Abstract
BACKGROUND AND PURPOSE: The radiologic diagnosis of idiopathic intracranial hypertension (IIH) is one of exclusion, with no reproducible positive features described in the imaging literature. Because MR venography is prone to flow artifacts, diagnosis of secondary intracranial hypertension (SIH) can also be problematic. Vascular hydraulics can be useful for diagnosis of these conditions when measured by invasive or sonographic means. The purpose of this study was to measure vascular flow and pulsatility characteristics with a noninvasive MR imaging method.
METHODS: Twelve patients with clinical and lumbar puncture findings of IIH or SIH and 12 control subjects were examined with MR venography and MR flow quantification studies of the cerebral arteries and veins. Total cerebral, superior sagittal sinus (SSS), and straight sinus blood flows were measured. Pulsatility indices from the arterial and venous flow for all patients were compared using the Student t test.
RESULTS: MR venography confirmed that seven of the 12 patients had venous outflow obstruction, and thus, SIH. The remaining five patients had IIH. All patients showed reduced sinus pulsatility compared with that of the control group; reductions of 42% in the SSS and 32% in the straight sinus were noted (P = .0001 and .005, respectively). In the IIH group, total blood flow was 46% higher than that in the control group (P = .0002), and SSS flow was normal. In the SIH group, total blood flow was normal; however, SSS flow was reduced by 25% (P = .003).
CONCLUSION: Reduced venous sinus pulsatility is a marker of intracranial hypertension secondary to raised venous sinus pressure. When suspicion of IIH or SIH exists and the MR venogram is difficult to interpret, raised total blood flow indicates IIH, whereas reduced SSS flow indicates SIH.
The vascular hydraulics of the syndrome underlying idiopathic intracranial hypertension (IIH), also known as benign intracranial hypertension or pseudotumor cerebri, have defied an exact understanding. Clinically, idiopathic cases are classically seen in obese women of child-bearing years and in patients with headache and papilledema at presentation (1). CSF pressures are elevated at lumbar puncture, but by definition, the composition of the CSF is found to be normal. CT and MR images show either no abnormality or some reduction in ventricular size, but the imaging literature reports no consistent positive features of this disease process (1). Thus, IIH remains a diagnosis of exclusion.
IIH all too frequently leads to loss of visual function that may progress to blindness (2). A positive diagnosis is desirable, because therapies for the idiopathic and secondary causes of raised intracranial pressure are often different. The secondary causes usually are related to venous sinus outflow obstruction (1). Dural sinus thrombosis can be identified in up to 26% of patients with symptoms of typical IIH (3), but cerebral venous thrombosis is often overlooked when intracranial hypertension is isolated (4).
MR venography provides an excellent noninvasive way to view venous sinuses (5) and is useful for the diagnosis of the secondary causes of intracranial hypertension. However, pitfalls and artifacts limit the usefulness of this technique in subtle cases (5, 6). King et al (7) found that both the venous phase of cerebral angiography and MR venography were not totally reliable in diagnosing minor obstruction because of streaming artifacts. Thus, a noninvasive measure of the vascular hydraulics of intracranial hypertension is desirable.
Karahalios et al (8) have hypothesized that the universal underlying mechanism of IIH and secondary intracranial hypertension (SIH) is elevation of the venous sinus pressure, but sinus pressure cannot be measured noninvasively. The pulsatility found in the venous outflow of the brain is due to the compliance within the walls of the veins, with passage of CSF pulsation through the walls of the veins (9). An increase in the pressure within the veins should reduce the compliance of their walls, and thus, the pulse wave transfer. The venous pulsatility index is thought to be an indirect measure of the venous sinus pressure, and therefore, of the underlying pathologic mechanisms of IIH and SIH. Total cerebral, superior sagittal sinus (SSS), and straight sinus blood flow have been used to differentiate the two subgroups. Selective SSS and straight sinus outflow obstruction in SIH should reduce the respective venous sinus flow. Because of autoregulation, a commensurate increase in collateral flow should maintain total blood flow. Cordell et al (1) suggested that total blood flow is increased in IIH, but as yet to my knowledge, this has not been confirmed with MR flow quantification. The purpose of this study was to define how this increased flow may contribute to the underlying pathologic mechanisms of IIH.
