Abstract
BACKGROUND AND PURPOSE: Intravertebral clefts have long been considered as pathognomonic for avascular necrosis and as a rare cause of compression fracture. We have observed unsuspected clefts opacifying frequently during vertebroplasty. Our purpose in this study was to determine the incidence of these clefts in symptomatic osteoporotic compression fractures, assess the sensitivity of MR imaging and conventional radiography in the detection of these clefts, and determine whether there is any prognostic significance of these clefts in patients treated with vertebroplasty.
METHODS: Retrospective chart reviews were conducted of 135 vertebroplasty procedures performed during a 2-year period. MR images and conventional radiographs were reviewed for the presence of clefts defined as fluid-filled cavities on MR images or gas-filled cavities on conventional radiographs. Digital radiographs obtained at the time of the procedure were inspected for the presence of opacified clefts. Imaging findings were correlated with subjective pain scores documented before the procedure and at 1 week, 1 month, 6 months, and 12 months after vertebroplasty.
RESULTS: Two hundred thirty-six osteoporotic compression fractures were treated with polymethylmethacrylate in 125 patients. Thirty-one and eight-tenths percent of the fractures were noted to contain clefts at the time of vertebroplasty. Fluid-filled clefts were detected on preoperative MR images in only 52.8% of the fractures with opacified clefts at vertebroplasty. Gas-filled clefts were evident on preoperative conventional radiographs in only 11.4% of the fractures with opacified clefts at vertebroplasty. No significant difference was noted in numerical pain scores between the two populations at baseline or 1 week or 1 month after the procedure. Pain scores at 6 and 12 months after vertebroplasty showed a trend toward greater pain relief in patients with clefts, although the difference was not statistically significant. A sustained, statistically significant decrease in pain scores after treatment (P < .01) was noted in both groups.
CONCLUSION: Intravertebral clefts are much more common than previously described and probably represent fracture nonunions. Imaging is not sensitive in detecting these clefts before vertebroplasty. We advocate complete filling of the cleft with cement during vertebroplasty to maximize stabilization of the fracture fragments. There is a trend toward greater pain relief being achieved 6 and 12 months after the procedure in patients with clefts that are opacified at the time of vertebroplasty.
Intravertebral clefts have been well documented in the imaging literature for more than a century. Initial reports dealt exclusively with air-filled clefts within vertebral compression fractures evident on conventional radiographs and were considered to be pathognomonic of ischemic necrosis (1–3). Recently, several investigators described the MR imaging features of these clefts, which have a variable appearance, depending on whether they are filled with gas or fluid at the time they are imaged (4–6). We have observed several patients undergoing vertebroplasty with intravertebral clefts that went undetected until they were filled with opaque cement at the time of the procedure. We have undertaken this retrospective review to determine the incidence of these clefts in association with osteoporotic fractures, to establish the sensitivity of MR imaging and conventional radiography in the detection of these clefts, and to determine whether the presence of a cleft had any prognostic value in predicting the degree of pain relief after the procedure.
Methods
The charts and available imaging studies of 135 patients undergoing vertebroplasty between September 1999 and August 2001 were retrospectively reviewed. All patients underwent clinical evaluation performed by a neuroradiologist to determine whether their back pain was referable to one or more compression fractures. Clinical examinations were performed under bi-plane fluoroscopy to aid in localizing the point of maximal pain. The numerical rating scale, an 11-point scale with which patients subjectively rate their pain from 0 (no pain) to 10 (unbearable pain), was used. Numerical rating scale scores were established at initial evaluation and by telephone interview at 1 week, 1 month, 6 months, and 12 months after the procedure.
