Abstract
BACKGROUND AND PURPOSE: Rotational angiography (RA) and digital subtraction angiography (DSA) together may depict more intracranial aneurysms than DSA alone. We compared the diagnostic value of 3D RA and biplanar DSA in detecting, classifying, and planning treatment for ruptured intracranial aneurysms.
METHODS: A total of 53 patients with acute subarachnoid hemorrhage (Hunt and Hess grades I–V) underwent angiography with both methods. DSA was performed in two to six standard projections in every vascular territory. Three-dimensional RA datasets were evaluated by using surface-shaded display and maximum intensity projection. The usefulness of DSA images and 3D datasets in detecting aneurysms (number, configuration) and treatment planning were retrospectively analyzed in a blinded manner.
RESULTS: In 42 patients, 56 aneurysms were detected, (one to five per patient; size, 0.6–20.4 mm); no aneurysm was found in 11 patients. RA revealed seven aneurysms not seen at conventional DSA. RA failed to depict one aneurysm visible only in a compression series. Delineation of the aneurysmal neck improved with RA in 71% of cases; the parent vessel and its relationship to adjacent vessels was demonstrated better with RA than with DSA in 45% and 50%, respectively. Endovascular treatment was proposed in nine patients; microsurgical therapy, in 26. In seven patients, both options were rated as being equal. Actual treatment consisted of eight endovascular procedures and 30 neurosurgical operations. Four patients died before therapy.
CONCLUSION: Compared with DSA, 3D RA allows more exact depiction of anatomic details that are important in planning surgery and interventional therapy for intracranial aneurysms. RA depicted more aneurysms.
The diagnosis of subarachnoid hemorrhage (SAH) includes an investigation for an intracranial aneurysm as the underlying cause. The highest diagnostic accuracy is mandatory in analyzing the anatomic details of the aneurysm itself and its related vessels. The current standard of reference in the diagnostic workup of intracranial aneurysms is digital subtraction angiography (DSA), which can depict aneurysms with a diameter smaller than 3 mm (1). Competing imaging studies such as MR angiography and CT angiography are reliable in depicting aneurysms larger than 3 mm (2, 3). Because even small aneurysms 2 mm or smaller can rupture and because the presence of multiple aneurysms may change the therapeutic approach, DSA is preferred in cases of acute SAH (4). Additionally, the appropriate choice of either neurosurgical or neuroradiologic-interventional treatment is based on the optimal depiction of as many anatomic details of the aneurysm as possible.
Recently, 3D RA has entered clinical use, especially because of its shorter 3D reconstruction time and more robust equipment (5). Some authors have shown the application of RA in different fields and its effect on planning therapeutic strategies. Findings reported by Heautot et al (6) and others (7–11) support the hypothesis that RA and DSA together can depict more intracranial aneurysms than DSA alone. The objective of this blinded retrospective study was to evaluate the diagnostic benefit of RA in comparison with that of DSA in detecting, classifying, and planning treatment for intracranial aneurysms.
Methods
Subjects
Between September 1999 and September 2000, 53 consecutive patients (33 women, 20 men; mean age, 57.9 years; age range, 33–75 years) with acute SAH (Hunt and Hess grades I–V) were referred to our department for intraarterial angiography. Appropriate informed consent was obtained from the patients or their relatives according to German law. In all patients, CT had revealed SAH (Fischer grades II–IV).
Digital Subtraction Angiography
Conventional DSA and 3D DSA (Advantx-E; GE Medical Systems, Milwaukee, WI) were performed with the Seldinger method via the femoral artery. For DSA, contrast medium was manually administered by using a 10-mL syringe (6–8 mL of Solutrast 300; Byk-Gulden, Konstanz, Germany). For 3D DSA, contrast material was automatically administered by using a power injector (Angiomat Illumena; Liebel-Flarsheim, Cincinnati, OH) at a rate of 2.5 mL/s and a total amount of 13 mL. DSA series (2 frames per second) were obtained in two (posteroanterior and lateral) to six (additional right anterior or left anterior or both, oblique, and Towne) views. DSA series were rated as being insufficient for the diagnosis or exclusion of an aneurysm in nine patients (17%), because the left and right anterior oblique views were not obtained; the angiographic standard had become the acquisition of only the posteroanterior and lateral views, routinely followed by subsequent 3D DSA. Images were printed on film (Scopix Laser 2B; Agfa-Gevaert, Mortsel, Belgium) by using a laser printer (Linx LP 400; DuPont, Wilmington, DE).
