have been used to describe the clinical predominance of rest tremor over other motor symptoms 32 and this clinical subtype has been associated with slower progression and less cognitive decline compared to other clinical presentations.31–33 Clinical presentations with prominent postural instability and gait disorder have been classified as a postural instability and gait disturbances (PIGD) subtype characterised by a rapid decline of motor function as well as cognition.33 Problems with empirically defined subtypes include the fact that patients initially presenting with tremulous or non-tremulous Parkinson’s disease motor signs may change categories with longer follow-up.33,34
Recent cluster analyses have included non-motor features,35 and in one of these studies, mild cognitive impairment, RBD, and orthostatic hypotension at baseline identified the most rapidly progressive subtype,36 which was termed diffuse malignant because of the most severe expression of both motor and non-motor features. The slowest progression was seen in patients presenting with predominant motor features of mild severity (mild motor-predominant), with a third subtype being termed intermediate (between the two).36
The ultimate proof for the validity of clinically defined disease subtypes should come from objective biological measures or biomarkers, showing that such sub-entities reflect differences in underlying disease mechanisms or pathology.29 However, in a recent brain bank study in which 111 patients had been retrospectively classified into mild motor-predominant, intermediate, and diffuse malignant subtypes found no group differences in Lewy pathology and Alzheimer related pathology.37 Currently, only genetic subtyping of Parkinson’s disease has established biological underpinnings.
Lessons from genetics
The advent of the genomics era has led to rapid advances in our understanding of the genetic etiology of Parkinson’s disease. These discoveries have been driven by improvements in sequencing and genotyping technology and their successful application to ever-larger cohorts. International efforts have revealed that the genetic architecture of Parkinson’s disease is highly complex, with both common and rare risk variants contributing to the disease pathogenesis.5 Mutations in at least twenty genes are recognised as causes of familial parkinsonism, each providing a snapshot into the molecular basis of the neurodegenerative process. Perhaps even more interesting is that we now know over 90 genetic risk loci for the more common sporadic form of the disease.38 Though it is more challenging to unravel the precise biology disrupted by these variants, the disease-associated genes begin to coalesce into common pathways, including dysregulation of mitochondrial homoeostasis, impaired processes related to the cell death machinery, inflammatory signalling, intracellular trafficking, and endosomal-lysosomal dysfunction.39
Genetic testing for mendelian forms of Parkinson’s disease is increasingly performed in clinical practice, and should be considered in patients with early onset of disease (defined as onset before the age of 40 years), patients with a family history, and individuals from high-risk populations with a high prevalence of specific monogenic forms of disease (e.g., Ashkenazi Jewish patients, North African Berber Arabs).40 Knowledge of the underlying gene defect within a family enables more effective counselling of patients and allows for predictive testing within asymptomatic family members. Increasingly, clinical trials are targeting specific genetic forms of neurodegeneration, and the identification of the causative gene potentially opens up opportunities for the patient to participate in such studies.
Genetic information is also refining our fundamental understanding of the clinical entity that we know as Parkinson’s disease. An early lesson learned from studying monogenic patients is that Parkinson’s disease is phenotypically diverse, and there is more significant overlap with atypical parkinsonism than previously appreciated. For example, patients harboring a disease-causing mutation in the LRRK2 gene can manifest with protean clinical presentations that include typical levodopa responsive Parkinson’s disease in the majority of cases, progressive supranuclear palsy, and occasionally amyotrophy.15,41,42 Along the same lines, patients with mutations in the genes GBA, SNCA, or VPS13C can present with typical Parkinson’s disease, but more commonly develop progressive cognitive impairment consistent with Lewy body dementia.43–45 While these observations only relate to 5–40% of Parkinson’s disease cases (depending on ethnic background), these findings provide crucial insights into the central pathways associated with parkinsonism and highlight potential targets for disease-modifying interventions. Although monogenic Parkinson’s disease cases are increasingly defined on a molecular basis (e.g., PARK-LRRK2, PARK-SNCA), only PARK-LRRK2 and PARK-Parkin are relatively common in clinical practice, as is Parkinson’s disease associated with high-risk variants in GBA.41 (Table 1).
Table 1 -.
Monogenic subtypes of Parkinson’s disease
| Clinical Phenotype Relative to ‘Classical’ PD | ||||||
|---|---|---|---|---|---|---|
| Genetic Entity | Mutation(s) | Inh. | AAO | Clinical Features | Progression | Comment(s) |
| Classical Parkinsonism (Autosomal Dominant Subtypes) | ||||||
|
PARK-SNCA (PARK 1, 4) |
missense (PARK1) dup./triplication (PARK 4) |
AD | younger | similar, prominent NMS, early dementia | faster | rare |
|
PARK-LRRK2 (PARK 8) |
missense | AD | similar | similar (less RBD) | similar | common in Ashkenazi Jews, Basque country, North African Berbers; pleomorphic pathology |
|
PARK-VPS35 (PARK 17) |
missense | AD | similar | similar | similar | rare |
|
PARK-CHCHD2 (PARK 22) |
missense, splice site, nonsense |
AD | similar | similar | similar | rare, Asian patients |
| Early-Onset Parkinsonism (Autosomal Recessive Subtypes) | ||||||
|
PARK-Parkin (PARK 2) |
missense, loss-of-function, exonic duplication, deletion | AR | younger | common early leg involvement & dystonia at onset, frequent dyskinesia | slow | common (accounts for up to 20% of PD with onset before age 50; dementia uncommon; no Lewy bodies |
|
PARK-PINK1 (PARK 6) |
missense, loss-of-function, exonic deletion, duplication | AR | younger | similar to PARK-Parkin, common psychiatric features | slow | second most common recessive PD subtype after PARK-Parkin, dementia uncommon |
|
PARK-DJ1 (PARK 7) |
missense, loss-of-function, small duplication/deletions | AR | younger | similar to PARK-Parkin, psychiatric symptoms | slow | rare, dementia uncommon |
| High-Risk Gene | ||||||
| GBA | missense, loss-of-function, small insertions/deletions | RG | younger | similar to sporadic PD, greater dementia risk | faster | very common (5–25% of PD patients), particularly in Ashkenazi Jews |
Abbreviations: Inh: Inheritance; AD: autosomal dominant; AR: autosomal recessive; RG: risk gene; NMS: non-motor symptoms; RBD: REM sleep behavior disorder.
4. Diagnosing Parkinson’s disease - challenges and pitfalls
Making a diagnosis of idiopathic Parkinson’s disease can be a straightforward clinical exercise in cases with a classic history, typical asymmetric motor signs, no atypical features, and exclusion of alternative etiologies.
However, in routine clinical practice diagnostic misclassification is common with error rates ranging from 15% to 24% in different series.8,46,47 A recent meta-analysis found a pooled diagnostic accuracy for the clinical diagnosis of Parkinson’s disease of only 80.6% across eleven clinico-pathological studies.48 Even with the use of stringent clinical diagnostic criteria, 10% of cases diagnosed with Parkinson’s disease by neurologists had alternative pathologies. Common errors in clinical practice include non-Parkinson’s disease tremor disorders, such as essential tremor, as well as different types of secondary parkinsonism, which are summarised in table 2.
Table 2 -.
