Abstract
BACKGROUND AND PURPOSE: We repeated a proton echo-planar spectroscopic imaging (PEPSI) study to test the hypothesis that children with dyslexia and good readers differ in brain lactate activation during a phonologic judgment task before but not after instructional treatment.
METHODS: We measured PEPSI brain lactate activation (TR/TE, 4000/144; 1.5 T) at two points 1–2 months apart during two language tasks (phonologic and lexical) and a control task (passive listening). Dyslexic participants (n = 10) and control participants (n = 8) (boys and girls aged 9–12 years) were matched in age, verbal intelligence quotients, and valid PEPSI voxels. In contrast to patients in past studies who received combined treatment, our patients were randomly assigned to either phonologic or morphologic (meaning-based) intervention between the scanning sessions.
RESULTS: Before treatment, the patients showed significantly greater lactate elevation in the left frontal regions (including the inferior frontal gyrus) during the phonologic task. Both patients and control subjects differed significantly in the right parietal and occipital regions during both tasks. After treatment, the two groups did not significantly differ in any brain region during either task, but individuals given morphologic treatment were significantly more likely to have reduced left frontal lactate activation during the phonologic task.
CONCLUSION: The previous finding of greater left frontal lactate elevation in children with dyslexia during a phonologic judgment task was replicated, and brain activation changed as a result of treatment. However, the treatment effect was due to the morphologic component rather than the phonologic component.
Dyslexia is a language disorder characterized by poor reading due to a phonologic deficit (1, 2). Functional MR imaging (fMRI) has been established as an effective tool to measure brain activation on the basis of blood oxygenation level dependent (BOLD) contrast mechanisms during language processing and dyslexia (3–6). In vivo MR spectroscopy is a technique for detecting brain chemicals either with (1) or without (7) a functional language activation procedure. Although fMRI and functional MR spectroscopy (fMRS) are both sensitive to changes in cerebral blood flow and although they may depict activation in similar regions of the brain, each may signal different underlying neural mechanisms. The BOLD response may reflect a stage of the sequence in neural metabolism earlier than MR spectroscopic lactate activation, which reflects an end stage of metabolism and its efficiency (8).
In a previous study, patients with dyslexia and age- and intelligence quotient (IQ)-matched control boys aged 9–12 years differed in their regional lactate response, as determined by using fMRS during a phonologic judgment task (1). After an instructional treatment that had both phonologic and morphologic components (9), the patients with dyslexia no longer differed from the control subjects in terms of left frontal lactate activation during this same task (10). The initial brain activation difference between the poor readers and the good readers fit with a large body of research showing a phonologic core deficit in dyslexia (2, 11). The change in brain activation after treatment supported other research showing that training in alphabetic principle (spelling-phoneme correspondences) is effective instruction for beginning readers (12) and an effective treatment for dyslexia (13).
One purpose of the current research was to evaluate whether the prior proton MR spectroscopic imaging (PEPSI) findings before and after treatment could be replicated in another sample. Another purpose was to evaluate whether the treatment effect could be replicated if treatment were restricted to either phonologic or morphologic components rather than a combination of the two. In contrast to the prior study in which all children received the same treatment by combining phonologic and morphologic components (9), children in the current study were randomly assigned to one of two treatments: phonology or morphology. An additional purpose was to extend the prior studies, which relied solely on group analyses, and to analyze the effects of treatment on individual brains. We tested the hypothesis that initial differences in patients and control subjects in lactate activation during phonologic judgment is eliminated in response to phonologic judgment treatment.
Methods
Study Design
Ten dyslexic children (six boys, four girls) and eight normal-reading control subjects (six boys, two girls) were imaged by using PEPSI (14) while performing the same three language tasks used in the prior studies (1, 10). Patients with dyslexia were imaged before and after a 3-week treatment for dyslexia (15). The control subjects were also imaged at approximately the same two time points, but they did not receive treatment, with an average of 68 days between the imaging sessions. The imaging protocol, the language stimuli, and the language tasks were identical across repeated imaging sessions, and they were equally spaced for all participants.
Participant Characterization
The University of Washington Human Subjects Institutional Review Board approved this study, and each participant (as well as his or her parent or guardian) provided written informed consent. All participants were right handed with the exception of one with a history of ambidexterity. The following psychometric tests were used to evaluate the reading and language abilities of each participant: verbal IQ test, Word Identification and Word Attack subtests of the Woodcock Reading Mastery Test (16), Phonologic (17), RAN letters, RAN Switching (18, 19), and Orthographic Word Choice (20). The control subjects and patients with dyslexia differed significantly in age-corrected standard scores for reading real words on the Word Identification test, with t(16) = 5 .73 and P < .001, and for reading pseudowords on the Word Attack test, with t(16) = 6 .35 and P < .001.
Brain Stimulation Tasks During PEPSI
The same three language tasks—passive listening, phonologic judgment, and lexical judgment—were used in each scanning session. In each task, the children were asked to listen to a word or word pairs presented auditorily at a rate of one every 4 seconds. Four types of word pairs, crossed for lexical status (word vs nonword) and sound similarity (rhyming vs nonrhyming) were presented on language judgment tasks: word-word, nonrhyming (eg, fly-church); word-word, rhyming (eg, fly-eye); word-nonword, nonrhyming (eg, crow-treel); and word-nonword, rhyming (eg, meal-treel). All stimuli used are in the appendix of the article by Serafini et al (8). The order of word-pair types and stimulus words was randomized within a task. The stimuli were presented inside the machine through custom-built magnet-compatible earphones (Mark Mathis, unpublished data). EPRIME (Psychology Software Tools, Pittsburgh, PA) was used to present and synchronize the language stimuli to the imaging machine.