Rotational Angiography
Three-dimensional datasets were obtained from rotational series consisting of two rotations. The first provided the subtraction mask. The C-arm was rotated 200° within 5 seconds at an exposure rate of 8.8 frames per second. A total of 44 images with a 512 × 512 matrix were acquired. The second rotation was performed during the administration of contrast material. All 88 images were immediately transferred to an Advantage 3.1 workstation (GE Medical Systems) via a network. Subtraction images and a primary 3D model were generated within 8 minutes (1 minute for transfer, 7 minutes for reconstruction). These were stored on the hard disk to serve as the basis for the various reconstruction algorithms.
Evaluations
Retrospectively, these angiographic data were evaluated by a neuroradiologist (M.S.) and a neurosurgeon (U.S.) with more than 20 and 11 years of experience in their specialties, respectively. They were blinded to all clinical information, including the location of the SAH. For analysis, DSA images were presented on film, and 3D DSA images were presented on the monitor screen of the workstation. All DSA series of the vascular territory covered at 3D DSA (basilar artery or left or right internal carotid artery) were presented. Three-dimensional DSA images, as well as source images, were evaluated on the workstation by using the full range of postprocessing parameters. Algorithms used were surface-shaded display (SSD) and maximum intensity projection. 3D reconstruction was performed by using SSD with a mean threshold of 1278 HU (range, 707–1930 HU). To optimize the display, we adjusted the threshold and window levels, selected contiguous objects, and cut overlying structures. Three-dimensional data sets were made anonymously. DSA was evaluated first, and 3D DSA was evaluated after 4 weeks.
Evaluation of Quality.—Two observers (U.S., M.S.) reviewed the cases simultaneously and determined findings by consensus. The overall quality of the DSA with respect to the 3D DSA series was rated on a four-point scale, as follows: 1, excellent; 2, good; 3, sufficient; and 4, insufficient. They also determined whether the number of DSA series was sufficient for diagnosing or excluding an aneurysm, according to the standard of aneurysmal diagnosis with DSA. The observers assessed the usefulness of the series for selective catheterization, the adequacy of its depiction of all regions of a vascular territory with minimal overlapping, and its depiction of findings in the early arterial phase.
Evaluation of Aneurysms.—The following group of quantifiable characteristics was evaluated: presence of aneurysms, site of the aneurysm according to the International Subarachnoid Aneurysm Trial protocol (12), and 3D measurement of the size of the aneurysmal dome and neck. A second group of descriptive characteristics was assessed as well. The quality of the depiction of the aneurysmal neck was rated on a four-point scale as follows: 1, excellent; 2, good; 3, sufficient; and 4, insufficient. The spatial relation of an aneurysm to the parent vessel and adjacent vessels (involved vs not involved, overlying vs not overlying) and vascular characteristics (eg, vasospasm, concomitant vascular malformations, vascular elongation, arteriosclerotic disease).
Evaluation of Therapeutic Impact.—We evaluated the effect of DSA and 3D DSA results on therapeutic decision making (ie, endovascular treatment vs surgery), and we estimated the risk of the chosen procedure as follows: 1, low; 2, intermediate; and 3, high. In surgery, low risk meant that surgical access to the site of the aneurysm was simple, the chosen surgical strategy was not expected to result in complications, and clip placement in the aneurysm was secure. Intermediate risk meant that access to the aneurysm was more complex and that preparation of the aneurysm was more difficult because of the proximity of important neuronal structures, which also resulted in a higher risk of aneurysmal rupture during the procedure. High risk meant that access and preparation were difficult and that a risk of incomplete clipping was present. In endovascular treatment, low risk meant that access for catheter navigation was easy, the aneurysm had a defined narrow neck, and complete occlusion was expected. Intermediate risk described more difficult access to the aneurysm and a broader neck with the possibility of incomplete occlusion or the need for remodeling techniques such as balloon or stent protection. High risk indicated anatomically difficult access to the aneurysm, which had a broad neck or arteries arising from the aneurysmal sack or both.