Secondary parkinsonisms
| Etiology | Mechanism | Differential clinical features vs. PD | Diagnosis | Therapy |
|---|---|---|---|---|
| Drug-induced * | Interference with DA-signaling | Often symmetric, perioral tremor, co-existent tardive syndromes. | Consistent history of exposure. Normal DAT- SPECT |
Discontinue the offending drug. Temporary use of anti-PD drugs |
| Vascular | Disruption of striato-pallido-thalamo-cortical motor network | Acute or subacute onset (not obligatory). Frequently presenting with gait disorder (lower body parkinsonism) | Strategic infarcts and subcortical microvascular lesions on MRI, normal DAT-SPECT (not obligatory) | Trial of L-Dopa Physiotherapy, occupational therapy |
| Toxic (Co, Mn) | Basal ganglia lesions (putamen, pallidum) | Symmetric parkinsonism, co-existent dystonia, severe dysarthria, ‘cock-gait’ (Mn) | History of exposure, MRI findings | Trial of L-Dopa. Physiotherapy, speech therapy, occupational therapy |
| Infectious | Basal ganglia abscesses or granuloma (toxoplasmosis, cryptococcosis; tuberculosis); encephalitic (HIV, CJD,PML) or postencephalitic basal ganglia involvement | Additional movement disorders and other neurological signs common | Medical history, systemic signs, MRI findings, CSF analysis, specific serologies. | Treatment of underlying conditions. Trial of L-Dopa |
| Autoimmune | Antineuronal antibodies affecting basal ganglia motor circuits (e.g., D2R-, DPPX, NMDA-, IGLON-5, & Ma2/Ta-AB’s) | Additional movement disorders and other neurological signs common | Antibody detection. Search for associated neoplasms |
Immunotherapy (IVIG, plasmapheresis, immunosuppressants), treatment of associated tumor |
| Neoplastic | Invasion or indirect compressive effects (frontal meningioma) of basal ganglia circuitry | Additional focal neurological signs | MRI | Treatment of underlying conditions. Trial of L-Dopa |
| Metabolic | Basal ganglia involvement (e.g., Wilson’s disease, non-ketotic hyperglycemia, extrapontine myelinolysis, calcium dyshomeostasis, hypermagnesemia in liver disease, iron deposition in NBIA’s) | Additional movement disorders and other neurological, psychiatric and systemic signs common | Specific laboratory and imaging studies | Treatment of underlying conditions. Trial of L-Dopa |
| NPH | Compromised prefrontal motor connectivity | Small stepped & broad-based gait disorder with freezing, no rest tremor or upper limb involvement (‘lower body parkinsonism’) | Neuroimaging (brain CT or MRI) | CSF drainage (repeated LP, ventricular shunting) |
| Functional | Multifactorial, includes psychiatric comorbidity and impaired self-agency | Abrupt onset, spontaneous fluctuation, effortful demonstrative slowness, tremor with frequency variation and entrainment, no response to levodopa | History of psychiatric comorbidity, incongruent clinical presentation, remission with behavioral or psychotherapy | Counseling. Cognitive behavioral psychotherapy |
Most common offending drugs: DA receptor blockers including first generation (phenothiazines and butyrophenones) and second generation (e.g., olanzapine, risperidone, sulpiride, aripiprazole) antipsychotics as well as antiemetics ( metoclopramide, prochlorperazine and triflupromazine); DA depleting drugs (tetrabenazine or reserpine); Ca- antagonists (flunarizine, cinnarizine and verapamil); antiepileptics (valproate, carbamazepine or lamotrigine); antidepressants (SSRIs, combined noradrenergic -serotonergic reuptake inhibitors and antimuscarinics).
Abbreviations: DA Dopamine; DAT: dopamine transporter; SPECT: Single-photon emission computed tomography; CO Copper; MN Manganese; MRI magnetic resonance imaging; IVIG: intravenous immunoglobulin immunoglobulins; NBIA: neurodegeneration with brain iron accumulation; CT: computed tomography; NPH: normal pressure hydrocephalus; LP: lumbar puncture.
The greatest challenge, even for movement disorder specialists, is early diagnostic differentiation of Parkinson’s disease from atypical parkinsonian disorders. The term atypical parkinsonism is an umbrella term for a variety of neurodegenerative disorders in which a parkinsonian syndrome is a prominent clinical feature, but the full clinical spectrum, underlying pathology, progression, and prognosis fundamentally differ from Parkinson’s disease. The atypical parkinsonism syndromes include multiple system atrophy (MSA), which is pathologically defined by glial cytoplasmic inclusions of misfolded α-synuclein in oligodendrocytes, as well as the tauopathies progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD), defined by neuronal deposition of four-repeat phosphorylated tau aggregates.49,50 Early in the disease, all three conditions can be very difficult to distinguish from Parkinson’s disease as well as from each other. Clinico-pathological studies have revealed error rates in clinical assignment of patients with these different syndromes ranging from 7–35% of the cases.51–53
Clinical pointers that can inform the differential diagnosis between Parkinson’s disease and these main types of atypical degenerative parkinsonism syndromes are summarised in panel 2. These differentiating features can only evolve over time and, particularly the parkinsonian variants of MSA (MSA-P) and PSP (PSP-P), can be notoriously difficult to distinguish from Parkinson’s disease in early disease stages – including asymmetry (which is particularly striking in CBD) and levodopa-responsiveness.
Panel 2 -.
Clinical diagnostic pointers for atypical parkinsonism (‘red flags’)
| Multiple system atrophy |
| • Poor response to L-Dopa (initial responsiveness to L-Dopa in about 30% of cases) |
| • Severe & early autonomic failure (OH, male ED, post-void residual volume >100ml) in the first 5 years of disease |
| • Nocturnal stridor |
| • Early marked dysarthria |
| • Rapid disease progression |
| • Early postural instability |
| • Babinski sign or other pyramidal signs |
| • Cerebellar signs |
| • Jerky postural hand tremor (minipolymyoclonus) |
| • Disproportionate antecollis (“dropped head”) |
| • Orofacial dystonia induced by levodopa |
| Progressive supranuclear palsy |
| • Akinetic rigid parkinsonism with Poor L-Dopa response |
| • Slowing of vertical saccades |
| • Supranuclear downgaze palsy (often not present in the first year after onset) |
| • Square wave jerks |
| • Levator inhibition |
| • Blepharospasm |
| • Pseudobulbar crying |
| • Early dysarthria & dysphagia |
| • Early postural instability/falls |
| • Early Progressive gait freezing |
| • Early marked frontal dementia |
| Corticobasal degeneration |
| • L-Dopa resistant unilateral akinetic parkinsonism |
| • Cortical sensory loss (i.e., agraphesthesia, astereognosia with intact primary sensory modalities) |
| • Limb apraxia, alien limb phenomenon |
| • Focal arm myoclonus or dystonia |
| • Early cognitive impairment with frontal signs and language problems(i.e progressive non fluent aphasia) |
| • Early postural instability and falls |
Abbreviations: OH orthostatic hypotension; ED erectile dysfunction
Clinical diagnostic criteria for Parkinson’s disease
To enhance the diagnostic accuracy of a clinical diagnosis of Parkinson’s disease, the International Parkinson and Movement Disorder Society (MDS) has proposed a set of criteria that essentially represent a revised version of the Queens Square Brain Bank (QSBB) Criteria that have been the most commonly used over the past decades.54,55 These criteria rest on the expert clinical neurological examination showing of a parkinsonian syndrome defined by the presence of bradykinesia and at least one additional cardinal motor feature (rigidity or classical asymmetric 5-Hz resting tremor), plus the application of supportive and exclusionary features. In contrast to the QSBB criteria, the MDS criteria list a number of non-exclusionary clinical features that are unusual in Parkinson’s disease and should raise suspicion of potential alternative diagnoses (‘red flags’). Based on the presence of supportive and absence of exclusionary features, as well as the presence or absence of ‘red flags’, the MDS criteria operationalize two levels of diagnostic certainty for Parkinson’s disease, namely ‘clinically established’ and ‘clinically probable’. The first category establishes a set of criteria aimed to maximize specificity at the possible expense of sensitivity, while criteria for the second level aim for enhanced sensitivity (Suppl.1).
A validation study of the MDS criteria has shown excellent sensitivity (96%) and specificity (95%) for a diagnosis of ‘clinically probable Parkinson’s disease’. The specificity of a diagnosis of ‘clinically established Parkinson’s disease’ was even higher (98.5%), but – as anticipated – this was at the expense of reduced sensitivity (59.3%). For patients with a disease duration of less than 5 years, the specificity of a clinically probable Parkinson’s disease diagnosis was 87%.56 The MDS criteria incorporate two ancillary tests among the four supportive diagnostic criteria, but future diagnostic algorithms will need to incorporate additional tests and biomarkers to further enhance diagnostic accuracy and sensitivity for early or prodromal disease stages.
