Abstract
Summary: The Trispan device is a new tool designed for use in the endovascular treatment of wide-neck aneurysms with coils. We describe another application of this device to control coil deposition and to prevent coil migration during transvenous occlusion of high-flow arteriovenous fistulas.
Endovascular techniques have become the standard approach for the treatment of arteriovenous fistulas (AVFs) (1). AVFs can be treated with a variety of occlusive devices or products (eg, coils, glue) by arterial or venous approaches. One potential complication of the use of embolization techniques in high-flow situations is distal migration of the occlusive material. Controlled detachable coils are useful in this context, because they can be repositioned until a stable and safe position is obtained, and thus, they are commonly used as the initial occlusive agents (2). In cases of high-vascular-flow AVFs with no anatomic features to provide intrinsic support (eg, stenosis, vascular loop), coils may not be satisfactorily anchored, and its migration after its detachment is possible. We describe our initial experience with the Trispan device (Target Therapeutics, Fremont, CA) to control coil deposition and to prevent the distal migration of coils in patients with a high-flow AVF. Four patients, two children (one aged 10 months; the other, 10 years), and two adults (one aged 47 years; the other, 84 years) with intracranial high-flow fistulas (one vein of Galen malformation, three complex or multiple dural fistulas [Table]) were endovascularly treated with the assistance of one or several Trispan devices. In each case, planned treatment included deliberate coil occlusion of the venous outlet of the fistula. Herein we present two of these cases.
TABLE 1:
Case summaries: pathologic findings, treatments, and results
| Case No./ Patient Sex, Age | Pathologic Findings | Treatment | Result |
|---|---|---|---|
| 1/M, 10 mo | Macrocephalus. No cardiac failure. Vein of Galen malformation diagnosed in utero. | Two sessions of arterial and venous embolization with coils and glue. Trispan device deployed at the outlet of the venous pouch to prevent coil migration. | No complication related to treatment. Fistula almost closed. Normal development (at 6-mo follow-up). Cranial overgrowth reduced. Control angiography with embolization if necessary was scheduled. |
| 2/F, 84 y | Subarachnoid hemorrhage. Right transverse sinus dural fistula with reflux in the superior sagittal sinus and cerebral venous congestion. Benign left sigmoid dural fistula without reflux. | Transvenous occlusion of the right transverse sinus with coils. Trispan deployed just distal to the confluence of a right ambivalent vein of Labbé to avoid coil migration and occlusion of the outlet of the vein of Labbé. | No treatment related complication. Cure of the right transverse fistula. Marked improvement of the cerebral venous drainage and congestion. Left fistula unchanged. |
| 3/F, 47 y | Subarachnoid hemorrhage. Complex dural fistula of the vein of Galen region with deep cerebral venous reflux. | Arterial embolization via meningeal branches with coils and glue to reduce the shunt. Transvenous occlusion of a deep cerebral venous pouch draining the fistula. Two Trispan devices used to prevent coil migration outside the pouch to the straight sinus. | No complication related to treatment. Good recovery. Fistula dramatically reduced and transformed into a benign type (no cerebral venous reflux) at 3-mo follow-up angiography. |
| 4/F, 10 y | Multiple, very high-flow complex and evolving dural fistulas of the left transverse, torcular, and right sigmoid sinuses. Left jugular vein occluded. Right jugular vein stenotic at the foramen. Increasing headaches. Four endovascular treatments. Intraventricular hemorrhage without sequela 2 wk prior to latest treatment. | Surgical exposure and direct puncture of the right transverse sinus. Occlusion of the straight sinus and superior sagittal sinus with balloons to disconnect fistula from cerebral venous drainage. Trispan device deployed via right jugular vein to prevent coil migration. Coils and glue in the left transverse sinus and torcular. | No technical complication. Left transverse fistula cured. Torcular fistula dramatically reduced. Right sigmoid sinus fistula unchanged. Cerebellar ataxia and dysphagia after embolization unexplained (MR imaging findings unchanged). Complete recovery within 10 d. |
Case Reports
Case 1
A 10-month-old boy had a prenatal sonographic diagnosis of a vein of Galen malformation. Findings from two postnatal MR imaging and MR angiographic examinations confirmed this diagnosis by depicting a 2.5-cm dilatation of the medial prosencephalic vein with arteriovenous shunts. The ventricles and CSF spaces were moderately enlarged. The brain was otherwise normal.