The passive listening task required children to listen to a word (from a list of word pairs on the language judgment tasks) without making any response. During the phonologic task, participants listened to the word pairs and judged whether they rhymed; whether the words were real was irrelevant, and meaning had to be ignored. During the lexical task, participants listened to the same word pairs and judged whether the word pairs contained two real, meaningful words; whether the words rhymed was irrelevant, and phonology had to be ignored.
The same stimulus lists were used for the lexical and phonologic tasks; only the task instructions were changed. Participants indicated their rhyme and lexical decisions by pressing a button held in their right hand if the answer was yes (ie, the words rhymed in the phonologic task or were both real words in the lexical task). All participants practiced the phonologic and lexical judgment tasks outside the imaging machine during a pre-imaging training session to ensure that they understood the language task before they were examined. Accuracy and reaction times were recorded during the practice sessions and each imaging session.
MR Imaging and Spectroscopy
Conventional MR imaging and PEPSI were performed on a clinical 1.5-T MR imaging system (Signa; GE Medical Systems, Milwaukee, WI) equipped with version 5.8 software and a custom-built RF head coil combined with an audiovisual display system. MR images were acquired in the sagittal plane by using a spin-echo sequence (TR/TE, 400/8) and in the axial plane by using a fast-spoiled gradient-recalled acquisition in the steady state (GRASS) (200/4) sequence. The coordinates of the Sylvian fissure and surrounding language-related structures were determined on the sagittal images, which were co-registered with the axial images for both MR imaging and spectroscopic imaging. The single PEPSI section was oriented to encompass the frontal operculum and the posterior portion of the superior temporal gyrus. Deeper subcortical structures were also included; these are associated (through neuronal connectivity) with the cortical areas. Parameters for PEPSI (14) data acquisition included the following: TR/TE/NEX, 4000/144/2; spatial matrix, 32 × 32; echoes in the echo-planar acquisition, 512 (256 points for chemical shift after sorting); complex points per echo, 32; half-echo acquisition; field of view, 24 cm; and section thickness, 20 mm. The voxel size was approximately 1 cm3, and the acquisition time for each PEPSI examination was approximately 4.5 minutes. Three PEPSI images were acquired at each session by using the three tasks described previously. Participants always performed the passive listening task first, and then the order of the lexical and phonologic tasks was counterbalanced across participants. For individual participants, we used the same order in the first and second imaging sessions.
PEPSI Lactate Processing
Software was developed to perform five steps on the PEPSI images acquired during each of three tasks. These steps were user-independent and automated for the 32 × 32 matrix of the spectra and were the following: 1) 3D Fourier transformation of raw PEPSI data, 2) phasing of each spectrum, 3) baseline correction, 4) detection of valid proton spectra within the brain, and 5) lactate resonance detection and quantification.
To ensure that the 1.3-ppm resonance was that of lactate (step 5), we used a TE of 144 milliseconds because, at this TE, the lactate resonance is inverted (due to its J-coupling properties), and this feature allows discrimination between lactate and residual lipids. We also applied a narrow integration window of 1.2–1.4 ppm.
To assess focal brain activation during either phonologic or lexical judgment, as compared with passive listening, Z-score maps were created from the lactate/N-acetylaspartate (NAA) ratios. This comparison removed the lower-level language and acoustic stimulus effects from the activation related to phonologic or lexical judgments. The following equation was used to create the Z maps for comparing a language judgment with passive listening: [lactate/NAA (task) - lactate/NAA (passive listening)]/[SD of lactate/NAA (passive listening)], where (task) in the equation refers to data from either the phonologic task or the lexical task. The Z maps were based on all voxels that were valid for both the passive listening task and language judgment tasks. The SD of lactate/NAA was calculated for each participant by using all valid spectra from the control task (passive listening). This Z score was calculated for all voxels that contained valid spectra for each language condition. The number of valid voxels for the dyslexic group was not significantly different from that of the control group.
The PEPSI data were analyzed to sum the number of activated voxels (with elevated lactate above the threshold) in each of four quadrants of the brain. The definition of the threshold for lactate elevation indicating brain activation was based on Z scores greater than 2.0 on a voxel-by-voxel basis for the language task (either phonologic or lexical) compared with passive listening. Because regional specificity of lactate response is not well established and also because of the large variability between participants in the spatial location of the lactate response, we chose to analyze the data in four quadrants rather than to try to specify particular structures or regions in the brain. The four quadrants were defined by dividing the brain along the midline of the axial and MR image and along the perpendicular line that crossed the midpoint of the thalamus (division lines are also shown in Fig 1). Overall, patients with dyslexia and control subjects did not differ in number of valid activated voxels. An analysis of variance (ANOVA) was used to test for differences in the number of activated voxels between control subjects and patients with dyslexia in each quadrant of the brain.