Diagnosis in the ‘pre-diagnostic’ stage
There is consensus that the process leading to clinically defined Parkinson’s disease starts much earlier than can be captured by current diagnostic criteria (Figure). To date, there are no biomarkers that would enable a confident diagnosis of any of these conceptual phases of ‘pre-diagnostic’ Parkinson’s disease with high sensitivity and specificity. This is particularly problematic when it comes to counselling individuals who present with one or more features associated with an increased risk of developing clinically defined Parkinson’s disease, such as a positive family history or asymptomatic carrier status for disease-associated mutations plus non-motor features of prodromal Parkinson’s disease like hyposmia or RBD.
In a research setting, identifying individuals at risk for Parkinson’s disease is important to understand the progression of pre-clinical and prodromal disease and to recruit participants for clinical trials of potentially disease-modifying therapies26. Several cross-sectional and prospective case-control studies have attempted to define the predictive value of prodromal clinical, non-genetic and genetic Parkinson’s disease risk factors, and neuroimaging tests, to determine the probability of conversion to clinically manifest Parkinson’s disease.23,25, 28 RBD stands out among the clinical markers of Parkinson’s disease risk in that more than 90% of individuals with isolated RBD will eventually develop neurodegenerative parkinsonism – most commonly Parkinson’s disease or Parkinson’s disease-dementia.57,58 The latent period from RBD onset to the development of Parkinson’s disease is variable and usually greater than 10 years,57 although the presence of olfactory dysfunction, abnormalities on the DAT SPECT, or transcranial sonography have been shown to identify those closer to clinical conversion.58–59
The International Parkinson and Movement Disorder Society has proposed criteria for a research diagnosis of prodromal Parkinson’s disease. These attempts provide an evidence-based framework to statistically estimate the likelihood for future Parkinson’s disease at an individual level based on a large set of well-characterised markers of Parkinson’s disease risk.60,61 Prospective cohort-studies have provided evidence for the validity of these criteria in population-based samples.62,63 A similar algorithm has been developed in the format of an online tool to assess Parkinson’s disease risk and has been tested and validated in the PREDICT-Parkinson’s disease study, a prospective community-based population study involving more than 1000 participants.64,65
Although current research algorithms may provide an opportunity for earlier detection of Parkinson’s disease than is currently possible in clinical practice, their sensitivities and predictive values are still suboptimal, and there is an urgent need for sensitive and reliable Parkinson’s disease biomarkers.
Diagnostic Testing – from Clinical Routine to Future Biomarkers
Until now, clinicians have to rely on the judicious use of a limited number of diagnostic tests to solidify a clinical diagnosis of Parkinson’s disease. Their use follows principles of cost-effectiveness, and diagnostic yield is context-dependent. Table 3 highlights broadly available ancillary tests that have been established to support Parkinson’s disease or an alternative diagnosis.
Table 3 -.
Useful diagnostic tests in patients presenting with parkinsonism
| Test | Outcome | Interpretation |
|---|---|---|
| Olfactory Function (UPSIT; Sniffin Sticks) | Normosmia | Questions a PD diagnosis |
| Hyposmia | Consistent with PD | |
| Imaging | ||
| Structural MRI | Normal or signs of unrelated co-morbidity | Consistent with PD |
| Structural basal ganglia pathology (e.g., infarcts, hematoma, abscess, calcification, iron deposition), frontal meningioma, normal pressure hydrocephalus | Secondary parkinsonism | |
| Putamenal atrophy and hypointensity, putamenal rim sign, pontocerebellar atrophy, MCP atrophy, hot cross bun sign | Suggestive of MSA | |
| Midbrain atrophy (hummingbird sign), dilated 3rd ventricle, SCP atrophy | Suggestive of PSP | |
| Asymmetric parietal cortical atrophy | Suggestive of CBD | |
| MR-DWI | Normal | Consistent with PD |
| Increased putamenal diffusivity | Suggestive of MSA (may also be seen in PSP) | |
| Increased diffusivity in middle cerebellar peduncle (MCP) | Suggestive of PSP | |
| DAT-SPECT | Abnormal (asymmetric reduction of striatal tracer binding) | Consistent with PD or other degenerative parkinsonism |
| Normal | Excludes PD or other degenerative parkinsonism | |
| MIBG-SPECT | Reduced cardiac MIBG-uptake | Consistent with PD (inconclusive in early disease) |
| Normal | Suggestive of non-PD parkinsonism (inconclusive in early disease) or secondary parkinsonisms | |
| FDG-PET | Putamenal hypermetabolism (+ occipital & parietal hypometabolism) | Consistent with PD |
| Putamenal&cerebellar hypometabolism | Suggestive of MSA | |
| Frontal, caudate & brainstrem hypometabolism | Suggestive of PSP | |
| Asymmetric striatal & parietal hypometabolism | Suggestive of CBD | |
| Transcranial Ultrasound | Midbrain Hyperechogenicity & Basal Ganglia Normoechogenicity | Consistent with PD |
| Midbrain Normoechogenicity & Basal Ganglia Hyperechogenicity | May be suggestive of non-PD parkinsonism | |
| Genetic Testing | Pathogenic mutation in known PD gene | Confirms PD |
| Absence of a pathogenic mutation in known PD genes | Does not rule out PD | |
| Pathogenic mutation in a neurodegenerative disease gene other than PD genes | Suggestive of a non-PD mimic syndrome | |
Abbreviations: MSA: multiple system atrophy; PSP: progressive supranuclear palsy; CBD: corticobasal degeneration; MCP, middle cerebellar peduncle; SCP, superior cerebellar peduncle; MRI: magnetic resonance imaging; DWI: diffusion weighted imaging; DAT: dopamine transporter; SPECT: Single-photon emission computed tomography; MIBG: Meta-iodobenzylguanidine (myocardial scintigraphy). FDG-PET: Fluorodeoxyglucose positron emission tomography.
Olfactory function testing using the UPSIT or Sniffin Stick tests has been extensively studied in Parkinson’s disease and other parkinsonian syndromes. Hyposmia or anosmia have been consistent findings in about 90% of patients with Parkinson’s disease, while normosmia is the rule in the early stages of atypical degenerative or secondary parkinsonisms.66 The MDS criteria for Parkinson’s disease list hyposmia as one of four supportive criteria of a Parkinson’s disease diagnosis, and although on their validation study56 the olfactory testing only achieved 63.4% specificity, this feature has shown high diagnostic accuracy for distinguishing Parkinson’s disease from MSA and PSP in other studies.67 Given the low cost and easy applicability, olfactory testing should be part of the initial clinical workup of people with suspected Parkinson’s disease.
Imaging markers
Structural magnetic resonance imaging (MRI) is usually unremarkable in Parkinson’s disease. Nonetheless, it should also be part of the routine diagnostic process to distinguish Parkinson’s disease from secondary or atypical parkinsonian syndromes (Table 3) since several MRI features are highly specific for atypical parkinsonisms, although sensitivity is low at around 50%.