Despite moderate macrocephaly, this patient achieved normal developmental milestones at 6 months of age, with no cardiac complications. Elective endovascular treatment was planned after that time.
Angiography performed when the patient was aged 7 months revealed a mural-type vein of Galen malformation. This first endovascular session, which involved transarterial embolization with the placement of coils and glue proximal to the venous pouch, led to suboptimal results. Subsequently, the dilated venous system was accessed through a large left posterior choroidal artery. Attempted embolization with a 30 × 100-mm vein of Galen coil (Target Therapeutics) resulted in the migration of the coil into the left transverse sinus, and its retrieval by a left transjugular approach was required.
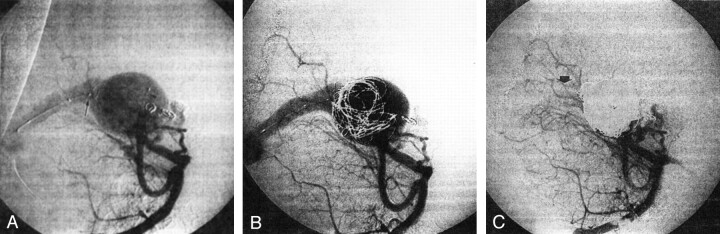
When the patient was 9 months old, a Renegade catheter (Target Therapeutics) was transarterially inserted through the shunt for coil delivery inside the venous pouch. A FasTracker-18 catheter (Target Therapeutics) was positioned in the venous dilatation via retrograde venous approach. A 16-mm Trispan device (Target Therapeutics) was deployed at the exit of the venous pouch through the FasTracker catheter, to retain the deployment of the coils through the transarterial microcatheter (Fig 1A and B). Numerous platinum coils were detached without incident. Because of a persistent shunt, glue (30–50% Histoacryl [Braun, Tuttlingen, Germany] and 70–50% Lipiodol Ultra-Fluid [Therapex, Montreal, Canada]) was injected into the pouch with transarterial and transvenous microcatheters. At the end of the procedure, the malformation was nearly completely closed (Fig 1C).
Fig 1.
Case 1.
A, Vertebral angiogram in the oblique view shows a mural-type vein of Galen malformation. The microcatheter used for coil deployment (arrowhead) was introduced through a posterior choroidal artery. The Trispan device (arrow) is deployed at the outlet of the venous pouch by retrograde venous approach. Two coils from the previous transarterial embolization procedure are depicted.
B, Angiogram obtained during embolization shows several coils detached in the venous pouch under the protection afforded by the Trispan device.
C, Final angiogram (same projection as in A) shows nearly complete obliteration of the malformation. Note a minimal residual opacification of the straight sinus (arrow).
At 15 months of age, the patient was clinically intact, with good psychomotor development. The macrocephaly had diminished, and MR images revealed a smaller pouch without flow void artifacts. Follow-up angiography, with embolization if needed for definitive cure, was planned.