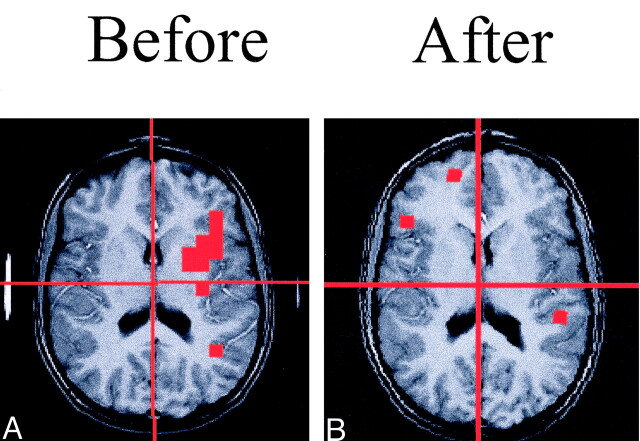
Fig 1.
Spoiled GRASS MR images (200/5) with PEPSI lactate activation overlay from a participant with dyslexia. Note the large area of lactate activation in the left frontal region that is no longer observed after treatment.
A, Image before treatment.
B, Image after treatment.
Instructional Treatment
Patients with dyslexia were randomly assigned to the treatments, which has been discussed in detail previously (15). Of the 10 patients with dyslexia, four were in the phonology treatment group and six were in the morphology treatment group.
Results
Psychometric Results
At the initial imaging session, the patients with dyslexia were reading, on average, about 1 SD below the population mean for age on the Word Identification and Word Attack subtests of the Woodcock Reading Mastery Test (16), and the control subjects were reading well above the population mean on these same tests (See Table 1). The control subjects and patients with dyslexia differed significantly in age-corrected standard scores for reading real words on the Word Identification test, with t(16) = 5 .73 and P < .001, and for reading pseudowords on the Word Attack test, with t(16) = 6 .35 and P < .001.
TABLE 1:
Language measures
| Measure | Patients with Dyslexia |
Control Subjects |
||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Verbal IQ | 115.5 | 10.2 | 120.1 | 15.5 |
| Word identification* | 83.5 | 11.5 | 109.3 | 5.9 |
| Word attack† | 86.8 | 7.7 | 107.3 | 5.3 |
| Phonologic‡ | 8.8 | 1.6 | 11.3 | 1.4 |
| RAN letters§ | 2.2 | 1.9 | −0.7 | 0.6 |
| RAN switching letters/numbers§ | 3.9 | 3.2 | −0.4 | 0.8 |
| Orthographic word choice‖ | 33.3 | 20.0 | 71.3 | 11.3 |
Real words were used.
Pronounceable pseudowords were used.
The task was to delete sounds in spoken words. The frequency of correct responses was recorded.
The task was to rapidly but accurately name rows of continuous letters or alternating letters and digits. Z scores were based on grade norms for time (in seconds), with positive scores indicating performance slower and below the mean and negative scores indicating performance faster and above the mean.
The task was to choose correctly spelled words from three choices pronounced the same or nearly the same. The percentage of correct responses was recorded.
The patients with dyslexia were deficient in three skills: phonologic (elision) (17), rapid automated naming of letters and of letters or numbers (18, 19), and an orthographic choice (pseudohomonym choice) task (20) that predict the ease of learning to read and the response to intervention (21) (Table 1). The patients with dyslexia and the control subjects differed significantly on the phonologic elision measure, with t(15) = 3 .43 and P = .004; on the RAN letter test, with t(15) = −4.15 and P < .001; on the RAN letters and numbers switching test, with t(15) = −3.73 and P = .002; and on the orthographic choice test, with t(15) = 4 .73 and P < .001. These results indicate that this sample of patients with dyslexia had a triple deficit in the language phenotype markers for dyslexia (21).
However, the control subjects (mean age, 136.0 months; SD, 10.9) and patients with dyslexia (mean age, 139.4.0 months; SD, 8 .19) did not differ in age at the time of the initial imaging session, with t(16) = −0.76 and P = .460. Likewise, at the time of the initial session, the groups did not differ in verbal IQ scores, with t(16) = 1 .42 and P = .175) (Table 1). All values were based on findings in the 10 patients with dyslexia and in the eight control subjects that had usable imaging data at both pre-testing and post-testing.
Behavioral Measurements During Imaging
Table 2 contains the mean accuracies and reaction times of the patients with dyslexia and the control subjects in response to phonologic and lexical judgment tasks.
TABLE 2:
Accuracy and reaction time of patients with dyslexia and control subjects in response to phonologic and lexical judgment tasks
| Session and Task | Patients with Dyslexia |
Control Subjects |
||
|---|---|---|---|---|
| Accuracy | Reaction Time | Accuracy | Reaction Time | |
| Training before first session | ||||
| Phonologic | 91 ± 8 | 1731 ± 245 | 94 ± 8 | 1179 ± 453 |
| Lexical | 95 ± 5 | 1675 ± 307 | 89 ± 7 | 1247 ± 264 |
| During first session | ||||
| Phonologic | 85 ± 16 | 1475 ± 165 | 97 ± 2 | 1178 ± 45 |
| Lexical | 84 ± 9 | 1614 ± 272 | 89 ± 7 | 1440 ± 245 |
| During second session | ||||
| Phonologic | 85 ± 16 | 1546 ± 238 | 94 ± 11 | 1378 ± 286 |
| Lexical | 84 ± 15 | 1568 ± 205 | 88 ± 8 | 1512 ± 182 |
Accuracy.—
The patients with dyslexia and the control subjects did not differ significantly in accuracy with the lexical task during the training outside the magnet or during the first or second imaging sessions. With the phonologic task, control subjects performed more accurately during the first session, with F(1, 16) = 4 .41 and P = .05), but patients with dyslexia and control subjects did not differ significantly during training or the second imaging session. Overall, no floor or ceiling effects were found for either group with either task, and performance was well above that due to chance. Thus, the results were not compromised by the task being too easy or too difficult.