Statistical Analysis
The depiction of intracranial aneurysms was statistically evaluated by using logistic regression (χ2 test) to test the significance of differences between 3D DSA and DSA. The results of the evaluation were compared with those of the real clinical therapy and the course in each case. The estimation of the risk of the therapeutic procedures, as determined during the reading of the DSA and 3D DSA images, was correlated with the real risk of surgery or neurointervention by reviewing the surgical or interventional report and by classifying the risk of the procedures as low, intermediate, or high as just described. The surgical and interventional reports were used as the criterion standard for the findings at angiography.
Results
No procedure-related morbidity or mortality occurred with 3D RA, and the examination was well tolerated by all 53 patients. In 28 (53%) patients examined without general anesthesia, complaints centered on the automated administration of contrast medium, which caused a prolonged feeling of heat, in comparison with the manual administration of contrast material at DSA.
Evaluation of Quality
The overall quality of DSA (mean rating, 1.2) as well as 3D DSA (mean rating, 1.3) was excellent. The quality of three DSA and five 3D DSA examinations was rated as only sufficient because of movement artifacts. All five sufficient 3D DSA examinations were performed without general anesthesia.
Evaluation of Aneurysms
Quantifiable Characteristics: Presence, Site, and Dimensions of Aneurysms.—In 42 patients (79%), 56 aneurysms were detected (one to five per patient). Of the 56 aneurysms, 19 (34%) were located at the internal carotid artery; 16 (29%), at the middle cerebral artery; 11 (20%), at the anterior communicating artery; three (5%) at the anterior cerebral artery; three (5%), at the posterior communicating artery; and four (7%), in the posterior circulation (three basilar arteries, one posterior cererbral artery).
In the remaining 11 patients, neither DSA nor 3D DSA revealed an aneurysm. One aneurysm depicted at DSA was not seen at 3D DSA because of its site. It was located at the posterior communicating artery and was seen in the DSA series only with an injection into the vertebral artery during manual compression of the ipsilateral common carotid artery, as is routinely done to show the circle of Willis. Filling of the posterior communicating artery via the internal carotid artery was not possible, and 3D DSA in this case had been performed during an injection into the internal carotid artery. Seven aneurysms that were not seen with DSA were detected with 3D DSA (Table; Figs 1 and 2). In two of these cases, the DSA series was rated as insufficient for the exclusion or diagnosis of an aneurysm. Four of the aneurysms that were seen at 3D DSA and not at DSA were surgically proven, and two were proven at repeat angiography. One aneurysm was seen in a patient who died before therapy.
Location and size of aneurysms detected only at 3D RA
| No. | Location* | Width × Length × Neck Size, mm | Status | DSA |
|
|---|---|---|---|---|---|
| Quality† | Series Sufficient‡ | ||||
| 1 | Ophthalmic | 1.4 × 2.3 × 2.3 | Unruptured | Excellent | Yes |
| 2 | Ophthalmic | 1.1 × 0.9 × 0.9 | Unruptured | Excellent | Yes |
| 3 | A2 segment of the ACA | 1.4 × 1.7 × 1.7 | Unruptured | Excellent | Yes |
| 4 | MCA bifurcation | 2.2 × 2.2 × 1.6 | Ruptured | Excellent | Yes |
| 5 | MCA bifurcation | 1.6 × 1.5 × 1.6 | Unruptured | Excellent | Yes |
| 6 | MCA bifurcation | 2.3 × 2.2 × 2.7 | Ruptured | Excellent | No |
| 7 | M1 segment of the MCA | 1.1 × 1.5 × 1.5 | Unruptured | Excellent | No |
ACA indicates anterior cerebral artery; MCA, middle cerebral artery.
The overall quality of the DSA images with respect to the 3D DSA series was graded as excellent, good, sufficient, or insufficient.
The sufficiency of the number of DSA series in diagnosing or excluding an aneurysm was assessed in terms of the following: usefulness for selective catheterization, adequacy in the depiction of all regions of a vascular territory with minimal overlapping, and depiction of the early arterial phase.
Fig 1.
Angiograms in a patient with SAH Hunt and Hess grade I.
A, SSD reconstruction,
B, DSA series in a small, middle cerebral arterial aneurysm accompanied by a second developing aneurysm, which was identified only at RA.
Fig 2.