Methods
Subjects
This investigation spanned June 1999 to February 2001. Twelve patients fulfilled the clinical criteria for IIH: headache at presentation, with or without visual obscuration; raised CSF pressure (>18 cm H2O); and no abnormalities of CSF composition. This patient group comprised 10 female and two male patients with mean age of 34 ± 14 (SD) years (range, 13–52 years). This group was subdivided on the basis of the MR venographic findings into seven patients (five female and two male) who had evidence of significant venous outflow obstruction (ie, the SIH group) and the remaining five patients (all female) with IIH (ie, the IIH group). A control group was selected from patients referred to the MR unit for diagnostic studies for conditions not related to raised intracranial pressure, chronic headache, or ischemia and comprised 10 female and two male patients with mean age of 33 ± 12 (SD) years (range, 13–53 years). Informed consent was obtained from all patients, and the hospital ethics committee reviewed the study protocol.
MR Imaging and Analysis
All patients were examined on an MR unit (Magnetom Vision; Siemens Medical Systems, Erlangen, Germany) with a 1.5-T superconducting magnet. A retrospectively cardiac-gated, phase contrast, flow quantification sequence was used with 29/7/1 (TR/TE/NEX), 30° flip angle, 6-mm section thickness, 192 × 512 matrix, and 200-cm field of view. Velocity encoding values of 40 and 75 cm/s were used. The lower velocity encoding value was selected to maximize measurement of venous structures, with the higher value used to maximize the arterial measurements. Section plane was selected to intersect the SSS approximately 2 cm above the confluence of sinuses and to continue through the straight sinus and pass through the basilar artery and the cavernous portion of the internal carotid arteries, as per the literature (9). Regions of interest were placed around the SSS, the straight sinus, the carotid arteries, and basilar artery in each patient. Care was taken to exclude aliasing by retrospectively manipulating the baselines of each resultant graph, giving an effective venous upper flow limit of 80 cm/s and arterial flow limit of 125 cm/s.
The addition of the flow in both the carotid and the basilar arteries gave the total blood flow for each patient. The SSS and straight sinus flows were calculated from the respective regions of interest. Pulsatility indices for the arterial input and straight sinus and SSS outputs were obtained. Pulsatility indices were derived at each site by subtracting the end diastolic flow from the peak systolic flow and dividing by the mean flow. Mean and standard deviations were obtained for each group of patients.
Analysis of variance statistics were performed. Differences between the groups were tested by use of an unpaired t test. A P value of less than .05 was considered to indicate a statistically significant difference.
Results
Findings are summarized in the Table.
Blood flow and pulsatility
| Group | Volume (mL/min) |
Pulsatility Index | ||||
|---|---|---|---|---|---|---|
| SSS | Straight Sinus | Total | Arterial | SSS | Straight Sinus | |
| Control (n = 12) | 400 (65) | 117 (35) | 855 (150) | 0.72 (0.16) | 0.41 (0.11) | 0.50 (0.15) |
| IIH (n = 5) | 467 (94) | 162 (50) | 1248 (147) | 0.58 (0.16) | 0.25 (0.02) | 0.35 (0.05) |
| SIH (n = 7) | 300 (49) | 113 (51) | 853 (77) | 0.72 (0.37) | 0.23 (0.05) | 0.34 (0.10) |
| ANOVA P Value* | .001 | .012 | <.001 | .507 | <.001 | .019 |
Note.—Data are the mean. Numbers in parentheses are standard deviations.
ANOVA indicates analysis of variance.
Mean Blood Flow
Mean total blood flow in the IIH group was 46% greater than that in the control group (P = .0002), and straight sinus blood flow was 38% greater (P = .05). Although the SSS blood flow in the IIH group was 17% greater than that in the control group, this failed to reach significance. The SIH group showed no significant difference from the control group in terms of total blood flow or straight sinus blood flow. The SIH group showed a 25% reduction in SSS flow compared with that in the control group (P = .003). Comparison between the IIH group and the SIH group showed that total blood flow for the IIH group was 46% higher and SSS flow was 56% higher than those values in the SIH group (P = .0001 and P = .02, respectively). Although the straight sinus flow was 43% higher, this failed to reach significance.
Vascular Pulsatility
No significant difference was noted between the IIH and SIH groups for any of the pulsatility indices measured. Taken as a group, the patients with raised intracranial pressure showed a reduction in SSS pulsatility of 41% and a reduction in straight sinus pulsatility of 32% compared with the control group (P = .0001 and P = .005, respectively). The arterial pulsatility was not significantly different between the groups.
