Abstract
BACKGROUND AND PURPOSE: Time-of-flight (TOF) magnetic resonance angiography (MRA) is commonly used to visualize the carotid arteries; however, flow void artifacts can appear. Our purpose was to determine the frequency and diagnostic meaning of flow voids by using real patient data, as part of a larger study of MRA compared with the criterion standard, digital subtraction angiography (DSA).
METHODS: In 1997–2000, 390 consecutive patients with sonographic findings suggestive of carotid artery stenosis were included in this study. All patients subsequently underwent three-dimensional (3D) TOF MRA and conventional DSA. The frequency of flow void artifacts on 3D TOF MRA images were compared with stenosis measurements on DSA images.
RESULTS: We recorded 107 flow voids (16%) during 3D TOF MRA of 662 carotid arteries. DSA images were available for comparison in 102 cases. The median percentage of stenosis in this subgroup of flow voids on MRA images was 80%, compared with measurements on DSA images according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria. Stenoses ranged from 36% to 100% (occlusion). Three flow voids (2.9%) were in the 0–49% range; 11 (10.8%), in the 50–69% range; and 86 (84.3%), in the 70–99% range. Two flow voids (2.0%) represented occlusions. The positive predictive value of a flow void artifact for the presence of severe (70–99%) stenosis was 84.3% (95% CI: 77.3%, 91.4%).
CONCLUSION: Flow void artifacts represented severe stenosis in most of the arteries. According to our data, the assumption that flow voids on 3D TOF MRA images represent severe stenosis is justified.
Digital subtraction angiography (DSA) has been widely used to visualize the carotid arteries. On the basis of findings from randomized trials, it became the criterion standard in selecting patients for carotid endarterectomy (1–4). DSA, however, has a relatively high risk of morbidity and mortality (5, 6). However, complication rates of 0.5% for stroke and 0.4% for transient ischemic attack (TIA) have recently been reported for DSA (7). Noninvasive tests such as duplex ultrasonography (DUS) and magnetic resonance angiography (MRA) are therefore increasingly used in the diagnosis of carotid artery stenosis to avoid the risks associated with DSA (7–12). The most commonly used technique is time-of-flight (TOF) MRA. New techniques, such as contrast-enhanced MRA, seem promising for the future (13–15). To date, however, only a few groups have reported the results of contrast-enhanced MRA validated against the criterion standard of DSA (12).
One of the main problems in imaging the carotid arteries with TOF MRA is the possibility of flow void artifacts (signal intensity loss near the stenosis) (16). This phenomenon is believed to occur especially in the stenotic lumen of arteries because of turbulence of the blood flow. In imaging the carotid arteries, the frequency of flow voids might be 10–20%. Therefore, most diagnostic studies of MRA use the assumption that a flow void represents severe (70–99%) stenosis (17–20). This assumption is mostly based on technical explanations rather than on the results of clinical studies. One group (21) studied the appearance of flow void artifacts by using DSA as the criterion standard in a series of 50 patients. They observed good accuracy regarding concordant DUS findings and the presence of a void on MRA images in the recognition of severe stenosis.
In our opinion, the correct interpretation and estimation of the degree of stenosis of a flow void artifact is of crucial importance in the interpretation of TOF MRA test results in clinical practice. In the present study, our purpose was to determine the frequency and diagnostic meaning of flow voids by using real patient data, as part of a larger study in which MRA was compared with the criterion standard of DSA (22). However, in deciding whether noninvasive testing can replace DSA in providing a valid estimate of the diagnostic value of MRA and DUS, a cost-effectiveness analysis should be performed (7, 22).
TOF MRA
TOF MRA is a gradient-echo technique in which contrast material is obtained with the inflow of fresh unsaturated blood through an image section with (pre)saturated static tissue. This process makes visualization of the lumen of an artery possible. Both two-dimensional (2D) and three-dimensional (3D) techniques are applied. 2D TOF MRA uses very thin sections as imaging volumes. The signal intensity of blood is high, making the technique sensitive in distinguishing slow flow from occlusions. The imaged volume in 3D TOF MRA is larger. Compared with 2D TOF MRA, 3D techniques have higher spatial resolution, a greater signal-to-noise-ratio, and lower sensitivity for voids because of the smaller voxels and shorter echo time. With 3D TOF MRA, the degree of stenosis can be measured according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria (1) by using postprocessing maximum-intensity projections (MIPs) of the bifurcation of the carotid artery.