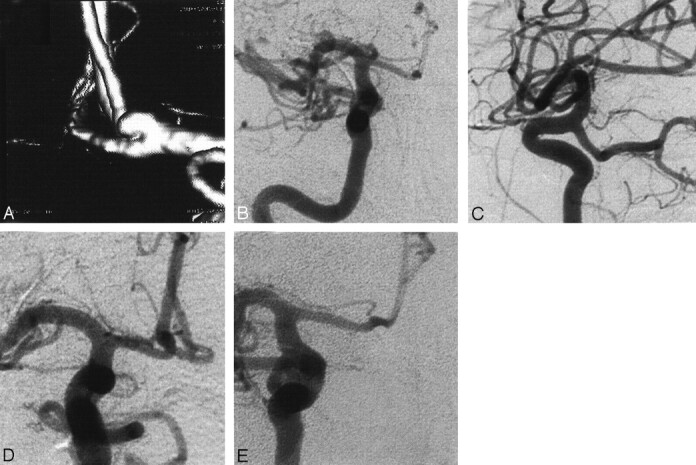
Angiograms in a patient with SAH Hunt and Hess grade II.
A, SSD reconstruction.
B–D, DSA views of an anterior communicating arterial aneurysm that was visualized only at RA.
Overall, the maximum diamter of the aneurysms was 0.6–20.4 mm, the mean size was 3.8 × 6.1 mm, and the mean neck width was 3.8 mm (range, 0.6–10.1 mm). All aneurysms not detected at DSA (mean size, 1.9 mm) were located in the anterior circulation. The DSA series was rated as being insufficient for two aneurysms; therefore, only five aneurysms can be regarded as being truly nondiagnostic at DSA.
Descriptive Characteristics: Quality and Therapeutic Risk and Strategy.—A total of 49 aneurysms were depicted with both methods. The quality of the depiction of the aneurysmal neck at DSA was equal with that of 3D DSA in 23% of patients, depiction was better with 3D DSA than with DSA in 71%, and depiction was worse with 3D DSA than with DSA in 7% because of motion artifacts. The mean improvement in depiction was 1.02 on the aforementioned four-point scale. The mean quality was good to sufficient (score, 2.7) for DSA and excellent to good (score, 1.6) for 3D DSA (P < .001). In 11 DSA examinations, the depiction was rated as insufficient for treatment planning; in nine patients, an insufficient number of DSA views had been obtained.
In 45% of all cases, better depiction of the parent vessel was achieved by using 3D DSA, and an involvement could be excluded or confirmed definitely. In 50% the location of the aneurysm with respect to the neighboring vessels was clarified (Figs 3, 4). Five (9%) of 53 patients had vasospasm at examination.
Fig 3.
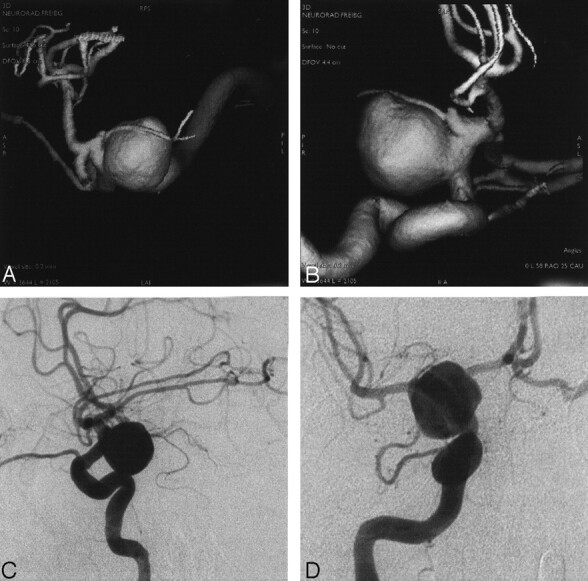
Angiograms in a patient with SAH Hunt and Hess grade IV.
A and B, SSD-reconstructions.
C and D, DSA series in a large internal carotid arterial aneurysm. Its relation to the parent vessel and neighboring vessels were superiorly visualized at RA.
Fig 4.
