Abstract
Little documentation of the correlation between MR imaging findings in isolated cerebellar cortical dysplasia (CCD) and its neuropathologic characteristics exists in the recent literature. We documented a postmortem neuropathologic study of a clinically and radiologically well-documented case of CCD in a neonate with severe hypotonia and status epilepticus. MR imaging revealed a global vermian hypoplasia with marked cortical dysplasia. CCD was associated with a voluminous heterotopic mass. The postmortem neuropathologic study confirmed vermian hypoplasia and CCD, which consisted of right cerebellar cortical polymicrogyria with subcortical heterotopia. CCD is a pathologic entity that could be well diagnosed with MR imaging even in the neonatal period.
In histologic studies, Rorke et al (1) showed that a high proportion of apparently normal infant brains contain one or more cerebellar heterotopias or minor dysplasia within the deep white matter. These conditions are found at the cerebellar midline or in the nodulus of the vermis and are defined as compact groups of mature neurons, focal or perivascular immature granule cell collections, mixed cell rests containing both mature neurons and immature granule cells, and heterotopias. Friede (2) defined cerebellar cortical dysplasias (CCDs) as the presence of heterotopias associated with disturbances of cortical layering or folia formation of the cerebellum. CCD has been reported in chromosomal abnormalities of the trisomy type (1, 2), congenital muscular dystrophies, and related syndromes (3); in intrauterine infections (4, 5); in gamma-radiation (6); in ethanol exposition (7); and in cases with widespread brain malformations (8). Isolated cases are infrequent (8–10).
Case Report
Clinical Data
The neonate was born prematurely at 36 weeks and 6 days. The clinical background during pregnancy revealed maternal bleeding during the first trimester and a menace of premature deliverance at 29 weeks. Signs of fetal distress led to cesarean section. Extraction was difficult because of an umbilical cord blood banking, and Apgar scores were 5, 6, and 7 at 1, 5, and 10 minutes, respectively. Cyanosis and respiratory acidosis were present, and mechanical ventilation and reanimation were necessary. Birth weight was 2655 g, and head circumference at birth was 36 cm. The child had severe hypotonia and severe difficulty in swallowing without neurologic deficit. The otorhinolaryngology examination revealed overture default of the glottis, suggesting a central neurologic disease or malformation. The clinical course was complicated, and the neurologic status was quickly impaired with severe hypotonia and tetrapyramidal signs.
Electroencephalographic tracing revealed a pattern of status epilepticus and severe and diffuse brain injury. Cerebral sonography showed an abnormally enlarged fourth ventricle, without parenchymal hypoxic or hemorrhagic lesions. MR imaging was performed to assess if cerebral malformations or brain lesions resulting from hypoxia-ischemia were present. Further clinical, metabolic, and genetic investigations were performed, including those for infection, metabolism, and conventional karyotype. The neonate survived for only 2 weeks, and a necropsy with neuropathologic study was performed.
MR Imaging Findings
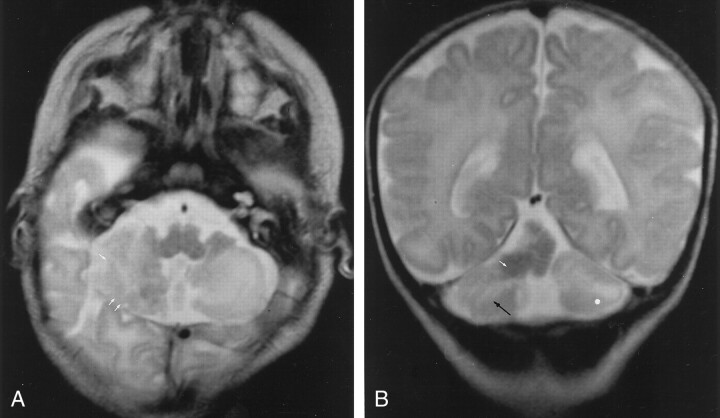
An MR imaging study was performed 1 week after birth and showed marked cortical dysplasia that affected the posteroinferior portion of the right cerebellar cortex (Fig 1A) and the anterosuperior portion of the vermis, which was hypoplasic and associated with a voluminous heterotopic mass (Fig 1B). Abnormalities of the subcortical white matter in cerebellar hemispheres were also seen (Fig 1B).
Fig 1.
MR images in CCD associated with heterotopic and vermian hypoplasia.
A, Axial T2-weighted image (5000/12/2 [TR/TE/NEX]) shows vertically oriented and disorganized folia (white arrows) in the posteroinferior part of the hemisphere.