Novel MR imaging techniques, including neuromelanin imaging (NMI), quantitative susceptibility mapping (QSM), or visual assessment of dorsal nigral hyperintensity, have the potential to assess nigral pathology in Parkinson’s disease and have been a major focus of recent research efforts. NMI exploits the paramagnetic properties of neuromelanin while QSM enables quantification of iron deposition in the SN.68,69 NMI has shown greater than 80% sensitivity and specificity to distinguish Parkinson’s disease from controls70 and could have potential to show alterations in prodromal Parkinson’s disease.71 QSM assessments of increased iron content in the SN have shown broadly similar performance in separating Parkinson’s disease from controls.72,73 Visual assessment of an area of dorsal nigral hyperintensity, which has been postulated to correspond to Nigrosome-1 and is lost in Parkinson’s disease, has shown a pooled sensitivity of 98% and a pooled specificity of 95% in distinguishing Parkinson’s disease from controls in a recent meta-analysis of 10 case-control studies, including 364 Parkinson’s disease subjects and 231 controls,74 and has also been suggested as a potential MRI biomarker in prodromal Parkinson’s disease.75
While these novel MRI techniques may hold potential as biomarkers of early or even prodromal Parkinson’s disease, they generally cannot distinguish between Parkinson’s disease and other types of degenerative parkinsonism since nigral pathology is common to all of these. This is different for a variety of novel MR diffusion tensor imaging techniques like free water imaging and neurite orientation dispersion, and density imaging (NODDI) that enable differentiation between Parkinson’s disease and atypical parkinsonism based on more widespread tissue integrity changes in MSA and PSP compared to Parkinson’s disease.76,77 Recent reports have also suggested high discriminative accuracy between Parkinson’s disease and MSA and PSP using observer-independent machine learning approaches using automated volumetry or automated voxel-based diffusivity 78,79 or multimodal MR imaging combining several MR parameters.80
A variety of radionuclide tracers are available to examine pre-synaptic and post-synaptic striatal dopaminergic function using PET or SPECT imaging.81 Among these, only DAT-SPECT has an established role in clinical routine due to its availability and moderate cost. Ligands of the presynaptic monoamine transporter (Ioflupane, Trodat) used in DAT-SPECT are sensitive to detect dysfunction or loss of striatal dopaminergic terminals and enable the identification of parkinsonian syndromes with nigral neurodegeneration like Parkinson’s disease and non-degenerative phenocopies, such as essential tremor, psychogenic, or vascular parkinsonism.82 Some studies have attempted to use DAT-SPECT to distinguish atypical parkinsonism from Parkinson’s disease by measuring the asymmetry index or the caudate to putamen binding ratio; however, these have not proved to be useful in clinical practice, and DAT-SPECT should not be considered a tool on the differential diagnosis between neurodegenerative parkinsonisms.83 While dopaminergic radiotracer imaging using DAT-SPECT or metabolic imaging with FDG-PET have shown sensitivity for prodromal stages of Parkinson’s disease,58,84 there is reason to expect that sensitive and specific radiotracer probes enabling visualization and quantification of a-synuclein deposits in the brain or peripheral autonomic nervous system via PET or SPECT imaging might significantly enhance early or even pre-clinical diagnosis of Parkinson’s disease and other synucleinopathies. Several candidates are in preclinical development, but none has yet reached the stage of clinical diagnostic testing in Parkinson’s disease.85
Fluid and tissue a-synuclein markers
Pathological a-synuclein species are also the major candidate in the search for sensitive and specific fluid and tissue Parkinson’s disease biomarkers. A variety of biopsy studies have suggested that immunohistochemical assessment for the presence of phosphorylated and aggregated a-synuclein in the enteric nervous system, autonomic nerve fibers in the salivary glands, or skin can distinguish Parkinson’s disease from healthy controls and, more importantly, might serve as a biomarker for prodromal disease stages.86–88
The availability of in vitro conversion assays with ultra-high sensitivity for amyloidogenic proteins like Real-Time Quaking Induced Conversion (RT-QuIC) and Protein Misfolding Cyclic Amplification (PMCA) has significantly impacted the search for molecular biomarkers. A number of recent case-control studies have found sensitivities and specificities of RT-QuIC or PMCA analyses of a-synuclein seeding activity in the CSF of above 90% to distinguish Parkinson’s disease from healthy controls or patients with tauopathies.89–92 This has recently been also shown for RT-QuIC or PMCA analyses of skin biopsies.93 Additionally, a-syn seeding activity has also been found using RT-QuIC in the CSF of non-manifesting carriers of a LRRK2 gene mutation94 and in the CSF and olfactory mucosa of patients with RBD.95,96 This suggests that protein misfolding assays for a-synuclein might have a role in detecting prodromal or pre-clinical stages of Parkinson’s disease. Intriguingly, a recent CSF PMCA study that encompassed 439 samples, including PD (n=71) and MSA (n=33) cases, provided evidence for distinctive strains of a-synuclein in Parkinson’s disease and MSA and thus the potential to distinguish between different synucleinopathies.91 Table 4 provides an overview of the currently most promising biomarker candidates that could help to identify people at-risk and in the prodromal stages of disease, enhance differential diagnostic accuracy in established clinical parkinsonism, and enable monitoring of disease progression.
Table 4 -.
Candidate biomarkers for Parkinson’s disease
| Modality | Biomarker | Diagnostic Potential | Comments | |||
|---|---|---|---|---|---|---|
| Prodromal PD | Manifest PD | DD Non-PD |
Progression | |||
| MRI | Substantia nigra neuromelanin 68,70,71 | +? | + | - | + | Insufficiently studied in prodromal PD |
| Dorsolateral nigral hyperintensity74,75 | +? | + | - | - | Insufficiently studied in prodromal PD No differentiation PD vs atypical PD |
|
| Quantitative Susceptibility Mapping (QSM) 69,72,73 | ? | + | - | ? | Limited number of studies Use in DD unclear No progression data |
|
| Tensor Imaging (Free Water MRI; NODDI) 76,77 | ? | + | + | ? | Role in early/prodromal Dx unclear No progression data |
|
| Automated Volumetry 78,79 | - | - | + | + | Insufficiently studied in prodromal PD | |
| Mutlimodal MRI80 | ? | - | + | ? | Insufficiently studied in prodromal PD No progression data |
|
| Radiotracer Imaging | A-syn | + | + | +/− | + | Tracers not yet available |
| Tau-protein 97 | - | - | + | - | Mostly studied in AD & PSP/CBD, limited data for PD | |
| Blood | Nfl 98 | - | - | + | - | Non-specific marker for neurodegeneration. Insufficiently studied in prodromal PD. |
| CSF |
a-syn seeding activity (RTQuiC, PMCA) 89–92,94,95 |
+ | + | + | - | May distinguish different a-syn strains in PD vs MSA |
| Nfl 99 | - | - | + | - | Non-specific marker for neurodegeneration. May differentiate PD from Atypical parkinsonism at group level Insufficiently studied in prodromal PD. |
|
| Tissue Biopsies | Dermal a-syn 86 (IHC) | + | + | - | ? | Requires multi-site sampling, limited sensitivity, not specific to PD vs other synucleinopathies |
| Dermal a-syn seeding activity (RT-QuIC; PMCA) 93 | ? | + | + | ? | Limited in-vivo information | |
| Olfactory Mucosa a-syn seeding activity (RT-QuIC) 96 | +? | + | ? | ? | Limited number of studies | |
| GI a-syn 87 | ? | +/− | - | ? | Invasive, Requires multi-site sampling, limited sensitivity | |
| Salivary gland a-syn 87,88 | + | + | - | - | Invasive, limited sensitivity | |
| Faeces | Gut microbiota | ? | +/− | - | - | Variable results ref different composition of bacterial microbiome between PD and controls |
| Digital biomarkers | Multiple motor and non-motor assessments / wearable devices100 | +? | - | - | + | Insufficiently studied in prodromal PD No differentiation PD vs atypical PD |
Abbreviations: DD: differential diagnosis; MRI magnetic resonance imaging; NODDI: neurite orientation dispersion and density imaging; a-syn: alpha-synuclein; Nfl neurofilament; RT-QuIC: Real-time quaking-induced conversion; PMCA: protein misfolding cyclic amplification; IHC: Immunohistochemistry; GI gastrointestinal
5. Conclusions and future directions
The diagnosis of Parkinson’s disease has profound implications for patients and their families, and despite important advances, it remains a challenge. It is anchored on well-defined criteria that have shown excellent sensitivity and specificity in clinical series,56 but diagnostic accuracy at a patient’s first visit is well below 100%, even in the hands of specialised movement disorder neurologists. This scenario will improve over the next decade as new Parkinson’s disease-specific biomarkers become available. Observer-independent machine-learning approaches to MRI data can distinguish Parkinson’s disease from its atypical mimics like MSA or PSP,78 and further advances in imaging markers for Parkinson’s disease, including radiotracer imaging of alpha-synuclein, are on the horizon.85
Genetics will play an essential role in the future of Parkinson’s disease diagnosis. Additional monogenic forms of Parkinson’s disease may still be identified. Aside from monogenic Parkinson’s disease, large-scale genome-wide association studies show us that the genetic etiology in most patients is complex, with multiple susceptibility variants driving disease risk within each patient. Under this paradigm, individual patients have numerous genetic risk variants for Parkinson’s disease that act synergistically with stochastic and environmental or lifestyle factors to tip the patient into disease. These insights give rise to the possibility of using polygenic risk scores or machine-learning algorithms to differentiate patients from healthy controls and to predict patient subgroups, age at onset, and clinical features.38,101,102 These advances will also have an impact on the development and application of disease modification therapies, as already exemplified by ongoing clinical trials targeting the GBA pathway or LRRK2 function.15,103
As soon as disease-modifying interventions become available, there will be enormous pressure to test and apply them to individuals in prodromal stages or to individuals with an increased risk for Parkinson’s disease. Such ‘pre-diagnostic’ stages of Parkinson’s disease will only be diagnosed through biomarkers, and several candidate approaches, including imaging, synuclein assays, tissue biopsies, and genetic biomarkers, are currently being studied.