Reaction Time.—
During training, the patients with dyslexia were significantly slower than the control subjects during both the lexical and phonologic tasks, with t(14) = −2.991 and P = .01 and with t(15) = −3.179 and P = .006, respectively. During the first imaging session, the patients with dyslexia were significantly slower than the control subjects during the phonologic task, with t(12) = −4.239 and P = .001, but not during the lexical task. The groups did not differ significantly in reaction time with either task during the second imaging session.
Reading and Language Improvement
Both treatment groups (morphologic and phonologic) significantly improved in standardized measures of accuracy and rate of phonologic decoding, morphologic awareness, accuracy of decoding words with morphologic units, and silent reading comprehension (15). However, the only treatment-specific effect (one treatment group improved relatively more) was that the morphology treatment group improved significantly more in speed of phonologic decoding than did the phonologic treatment group (15). Thus, morphologic treatment improved efficiency in the phonologic decoding of written words.
MR Spectroscopic Imaging Changes
Left Frontal Brain Region.—
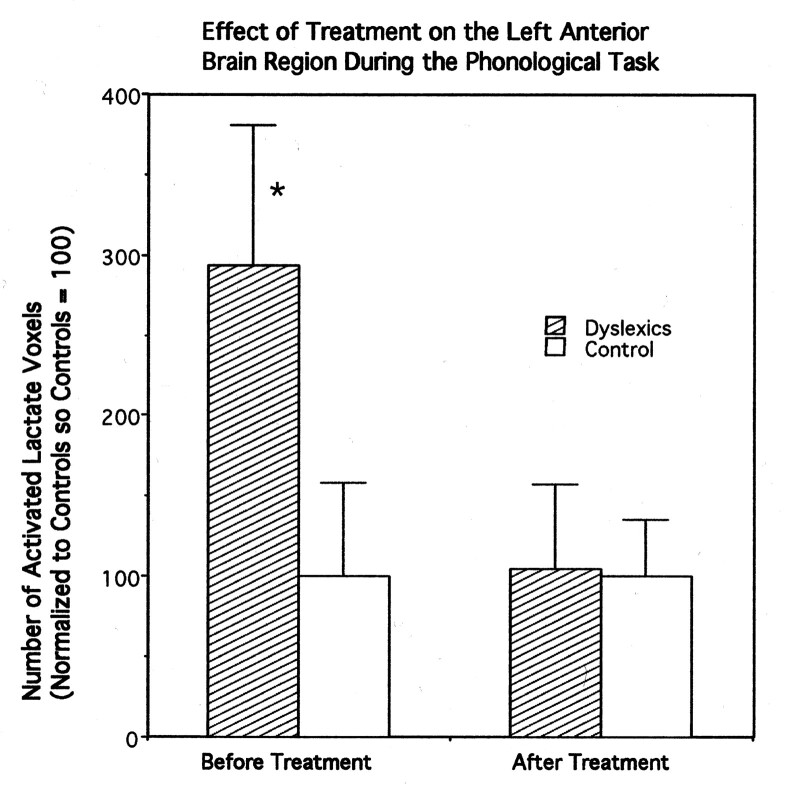
Before treatment, patients with dyslexia had significantly more brain voxels with elevated MR lactate levels (293.7 + SE 87 as a normalized percentage) compared with the control group (100 + SE 58 as a normalized percentage) during the phonologic task in the left frontal quadrant, with F(1, 16) = 3 .055 and P = .05 (directional hypothesis) (Fig 2). After treatment, with the same phonologic task and in the same region of brain, the patients with dyslexia (104 + SE 52 as a normalized percentage) did not significantly differ from the control subjects (100 + SE 35 as a normalized percentage), with F(1, 16) = 0.004 and P = .47 (Fig 2). No differences between groups were found with the lexical task in this region.
Fig 2.
Bar graph of the number of averaged activated voxels (normalized to control values), as defined by increases in lactate concentration in the left frontal brain quadrant during MR spectroscopy, in both patients with dyslexia and in control subjects. Error bars indicate the standard error of the mean. The asterisk indicates that the results of comparisons between patients and control subjects were significantly different. The data were collected by using PEPSI (TR/TE, 4000/144).