Discussion
A noninvasive test is needed for the diagnosis of IIH, which ideally should be based on an understanding of underlying vascular hydraulics. Despite much recent knowledge, the exact mechanisms of IIH remain unclear. The most commonly mentioned causative factors are increased resistance to CSF outflow, increased cerebral blood volume, and diffuse cerebral edema (10). In a effort to link these causative factors, Johnson and Patterson (11) suggested that IIH is caused by altered CSF resorption, owing to increased sagittal sinus venous pressure, which reverses the CSF to the sinus pressure gradient necessary to drive bulk flow of CSF. Karahalios et al (8) expanded on the above hypothesis, stating that elevated intracranial venous pressure is the underlying universal mechanism in all “pseudotumor cerebri.” They showed that the SSS pressure averaged 17 mm Hg in both IIH and SIH, with normal pressure ranging from 4 to 10 mm Hg. The patients with evidence of venous obstruction (SIH) had a focal pressure gradient at the obstruction, whereas the patients with normal anatomy (IIH) had elevated right atrial pressures that were associated with high sinus pressures (8). Pressure measurement requires direct invasive techniques; in this study, I sought to find a noninvasive alternative to measure the same vascular hydraulics.
Pulsatility
The venous outflow from the cranial sinuses is pulsatile. Stolz et al (12), by using transcranial Doppler sonography, found the mean resistive index in the SSS to be 0.36. The pulsatile nature of the venous outflow is shown in Figure 1, in which the arterial waveform is compared with the venous waveform in a healthy subject. The resistive index, and thus pulsatility, is always higher in the venous sinuses than in the cortical veins (12).
Fig 1.
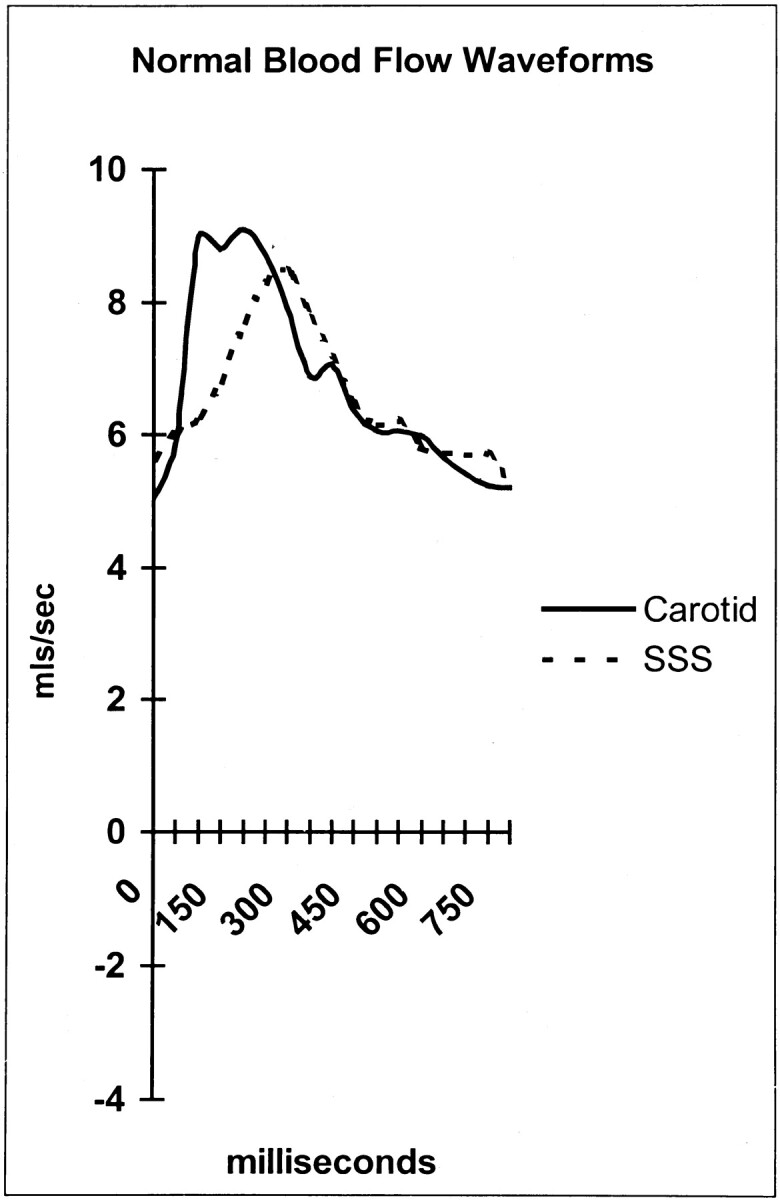
Arterial and SSS waveforms in a healthy subject. mls indicates milliliters.