Parkinson’s disease will evolve from a purely clinical to a biomarker-supported diagnostic entity, and new opportunities for early diagnosis will arise, and the diagnostic accuracy at the first neurological consultation will be significantly higher than today. Future generations of neurologists may no longer view ‘Parkinson’s disease’ as a single nosological entity but will be able to confidently diagnose subtypes with different prognoses and treatment responses.40 But there will be new challenges – most momentous when a diagnosis could be made in individuals free of symptoms but without the prospect of preventive therapy. Fortunately, the number of candidate drugs for disease-modification in clinical development has never been as large as today.104
Supplementary Material
Acknowledgments
Alicia Garrido’s effort is supported by Michael J. Fox Foundation (MJFF) for Parkinson’s Research; has additionally received honoraria from TEVA Pharma as well as travel/meeting expenses from the International Movement Disorders Society.
Sonja Scholz research is supported in part by the intramural research program of the National Institutes of Health (National Institute of Neurological Disorders and Stroke; project number: 1ZIANS003154).
SWS serves on the Scientific Advisory Council of the Lewy Body Dementia Association and is an editorial board member for the Journal of Parkinson’s Disease and JAMA Neurology.
Werner Poewe reports personal fees from AbbVie, Affiris, AstraZeneca, BIAL, Boston Scientific, Britannia, Intec, Ipsen, Lundbeck, Neuroderm, Neurocrine, Denali Pharmaceuticals, Novartis, Orion Pharma, Teva, UCB and Zambon (consultancy and lecture fees in relation to clinical drug development programmes for Parkinson’s disease and has received grant support from the Michael J. Fox Foundation and the EU FP7 & Horizon 2020 programmes
Footnotes
Financial disclosures
Eduardo Tolosa has received honoraria for consultancy from TEVA, Bial, Prevail, Boehringer Ingelheim, Roche and BIOGEN and has received funding for research from Spanish Network for Research on Neurodegenerative Disorders (CIBERNED) - Instituto Carlos III (ISCIII), and The Michael J. Fox Foundation (MJFF) for Parkinson’s Research.
Search strategy and selection criteria
References for this Review were identified by searches of PubMed between 2006 and December, 2020 by use of the following terms: parkins*[title] AND “diagnosis”, “epidemiology”, “risk factor”, “genetic”, “premotor”,“prodromal”, “atypical”. Bibliographies of papers were also reviewed. Papers published in English and German were considered. The final reference list was generated on the basis of relevance to the topics covered in this Review.
References:
- 1.GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019. May;18(5):459–480. doi: 10.1016/S1474-4422(18)30499-X. Epub 2019 Mar 14. PMID: 30879893; PMCID: PMC6459001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorsey ER, Sherer T, Okun MS, Bloem BR. The Emerging Evidence of the Parkinson Pandemic. J Parkinsons Dis. 2018;8(s1):S3–S8. doi: 10.3233/JPD-181474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rüb U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). J Neurol. 2002;249 Suppl 3:III/1–III/5. doi: 10.1007/s00415-002-1301-4 [DOI] [PubMed] [Google Scholar]
- 4.Steiner JA, Quansah E, Brundin P. The concept of alpha-synuclein as a prion-like protein: ten years after. Cell Tissue Res. 2018. Jul;373(1):161–173. doi: 10.1007/s00441-018-2814-1. Epub 2018 Feb 26. PMID: 29480459; PMCID: PMC6541204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blauwendraat C, Nalls MA, Singleton AB. The genetic architecture of Parkinson’s disease. Lancet Neurol. 2020;19(2):170–178. doi: 10.1016/S1474-4422(19)30287-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3 [DOI] [PubMed] [Google Scholar]
- 7.Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013. Published 2017 Mar 23. doi: 10.1038/nrdp.2017.13 [DOI] [PubMed] [Google Scholar]
- 8.Rajput AH, Rajput A. Accuracy of Parkinson disease diagnosis unchanged in 2 decades. Neurology. 2014;83(5):386–387. doi: 10.1212/WNL.0000000000000653 [DOI] [PubMed] [Google Scholar]
- 9.Tolosa E, Gaig C, Santamaría J, Compta Y. Diagnosis and the premotor phase of Parkinson disease. Neurology. 2009;72(7 Suppl):S12–S20. doi: 10.1212/WNL.0b013e318198db11 [DOI] [PubMed] [Google Scholar]
- 10.Mahlknecht P, Seppi K, Poewe W. The Concept of Prodromal Parkinson’s Disease. J Parkinsons Dis. 2015;5(4):681–697. doi: 10.3233/JPD-150685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parkinson’s disease subtypes: lost in translation? Marras C, Lang A. J Neurol Neurosurg Psychiatry. 2013. Apr;84(4):409–15. doi: 10.1136/jnnp-2012-303455. Epub 2012 Sep 5. PMID: 22952329 Review. [DOI] [PubMed] [Google Scholar]
- 12.Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB. Clinical criteria for subtyping Parkinson’s disease: biomarkers and longitudinal progression. Brain. 2017;140(7):1959–1976. doi: 10.1093/brain/awx118 [DOI] [PubMed] [Google Scholar]
- 13.Faghri F, Hashemi SH, Leonard H, et al. Predicting onset, progression, and clinical subtypes of Parkinson disease using machine learning. Preprint: doi: 10.1101/338913. [DOI] [Google Scholar]
- 14.Poewe W, Seppi K, Marini K, Mahlknecht P. New hopes for disease modification in Parkinson’s Disease. Neuropharmacology. 2020;171:108085. doi: 10.1016/j.neuropharm.2020.108085 [DOI] [PubMed] [Google Scholar]
- 15.Tolosa E, Vila M, Klein C, Rascol O. LRRK2 in Parkinson disease: challenges of clinical trials. Nat Rev Neurol. 2020;16(2):97–107. doi: 10.1038/s41582-019-0301-2 [DOI] [PubMed] [Google Scholar]
- 16.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–376. doi: 10.1136/jnnp.2007.131045 [DOI] [PubMed] [Google Scholar]
- 17.Gelpi E, Navarro-Otano J, Tolosa E et al. Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov Disord. 2014. Jul;29(8):1010–8. doi: 10.1002/mds.25776. Epub 2014 Jan 2. PMID: 24395122. [DOI] [PubMed] [Google Scholar]
- 18.Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18(8):509. doi: 10.1038/nrn.2017.91 [DOI] [PubMed] [Google Scholar]
- 19.Pont-Sunyer C, Hotter A, Gaig C, et al. The onset of nonmotor symptoms in Parkinson’s disease (the ONSET PD study). Mov Disord. 2015;30(2):229–237. doi: 10.1002/mds.26077 [DOI] [PubMed] [Google Scholar]
- 20.Zis P, Martinez-Martin P, Sauerbier A, et al. Non-motor symptoms burden in treated and untreated early Parkinson’s disease patients: argument for non-motor subtypes. Eur J Neurol. 2015. new Aug;22(8):1145–50. doi: 10.1111/ene.12733. Epub 2015 May 15. PMID: 25981492. [DOI] [PubMed] [Google Scholar]