B, Coronal T2-weighted image (5000/120/2) shows vertically oriented fissures (black arrow) in the cerebellar cortex, consistent with the diagnosis of CCD. Note cerebellar white matter hyperintensity (white dot) and heterotopia (white arrow).
Neuropathologic Data
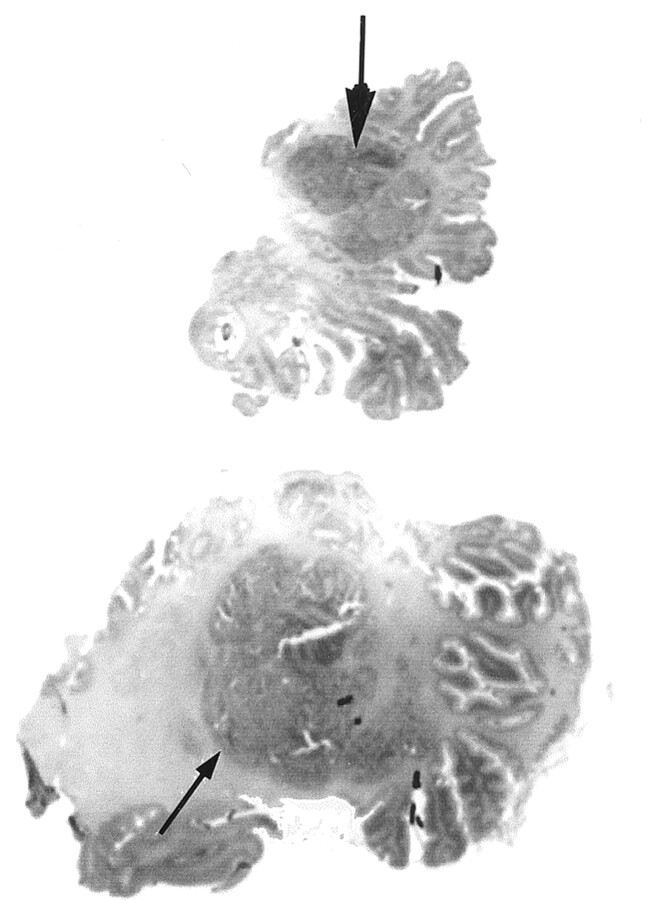
Grossly, the posterior view of the cerebellum disclosed a vermian hypoplasia. The vermis seemed to correspond to a large multinodular conglomerate made of nodules from the vermis and the internal part of the posterior right and left cerebellar hemispheres (Fig 2). In addition, the superior, inferior, and lateral parts of the hemispheres presented a primitive foliation (Fig 3A and B), and the ventricular cavity was irregular because of a large subependymal heterotopia. The brain stem did not show any obvious abnormality.
Fig 2.
Coronal microscopic sections (posterior to anterior view) of the cerebellar lesions at vermian (top) and hemispheric (bottom) levels show vermian hypoplasia with irregularly shaped foliation. A large central multinodular conglomerate (arrows) corresponding to juxtaposed ectopic polymicrogeria nodules is seen in the right hemisphere and vermis.
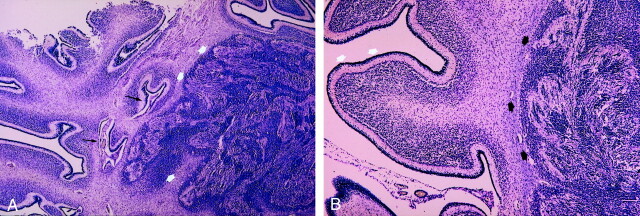
Fig 3.
Histologic sections in CCD associated with heterotopic and vermian hypoplasia.
A, Vermis and right cerebellar hemisphere show abnormal cerebellar lamella associated with typical polymicrogyria: deep folding of the cerebellar surface (black arrows). Note the heterotopic aspect of polymicrogyria in the white matter: cavities appear obliterated (white arrows). (Luxor blue stain, original magnification ×25.)
B, Right cerebellar hemisphere shows abnormally large cerebellar folia (as in premature cerebellum) (white arrows) associated with a large nodule of compact polymicrogyria with obliterated cavities and containing mixed cells (spindle, granular cells, and large neurons) (black arrows). (Luxol blue stain, original magnification ×50.)