Case 2
An active 84-year-old woman had a sudden headache without neurologic deficit. CT scanning performed in the emergency department showed blood in the subarachnoid space. Her medical history was unremarkable except for high blood pressure and mild angina pectoris, and both were medically controlled. Angiography performed the following day demonstrated two dural fistulas. One was near the left jugular foramen and was draining without reflux into the left jugular vein. The other involved the right transverse sinus (Fig 2A), with reflux into the straight and superior sagittal sinuses (Fig 2B). Ambivalent flow was present in the right vein of Labbé (Fig 2B and C). The right sigmoid sinus was stenotic and drained only the fistula. Generalized cerebral venous congestion was demonstrated. After angiography, auscultation of both mastoids revealed an obvious thrill. When questioned, the patient reported a history of pulsatile tinnitus lasting several years. One week after the hemorrhage, the patient was transferred to our institution for endovascular treatment. A repeat angiogram showed that the findings were unchanged. Using a retrograde venous approach through the left jugular vein, two microcatheters (Excelsior; Target Therapeutics) were placed in the right transverse sinus. The first microcatheter used for coil deposition was placed at the distal end of the fistula and incrementally withdrawn while coil packing was performed until an angiographic cure was obtained. The second was placed at the proximal end of the fistula, and a Trispan device was deployed (Fig 2D). The device was used to avoid coiling of the confluence of the right vein of Labbé, which was close to the fistula. The left jugular foramen dural fistula without reflux was not treated. The patient’s postoperative course was unremarkable. An angiogram obtained 3 months later confirmed cure of the right transverse dural fistula (Fig 2E) and patency with antegrade flow in the right vein of Labbé (Fig 2F). The dural fistula in the left jugular foramen was unchanged. The global cerebral venous congestion was markedly improved.
Fig 2.
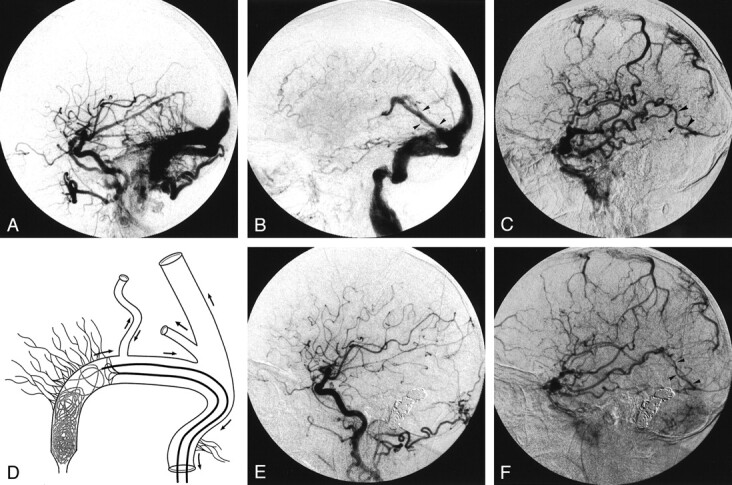
Case 2.
A and B, Lateral right common carotid angiograms. Early phase image (A) shows the right transverse dural fistula. Parenchymal phase image (B) shows reflux in the straight, superior sagittal, and left transverse sinuses from the right transverse dural fistula. The right vein of Labbé (arrowheads in B) is opacified in a retrograde fashion.
C, Lateral venous phase angiogram obtained with a right internal carotid injection shows cerebral venous congestion. Despite retrograde flow in the right vein of Labbé (see image in B), antegrade flow (arrowheads) persists.
D, Schematic drawing of the cerebral venous drainage (left anterior oblique view) in patient 2 during embolization. The Trispan device is deployed to prevent loops of coils from being positioned in front of the right vein of Labbé.
E and F, Lateral angiograms obtained with a right common carotid injection 3 months after endovascular treatment. Arterial phase image (E) depicts cure of the right transverse dural fistula. Venous phase image (F) shows improvement in cerebral venous drainage and antegrade flow in the right vein of Labbé (arrowheads).
Discussion
The ideal treatment of AVFs is to close the shunt without affecting normal vascular structures on the arterial or venous side. With current endovascular devices, this result is occasionally possible in rare anatomic situations such as vertebrovertebral or carotid-jugular fistulas treated with covered stent (3).