- 21.Barone P, Erro R, Picillo M. Quality of Life and Nonmotor Symptoms in Parkinson’s Disease. Int Rev Neurobiol. 2017;133:499–516. doi: 10.1016/bs.irn.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 22.Safarpour D, Thibault DP, DeSanto CL, et al. Nursing home and end-of-life care in Parkinson disease. Neurology. 2015;85(5):413–419. doi: 10.1212/WNL.0000000000001715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross GW, Abbott RD, Petrovitch H, Tanner CM, White LR. Pre-motor features of Parkinson’s disease: the Honolulu-Asia Aging Study experience. Parkinsonism Relat Disord. 2012;18 Suppl 1:S199–S202. doi: 10.1016/S1353-8020(11)70062-1 [DOI] [PubMed] [Google Scholar]
- 24.Siderowf A, Lang AE. Parkinson’s disease: concepts and definitions. Mov Disord. 2012;27(5):608–616. doi: 10.1002/mds.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrag A, Horsfall L, Walters K, Noyce A, Petersen I. Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study. Lancet Neurol. 2015;14(1):57–64. doi: 10.1016/S1474-4422(14)70287-X [DOI] [PubMed] [Google Scholar]
- 26.Salat D, Noyce AJ, Schrag A, Tolosa E. Challenges of modifying disease progression in prediagnostic Parkinson’s disease. Lancet Neurol. 2016;15(6):637–648. doi: 10.1016/S1474-4422(16)00060-0 [DOI] [PubMed] [Google Scholar]
- 27.Pont-Sunyer C, Tolosa E, Caspell-Garcia C, et al. The prodromal phase of leucine-rich repeat kinase 2-associated Parkinson disease: Clinical and imaging Studies. Mov Disord. 2017;32(5):726–738. doi: 10.1002/mds.26964 [DOI] [PubMed] [Google Scholar]
- 28.Mahlknecht P, Stockner H, Marini K, et al. Midbrain hyperechogenicity, hyposmia, mild parkinsonian signs and risk for incident Parkinson’s disease over 10 years: A prospective population-based study. Parkinsonism Relat Disord. 2020;70:51–54. doi: 10.1016/j.parkreldis.2019.12.008 [DOI] [PubMed] [Google Scholar]
- 29.Fereshtehnejad SM, Postuma RB. Subtypes of Parkinson’s Disease: What Do They Tell Us About Disease Progression?. Curr Neurol Neurosci Rep. 2017;17(4):34. doi: 10.1007/s11910-017-0738-x [DOI] [PubMed] [Google Scholar]
- 30.Schrag A, Ben-Shlomo Y, Brown R, Marsden CD, Quinn N. Young-onset Parkinson’s disease revisited-clinical features, natural history, and mortality. Mov Disord. 1998;13(6):885–894. doi: 10.1002/mds.870130605 [DOI] [PubMed] [Google Scholar]
- 31.Selikhova M, Williams DR, Kempster PA, Holton JL, Revesz T, Lees AJ. A clinico-pathological study of subtypes in Parkinson’s disease. Brain. 2009;132(Pt 11):2947–2957. doi: 10.1093/brain/awp234 [DOI] [PubMed] [Google Scholar]
- 32.Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC. How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov Disord. 2013;28(5):668–670. doi: 10.1002/mds.25383 [DOI] [PubMed] [Google Scholar]
- 33.Simuni T, Caspell-Garcia C, Coffey C, et al. How stable are Parkinson’s disease subtypes in de novo patients: Analysis of the PPMI cohort? Parkinsonism Relat Disord. 2016;28:62–67. doi: 10.1016/j.parkreldis.2016.04.027 [DOI] [PubMed] [Google Scholar]
- 34.Lee JW, Song YS, Kim H, Ku BD, Lee WW. Alteration of Tremor Dominant and Postural Instability Gait Difficulty Subtypes During the Progression of Parkinson’s Disease: Analysis of the PPMI Cohort. Front Neurol. 2019;10:471. Published 2019 May 7. doi: 10.3389/fneur.2019.00471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mu J, Chaudhuri KR, Bielza C, de Pedro-Cuesta J, Larrañaga P, Martinez-Martin P. Parkinson’s Disease Subtypes Identified from Cluster Analysis of Motor and Non-motor Symptoms. Front Aging Neurosci. 2017;9:301. Published 2017 Sep 20. doi: 10.3389/fnagi.2017.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fereshtehnejad SM, Romenets SR, Anang JB, Latreille V, Gagnon JF, Postuma RB. New Clinical Subtypes of Parkinson Disease and Their Longitudinal Progression: A Prospective Cohort Comparison With Other Phenotypes. JAMA Neurol. 2015;72(8):863–873. doi: 10.1001/jamaneurol.2015.0703 [DOI] [PubMed] [Google Scholar]
- 37.De Pablo-Fernández E, Lees AJ, Holton JL, Warner TT. Prognosis and Neuropathologic Correlation of Clinical Subtypes of Parkinson Disease. JAMA Neurol. 2019. April 1;76(4):470–479. doi: 10.1001/jamaneurol.2018.4377. PMID: 30640364; PMCID: PMC6459129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nalls MA, Blauwendraat C, Vallerga CL, et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet Neurol. 2019;18(12):1091–1102. doi: 10.1016/S1474-4422(19)30320-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bandres-Ciga S, Saez-Atienzar S, Kim JJ, et al. ; International Parkinson Disease Genomics Consortium. Large-scale pathway specific polygenic risk and transcriptomic community network analysis identifies novel functional pathways in Parkinson disease. Acta Neuropathol. 2020. Sep;140(3):341–358. doi: 10.1007/s00401-020-02181-3. Epub 2020 Jun 29. PMID: 32601912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berardelli A, Wenning GK, Antonini A, et al. EFNS/MDS-ES/ENS [corrected] recommendations for the diagnosis of Parkinson’s disease [published correction appears in Eur J Neurol. 2013 Feb;20(2):406]. Eur J Neurol. 2013;20(1):16–34. doi: 10.1111/ene.12022 [DOI] [PubMed] [Google Scholar]
- 41.Blauwendraat C, Pletnikova O, Geiger JT, et al. Genetic analysis of neurodegenerative diseases in a pathology cohort. Neurobiol Aging 2019;76:214.e1–214.e9. doi: 10.1016/j.neurobiolaging.2018.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez-Contreras M, Heckman MG, Tacik P, et al. Study of LRRK2 variation in tauopathy: Progressive supranuclear palsy and corticobasal degeneration. Mov Disord. 2017;32(1):115–123. doi: 10.1002/mds.26815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361(17):1651–1661. doi: 10.1056/NEJMoa0901281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045 [DOI] [PubMed] [Google Scholar]
- 45.Lesage S, Drouet V, Majounie E, et al. Loss of VPS13C Function in Autosomal-Recessive Parkinsonism Causes Mitochondrial Dysfunction and Increases PINK1/Parkin-Dependent Mitophagy. Am J Hum Genet. 2016;98(3):500–513. doi: 10.1016/j.ajhg.2016.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schrag A, Ben-Shlomo Y, Quinn N. How valid is the clinical diagnosis of Parkinson’s disease in the community?. J Neurol Neurosurg Psychiatry. 2002;73(5):529–534. doi: 10.1136/jnnp.73.5.529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: A systematic review and meta-analysis. Neurology. 2016;86(6):566–576. doi: 10.1212/WNL.0000000000002350 [DOI] [PubMed] [Google Scholar]
- 49.Wenning GK, Litvan I, Tolosa E. Milestones in atypical and secondary Parkinsonisms. Mov Disord. 2011;26(6):1083–1095. doi: 10.1002/mds.23713 [DOI] [PubMed] [Google Scholar]