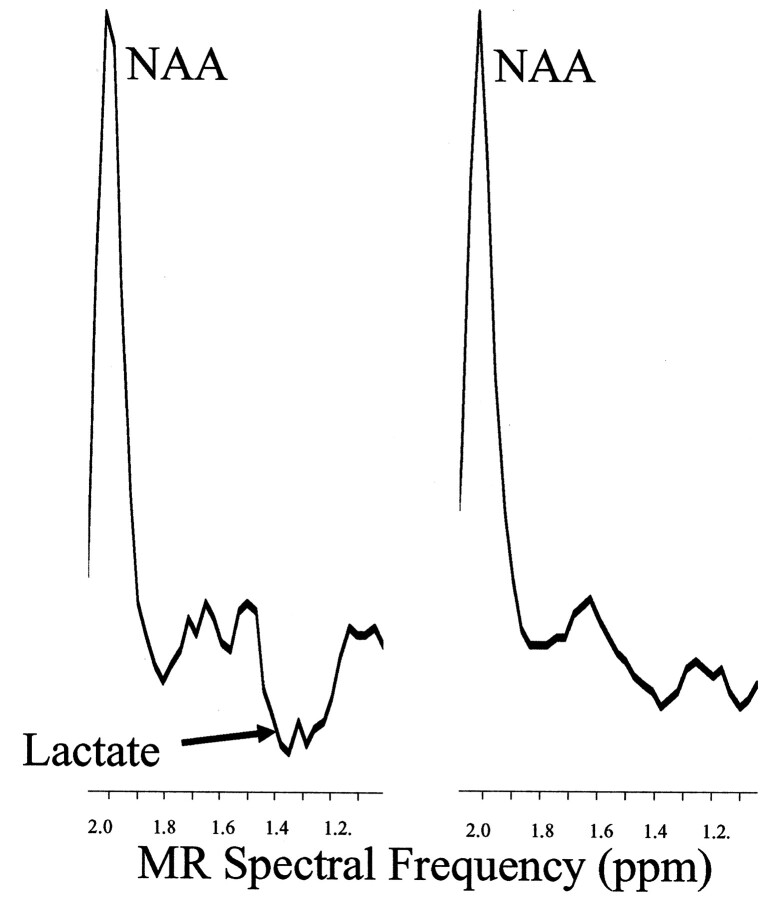
For the individual participant analyses of the phonologic task in the left frontal region, the lactate change was sensitive to the type of treatment. Of the six who received morphologic treatment, the lactate activation decreased in five, including the ambidextrous subject (average decrease, −5.28). Of the four who received phonologic treatment, all stayed the same or increased (average increase, +2.65). The Fischer exact test, based on the binomial distribution (6.148), showed a significant effect (P < .05) related to the type of treatment (Table 3). Sample data from a spectrum of a dyslexic child are shown in Figure 3, and the lactate overlay from this same child is shown in Figure 1.
TABLE 3:
Change in lactate activation in the left frontal region during the phonological task as a function of treatment
| Change in Lactate Activation | Phonologic Treatment | Morphologic Treatment |
|---|---|---|
| Decrease | 0 | 5 |
| Increase | 4 | 1 |
Fig 3.
Baseline-corrected proton spectra from a dyslexic participant in the left frontal region of the brain shows significant lactate activation during the phonologic task. The inverted lactate proton doublet can be observed at 1.3 ppm during the phonologic task (left) but not during passive listening (right). The other resonance that can be observed in the spectra is NAA.
Right Parietal Brain Region.—
Before treatment, the dyslexic patients (436 + SE 118 as a normalized percentage) also had significantly more brain lactate voxels above the threshold, compared with control subjects (100 + SE 33 as a normalized percentage), in the right parietal region of the brain for the phonologic task, with F(1, 16) = 6 .04 and P < .02. Patients with dyslexia (553 + SE 154 as a normalized percentage) also had significantly more brain lactate voxels, compared with control subjects (100 + SE 28 as a normalized percentage), in the right parietal region of the brain for the lexical task, with F(1, 16) = 6 .6 and P < .02. However, neither of these differences was significant after treatment. For the individual brain analysis of the right parietal region, treatment was more likely to result in lactate reduction (probability that this effect was not due to chance on the basis of a binomial distribution, P = .05). However, the effect was not treatment-specific. Both kinds of language treatment were effective in reducing lactate activation during the lexical task (see Table 4). In contrast, after either treatment, lactate was as likely to increase as decrease during the phonologic task in this region.
TABLE 4:
Change in lactate activation in the right parietal region during lexical task as a function of treatment
| Change in Lactate Activation | Phonologic Treatment | Morphologic Treatment |
|---|---|---|
| Decrease | 4 | 4 |
| Increase | 0 | 2 |
Left Parietal and Right Frontal Regions.—
For the phonologic and lexical judgment tasks, no differences in lactate activation were found between patients with dyslexia and control subjects in left parietal and right frontal regions.
Discussion
The result in the left frontal region of the brain before treatment is consistent with that reported in previous research (3). The number of activated brain lactate voxels during phonologic judgment was significantly higher in dyslexic patients compared with control subjects before treatment. However, in comparison to the treatment in the prior study that combined phonologic and morphologic components (9), in this study, we compared the effects of treatments involving either the phonologic or the morphologic component. On the basis of the analysis of individual brains, morphologic treatment was significantly more likely than phonologic treatment to decrease lactate activation in left frontal regions during a phonologic judgment task. Because of the small numbers of subjects, this initial report of the advantage of morphologic treatment for dyslexic children in grades 4–6 requires further research.