William Harvey (13) noted that the blood flowing out of the capillaries and the smallest veins does not pulsate, and he suggested that systemic arteries decrease flow pulsation within the great vessels. This raises the question, if the arteries decrease in pulsation before the capillary bed, how do the pulsations reemerge and appear to increase as the blood passes into the venous sinuses? The answer seems to be a consequence of the way the arterial tree filters the pulsation. The pulsation is passed into the CSF space because of arterial wall compliance (ie, expansion and contraction of vessel walls). The Monroe-Kellie doctrine states that, as the intracranial volume is fixed and as the brain and arterial tree expand in systole, then a volume, equal to this increase, must be expelled from the cranial cavity (14). The Monroe-Kellie doctrine predicts that the volume expelled is essentially a combination of CSF vented by the foramen magnum and venous blood (14). CSF is known to be vented at the beginning of systole, and the venous blood pulsation is vented on average 90–100 ms later in mid-to-late systole (9); note the delay between arterial and venous peaks in Figure 1. Thus, instead of passing through the capillary bed, the pulsatile components of the blood supply pass into the CSF from the arteries down into the spinal canal, and finally, out into the venous outflow. The passage of the pulsation through the venous walls is, as shown by the transcranial Doppler sonography data presented herein (12), preferentially through the venous sinus walls rather than through the cortical veins, which seems counterintuitive.
Greitz et al (15) suggested that the high compliance afforded by the walls of the venous sinuses is a function of the arachnoid granulations. This compliance depends on pressures on either side of the venous wall being normal. If, as in IIH and SIH, the sinus pressure is greatly elevated, then the sinus wall compliance is expected to decrease, and consequently, the pulsatility of the sinus blood flow is also expected to decrease. These were the findings in both patient groups, with a mean SSS pulsatility reduction of 41% (P = .0001). This finding is depicted in Figure 2, in which the arterial waveform is compared with the reduced (flattened) venous waveform in a patient with SIH. The venous pulsatility is an indirect, noninvasive indication of the relative reduction in sinus wall compliance in the brain in the same way that hepatic vein pulsatility can be used to gauge a reduction in wall compliance in liver disease (16). Dampening of the hepatic vein signal intensity on sonograms has been observed in acute and chronic liver disease and has been attributed to reduced hepatic compliance (16). In this instance, the reduction in compliance is due to raised sinus pressure. The pulsatility is reduced in both the SSS and straight sinus, suggesting reduced compliance and increased pressure in both the superficial and deep portions of the brain. Ventricular dilatation in hydrocephalus is a consequence of a compliance and pressure gradient developing across the brain parenchyma (9). The lack of such a gradient in IIH and SIH may be the reason why ventricular dilatation does not develop in these two conditions, despite raised CSF pressure.
Fig 2.
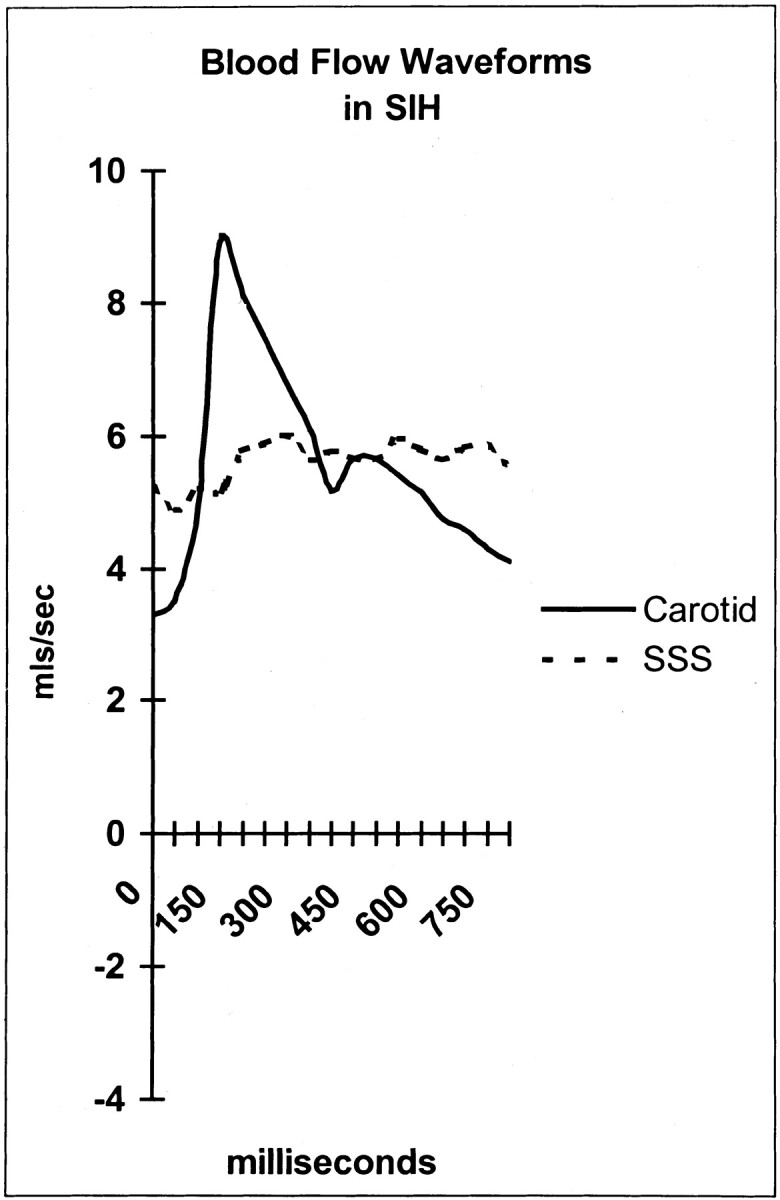
Arterial and SSS waveforms in a patient with SIH. Note the flat venous flow. mls indicates milliliters.