- 50.Höglinger GU, Respondek G, Stamelou M, et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord. 2017;32(6):853–864. doi: 10.1002/mds.26987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adler CH, Beach TG, Hentz JG, et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology. 2014;83(5):406–412. doi: 10.1212/WNL.0000000000000641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125(Pt 4):861–870. doi: 10.1093/brain/awf080 [DOI] [PubMed] [Google Scholar]
- 53.Koga S, Aoki N, Uitti RJ, et al. When DLB, PD, and PSP masquerade as MSA: an autopsy study of 134 patients. Neurology. 2015;85(5):404–412. doi: 10.1212/WNL.0000000000001807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30(12):1591–1601. doi: 10.1002/mds.26424 [DOI] [PubMed] [Google Scholar]
- 55.Lees AJ, Hardy J, Revesz T. Parkinson’s disease [published correction appears in Lancet. 2009 Aug 29;374(9691):684]. Lancet. 2009;373(9680):2055–2066. doi: 10.1016/S0140-6736(09)60492-X [DOI] [PubMed] [Google Scholar]
- 56.Postuma RB, Poewe W, Litvan I, et al. Validation of the MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2018;33(10):1601–1608. doi: 10.1002/mds.27362 [DOI] [PubMed] [Google Scholar]
- 57.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol. 2013;12(5):443–453. doi: 10.1016/S1474-4422(13)70056-5 [DOI] [PubMed] [Google Scholar]
- 58.Dauvilliers Y, Schenck CH, Postuma RB, Iranzo A, Luppi PH, Plazzi G, Montplaisir J, Boeve B. REM sleep behaviour disorder. Nat Rev Dis Primers. 2018. August 30;4(1):19. doi: 10.1038/s41572-018-0016-5. PMID: 30166532. [DOI] [PubMed] [Google Scholar]
- 59.Mahlknecht P, Iranzo A, Högl B, et al. Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD. Neurology. 2015;84(7):654–658. doi: 10.1212/WNL.0000000000001265 [DOI] [PubMed] [Google Scholar]
- 60.Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2015;30(12):1600–1611. doi: 10.1002/mds.26431 [DOI] [PubMed] [Google Scholar]
- 61.Heinzel S, Berg D, Gasser T, et al. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2019;34(10):1464–1470. doi: 10.1002/mds.27802 [DOI] [PubMed] [Google Scholar]
- 62.Mahlknecht P, Gasperi A, Djamshidian A, et al. Performance of the Movement Disorders Society criteria for prodromal Parkinson’s disease: A population-based 10-year study. Mov Disord. 2018;33(3):405–413. doi: 10.1002/mds.27281 [DOI] [PubMed] [Google Scholar]
- 63.Pilotto A, Heinzel S, Suenkel U, et al. Application of the movement disorder society prodromal Parkinson’s disease research criteria in 2 independent prospective cohorts. Mov Disord. 2017;32(7):1025–1034. doi: 10.1002/mds.27035 [DOI] [PubMed] [Google Scholar]
- 64.Noyce AJ, Bestwick JP, Silveira-Moriyama L, et al. PREDICT-PD: identifying risk of Parkinson’s disease in the community: methods and baseline results. J Neurol Neurosurg Psychiatry. 2014;85(1):31–37. doi: 10.1136/jnnp-2013-305420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noyce AJ, R’Bibo L, Peress L, et al. PREDICT-PD: An online approach to prospectively identify risk indicators of Parkinson’s disease. Mov Disord. 2017;32(2):219–226. doi: 10.1002/mds.26898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Katzenschlager R, Lees AJ. Olfaction and Parkinson’s syndromes: its role in differential diagnosis. Curr Opin Neurol. 2004;17(4):417–423. doi: 10.1097/01.wco.0000137531.76491.c2 [DOI] [PubMed] [Google Scholar]
- 67.Krismer F, Pinter B, Mueller C, et al. Sniffing the diagnosis: Olfactory testing in neurodegenerative parkinsonism. Parkinsonism Relat Disord. 2017;35:36–41. doi: 10.1016/j.parkreldis.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 68.Ohtsuka C, Sasaki M, Konno K, et al. Changes in substantia nigra and locus coeruleus in patients with early-stage Parkinson’s disease using neuromelanin-sensitive MR imaging. Neurosci Lett. 2013. Apr 29;541:93–8. doi: 10.1016/j.neulet.2013.02.012. Epub 2013 Feb 18. PMID: 23428505. [DOI] [PubMed] [Google Scholar]
- 69.Langkammer C, Schweser F, Krebs N, et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study. Neuroimage. 2012. Sep;62(3):1593–9. doi: 10.1016/j.neuroimage.2012.05.049. Epub 2012 May 24. PMID: 22634862; PMCID: PMC3413885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X, Zhang Y, Zhu C, et al. The diagnostic value of SNpc using NM-MRI in Parkinson’s disease: meta-analysis. Neurol Sci. 2019. Dec;40(12):2479–2489. doi: 10.1007/s10072-019-04014-y. Epub 2019 Aug 7. Erratum in: Neurol Sci. 2019 Aug 28;: PMID: 31392640. [DOI] [PubMed] [Google Scholar]
- 71.Ehrminger M, Latimier A, Pyatigorskaya N et al. The coeruleus/subcoeruleus complex in idiopathic rapid eye movement sleep behaviour disorder. Brain. 2016. Apr;139(Pt 4):1180–8. doi: 10.1093/brain/aww006. Epub 2016 Feb 26. PMID: 26920675. [DOI] [PubMed] [Google Scholar]
- 72.Murakami Y, Kakeda S, Watanabe K, et al. Usefulness of quantitative susceptibility mapping for the diagnosis of Parkinson disease. AJNR Am J Neuroradiol. 2015. Jun;36(6):1102–8. doi: 10.3174/ajnr.A4260. Epub 2015 Mar 12. PMID: 25767187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takahashi H, Watanabe Y, Tanaka H, et al. Comprehensive MRI quantification of the substantia nigra pars compacta in Parkinson’s disease. Eur J Radiol. 2018. Dec;109:48–56. doi: 10.1016/j.ejrad.2018.06.024. Epub 2018 Oct 28. PMID: 30527311. [DOI] [PubMed] [Google Scholar]
- 74.Mahlknecht P, Krismer F, Poewe W, Seppi K. Meta-analysis of dorsolateral nigral hyperintensity on magnetic resonance imaging as a marker for Parkinson’s disease. Mov Disord. 2017. Apr;32(4):619–623. doi: 10.1002/mds.26932. Epub 2017 Feb 2. PMID: 28151553. [DOI] [PubMed] [Google Scholar]
- 75.De Marzi R, Seppi K, Högl B, et al. Loss of dorsolateral nigral hyperintensity on 3.0 tesla susceptibility-weighted imaging in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol. 2016. Jun;79(6):1026–30. doi: 10.1002/ana.24646. Epub 2016 Apr 22. PMID: 27016314. [DOI] [PubMed] [Google Scholar]
- 76.Planetta PJ, Ofori E, Pasternak O, et al. Free-water imaging in Parkinson’s disease and atypical parkinsonism. Brain. 2016. Feb;139(Pt 2):495–508. doi: 10.1093/brain/awv361. Epub 2015 Dec 24. PMID: 26705348; PMCID: PMC5790142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitchell T, Archer DB, Chu WT, et al. Neurite orientation dispersion and density imaging (NODDI) and free-water imaging in Parkinsonism. Hum Brain Mapp. 2019. Dec 1;40(17):5094–5107. doi: 10.1002/hbm.24760. Epub 2019 Aug 12. PMID: 31403737; PMCID: PMC6865390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scherfler C, Göbel G, Müller C, et al. Diagnostic potential of automated subcortical volume segmentation in atypical parkinsonism. Neurology. 2016;86(13):1242–1249. doi: 10.1212/WNL.0000000000002518 [DOI] [PubMed] [Google Scholar]