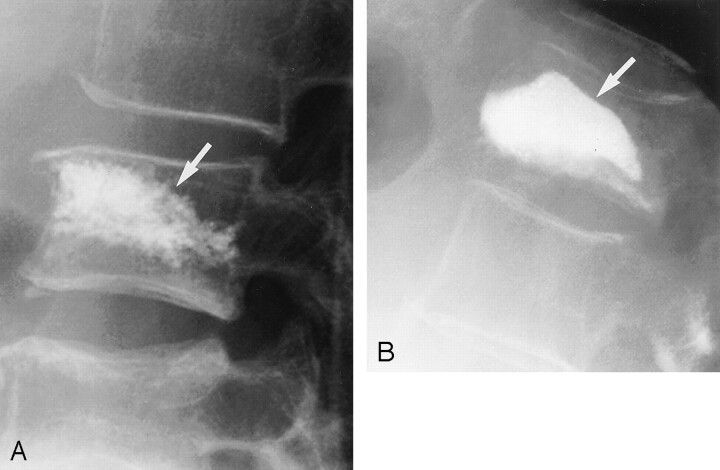
Preprocedural MR images and conventional radiographs were evaluated for the presence of clefts within vertebral compression fractures by two neuroradiologists (J.I.L., T.P.M.) who were blinded to the results of vertebroplasty. Fluid-filled clefts were defined as well-demarcated, linear or ellipsoid areas of T2 prolongation, which were isointense relative to CSF on T2-weighted images acquired in the sagittal plane. Preprocedural conventional radiographs and fluoroscopic images were reviewed for evidence of sub-end plate gas. Imaging findings were correlated with the presence of point tenderness on manual palpation elicited and recorded during the vertebroplasty evaluation. Distribution of radiopaque polymethylmethacrylate mixed with barium sulfate on postprocedural fluoroscopic digital radiographs was classified as trabecular opacification or cleft opacification (Fig 1). The presence of a cleft opacified with polymethylmethacrylate was correlated with clinical response to the procedure and compared with those patients without clefts.
Fig 1.
Lateral radiographs show two patterns of opacification after polymethylmethacrylate cement injection. Corresponding MR image is shown.
A, Trabecular pattern of opacification represents opaque cement interspersed throughout the trabecular space (arrow).
B, Cleft pattern of opacification represents opaque cement filling a large sub-end plate cavity (arrow).
Results
One hundred thirty-five cases were reviewed as part of this retrospective evaluation. Ten cases were ultimately excluded: one involved a kyphoplasty procedure, two involved prophylactic cement injections before spinal fusion procedures, and seven involved pathologic fractures. The 125 remaining patients included 89 female and 36 male patients with a mean age of 75 years. Two hundred thirty-six osteoporotic compression fractures were injected with polymethylmethacrylate mixed with opaque barium powder under bi-plane fluoroscopy by using a unipedicular or bipedicular approach as described by Jensen et al (7). Operative vertebral levels are listed in Table 1. Seventy opacified clefts were observed in 62 patients. The distribution of these clefts is listed in Table 2. MR images were available for review for 48 of the 62 patients. Of 53 opacified clefts in 48 patients, 28 fluid-filled clefts in 28 patients were detected on MR images (52.8%) (Fig 2). MR imaging helped detect five additional clefts at symptomatic levels in five patients that did not subsequently opacify when injected with cement and seven additional clefts at asymptomatic levels in seven patients that were not treated. Standard and fluoroscopic radiographs helped detect eight (11.4%) gas-filled clefts at symptomatic levels in eight patients (Fig 3). All gas-filled clefts opacified with cement injection. Considering only the treated compression fractures identified in this series, 75 (31.8%) of 236 compressed vertebral bodies were noted to contain a cleft at the time of vertebroplasty.