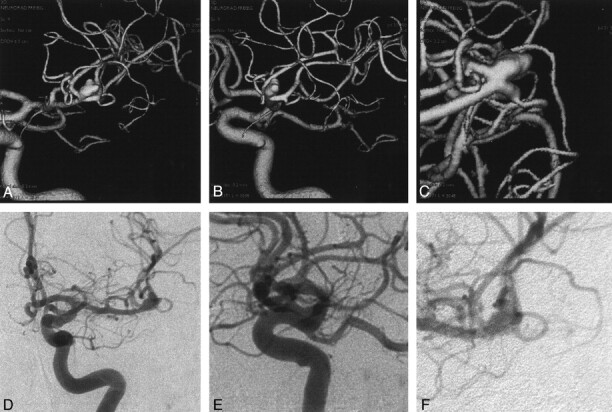
Angiograms in a patient with SAH Hunt and Hess grade II.
A–C, SSD-reconstructions.
D–F, DSA series in an aneurysm in the bifurcation of the middle cerebral artery. The extent, neck, and spatial relationship of the aneurysm to the neighboring and parent vessels was reliably demonstrated at RA.
Evaluation of Therapeutic Impact
Because of the different estimated risks of the therapeutic regimens, in nine of 42 patients a neuroradiologic-endovascular procedure was recommended, and in 26 of 42 a microsurgical procedure was recommended. In seven patients, the options for microsurgical versus endovascular were rated as being equal, according to the observers’ opinions and the standards for treatment at our hospital. In two patients, an aneurysm was assumed to be present after DSA images were reviewed, but these could not be diagnosed with sufficient confidence. Without 3D DSA, surgical exploration would have been recommended. However, 3D DSA depicted the aneurysms; therefore, a clear therapeutic decision could be made.
In seven of 42 patients, knowledge of 3D DSA findings changed the therapy, in comparison with the decision made by reviewing only the DSA series.
No therapy could be established in four patients because of their rapid clinical deterioration and death; therefore, the proposed and actual therapies could not be compared. Therefore, actual therapy consisted of eight endovascular procedures and 30 neurosurgical operations.
The estimation of the risk of a procedure led to the correct classification of the risk in 19 (45%) of 42 procedures after 3D DSA images were reviewed. Six cases had a low procedural risk; nine cases, intermediate risk; and four cases, high risk. The procedural risk in only nine (21%) of 42 procedures were correctly classified after DSA images were reviewed: Three cases had a low risk; four cases, intermediate risk; and two cases, high risk. No tendency to under- or overestimate risks with either of the methods was present, when compared with the real situation at surgery or intervention.
Discussion
Despite earlier proposals to use 3D-like modalities to overcome the problems of conventional angiography in precisely depicting intracranial vasculature (6, 13), 3D RA has only recently become a clinically practical tool. The main advantage, as other authors (5, 10, 14, 15) reported, is the 3D visualization of complex anatomic vascular patterns. Therefore, both therapeutic strategies benefit from improved depiction of the spatial relationship of the aneurysm to the aneurysm-bearing vessel and the surrounding vascular structures (16). The better understanding of the detailed anatomic situation facilitates the planning of safe treatment. In particular, interventional procedures benefit from the exact (or, in this study, significantly better) visualization of the neck of the aneurysm, which helps in deciding whether an aneurysm is generally suited for coil placement (17). The more intimate knowledge of the anatomic details is also helpful in determining the right site, the shape of the coils to be used for endovascular packing, and the angiographic working position during coil deployment. The improvement was considerable in our study. The size of the aneurysm and the diameter of the neck can be correctly measured with 3D DSA, as was proved in a recent in vitro study (18). The difference between real and measured size of the object does not exceed 0.4 mm. In comparison, despite standardization with catheter calibration, the measurement with DSA is less precise.
A drawback of the present study was the incomplete number of conventional DSA series in nine patients. However, only two of the seven aneurysms were not detected at DSA; the reason was an insufficient number of views. Even if we exclude these two patients, several aneurysms (five [9%]) were detected only with 3D DSA. Thus, the rate of false-negative results can generally be reduced by using 3D DSA, particularly in small aneurysms that can be obscured by the parent vessel at DSA, even with the use of different views. Nevertheless, although aneurysms smaller than 1 cm have a lower risk of bleeding (19–21), two such aneurysms bled.