- 79.Huppertz HJ, Möller L, Südmeyer M, et al. Differentiation of neurodegenerative parkinsonian syndromes by volumetric magnetic resonance imaging analysis and support vector machine classification. Mov Disord. 2016;31(10):1506–1517. doi: 10.1002/mds.26715 [DOI] [PubMed] [Google Scholar]
- 80.Péran P, Barbagallo G, Nemmi F, et al. MRI supervised and unsupervised classification of Parkinson’s disease and multiple system atrophy. Mov Disord. 2018. Apr;33(4):600–608. doi: 10.1002/mds.27307. Epub 2018 Feb 23. PMID: 29473662. [DOI] [PubMed] [Google Scholar]
- 81.Strafella AP, Bohnen NI, Perlmutter JS, et al. Molecular imaging to track Parkinson’s disease and atypical parkinsonisms: New imaging frontiers. Mov Disord. 2017;32(2):181–192. doi: 10.1002/mds.26907 [DOI] [PubMed] [Google Scholar]
- 82.Scherfler C, Schwarz J, Antonini A, et al. Role of DAT-SPECT in the diagnostic work up of parkinsonism. Mov Disord. 2007;22(9):1229–1238. doi: 10.1002/mds.21505 [DOI] [PubMed] [Google Scholar]
- 83.Matesan M, Gaddikeri S, Longfellow K, Miyaoka R, Elojeimy S, Elman S, Hu SC, Minoshima S, Lewis D. I-123 DaTscan SPECT Brain Imaging in Parkinsonian Syndromes: Utility of the Putamen-to-Caudate Ratio. J Neuroimaging. 2018. Nov;28(6):629–634. doi: 10.1111/jon.12530. Epub 2018 Jun 14. PMID: 29905019. [DOI] [PubMed] [Google Scholar]
- 84.Meles SK, Renken RJ, Janzen A, et al. ; REMPET Study Group. The Metabolic Pattern of Idiopathic REM Sleep Behavior Disorder Reflects Early-Stage Parkinson Disease. J Nucl Med. 2018. Sep;59(9):1437–1444. doi: 10.2967/jnumed.117.202242. Epub 2018 Feb 23. PMID: 29476004. [DOI] [PubMed] [Google Scholar]
- 85.Helmich RC, Vaillancourt DE, Brooks DJ. The Future of Brain Imaging in Parkinson’s Disease. J Parkinsons Dis. 2018;8(s1):S47–S51. doi: 10.3233/JPD-181482. PMID: 30584163; PMCID: PMC6311365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Donadio V, Incensi A, Leta V, et al. Skin nerve α-synuclein deposits: a biomarker for idiopathic Parkinson disease. Neurology. 2014;82(15):1362–1369. doi: 10.1212/WNL.0000000000000316 [DOI] [PubMed] [Google Scholar]
- 87.Tsukita K, Sakamaki-Tsukita H, Tanaka K, Suenaga T, Takahashi R. Value of in vivo α-synuclein deposits in Parkinson’s disease: A systematic review and meta-analysis. Mov Disord. 2019. Oct;34(10):1452–1463. doi: 10.1002/mds.27794. Epub 2019 Jul 19. PMID: 31322768. [DOI] [PubMed] [Google Scholar]
- 88.Vilas D, Iranzo A, Tolosa E, et al. Assessment of α-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol. 2016;15(7):708–718. doi: 10.1016/S1474-4422(16)00080-6 [DOI] [PubMed] [Google Scholar]
- 89.Fairfoul G, McGuire LI, Pal S, et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol. 2016;3(10):812–818. Published 2016 Aug 28. doi: 10.1002/acn3.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shahnawaz M, Tokuda T, Waragai M, et al. Development of a Biochemical Diagnosis of Parkinson Disease by Detection of α-Synuclein Misfolded Aggregates in Cerebrospinal Fluid. JAMA Neurol. 2017;74(2):163–172. doi: 10.1001/jamaneurol.2016.4547 [DOI] [PubMed] [Google Scholar]
- 91.Rossi M, Candelise N, et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 2020. Jul;140(1):49–62. doi: 10.1007/s00401-020-02160-8. Epub 2020 Apr 27. Erratum in: Acta Neuropathol. 2020 Aug;140(2):245. PMID: 32342188; PMCID: PMC7299922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Rumund A, Green AJE, Fairfoul G, Esselink RAJ, Bloem BR, Verbeek MM. α-Synuclein real-time quaking-induced conversion in the cerebrospinal fluid of uncertain cases of parkinsonism. Ann Neurol. 2019. May;85(5):777–781. doi: 10.1002/ana.25447. Epub 2019 Mar 25. PMID: 30801759; PMCID: PMC6593725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Z, Becker K, Donadio V, Siedlak S, et al. Skin α-Synuclein Aggregation Seeding Activity as a Novel Biomarker for Parkinson Disease. JAMA Neurol. 2020. Sep 28:e203311. doi: 10.1001/jamaneurol.2020.3311. Epub ahead of print. PMID: 32986090; PMCID: PMC7522783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garrido A, Fairfoul G, Tolosa ES, Martí MJ, Green A; Barcelona LRRK2 Study Group. α-synuclein RT-QuIC in cerebrospinal fluid of LRRK2-linked Parkinson’s disease. Ann Clin Transl Neurol. 2019. May 9;6(6):1024–1032. doi: 10.1002/acn3.772. PMID: 31211166; PMCID: PMC6562027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iranzo A MD, Fairfoul G Na Ayudhaya AC et al. Cerebrospinal fluid α-synuclein detection by RT-QuIC in patients with isolated rapid-eye-movement sleep behaviour disorder. Lancet Neurol in press [DOI] [PubMed] [Google Scholar]
- 96.Stefani A, Iranzo A, Holzknecht E, et al. Alpha-synuclein seeds in olfactory mucosa of isolated rapid-eye-movement sleep behaviour disorder. Brain 2020, in press. [DOI] [PubMed] [Google Scholar]
- 97.Schönecker S, Brendel M, Palleis C, et al. PET Imaging of Astrogliosis and Tau Facilitates Diagnosis of Parkinsonian Syndromes. Front Aging Neurosci. 2019;11:249. Published 2019 Sep 11. doi: 10.3389/fnagi.2019.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hansson O, Janelidze S, Hall S, et al. Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017;88(10):930–937. doi: 10.1212/WNL.0000000000003680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Herbert MK, Aerts MB, Beenes M, et al. CSF Neurofilament Light Chain but not FLT3 Ligand Discriminates Parkinsonian Disorders. Front Neurol. 2015;6:91. Published 2015 May 5. doi: 10.3389/fneur.2015.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Espay AJ, Bonato P, Nahab FB, et al. Technology in Parkinson’s disease: Challenges and opportunities. Mov Disord. 2016;31(9):1272–1282. doi: 10.1002/mds.26642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nalls MA, McLean CY, Rick J, et al. Diagnosis of Parkinson’s disease on the basis of clinical and genetic classification: a population-based modelling study. Lancet Neurol 2015; 14(10): 1002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Escott-Price V, International Parkinson’s Disease Genomics C, Nalls MA, et al. Polygenic risk of Parkinson disease is correlated with disease age at onset. Ann Neurol 2015; 77(4): 582–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McFarthing K, Buff S, Rafaloff G, Dominey T, Wyse RK, Stott SRW. Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2020. J Parkinsons Dis. 2020;10(3):757–774. doi: 10.3233/JPD-202128. PMID: 32741777; PMCID: PMC7458531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Greenland JC, Williams-Gray CH, Barker RA. The clinical heterogeneity of Parkinson’s disease and its therapeutic implications. Eur J Neurosci. 2019;49(3):328–338. doi: 10.1111/ejn.14094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.