Flow Voids
In imaging the carotid arteries, the frequency of flow voids on TOF MRA images might be 10–20%. Flow voids are considered to be caused by blood flow disturbances near a stenosis. On the basis of the technical explanations, however, the exact determination of the degree of stenosis remains impossible. When reading TOF MRA images, one should realize that this technique does not provide purely morphologic images. Instead, it relies on the signal intensity created by flowing blood. For this reason, a direct comparison of DSA and TOF MRA is not at all straightforward. A common problem with 2D or 3D TOF MRA is the local reduction of signal intensity or even the total loss of signal intensity due to spin dephasing caused by complex flow patterns distal to high-grade stenoses (23). The severity of such dephasing artifacts have been shown to depend on the gradient wave forms of the imaging sequence (24) and on many parameters related to the hemodynamics of the stenosis, such as its geometry, the average blood flow velocity, the Reynolds number, the turbulence intensity, and the turbulent fluctuation velocity (25). Because of the complex nature of the appearance of the poststenotic signal intensity on TOF MR angiograms and especially because of the hemodynamic aspects involved, accurate grading of stenoses on MR images remains difficult from a technical point of view, even with knowledge of the flow rate. Still, flow-induced signal intensity loss (also called a flow void) distal to a stenosis in the internal carotid artery on TOF angiograms is assumed to reflect the presence of a severe (70–99%) stenosis.
In this study, we tested the assumption that the conclusion of severe (70–99%) stenosis is appropriate when a flow void is observed with TOF MR. Because of the clinical relevance of selecting the appropriate patients for carotid endarterectomy, we studied the incidence of this phenomenon and validated the appearance of flow voids in relation to stenosis measurements on DSA.
Methods
Study Population
From January 1997 to November 2000, 390 consecutive patients with carotid artery stenosis at DUS and scheduled to undergo DSA before possible carotid endarterectomy were included in the prospective diagnostic study (296 male, 94 female; mean age, 67 years; age range, 39–88 years). Subsequently, all patients underwent MRA. Ninety-one percent of the patients had symptoms of carotid artery disease (TIA, minor disabling ischemic stroke, or amaurosis fugax) in the prior 6 months, and 9% of the patients were asymptomatic. We excluded patients who had contraindications for MRA, such as metal implants not suitable for MR imaging or claustrophobia. All patients provided written informed consent. Our study was approved by the medical ethics committees of the participating hospitals. The baseline characteristics of the patients are listed in Table 1. The accuracies of MRA and DUS in comparison with DSA from the total study population will be published elsewhere (22).
TABLE 1:
Baseline Characteristics of the Study Population
| Characteristic | Value |
|---|---|
| Age (y) | |
| Mean | 67 |
| Range | 39–88 |
| Sex (N = 390) (%) | |
| Male | 76 |
| Female | 24 |
| Symptoms* (%) | |
| Amaurosis fugax, retinal | 20 |
| Transient ischemic attack | 38 |
| Stroke | 33 |
| Asymptomatic | 9 |
| Hypertension (%) | 48 |
| Diabetes (%) | 15 |
| Cardiac history (%) | |
| Angina | 18 |
| Myocardial infarction | 16 |
| Heart failure | 6 |
| Bypass-surgery or PTCA† | 11 |
| Peripheral arterial disease (%) | |
| Claudication | 15 |
| Surgery or PTA‡ | 8 |
| Smoker, % | |
| Yes | 49 |
| Former | 34 |
Present 0–6 months prior to the patient’s inclusion into the study.
Percutaneous transluminal coronary angioplasty.
Percutaneous transluminal angioplasty.
Diagnostic Tests
All patients underwent DUS, DSA, and MRA within 1 month. The degree of stenosis on DUS was determined on the basis of the peak systolic velocity (PSV) in the proximal part of the ICA, which is the most accurate estimator of the degree of stenosis at DUS (26). We used a PSV of 270 cm/s as the threshold representing 70% stenosis and a PSV of 210 cm/s to represent 50% stenosis, as assessed with DSA (27).
DSA was performed with the selective positioning of an intraarterial catheter in both common carotid arteries by using the Seldinger technique. Two or three projections—lateral, posteroanterior, or ipsilateral oblique—were acquired in each carotid bifurcation.
MRA was performed with a 1.5-T MR imaging system by using the 3D TOF technique. A 3D spoiled gradient-echo acquisition was applied by using the following parameters: TR/TE/NEX, 30/6.9/3; excitation angle, 15°; field of view, 96 × 120; acquisition matrix, 147 × 256; and 50 sections with a 1.0-mm thickness reconstructed to 100 0.5-mm-thick sections. A quadrature head coil was used as a receiver coil. The imaging duration was about 9 minutes. Postprocessing subvolumes were generated to isolate each carotid artery and to create 12 MIP images that were radially projected at 15° increments (rotation about the long axis of the body).