This change in brain activation after treatment converges with the change on a standardized measure of efficiency of phonologic decoding after treatment (15). On the standardized measure, the rate of phonologic coding increased significantly more in children receiving morphologic treatment than in those who received only phonologic treatment. Increased speed may be related to greater verbal efficiency. Individual children receiving the morphologic component also showed a decrease in lactate activation during phonologic judgment, which possibly indicated greater metabolic efficiency in performing the phonologic task (8). We propose that helping dyslexic children in the upper grades to become aware of the morphologic units in words may help them to use phonologic information more efficiently. Early in reading development, intensive phonologic training helps dyslexic readers, but later in their reading development, morphologic training becomes beneficial (15). Although phonologic training continues to be important for older developmental dyslexic readers, morphologic training may also add benefits when combined with alphabet principle training to improve the efficiency of phonologic judgments (fMRS data) and phonologic decoding in dyslexics in the upper elementary grades. However, we acknowledge that further research is needed to draw firm conclusions about the effect of these treatments.
Differences between patients with dyslexia and control subjects in the right parietal region of the brain had not been observed in earlier spectroscopic studies. However, these findings are consistent with MEG results reported by Simos et al (22) in dyslexic children during the second stage of activation. In dyslexic children, activation profiles during the printed word recognition task consistently featured initial activation of the left basal temporal cortices (includes the inferior temporal gyrus and possibly the fusiform gyrus), followed by activation of the right temporoparietal areas (including the angular gyrus). Non-impaired readers showed predominant activation of left basal followed by left temporoparietal activation. From these results, they hypothesized that reading difficulties in developmental dyslexia were associated with an initial aberrant pattern of functional connectivity between brain areas normally involved in reading early in processing, namely ventral visual association cortex and temporoparietal areas in the left hemisphere, but a difference between dyslexic readers and good readers subsequently occurred in the right parietal regions. PEPSI lactate activation appeared to be sensitive to that right parietal difference.
Serafini et al have reviewed several aspects of lactate as a metabolite during brain activation based on decades of research on this issue (8). Since the 1940s, we have recognized that elevated tissue lactate levels can be attributed to hypoxia and anaerobic energy metabolism (23, 24). However, since that time, many studies have demonstrated that lactate production and nonoxidative glucose metabolism occur during physiologic stimulation in brain tissue in both animals (25–28) and humans (29–32). These findings suggest that changes in neural activity are supported by glycolysis. In vitro, brain tissue has been shown to not only produce lactate under aerobic conditions but also to support synaptic function in rat hippocampal sections (33, 34). Also, lactate is a preferred substrate to glucose in sympathetic ganglia (35). Other reports from animal and cell culture studies have demonstrated that the heightened energy demands of activated neurons are met through increased glial glycolytic flux, and lactate may be a crucial aerobic energy substrate that allows neurons to endure activation (33, 36). Menon et al (37) and Frahm et al (30) have both presented evidence that an initial positive correlation exists between regional lactate production and fMRI (BOLD) signal intensity.
MR spectroscopy has also been used to demonstrate neurochemical abnormalities in dyslexic brains, even when the participants are resting and do not have to perform a task. Rae et al (7) found MR spectroscopy-detectable differences in the ratio of choline-containing compounds to NAA between dyslexic men and control subjects in the left temporoparietal lobe and right cerebellum. Richardson et al (38) found an elevated phosphomonoesters peak area in patients with dyslexia compared with control subjects by using phosphorus-31 MR spectroscopy.
Corina et al (4) used fMRI to compare dyslexic children and control subjects during the same aurally presented phonologic and lexical judgment tasks used in the current study; a tone judgment task was used instead of passive listening as the control task in that study. When fMRI data were analyzed with tone as the main task (“on condition”), there was no significant difference between dyslexic patients and control subjects; these findings were consistent with those of an fMRS study using PEPSI (1). When the two groups (dyslexic patients and control subjects) were compared with the two language tasks (phonologic and lexical judgment) and the two hemispheres (right and left), four regions—middle frontal gyrus, inferior temporal gyrus, precentral gyrus, and orbital frontal cortex—had a significant interaction between language task and group. The last three regions had a significant three-way interaction with task, group, and hemisphere. Children with dyslexia and good readers differed in right inferior temporal gyrus; this finding provided more localized evidence with the same phonologic and lexical tasks than that possible with PEPSI. Sound may be mapped onto meaning in this region (see reference 40 for a review of the research evidence). This result suggests that dyslexic patients have difficulty not only in phonologic judgments but also in judgments that require the coordination of sound and meaning while ignoring one and attending to the other.
Both fMRI and MEG have been used to detect differences in left parietal regions between dyslexic children and control subjects (6, 39,41) that PEPSI lactate activation does not detect with the auditory language tasks used in this study. During letter matching, normal-reading children showed activity throughout extrastriate cortex, especially in occipitoparietal regions, whereas dyslexic children had little activity in extrastriate cortex during this task (6). Temple and colleagues (6) concluded that dyslexia in childhood may be characterized by disruptions in the neural bases of both phonologic and orthographic processes that are important for reading. Considering that our tasks did not use orthographic stimuli, orthographic tasks (visual word forms) may be needed to detect the difference in the left parietal regions between dyslexic readers and good readers.