Intracranial AVFs can be treated by selective occlusion of the feeding artery or arteries close to the shunt; this procedure preserves the normal branches and prevents collateral circulation recruitment. However, arterial occlusion close to the shunt is often difficult, and treatment remains incomplete in many cases (ie, dural fistula on a long sinus segment).
Endovascular occlusion of the vein at the site of the fistula has been more recently described and is more frequently curative. Indications for the venous approach are now established for dural fistulas (4) and vein of Galen malformations (5) when the venous segment to be occluded drains only the fistula. Nevertheless, in the setting of high-flow AVFs, stabilization of the embolic material can be challenging, even with controlled detachable coils.
The Trispan device is a new tool designed for the endovascular treatment of wide-neck aneurysms (6, 7). It is made of three nitinol loops partly covered by radiopaque platinum that is centrally fixed at the struts of the loops. To assist coil embolization of an aneurysm, this device is positioned within the sac by using a 0.018-inch catheter to form a scaffold across the aneurysmal ostium. A second microcatheter is then inserted into the aneurysmal sac for coil deposition. Initial experimental and clinical results are encouraging with regard to this indication. (3, 4).
Another indication for the use of the Trispan device in assisting the endovascular treatment of AVFs is described. The device is used as a supporting structure on which coils could loop and form a stable mesh before their detachment. The Trispan device also acts as a landmark to limit the extension of the coil mass during embolization. Therapeutic venous occlusions should protect the normal cerebral venous drainage. The Trispan device can protect the confluence of an antegrade vein or sinus by preventing its obstruction by loops of coils (ie, case 2 [Fig 2D]). Finally, the Trispan device is also a filter that could eventually stop the migration of a coil after its detachment. The sizing of Trispan device is not as crucial in fistulas as it is in aneurysms. Ideally, the device should be larger than the diameter of the sinus or venous pouch to be occluded and should maintain contact with the wall of those structures.
Drawbacks and limitations of this strategy include the need to use two microcatheters, with their consequent technical difficulties. The largest Trispan device measures 16 mm, which may be too small to fit a large venous pouch or sinus (eg, as in case 4 [Table]).
Conclusion
In the treatment of intracranial high-flow AVFs by means of venous occlusion, the Trispan device can create an artificial obstacle on which coils can loop and be retained. Additionally, it can help prevent occlusion or impairment at the confluence of an antegrade vein or sinus and thus protect cerebral venous drainage.
References
- 1.Duckwiler G. Dural arteriovenous fistula. Neuroimaging Clin N Am 1992;2:291–307 [Google Scholar]
- 2.Nesbit GM, Barnwell SL. The use of electrolytically detachable coils in treating high-flow arteriovenous fistulas. AJNR Am J Neuroradiol 1998;19:1565–1569 [PMC free article] [PubMed] [Google Scholar]
- 3.Redekop G, Marotta T, Weill A. Treatment of traumatic aneurysms and arteriovenous fistulas of the skull base using endovascular stents. J Neurosurg 2001;95:412–419 [DOI] [PubMed] [Google Scholar]
- 4.Roy D, Raymond J. The role of transvenous embolization in the treatment of intracranial dural arteriovenous fistulas. Neurosurgery 1997;40:1133–1141 [DOI] [PubMed] [Google Scholar]
- 5.Halbach VV, Dowd CF, Higashida RT, Balousek PA, Ciricillo SF, Edwards MS. Endovascular treatment of mural-type vein of Galen malformations. J Neurosurg 1998;89:74–80 [DOI] [PubMed] [Google Scholar]
- 6.Turk AS, Rappe AH, Villar F, Virmani R, Strother CM. Evaluation of the Trispan Neck Bridge device for the treatment of wide-necked aneurysms: An experimental study in canines. Stroke 2001;32:492–497 [DOI] [PubMed] [Google Scholar]
- 7.Raymond J, Guilbert F, Roy D. Neck-bridge device for endovascular treatment of wide neck bifurcation aneurysms: initial experience. Radiology 2001;221:318–326 [DOI] [PubMed] [Google Scholar]