Microscopically, the most impressive lesions were located in the medial part of the cerebellum. The lateral part was recovered with a normal layered pattern despite a primitive architecture of the folia. Nodules of chaotic appearance formed the vermis that corresponded to polymicrogyric cortical invaginations (Fig 3A). The folia were scrambled together with apparent fusing of apposed molecular layers and surrounding small cavities with meningeal vessels. Superficially, the folial pattern was completely obliterated (Fig 3A and B) but presented some areas of successive splits that always remained overlapped by thickened meninges. The right hemisphere was atrophic and more involved with polymicrogyria (Fig 2). No right nucleus dentatus was clearly identifiable. At the left hemisphere, a large heterotopic nodule of Purkinje cells was deep within the album, close to the left wall of the ventricule (not shown). The left nucleus olivary inferior was better developed than the right nucleus. At the cerebral level, no lesions were found. With von Kossa and Perls stainings, we did not find any calcification or hemosiderosis, as is often seen in inflammatory or ischemic processes. Similarly, immunohistochemical findings demonstrated absence of macrophages. No astrocytic response or glial repair was noticed.
Other postmortem findings were horseshoe-shaped kidneys and marked bilateral adrenal gland hypoplasia. Results of cerebral cortex, ocular, and muscle examinations were normal.
Discussion
To our knowledge, this is the first study of neuropathologic correlation to MR imaging findings in a neonatal case of isolated CCD. A meticulous MR imaging technique allows a good delineation of the CCD even in the neonatal period. MR imaging consisted of thin-section spin-echo T1-weighted and spin-echo T2-weighted images with a high-spatial-resolution technique, performed in coronal, axial, and sagittal planes, by using 4-mm or 3-mm section thickness, particularly for sagittal views, to obtain a good identification of the vermis and cerebellar hemispheres. In this case, marked vermian hypoplasia was associated with a voluminous heterotopic mass. The diagnosis of a tumor was inconsistent with its isointensity compared with other myelinated parts of the brain and with the absence of mass effect. Nonetheless, the right cerebellar cortex had defective folia and disorganized and vertically orientated fissures. Cortical findings were similar to those reported in previous MR imaging studies in CCD and consisted of defective, large, or vertical abnormal fissures; irregular gray matter-white matter junction; lack of normal arborization of the white matter; and heterotopia in cerebellar hemispheres, leading to disorganized foliation (8). However, our case revealed that both disorganized foliation at the cerebellar level, which was considered to be CCD, and a heterotopic mass correlated histologically to cerebellar polymicrogyria. In the superior part of the vermis and the internal part of the posterior right hemisphere, polymicrogyria has not been easily recognized. Moreover, heterotopia of Purkinje cells at the level of the left lateral wall of the fourth ventricle was not seen at MR imaging.
The abnormalities in the present case were seen only at the leptomemenigeal and external granular cell layers. These data inevitably led us to wonder whether microgyria could be the result of a pathologic process involving both leptomeninges and the external granular cell layer. Accordingly, De Leon et al (11) showed that the presence of superficially located areas of interfolial and interlamellar fusion with sparing of the deep parts of the cortex suggest a secondary nature to the malformation affecting the cerebellum after the cerebellar lamellae and folia formation. Nevertheless, Gelot et al (12) reported, in a neuropathologic study of five cases of type II lissencephaly, that CCD was present at 23 weeks. They suggested that the vasculomeningeal proliferation partially fused with the underlying external granular layer at 18 weeks. This could be the first pathologic sign followed at 20 weeks by invagination of the dysplastic cortex in the white matter and, at 23 weeks, the invagination of the deep surface that includes cortical and mesenchymal material. As the meningeal proliferation in the cerebellum is an early and transitory process during ontogenesis and as the migration of the external granular cells is a later and protracted developmental event, the migration of these cells is not dramatically affected in cerebellar polymicrogyria.
Other reports have also suggested the role of the leptomeninges in the pathogenesis of the cortical dysplasia (13). Accordingly, Mercier and Hatton (14) describe in a recent report a meningeoglial cellular network that courses through all layers of meninges bordering or joining the vasculature and extends into the periventricular subependymal layer. They suggested that all the cell types of this cooperative network may communicate and control cellular proliferation, growth, and differentiation. Because neurogenesis and gliogenesis occur within this layer, the significance of an alteration of the meningeal expansions could be important in pathologic processes such as dysplasias, glial or meningeal tumors, and neurodegenerative diseases (15).