Seki et al (41) used fMRI to study Japanese dyslexic children (aged 9–12 years) while they read Japanese kana. All control subjects showed activation of the left middle temporal gyrus. In the dyslexic children, the activation of the left middle temporal gyrus was rather vague. However, other distinctively activated regions were detected as follows: the bilateral occipital cortex was detected in two dyslexic children; the inferior part of the frontal regions, in two others; and both the bilateral occipital cortex and the inferior part of precentral gyrus, in the remaining one. They suggested that these results indicate compensatory management processes for the unskilled reading ability of the dyslexic children; this observation is consistent with our prior spectroscopic findings in the left frontal regions in children (10) and with prior fMRI findings in the precentral gyrus (4). Taken together, results across studies point to underactivation of dyslexic brains to written words in the left posterior region (42), with overactivation or compensation in the right posterior and left frontal regions (40).
Contrasting results across studies may reflect differences in the language task given during the examination, the type of brain imaging technique used, the regions covered by the images, and the age and reading level of the patients with dyslexia. Ultimately, however, the differences between developmental dyslexics and good readers will be defined along circuits rather than individual neural structures. Although the fMRI BOLD response is excellent in localizing the activated neural circuits in the brain, PEPSI lactate activation provides additional, albeit less localized information, about the efficiency of the metabolic regulation in those circuits (8).
Conclusion
The results reported here are consistent with those of our prior report and suggest that instructional treatment (environmental effects) can affect brain processing. The sensitivity of left frontal regions to morphologic treatment with a phonologic task and that of the right parietal regions to both phonologic and morphologic treatment with a lexical task provides empirical evidence for nature-nurture interactions (40). Our results should not be construed to suggest that dyslexia can be cured with a short-term treatment, but they do provide cautious optimism that language-based treatments may benefit dyslexic children, as demonstrated by their performance on traditional standardized measures of reading, and on newer methods of assessing brain function.
Acknowledgments
The authors gratefully acknowledge the help of the Diagnostic Imaging Science Center Director Dr Kenneth Maravilla and MR technicians Gerald Ortiz and Denise Echelard. The authors also acknowledge the help of electrical engineers Cecil Hayes and Mark Mathis. We thank Dr David Corina who designed the language tasks.
Footnotes
Presented in part at the 52nd Annual Conference of the International Dyslexia Association, Albuquerque, New Mexico, October 24–27, 2001.
Support provided by the National Institute of Child Health and Human Development grant number HD33812.
References
- 1.Richards TL, Dager SR, Corina D, et al. Dyslexic children have abnormal brain lactate response to reading- related language tasks. AJNR Am J Neuroradiol 1999;20:1393–1398 [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner R, Torgesen J. The nature of phonological processing and its causal role in the acquisition of reading skills. Psych Bull 1987;101:192–212 [Google Scholar]
- 3.Shaywitz SE, Shaywitz BA, Pugh KR, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proc Nat Acad Sci 1998;95:2636–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corina DP, Richards TL, Serafini S, et al. fMRI auditory language differences between dyslexic and able reading children. Neuroreport 2001;12:1195–201 [DOI] [PubMed] [Google Scholar]
- 5.Eden GF, Zeffiro TA. Neural systems affected in developmental dyslexia revealed by functional neuroimaging. Neuron 1998;21:279–282 [DOI] [PubMed] [Google Scholar]
- 6.Temple E, Poldrack RA, Salidis J, et al. Disrupted neural responses to phonological and orthographic processing in dyslexic children: an fMRI study. Neuroreport 2001;12:299–307 [DOI] [PubMed] [Google Scholar]
- 7.Rae C, Lee MA, Dixon RM, et al. Metabolic abnormalities in developmental dyslexia detected by 1H magnetic resonance spectroscopy. Lancet 1998;351:1849–1852 [DOI] [PubMed] [Google Scholar]
- 8.Serafini S, Steury K, Richards T, Corina D, Abbott R, Berninger V. Comparison of fMRI and fMR spectroscopic imaging during language processing in children. Magn Reson Med 2001;45:217–225 [DOI] [PubMed] [Google Scholar]
- 9.Berninger VW. Dyslexia an invisible, treatable disorder: the story of Enstein’s ninja turtles. Learning Disabil Q 2000;23:175–195 [Google Scholar]
- 10.Richards TL, Corina D, Serafini S, et al. The effects of a phonologically-driven treatment for dyslexia on lactate levels as measured by proton MRSI. AJNR Am J Neuroradiol 2000;21:916–922 [PMC free article] [PubMed] [Google Scholar]
- 11.Morris RD, Stuebing KK, Fletcher JM, et al. Subtypes of reading disability: variability around a phonological core. J Educ Psychol 1998;90:347–373 [Google Scholar]
- 12.Adams M. Beginning to Read: Thinking and Learning About Print. Cambridge: MIT Press;1990