TABLE 1:
Distribution of all vertebroplasty procedures
| Level | Frequency | Percent |
|---|---|---|
| T4 | 2 | 0.8 |
| T5 | 4 | 1.7 |
| T6 | 8 | 3.4 |
| T7 | 11 | 4.7 |
| T8 | 9 | 3.8 |
| T9 | 10 | 4.2 |
| T10 | 9 | 3.8 |
| T11 | 19 | 8.1 |
| T12 | 34 | 14.4 |
| L1 | 34 | 14.4 |
| L2 | 32 | 13.6 |
| L3 | 22 | 9.3 |
| L4 | 28 | 11.9 |
| L5 | 14 | 5.9 |
| Total | 236 | 100 |
TABLE 2:
Distribution of intravertebral clefts at time of vertebroplasty
| Level | Frequency | Percentage |
|---|---|---|
| T5 | 1 | 1.4 |
| T6 | 2 | 2.9 |
| T7 | 4 | 5.7 |
| T8 | 5 | 7.1 |
| T9 | 2 | 2.9 |
| T10 | 4 | 5.7 |
| T11 | 10 | 14.3 |
| T12 | 18 | 25.7 |
| L1 | 8 | 11.4 |
| L2 | 10 | 14.3 |
| L3 | 2 | 2.9 |
| L4 | 3 | 4.3 |
| L5 | 1 | 1.4 |
| Total | 70 | 100 |
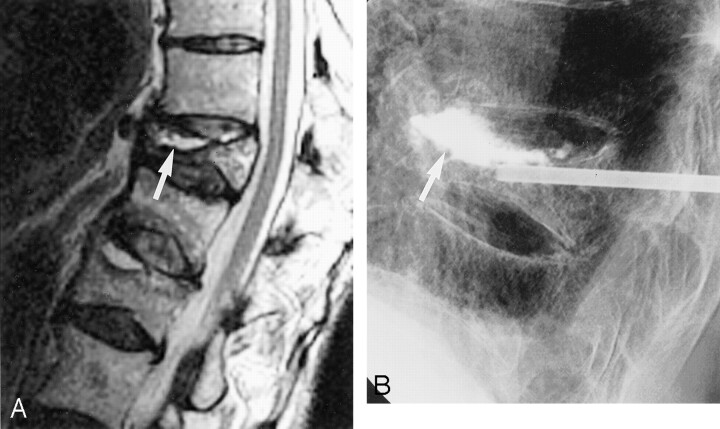
Fig 2.
Fluid-filled sub-end plate cleft.
A, Sagittal T2-weighted MR image shows cleft in association with a vertebral compression fracture (arrow).
B, Lateral radiograph shows opacification of cleft after cement injection (arrow). Note small amount of prevertebral extravasation of cement.
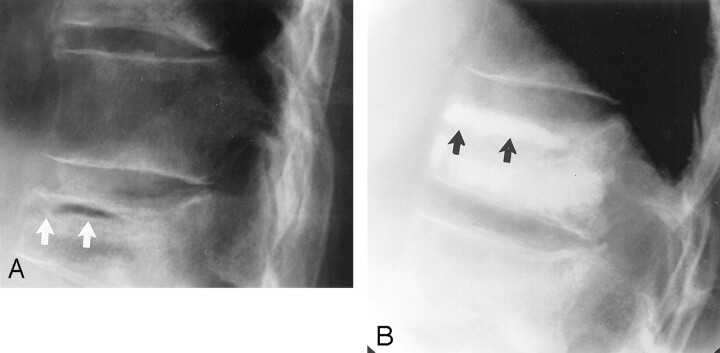
Fig 3.
Air-filled sub-end plate cleft.
A, Lateral radiograph obtained before vertebroplasty shows cleft associated with compression fracture (white arrows).
B, Lateral radiograph shows opacification of this cleft after polymethylmethacrylate injection (black arrows).