One observer in each hospital (including A.v.d.L.) read the DSA and MRA test results. The observer was blinded to the clinical information and to the results of the other tests. The DSA and MRA results were independently read, with a period of at least 1 month between the readings. The observers examined hard-copy DSA and MRA images. The stenoses were measured on both DSA and MRA images according to the NASCET criteria (1). Flow voids were defined as the complete loss of signal intensity in the internal carotid artery for a minimal length of 1 mm on the MIPs of both 2D and 3D TOF MRA images. By definition, the signal intensity had to reappear distal from the flow void in the ICA. To assess the interobserver variability in recognizing a flow void artifact, a second observer read the images of a subseries of 200 consecutive patients.
The quality of all DSA examinations was assessed by using scores of 1–3 in which 1 indicated good; 2, moderate; and 3, inferior. The overall quality of the examination as well as more detailed items such as over-projection and applicability to measure the degree of stenosis was incorporated.
Analysis
The frequency of flow voids at MRA was calculated in the total study population. From the flow voids observed at MRA, the corresponding estimated stenosis at DSA and DUS was recorded. Proportions from the voids for the categories of stenosis (0–49%, 50–69%, 70–99%, 100%) were calculated. The positive predictive value of flow voids for presence of severe stenosis was estimated by using DSA as the criterion standard. Interobserver variability in recognizing a flow void was assessed by estimating the ê value. We also studied whether the degree of stenosis at DSA (in case of a flow void at MRA) was related to the quality of the test by using the χ2 test.
Results
From the total of 390 patients, the following numbers of stenosis measurements from the symptomatic side were interpretable and could be included in the analyses: from DSA, 360; DUS, 372; and MRA, 341. Some values were missing because 1) it was not always feasible to perform all three tests before surgery, 2) some patients withdrew from the study after completing one or two tests, and 3) the test was not always correctly performed according to our study protocol. With DUS, the PSV was not measured on some occasions. Finally, the stenosis could not be measured in 10 and seven patients because of poor quality and reliability of the recordings made at MRA and DSA, respectively.
TOF MRA images of sufficient quality were available in 662 carotid arteries. During the readings of these images, 107 flow voids (16.2%) were recorded. DSA images were available in 102 of the 107. The median percentage of stenosis from the flow voids was 80%, and the stenoses ranged from 36% to 100% (occlusion) when they were compared with the stenosis measurements on DSA images, according to the NASCET criteria. In comparison with DSA, three flow voids (2.9%) were the 0–49% range of stenosis; 11 (10.8%), in the 50–69% range; 86 (84.3%) in the 70–99% range. Two (2.0%) represented an occlusion (Table 2). In the group of severe (70–99%) stenosis, nine cases of slow flow or the string sign were recorded, according to the DSA findings. These were graded as 99% stenoses.
TABLE 2:
Degree of Stenosis at DSA in 102 Flow Voids on Time-of-flight MRA Images
| Degree of Stenosis at DSA | Flow Voids (n = 102) |
|---|---|
| 0–49% | 3 (2.9) |
| 50–69% | 11 (10.8) |
| 70–99%* | 86 (84.3) |
| 100% (occlusion) | 2 (2.0) |
Note.—Data in parentheses are percentages.
In the group of severe (70–99%) stenosis, nine cases of slow flow or the string sign were recorded, according to the DSA findings. These were graded as 99% stenoses.
The positive predictive value of a flow void artifact for presence of severe (70–99%) stenosis, according to DSA results, was 84.3% (95% CI: 77.3%, 91.4%). The ê statistic for interobserver variability in recognizing a flow void was 0.81 (95% CI: 0.76, 0.83).
MRA, DUS, and DSA results were assessed in 98 of 108 carotid arteries in which MRA showed a flow void artifact. Table 3 illustrates the concordant and discordant findings determined by tabulating DUS results versus DSA results. DSA did not reveal a severe (70–99%) stenosis in 16 cases in which MRA showed a flow void. In 10 of the 14 arteries in which DSA showed a stenosis of less than 70%, DUS did show severe stenosis.
TABLE 3:
Categorized degree of stenosis on DUS and DSA. All 98 carotid arteries in this table were recognized as flow voids on MRA
| Categories of Stenosis at DUS | Categories of Stenosis at DSA |
||||
|---|---|---|---|---|---|
| 0–49% | 50–69% | 70–99% | 100% | Total | |
| 0–49% | 0 | 0 | 0 | 0 | 0 |
| 50–69% | 0 | 1 | 2 | 0 | 3 |
| 70–99% | 3 | 10 | 79 | 1 | 93 |
| 100% | 0 | 0 | 1 | 1 | 2 |
| Total | 3 | 11 | 82 | 2 | 98 |
A trend was observed between the quality of DSA images and the proportion of the carotid arteries in which the angiogram showed severe stenosis. The proportion was 87.5% in the group of DSA images with good quality and decreased to 78.3% in the group with moderate quality and to 71.4% in the group with inferior quality (Table 4). However, this trend was not significant (P = .15, χ2 test).