Blood Flow
Normal blood flow to the brain has been previously investigated with MR flow quantification. Marks et al (17) assessed normal total cerebral blood flow by using MR flow quantification with a technique similar to that used in this study and obtained a mean value of 858 mL/min. The mean normal blood flow in this series was 855 mL/min. The literature stresses that considerable variation exists in the mean blood flow in the SSS (10). Published flow rates vary from a low of 282 mL/min by Jordan et al (18), a mean value of 418 mL/min by Mattle et al (19), to a high value of 443 mL/min by Gideon et al (10). The mean value of 400 mL/min found in this series is within the published range, but the variation in normal shows the difficulty in basing a diagnostic decision on SSS flow alone. The variation in flow is not related to a variation in total flow, which is closely regulated by the brain on the basis of brain weight and metabolism. These latter variables do not vary as much as the SSS flow would seem to indicate. The variability would seem to depend on the degree to which collateral pathways such as the veins of Labbé, middle cerebral veins, or emissary veins come into play in any one patient. Little has been published on normal straight sinus flow. A series of patients with a mean age of 58 years had a mean flow of 78 mL/min (9); in this series of younger patients, the mean flow rate was 117 mL/min.
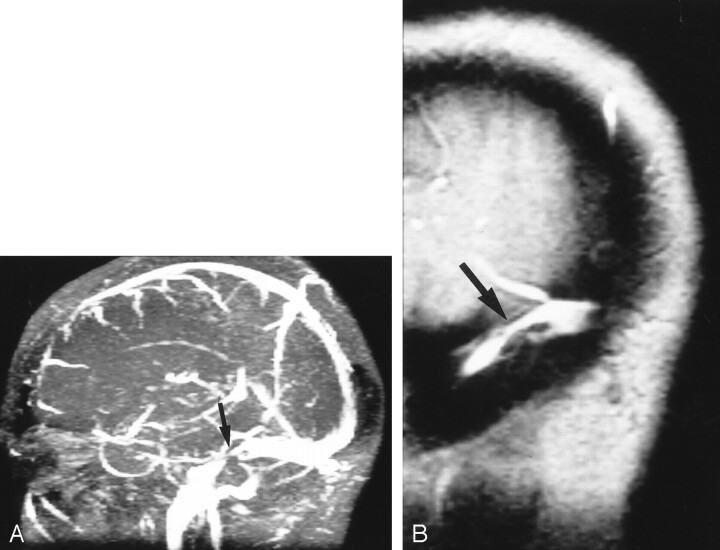
The SIH group had significant venous outflow obstruction. Five patients had focal thrombosis of the dominant transverse sinus; one, bilateral transverse sinus stenoses; and one, a very prominent arachnoid granulation that almost totally filled the posterior SSS. Figure 3 is an example of focal thrombosis, and Figure 4 shows bilateral stenoses. As would be expected, the mean SSS flow in the SIH group was reduced by 25% (P = .003), but the total blood flow was normal, indicating rapid autoregulation and rapid recruitment of venous collaterals.
Fig 3.
Focal thrombus.
A, MR venogram (30/5/1 [TR/TE/NEX]) shows acute thrombosis of the transverse sinus. Arrow indicates area of signal intensity loss.
B, Sagittal reconstruction shows nonocclusive thrombus (arrow) just distal to the vein of Labbé.
Fig 4.