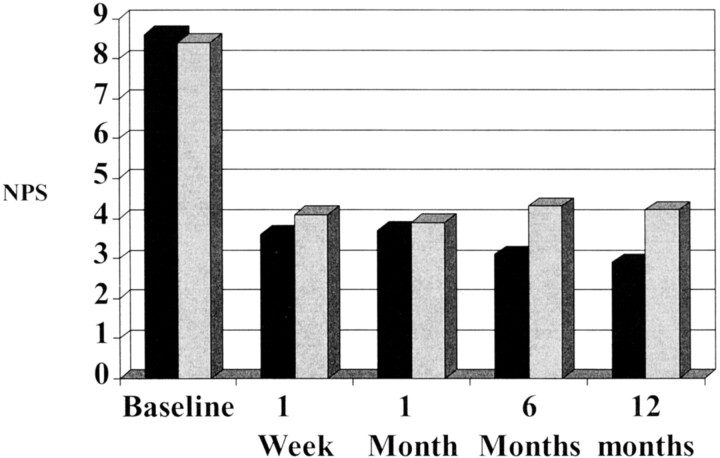
Numerical pain scores are displayed in the bar graph (Fig 4). Baseline numerical pain scores were available for 45 patients with clefts (mean = 8.6) and 71 patients without clefts (mean = 8.4) (P = .52). Postprocedural pain levels at 1 week were obtained for 45 patients with clefts (mean = 3.6) and 71 patients without clefts (mean = 4.1) (P = .22). Pain scores were obtained at 1 month for the 36 patients with clefts (mean = 3.7) and the 61 patients without clefts (mean = 3.9) (P = .71). Pain scores at 6 months were available for 29 patients with clefts (mean = 3.1) and 46 patients without clefts (mean = 4.3) (P = .20). Pain scores at 12 months were recorded for 18 patients with clefts (mean = 2.9) and 32 patients without clefts (mean = 4.2) (P = .18). Although there was a trend toward improved outcome in the cleft population, these differences were not statistically significant. Four of the five clefts evident on preoperative images that did not subsequently fill with cement had a return to preoperative baseline levels of pain at 6 to 12 months after the procedure (the fifth was lost to follow-up after 1 month).
Fig 4.
Graph shows mean numerical pain scores (NPS) recorded in intravertebral cleft versus non-cleft groups at preprocedural baseline, 1 week, 6 months, and 12 months after vertebroplasty. Black bars, cleft group; gray bars, non-cleft group.
Discussion
Intravertebral clefts associated with vertebral compression fractures, appreciated on conventional radiographs as an intraosseous vacuum phenomenon, have frequently been considered pathognomonic for avascular necrosis (Kummel’s disease) (1, 3, 8). Avascular necrosis is considered an uncommon cause for vertebral compression fracture, and there has been much debate regarding whether the ischemia precedes or occurs after the traumatic injury (2, 9, 10). Several reports in the literature have described the MR imaging appearance of these clefts, which can be filled with fluid or gas at the time of imaging (4, 6, 11). Malghem et al (6) found that prolonged periods in the supine position allowed many of the gas-filled clefts that were evident on conventional radiographs to fill with fluid and to be subsequently documented on T2-weighted MR images. The high incidence of clefts in our series of osteoporotic compression fractures would not support a causal association of these clefts with avascular necrosis, unless we are to assume that this cause is much more common than previously suspected.
The frequency of these clefts at the thoracolumbar junction is worthy of note. Because flexion and extension of the spine is most dynamic at the thoracolumbar junction (9, 12, 13), vertebral insufficiency fractures at these levels would be less likely to undergo complete healing when compared with other vertebral levels, which are comparatively immobile. Flexion and extension would be expected to produce pathologic motion along the plane of the compression fracture, preventing union of the fragments and predisposing to the formation of a connective tissue-lined cleft filled with serous fluid. Extension forces could produce further distraction of the fragments and thus produce transient accumulation of nitrogen gas within the cleft. We have noted distraction of the fracture fragments along the plane of the cleft with respiratory motion on at least three occasions with the patient in the prone (hyperextended) position at the time of fluoroscopy. Interfragmentary movement and size of fracture gap are regarded as the most important factors leading to nonunion and pseudarthrosis formation (14, 15).
On the basis of our observations during this retrospective review, we suggest that clefts actually represent fracture nonunions. Kumpan et al (9) and Shih et al (4) had reached a similar conclusion regarding clefts in their respective studies of osteoporotic compression fractures. If the cleft does represent an ununited fracture, filling the cleft during polymethylmethacrylate injection would seem to be a primary objective during vertebroplasty. This conclusion is further supported by the results obtained for the four patients with clefts evident on preoperative images that did not subsequently fill with cement; all four experienced a return to preoperative baseline levels of pain at 6 to 12 months after the procedure.