The advantages of 3D DSA may have an impact on the patient’s therapy, and 3D DSA may augment the role of DSA as the criterion standard in assessing intracranial aneurysms (22, 23). Untreated aneurysms that occur after the first SAH have bleeding risk of 0.5% per year (21); this fact is particularly important, because multiple aneurysms were found in seven (13%) of 53 patients. Nonetheless, the importance of DSA is well demonstrated in an aneurysm of the posterior communicating artery that was seen only on a compression series. In cases with ambiguous findings, 3D DSA can be performed with manual compression of the common carotid artery, as we tested in some patients. However, this technique was not performed in any of the patients in this study.
Image quality in five (9%) of 53 total 3D DSA examinations were rated as only sufficient because of inadvertant patient motion, which occured when the diagnostic examination was started without general anesthesia.
The evaluation was performed without knowledge of the clinical presentation and course to eliminate any influence on the analysis of the true anatomy and on the estimation of the treatment risk. Compared with DSA, 3D DSA enabled more realistic estimation of the therapeutic risk. The risk was correctly estimated in 19 of 42 examinations with 3D DSA, compared with nine of 42 examinations DSA. This finding clearly illustrates the effect of 3D angiography in the development of a therapeutic strategy.
An evaluation of 3D DSA always requires the postprocessing and review of data at an external workstation, which can exceed 15 minutes, including the time for data transfer, reconstruction, and anatomic analysis with multidirectional views. In the present study, we used SSD for reconstruction. In the widely applied postprocessing method for spiral CT data, volume rendering is the favored procedure for reconstruction (24, 25). However, the theoretical disadvantage of SSD, that is, losing information by defining a threshold of depicted Hounsfield units and by defining only contiguous objects for presentation, is offset by much faster postprocessing if different magnifications and many views are used.
Conclusion
RA in combination with DSA enabled the detection of more aneurysms than DSA alone. The depiction of the aneurysms themselves was substantially better, and a therapeutic decision could be made with more confidence. From the neurosurgeon’s point of view, the anatomic situation was better clarified, and this knowledge helped to eliminate surprises during surgical preparation. From the neuroradiologist’s point of view, the decision to perform coil placement could be made more easily and safely, because exact visualization of the aneurysmal neck was possible. Three-dimensional DSA may become an important add-on technique to preserve the role of DSA as the criterion standard in the detection of intracranial aneurysms.
Footnotes
Presented at the meeting of the European Society of Neuroradiology, Ancona, Italy, 2001, and at the meeting of the German Society for Neuroradiology, Munich, Germany, 2001.
References
- 1.Urbach H, Zentner J, Solymosi L. The need for repeat angiography in subarachnoid haemorrhage. Neuroradiology 1998;40:6–10 [DOI] [PubMed] [Google Scholar]
- 2.Metens T, Rio F, Baleriaux D, Roger T, et al. Intracranial aneurysms: detection with gadolinium-enhanced dynamic three-dimensional MR angiography-initial results. Radiology 2000;216:39–46 [DOI] [PubMed] [Google Scholar]
- 3.Korogi Y, Takahashi M, Katada K, et el. Intracranial aneurysms: detection with three-dimensional CT angiography with volume rendering—comparison with conventional angiographic and surgical findings. Radiology 1999;211:497–506 [DOI] [PubMed] [Google Scholar]
- 4.Schievink WI, Piepgras DG, Wirth FP. Rupture of previously documented small asymptomatic saccular intracranial aneurysms: report of three cases. J Neurosurg 1992;76:1019–1024 [DOI] [PubMed] [Google Scholar]
- 5.Bidaut LM, Laurent C, Piotin M, et al. Second-generation three-dimensional reconstruction for rotational three-dimensional angiography. Acad Radiol 1998;5:836–849 [DOI] [PubMed] [Google Scholar]