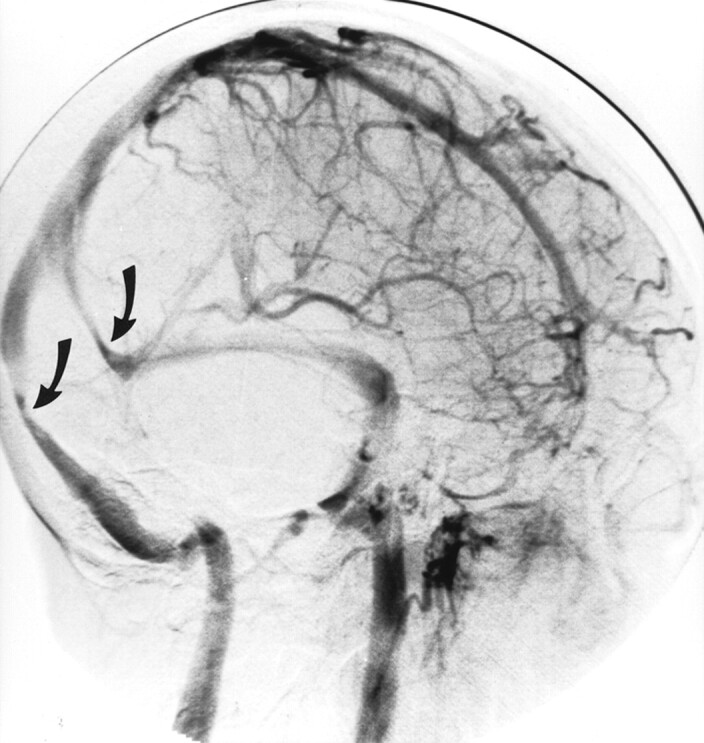
Oblique view of the venous phase of cerebral angiography shows bilateral transverse sinus stenoses (arrows).
The IIH group was well differentiated from the SIH group by the 46% (P = .0002) increase in total blood flow above normal and the 38% higher straight sinus flow above normal (P = .05). A 56% higher SSS flow compared with that of the SIH group (P = .002) was also noted. Foley (20) described increased cerebral blood flow in association with elevated intracranial pressure, and Mathew et al (21) found cerebral blood flow to be increased in IIH. The literature, however, is not unanimous on the finding of raised flow in IIH. Gjerris et al (22) found the total cerebral blood flow to be normal in IIH, but the criteria used for inclusion into their study saw five of the 14 patients with normal or borderline CSF pressures. Also unclear is how many of their patients had a secondary form of intracranial hypertension.
Cordell et al (1), by using a xenon radioisotope, found the cerebral blood flow to be elevated by 49% in IIH patients, which is very close to the 46% increase obtained in the current study. Thus, cerebral hyperemia of a significant degree is confirmed to be a component of IIH by this study. Cerebral hyperemia can only develop because of a derangement of the autoregulation provided by the arterioles. Arteriolar relaxation allows systemic pressure to gain access to the capillary bed, promoting cerebral edema through increased fluid leak. Abnormal MR diffusion signal intensity indicating interstitial brain edema and intracellular brain water accumulation in IIH has been demonstrated (23). Hyperemia also directly increases the venous sinus pressures, because the pressure gradient in a vessel segment is related to the resistance and the flow through that vessel segment: Pressure = Flow × Resistance. Increased flow through the SSS, straight sinus, and collateral veins, such as the veins of Labbé, would make a considerable increase in the total flow through the transverse sinuses. In this study, 619 mL/min of the mean cerebral flow is not accounted for by the SSS and straight sinus in the IIH group compared with 338 mL/min in the control subjects, confirming the much larger collateral flow.
King et al (7) showed that normally a pressure gradient of only 0–3 mm Hg exists between the SSS and internal jugular vein, but a pressure difference of at least 10 mm Hg always occurred in the lateral transverse sinuses in IIH. This occurred even when venograms showed only focal narrowing or tapering and in many cases no significant abnormality at all. The same group, using simultaneous venous sinus pressure monitoring and CSF removal, showed that the smooth tapering of the transverse sinuses associated with IIH was caused by direct compression of the sinuses by the raised CSF pressure (24). The increased pressure drop across the transverse sinuses is due to a combination of any increased resistance from the external compression and the increased blood flow. This pressure drop and the raised right atrial pressures in some patients (8) explain the raised venous pressures encountered. Raised venous pressures inhibit both CSF resorption by the arachnoid granulations and interstitial fluid resorption into the capillaries; thus, intracranial pressures must increase.