The use of the numerical rating scale has been previously validated in studies of patients with acute as well as chronic pain (16, 17). Berthier et al (16), in their comparison of several commonly used one-dimensional methods of pain intensity assessment, concluded that the numerical rating scale was preferable (over the visual analog and verbal rating scales) because of its low nonresponse rate, suitability for repeated measurements, and need for nothing more than a verbal response. This last advantage was particularly useful to us because all our follow-up data were collected by conducting telephone interviews. One-dimensional self-assessment pain scales have been studied as a measure of clinical outcome. Several studies have found that the minimum clinically significant difference between pre- and post-treatment pain scores is in the range of 13% when using the visual analog scale (18, 19). This minimum clinically significant difference has been documented to vary across the spectrum of pain severity. Bird and Dickson (20) found that a 28% difference in pre- and post-treatment scores was required to reach the minimum clinically significant difference in their patient cohort with pretreatment pain scores of 67 to 100 on a 100-point scale when using the visual analog scale. Using either the 13% or 28% benchmark and assuming similar responses with the numerical pain scores (difference of 1.1 or 2.4, respectively) used in this study, outcomes data for our population indicate a significant degree of pain reduction after vertebroplasty.
The large number of patients lost to follow-up at 6 and 12 months complicated our statistical analysis of pain scores. To achieve 80% power of detecting the minimum clinically significant difference between patients with clefts and those without clefts, a difference of 1 in the numerical pain scores was required at 1 week. Differences of 1.6, 2.3, and 2.6 in the numerical pain scores were required at 1, 6, and 12 months, respectively, to achieve the same statistical power. We can be reasonably confident that the slight differences in numerical pain scores at baseline, 1 week, and 1 month are not statistically significant (differences of 0.2, 0.5, and 0.2, respectively). We cannot be as confident regarding the lower post-procedural pain scores noted in the cleft population at 6 and 12 months (differences of 1.2 and 1.3, respectively). It is our hope that we will be able to resolve this issue as we continue to accumulate data for larger numbers of patients who have undergone vertebroplasty.
In our series, only 52.8% of the opacified clefts that underwent MR imaging were noted to be fluid filled. This fluid signal intensity was accentuated on T2-weighted MR images obtained using fat saturation. A much smaller number of these clefts (11.4%) were noted to be air filled at fluoroscopy. Many clefts went undetected, however, before opacification with radiopaque contrast material during venography. It had been our practice to include venography before cement injection. Persistent contrast within a cleft compromises our ability to fluoroscopically monitor cement injection. Because we are unable to detect clefts by MR imaging in almost half of all cases, we no longer advocate venography before cement administration. We also routinely leave all patients with opacified clefts in the prone (hyperextended) position for an additional 30 minutes after cement injection to prevent any unnecessary motion along the fracture plane. It is our hope that this will allow for maximal fracture stabilization and retention of any height restoration achieved after cleft distention.
Conclusion
Intravertebral clefts are common in patients with osteoporotic compression fractures treated with vertebroplasty. Imaging is not sensitive in preoperatively detecting these clefts. The high frequency of clefts observed in this series of compression fractures does not support the conventional association with avascular necrosis. In our opinion, it is much more likely that these clefts represent unhealed fractures. The preponderance of these clefts at the thoracolumbar junction suggests that flexion-extension forces along the plane of the fracture are probably responsible for the failure of these fractures to heal. We advocate complete filling of the cleft with cement during vertebroplasty to maximize stabilization of the fracture fragments. No significant differences in pain scores between patients with clefts and those without clefts were noted at baseline, 1 week, or 1 month. Although there was a trend toward greater pain relief in the patients with clefts at 6 and 12 months, these differences were not statistically significant.
Acknowledgments
We thank Sandra Everson, RN, and Barbara Buchanan, RN, for clinical support and Charles Rowland for statistical analysis.
Footnotes
Presented at the American Association of Neuroradiology 2002 Annual Meeting, Vancouver, British Columbia, Canada.