Most previous pathologic studies in CCD were performed in animal models or in cases associated with brain malformations. The present case adds new information concerning the MR imaging findings in neonatal isolated CCD. Similar findings were reported by Aida et al (3) and Takada and Nakamura (16) in Fukuyama-type congenital muscular dystrophy and related syndromes and by Sakata-Haga et al (7) in rats prenatally exposed to ethanol. Accordingly, we found fusion of sulci in their upper parts and small cavities with meningeal penetrating blood vessels within the cortex. Bilateral intermixed layers of molecular and granule cells or ectopic bundles of myelinated fibers over the malformed cortex were not found. We also found thickened meninges. A particular finding in our case was the variable features in polymicrogyria that depended on location. In the superficial layer of the vermis and right hemisphere, polymicrogyria was well defined and consisted of multiple cavities with a rich network of meningeal tissue. In the central white matter, most of these cavities were obliterated, and the differential diagnosis between polymicrogyria and a voluminous heterotopia was difficult to obtain (Fig 3A). Such data have not been reported in muscular dystrophy, in intrauterine infection (5), or in other types of cortical dysplasia. Our findings also differ from those of gamma-radiation dysplasia, which consists of granule cell necrosis and glial Bergman cell distortion that leads to ectopic granule cells and a reduction of the distance between the pial surface and the soma of Bergmann cells (6).
Cerebellar lamination may be disturbed by processes other than a pathologic process involving leptomeninges. Genetic influences may lead to aberrant migration and misorientation of Purkinje cells followed by abnormal perivascular aggregations of the external granule cells, or disturbed arrangement of glial fibers (17), white matter signals (18), or Bergmann glia that contribute to the positioning of fissures (17). Rats exposed to ethanol show defects in glia limitans on the cerebellar surface (the Bergmann glial end feet that contact the meningeal cells) that lead to a fusion of folia and also to heterotopia, because of the role of glia limitans in the arrest of neural migration (7). Other animal models have shown cerebellar alterations induced by chronic hypoxia, which in early periods of gestation could also induce cerebellar dysplasias (5, 19). In our study, CCD was isolated; we did not find congenital muscular dystrophy or related syndromes, or inflammatory or ischemic changes, and alcohol consumption was not confirmed. However, other abnormalities such as adrenal hypoplasia and horseshoe-shaped kidney were also found, leading us to wonder whether genetic influences could have had a role in the pathogenesis of this malformation responsible for aberrant migration and then foliation (20).
Minor forms of CCD have been considered as a normal variant, because they may be diagnosed in patients undergoing MR imaging for a variety of indications (10). Nevertheless, CCD is frequently found in patients with widespread cerebral malformations, severe neurocognitive defects, global or motor developmental delay, and hypotonia (8). However, even if recent evidence suggests two types of events involved in the genesis of CCD—a genetic disorder affecting the migration of cerebellar cells and a pathologic event secondary to infection, hypoxia, or toxins such as ethanol, affecting the glia limitans or a meningeoglial network—the embryogenesis of CCD and its functional significance remain poorly understood. Correlation of CCD with neurologic focal symptoms or developmental delay is difficult to confirm because, in most cases, CCD is associated with other supratentorial malformations. As suggested by Komatsu et al (21), in patients with fetal alcohol syndrome who have mental retardation, these morphologic changes may be involved in the failure of neural circuit organization in the cerebellum, and they should be carefully evaluated in the pathologic and clinical examination of cerebellar and brain dysfunction.
Acknowledgments
We are grateful to Mme le Professeur Antoinette Gelot for her review of the manuscript and her valuable comments and to Eric D’Haese for assistance in obtaining the MR images. Cooperation of the medical and technical staff of the Department of Anatomo-Pathology, Hôpital Jeanne de Flandre, and the Department of Neuropathology, Hôpital Roger Salengro are gratefully acknowledged.