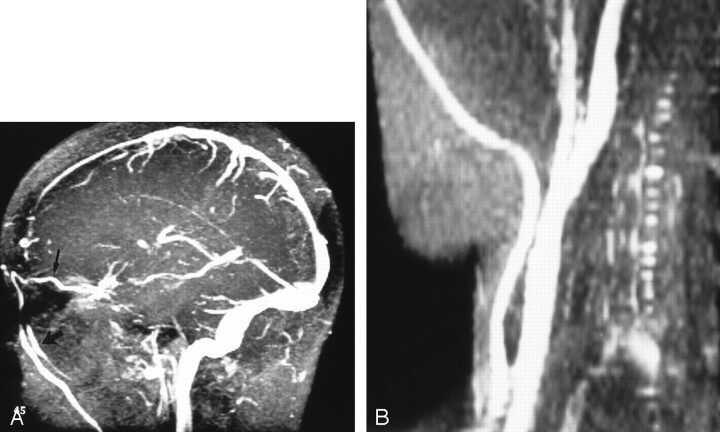
Figure 5A is the MR venogram obtained from a patient with IIH that shows extensive venous collateral flow through the orbits and face, and Figure 5B shows the distended great veins of the neck. The patient in Figure 5 was also noted to have iron deficiency anemia (hemoglobin 83 mg/L). Anemia would be expected to potentiate the hyperemia of IIH by producing an anoxic stress to the brain, further increasing the arteriolar relaxation. Treatment of iron deficiency anemia has resulted in resolution of the symptoms of IIH, and recurrence of IIH symptoms has occurred with relapse of anemia (1, 25).
Fig 5.
Patient with IIH.
A, MR venogram (30/5/1 [TR/TE/NEX]) shows a dilated ophthalmic vein (thin arrow) leading to distended facial veins (thick arrow).
B, MR venogram (30/5/1) shows distended veins of the right side of the neck.
A potential bias in this study exists owing to the reference standard used to differentiate IIH and SIH. MR venograms, as previously noted, can be difficult to interpret. In all idiopathic cases, the flow depicted in the SSS and transverse sinuses was clearly normal. In the SIH group, follow-up studies were obtained in those patients with presumed venous thrombosis after anticoagulant therapy, and improvement in the sinus lumen was used to confirm previous obstruction. The patient with bilateral venous sinus stenoses had an equivocal MR venogram, and cerebral angiography was performed to provide the reference standard in this instance.
Conclusion
Currently, the radiologic diagnosis of IIH is one of exclusion, with no positive features established with noninvasive imaging to aid diagnosis. In this study, MR flow quantification was assessed in this patient group, and reduced venous sinus pulsatility was found to be a marker of intracranial hypertension secondary to raised venous sinus pressure in both IIH and SIH. In cases in which the MR venogram is difficult to interpret, raised total blood flow indicates IIH, whereas reduced SSS flow indicates SIH, and a closer review of the venogram in the latter may be warranted. Cerebral hyperemia can explain all the pathologic mechanisms of IIH, and the 46% increase in blood flow to the brain would seem to be a primary failure of autoregulation. The findings of this study would indicate that the vascular hydraulics of IIH are closely related to cerebral hyperemia; thus, the name hyperemic intracranial hypertension may be a better descriptor in the subset of patients who have IIH with raised cerebral blood flow.
Footnotes
Presented in abstract form at the Royal Australian and New Zealand College of Radiologists annual scientific meeting, Melbourne, Australia, October 18, 2001.
References
- 1.Cordell E, Tranmer BI, Adey G, Kohut J. Increased cerebral blood flow in idiopathic pseudotumor cerebri. Neurol Res 1990;12:226–230 [DOI] [PubMed] [Google Scholar]
- 2.Wall M. Idiopathic intracranial hypertension. Semin Ophthalmol 1995;10:251–259 [DOI] [PubMed] [Google Scholar]
- 3.Leker RR, Steiner I. Features of dural sinus thrombosis simulating pseudotumor cerebri. Eur J Neurol 1999;6:601–604 [DOI] [PubMed] [Google Scholar]
- 4.Biousse V, Ameri A, Bousser MG. Isolated intracranial hypertension as the only sign of cerebral venous thrombosis. Neurology 1999;53:1537–1542 [DOI] [PubMed] [Google Scholar]
- 5.Wang AM. MRA of venous sinus thrombosis. Clin Neurosci 1997;4:158–164 [PubMed] [Google Scholar]
- 6.Ayanzen RH, Bird CR, Keller PJ, Mc Cully FJ, Theobald MR, Heiserman JE. Cerebral MR venography: normal anatomy and potential diagnostic pitfalls. AJNR Am J Neuroradiol 2000;21:74–78 [PMC free article] [PubMed] [Google Scholar]