References
- 1.Kummell H. Die rarefizierende Ostitis der Wirbelkrper. Deutsche Med 1895;21:180–181 [Google Scholar]
- 2.Resnick D, Niwayama G, Guerra, J, et al. Spinal vacuum phenomena: anatomic study and review. Radiology 1981;139:341–348 [DOI] [PubMed] [Google Scholar]
- 3.Maldague BE, Noel HM, Malghem JJ. The intravertebral vacuum cleft: a sign of ischemic vertebral collapse. Radiology 1978;129:23–29 [DOI] [PubMed] [Google Scholar]
- 4.Shih TT, Tsuang YH, Huang KM, Chen PQ, Su CT. Magnetic resonance imaging of vertebral compression fractures. J Formos Med Assoc 1996;95:313–319 [PubMed] [Google Scholar]
- 5.Naul LG, Peet GJ, Maupin WB. Avascular necrosis of the vertebral body: MR imaging. Radiology 1989;172:219–222 [DOI] [PubMed] [Google Scholar]
- 6.Malghem J, Maldague B, Labaisse MA, et al. Intravertebral vacuum cleft: changes in content after supine positioning. Radiology 1993;187:483–487 [DOI] [PubMed] [Google Scholar]
- 7.Jensen ME, Evans AJ, Mathis JM, Kallmes DF, Cloft HJ, Dion JE. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol 1997;18:1897–1904 [PMC free article] [PubMed] [Google Scholar]
- 8.Brower A, Downey EF. Kummel’s disease: report of a case with serial radiographs. Radiology 1981;141:363–364 [DOI] [PubMed] [Google Scholar]
- 9.Kumpan W, Salomonowitz E, Seidl G, Wittich GR. The intravertebral vacuum phenomenon. Skeletal Radiol 1986;15:444–447 [DOI] [PubMed] [Google Scholar]
- 10.Stojanovic J, Kovac V. Diagnosis of ischemic vertebral collapse using selective spinal angiography. Fortschr Geb Rontgenstr Nuklearmed Erganzungsband 1981;10:271–277 [DOI] [PubMed] [Google Scholar]
- 11.Dupuy DE, Palmer WE, Rosenthal DI. Vertebral fluid collection associated with vertebral collapse. AJR Am J Roentgenol 1996;167:1535–1538 [DOI] [PubMed] [Google Scholar]
- 12.Fang D, Leong JY, Ho EK, et al. Spinal pseudarthrosis in ankylosing spondylitis: clinicopathological correlation and the results of anterior spinal fusion. J Bone Joint Surg Br 1988;70:443–447 [DOI] [PubMed] [Google Scholar]
- 13.Lindh M. Biomechanics of the lumbar spine. In: Frankel H, Nordin M, eds: Basic Biomechanics of the Skeletal System. Philadelphia: Lea and Febiger;1981. :255–290
- 14.Claes L, Wolf S, Augat P. Mechanical factors influencing callus healing. Chirurg 2000;71:989–994 [DOI] [PubMed] [Google Scholar]
- 15.Rohe K, Bierther M, Becker W, et al. Connective tissue in non-union and pseudarthrosis of long bones: parts I, II and III. Z Orthop Ihre Grenzgeb 1980;118:937–967 [DOI] [PubMed] [Google Scholar]
- 16.Berthier F, Potel G, Leconte P, et al. Comparative study of methods of measuring acute pain intensity in an ED. Am J Emerg Med 1998;16:132–136 [DOI] [PubMed] [Google Scholar]
- 17.Strong J, Ashton R, Chant D. Pain intensity measurement in chronic low back pain. Clin J Pain 1991;7:209–218 [DOI] [PubMed] [Google Scholar]
- 18.Todd KH, Funk KG, Funk JP, et al. Clinical significance of reported changes in pain severity. Ann Emerg Med 1996;27:485–489 [DOI] [PubMed] [Google Scholar]
- 19.Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med 2001;38:633–638 [DOI] [PubMed] [Google Scholar]
- 20.Bird BB, Dickson EW. Clinically significant changes in pain along the visual analog scale. Ann Emerg Med 2001;38:639–643 [DOI] [PubMed] [Google Scholar]