References
- 1.Rorke LB, Fogelson MH, Riggs HE. Cerebellar heterotopia in infancy Dev Med Child Neurol 1968;10:644–650 [DOI] [PubMed] [Google Scholar]
- 2.Friede RL. Developmental neuropathology. 2nd ed. Berlin Heidelberg New York: Springer-Verlag;1989. :361–371
- 3.Aida N, Tamagawa K, Takada K, et al. Brain MR in Fukuyama congenital muscular dystrophy AJNR Am J Neuroradiol 1996;17:605–613 [PMC free article] [PubMed] [Google Scholar]
- 4.Sugita K, Ando M, Makino M, Takanashi J, Fujimoto N, Niimi H. Magnetic resonance imaging of the brain in congenital rubella virus and cytomegalovirus infections Neuroradiology 1991;33:239–242 [DOI] [PubMed] [Google Scholar]
- 5.Marques Dias MJ, Harmant-van Rijckeevorsel G, Landrieu P, et al. Prenatal cytomegalovirus disease and cerebral microgyria: evidence for perfusion failure, not disturbance of histogenesis, as the major cause of fetal cytomegalovirus encephalopathy Neuropediatrics 1984;15:18–24 [DOI] [PubMed] [Google Scholar]
- 6.Inouye M, Hayasaka S, Funahshi A, et al. Gamma-radiation produces abnormal Bergmann fibers and ectopic granule cells in mouse cerebellar cortex J Radiat Res 1992;33:275–281 [DOI] [PubMed] [Google Scholar]
- 7.Sakata-Haga H, Sawada K, Hisano S, Fukui Y. Abnormalities of cerebellar foliation in rats prenatally exposed to ethanol Acta Neuropathol (Berl) 2001;102:36–40 [DOI] [PubMed] [Google Scholar]
- 8.Soto-Ares G, Delmaire C, Deries B, et al. Cerebellar cortical dysplasia: MR findings in a complex entity Am J Neuroradiol 2000;21:1511–1519 [PMC free article] [PubMed] [Google Scholar]
- 9.Demarel PH, Lievel-Lagae PC, Baert AL. MR of cerebellar cortical dysplasia AJNR Am J Neuroradiol 1998;19:984–986 [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki M, Oikawa H, Ehara S, et al. Disorganised unilateral cerebellar folia: a mild form of cerebellar cortical dysplasia? Neuroradiology 2001;43:151–155 [DOI] [PubMed] [Google Scholar]
- 11.A de Leon G, Grover W-D, Morinigo Mestre G. Cerebellar microgyria Acta Neuropath 1976;35:81–85 [DOI] [PubMed] [Google Scholar]
- 12.Gelot A, Billette de Villemeur T, Bordarier C, et al. Developmental aspects of type II lissencephaly: comparative study of dysplastic lesions in fetal and postnatal brains Acta Naeuropathol 1995;89:72–84 [DOI] [PubMed] [Google Scholar]
- 13.J Sievers, FW Pehlemann, Gude S, Berry M. A time course study of the alterations in the developpment of the hamster cerebellar cortex after destruction of the overlying meningeal cells with 6-hydroxydopamine on the day of birth J Neurocytol 1994;23:117–134 [DOI] [PubMed] [Google Scholar]
- 14.Mercier F, Hatton G. Immunocytochemical basis for a meningeo-glial network J Comp Neurol 2000;420:445–465 [DOI] [PubMed] [Google Scholar]
- 15.Mercier F, Hatton G-I. Connexin 26 and basic fibroblast growth factor are expressed primarily in the subpial and subependymal layers in adult brain parenchyma: roles in stem cell proliferation and morphological plasticity J Comp Neurol 2001;431:88–104 [DOI] [PubMed] [Google Scholar]
- 16.Takada K, Nakamura H. Cerebellar micropolygyria in Fukuyama congenital muscular dystrophy: observations in fetal and pediatric cases Brain Dev 1990;12:774–778 [DOI] [PubMed] [Google Scholar]
- 17.Kuwamura M, Morikawa T, Yamate J, et al. Glial pathology in development of cerebellar dysplasia in the hereditary cerebellar vermis defects (CVD) rat Acta Neuropathol 2000;99:305–309 [DOI] [PubMed] [Google Scholar]
- 18.Engelkamp D, Rashbass P, Seawright A, van Heyningen V. Role of Pax6 development of the cerebellar system Development 1999;126:3585–3596 [DOI] [PubMed] [Google Scholar]
- 19.Lee C, Kim D-W, Jeon G-S, et al. Cerebellar alterations induced by chronic hypoxia: an immunohistochemical study using a chick embryonic model Brain Res 2001;901:271–276 [DOI] [PubMed] [Google Scholar]
- 20.Millen KJ, Millonig JH, Wingate RJT, et al. Neurogenetics of the cerebellar system J Child Neurol 1999;14:574–582 [DOI] [PubMed] [Google Scholar]
- 21.Komatsu S, Sakata-Haga H, Sawada K, Hisano S, Fukui Y. Prenatal exposure to ethanol induces leptomeningeal heterotopia in the cerebral cortex of the rat fetus Acta Neuropathol 2001;101:22–26 [DOI] [PubMed] [Google Scholar]