- 6.Heautot JF, Chabert E, Gandon Y, et al. Analysis of cerebrovascular diseases by a new 3-dimensional X-ray angiography system. Neuroradiology 1998;40:203–209 [DOI] [PubMed] [Google Scholar]
- 7.Schumacher M, Kutluk K, Ott D. Digital rotational radiography in neuroradiology. AJNR Am J Neuroradiol 1989;10:644–649 [PMC free article] [PubMed] [Google Scholar]
- 8.Unger B, Link J, Trenkler J, et al. Digital 3D rotational angiography for the preoperative and preinterventional clarification of cerebral arterial aneurysms. Röfo Fortschr Geb Röntgenstr Neuen Bildgeb Verfahr 1999;170:482–491 [DOI] [PubMed] [Google Scholar]
- 9.Biederer J, Link J, Peter D, et al. Rotational digital subtraction angiography of carotid bifurcation stenosis. Röfo Fortschr Geb Röntgenstr Neuen Bildgeb Verfahr 1999;171:283–289 [DOI] [PubMed] [Google Scholar]
- 10.Tanoue S, Kiyosue H, Kenai H, et al. Three-dimensional reconstructed images after rotational angiography in the evaluation of intracranial aneurysms: surgical correlation. Neurosurgery 2000;47:866–871 [DOI] [PubMed] [Google Scholar]
- 11.Elgersma OE, Wust AF, Buijs PC, et al. Multidirectional depiction of internal carotid arterial stenosis: three-dimensional time-of-flight MR angiography versus rotational and conventional digital subtraction angiography. Radiology 2000;216:511–516 [DOI] [PubMed] [Google Scholar]
- 12.International Subarachnoid Aneurysm Trial, Trial Protocol. ISAT Trial Vessel Identification Codes. Oxford, England:1997
- 13.Hering M, Wakhloo AK, Zwicker C, et al. Multi-project angiography in the imaging of cerebral aneurysm. Aktuelle Radiol 1998;8:169–175 [PubMed] [Google Scholar]
- 14.Anxionnat R, Bracard S, Picard L, et al. 3D Angiography first results in intracranial aneurysms. Riv Neurorad 1999;12(S2):85–87 [Google Scholar]
- 15.Tu RK, Cohen WA, Maravilla KR, et al. Digital subtraction rotational angiography for aneurysms of the intracranial anterior circulation: injection method and optimization. AJNR Am J Neuroradiol 1996;17:1127–1136 [PMC free article] [PubMed] [Google Scholar]
- 16.Ishihara S, Ross IB, Piotin M, et al. 3D Rotational angiography: recent experience in the evaluation of cerebral aneurysms for treatment. Intervent Neurorad 2000;6:85–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anxionnat R, Bracard S, Ducrocq X, et al. Intracranial aneurysms: clinical value of 3D digital subtraction angiography in the therapeutic decision and endovascular treatment. Radiology 2001;218:799–808 [DOI] [PubMed] [Google Scholar]
- 18.Husstedt H, Trousset Y, Klisch J, Schumacher M. Precision of 3D-DSA for imaging intracranial aneurysms. Eur Rad 2000;10(S1):121 [Google Scholar]
- 19.Nehls DG, Flom RA, Carter LP, et al. Multiple intracranial aneurysms: determining the site of rupture. J Neurosurg 1985;63:342–348 [DOI] [PubMed] [Google Scholar]
- 20.Juvela S, Porras M, Poussa K. Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. J Neurosurg 2000;93:379–387 [DOI] [PubMed] [Google Scholar]
- 21.ISUIA. Unruptured intracranial aneurysms: risk of rupture and risks of surgical intervention—International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med 1998; 10;339:1725–1733 [DOI] [PubMed] [Google Scholar]
- 22.Asari S, Ohmoto T. Natural history and risk factors of unruptured cerebral aneurysms. Clin Neurol Neurosurg 1993;95:205–214 [DOI] [PubMed] [Google Scholar]
- 23.Missler U, Hundt C, Wiesmann M, Mayer T, Brückmann H. Three-dimensional reconstructed rotational digital subtraction angiography in planning treatment of intracranial aneurysms. Eur Radiol 2000;10:564–568 [DOI] [PubMed] [Google Scholar]
- 24.Hong KC, Freeny PC. Pancreaticoduodenal arcades and dorsal pancreatic artery: comparison CT angiography with three-dimensional volume rendering, maximum intensity projection, and shaded-surface display. AJR Am J Roentgenol 1999;172:925–931 [DOI] [PubMed] [Google Scholar]
- 25.Addis KA, Hopper KD, Iyriboz TA, et al. CT angiography: in vitro comparison of five reconstruction methods. AJR Am J Roentgenol 2001;177:1171–1176 [DOI] [PubMed] [Google Scholar]