- 7.King JO, Mitchell PJ, Thompson KR, Tress BM. Cerebral venography and manometry in idiopathic intracranial hypertension. Neurology 1995;45:2224–2228 [DOI] [PubMed] [Google Scholar]
- 8.Karahalios DG, Rekate HL, Khayata MH, Apostolides PJ. Elevated intracranial venous pressure as a universal mechanism in pseudotumor cerebri of varying etiologies. Neurology 1996;46:198–202 [DOI] [PubMed] [Google Scholar]
- 9.Bateman GA. Vascular compliance in normal pressure hydrocephalus. AJNR Am J Neuroradiol 2000;21:1574–1585 [PMC free article] [PubMed] [Google Scholar]
- 10.Gideon P, Thomsen C, Gjerris F, Sorensen PS, Stahlberg F, Henriksen O. Measurement of blood flow in the superior sagittal sinus in healthy volunteers and in patients with normal pressure hydrocephalus and idiopathic intracranial hypertension with phase-contrast cine MR imaging. Acta Radiol 1996;37:171–176 [DOI] [PubMed] [Google Scholar]
- 11.Johnson I, Patterson A. Benign intracranial hypertension, II: CSF pressure and circulation. Brain 1974;97:301–312 [DOI] [PubMed] [Google Scholar]
- 12.Stolz E, Kaps M, Kern A, Babacan SS, Dorndorf W. Transcranial color duplex Doppler sonography of intracranial veins and sinuses in adults: reference data from 130 volunteers. Stroke 1999;30:1070–1075 [DOI] [PubMed] [Google Scholar]
- 13.O’Rourke MF. Hypertension is a myth. Aust NZ Med 1983;13:84–90 [DOI] [PubMed] [Google Scholar]
- 14.Bateman GA. Toward a better understanding of normal pressure hydrocephalus [letter]. AJNR Am J Neuroradiol 2001;22:596. [PMC free article] [PubMed] [Google Scholar]
- 15.Greitz D, Greitz T, Hindmarsh T. A new view on CSF circulation with potential for pharmacological treatment of childhood hydrocephalus. Acta Pediatr 1997;86:125–132 [DOI] [PubMed] [Google Scholar]
- 16.Bolondi L, Bassi SL, Gaiani S, et al. Liver cirrhosis: changes of Doppler waveform of hepatic veins. Radiology 1991;178:513–516 [DOI] [PubMed] [Google Scholar]
- 17.Marks MP, Pelc NJ, Enzmann DR. Determination of cerebral blood flow with a phase-contrast cine MR imaging technique: evaluation of normal subjects and patients with arteriovenous malformations. Radiology 1992;182:467–476 [DOI] [PubMed] [Google Scholar]
- 18.Jordan JE, Pelc NJ, Enzmann DR. Velocity and flow quantification in the superior sagittal sinus with ungated and cine (gated) phase-contrast MR imaging. J Magn Reson Imaging 1994;4:25–28 [DOI] [PubMed] [Google Scholar]
- 19.Mattle H, Edelman RR, Reis MA, Atkinson DJ. Flow quantification in the superior sagittal sinus using magnetic resonance. Neurology 1990;40:813–815 [DOI] [PubMed] [Google Scholar]
- 20.Foley J. Benign forms of intracranial hypertension: “toxic and otitic” hydrocephalus. Brain 1955;78:1–41 [DOI] [PubMed] [Google Scholar]
- 21.Mathew NT, Sterling-Meyer J, Ott EO. Increased cerebral blood volume in benign intracranial hypertension. Neurology 1975;25:646–649 [DOI] [PubMed] [Google Scholar]
- 22.Gjerris F, Soelberg Sorensen P, Vorstrup S, Paulson OB. Intracranial pressure, conductance to cerebrospinal fluid outflow, and cerebral blood flow in patients with benign intracranial hypertension (pseudotumor cerebri). Ann Neurol 1985;17:158–162 [DOI] [PubMed] [Google Scholar]
- 23.Sorensen PS, Thomsen C, Gjerris, Henriksen O. Brain water accumulation in pseudotumor cerebri demonstrated by MR-imaging of brain water self-diffusion. Acta Neurochir Suppl (Wein) 1990;51:363–365 [DOI] [PubMed] [Google Scholar]
- 24.King JO, Mitchell PJ, Thompson KR, Tress BM. Manometry combined with cervical puncture in idiopathic intracranial hypertension. Neurology 2002;58:26–30 [DOI] [PubMed] [Google Scholar]
- 25.Tugal O, Jacobsen R, Berezin S, et al. Recurrent benign intracranial hypertension due to iron deficiency anemia: case report and review of the literature. Am J Pediatr Hematol Oncol 1994;16:266–270 [DOI] [PubMed] [Google Scholar]