- 13.Lovett M, Bordon S, DeLuca T, Lacerenza L, Benson N, Brackstone D. Treating the core deficits of dyslexia: evidence of transfer of learning after phonologically- and strategy- based reading training programs. Develop Psych 1994;30:805–822 [Google Scholar]
- 14.Posse S, Dager SR, Richards TL, et al. In vivo measurement of regional brain metabolic response to hyperventilation using magnetic resonance proton echo planar spectroscopic imaging (PEPSI). Mag Res Med 1997;37:858–865 [DOI] [PubMed] [Google Scholar]
- 15.Berninger VW, Nagy W, Carlisle J, et al. Effective treatment for dyslexics in grades 4 to 6. In: Foorman B, ed. Preventing and Remediating Reading Difficulties: Bringing Science to Scale. Timonium, Md: York Press;2002
- 16.Woodcock R. Woodcock Reading Mastery Test—Revised. Circle Pine, MN: American Guidance Service;1987
- 17.Wagner R, Torgesen J, Rashotte C. Comprehensive Test of Phonological Skills. Austin: ProEd.2000
- 18.Wolf M, Bally H, Morris R. Automaticity, retrieval processes, and reading: a longitudinal study in average and impaired reading. Child Develop 1986;57:988–1000 [DOI] [PubMed] [Google Scholar]
- 19.Wolf M. Rapid alternation stimulus naming in the developmental dyslexias. Brain Lang 1986;27:360–379 [DOI] [PubMed] [Google Scholar]
- 20.Olson R, Wise B, Conners F, Rack J, Fulker D. Specific deficits in component reading and language skills: genetic and environmental influences. J Learning Disabil 1989;22:339–348 [DOI] [PubMed] [Google Scholar]
- 21.Berninger VW, Abbott RD, Thomas J, et al. Language phenotypes for reading and writing disability. SSR 2001;5:59–105 [Google Scholar]
- 22.Simos PG, Breier JI, Fletcher JM, et al. Brain activation profiles in dyslexic children during non-word reading: a magnetic source imaging study. Neurosci Lett 2000;290:61–65 [DOI] [PubMed] [Google Scholar]
- 23.Friedmann TE, Barbarka C. The significance of the ratio of lactic acid to pyruvic acid in blood after exercise. J Biol Chem 1941;141:993–994 [Google Scholar]
- 24.Halijamae H. Lactate metabolism. Intens Care World 1987;4:118–121 [Google Scholar]
- 25.Fellows LK, Boutelle MG, Fillenz M. Physiological stimulation increases nonoxidative glucose metabolism in the brain of the freely moving rat. J Neurochem 1993;60:1258–1263 [DOI] [PubMed] [Google Scholar]
- 26.Fray AE, Forsyth RJ, Boutelle MG, Fillenz M. The mechanisms controlling physiologically stimulated changes in rat brain glucose and lactate: a microdialysis study. J Physiol (Lond) 1996;496:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Y, Wilson GS. A temporary local energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J Neurochem 1997;69:1484–1490 [DOI] [PubMed] [Google Scholar]
- 28.Hyder F, Chase JR, Behar KL, et al. Increased tricarboxylic acid cycle flux in rat brain during forepaw stimulation detected with 1H[13C]NMR. Proc Natl Acad Sci USA 1996;93:7612–7617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science 1988;241:462–464 [DOI] [PubMed] [Google Scholar]
- 30.Frahm J, Krueger G, Merboldt KD, Kleinschmidt A. Dynamic NMR studies of perfusion and oxidative metabolism during focal brain activation. Adv Exp Med Biol 1997;413:195–203 [DOI] [PubMed] [Google Scholar]
- 31.Prichard J, Rothman D, Novotny E, et al. Lactate rise detected by H-1 NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci USA 1991;88:5829–5831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sappey MD, Calabrese G, Fein G, Hugg JW, Biggins C, Weiner MW. Effect of photic stimulation on human visual cortex lactate and phosphates using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab 1992;12:584–592 [DOI] [PubMed] [Google Scholar]
- 33.Schurr A, West CA, Rigor BM. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science 1988;240:1326–1328 [DOI] [PubMed] [Google Scholar]
- 34.Izumi Y, Benz AM, Zorumski CF, Olney JW. Effects of lactate and pyruvate on glucose deprivation in rat hippocampus slices. Neuroreport 1994;5:617–620 [DOI] [PubMed] [Google Scholar]
- 35.Larrabee MG. Lactate metabolism and its effects on glucose metabolism in an excised neural tissue. J Neurochem 1995;64:1734–1741 [DOI] [PubMed] [Google Scholar]
- 36.Magistretti PJ, Pellerin L, Martin JL. Brain energy metabolism: an integrated cellular perspective. In: Bloom F, Kufper D, eds. Psychopharmacology: the Fourth Generation of Progress. New York: Raven;1995. :675–670
- 37.Menon RS, Gati JS. Two second temporal resolution measurements of lactate correlate with EPI BOLD fMRI timecourses during photic stimulation. In: Proceedings of the International Society for Magnetic Resonance in Medicine. Berkeley: International Society for Magnetic Resonance in Medicine;1997. :152
- 38.Richardson AJ, Cox IJ, Sargentoni J, Puri BK. Abnormal cerebral phospholipid metabolism in dyslexia indicated by phosphorus-31 magnetic resonance spectroscopy. Nmr Biomed 1997;10:309–314 [DOI] [PubMed] [Google Scholar]
- 39.Georgiewa P, Rzanny R, Hopf JM, et al. fMRI during word processing in dyslexic and normal reading children. Neuroreport 1999;10:3459–3465 [DOI] [PubMed] [Google Scholar]
- 40.Berninger VW, Richards TL. Brain Literacy for Educators and Psychologists. New York: Academic Press;2002
- 41.Seki A, Koeda T, Sugihara S, et al. A functional magnetic resonance imaging study during sentence reading in Japanese dyslexic children. Brain Dev 2001;23:312–316 [DOI] [PubMed] [Google Scholar]
- 42.Shaywitz BA, Shaywitz SE, Pugh KR, et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiat 2002;52:101–110 [DOI] [PubMed] [Google Scholar]