TABLE 4:
Quality of DSA Images and the Degree of Stenosis Depicted
| Quality of DSA Images | No. of Arteries by Degree of Stenosis |
||
|---|---|---|---|
| <70% or 100% | 70–99%* | Total (n = 102) | |
| Good | 9 (12.5) | 63 (87.5) | 72 (100) |
| Moderate | 5 (21.7) | 18 (78.3) | 23 (100) |
| Inferior | 2 (28.6) | 5 (71.4) | 7 (100) |
Note.— All 102 carotid arteries in the table were recognized as flow voids at MRA. Data in parentheses are percentages.
Not significant linear-by-linear association (P = .15, χ2 test).
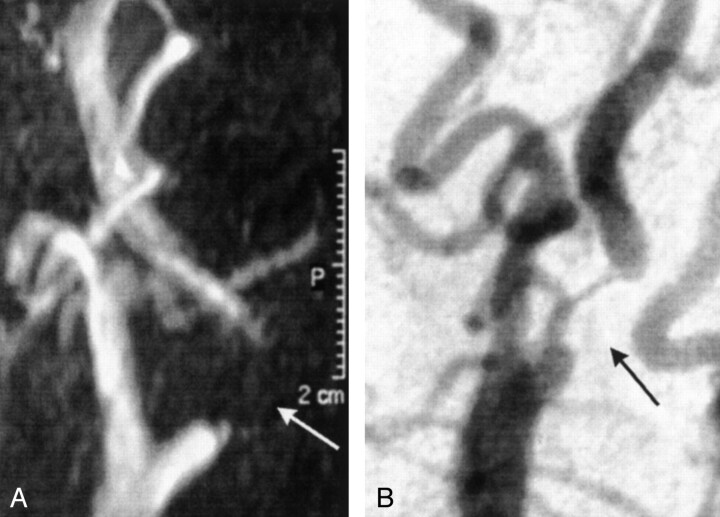
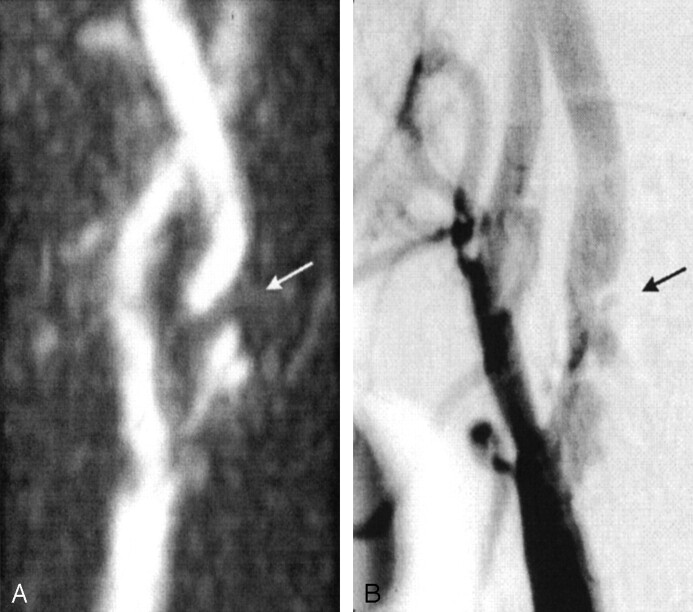
Figures 1 and 2 show examples of flow voids on 3D TOF MRA images with concordant (Fig 1) and discordant (Fig 2) DSA findings.
Fig 1.
Images in a 70-year-old man with a minor stroke of the right hemisphere.
A, MRA image shows a long flow-void artifact (arrow).
B, DSA image clearly shows a severe (70–99%) stenosis in the internal carotid artery (arrow).
Fig 2.
Images in a 64-year-old man with a TIA of the right hemisphere.
A, MRA image shows a short flow-void artifact distal to a visible irregular plaque in the internal carotid artery (arrow). All other available projections of the 3D TOF MRA images (except for two directions with over-projection that made the internal carotid artery invisible, 12 total) showed an interruption in the blood-flow signal intensity. The flow void was confirmed on the transversal 2D TOF MRA images.
B, DSA image also shows the irregular stenosis with an ulcerated plaque in the internal carotid artery. The measurement of the most severe stenosis with DSA, however, revealed a moderate (50–69%) stenosis (arrow).
Discussion
In visualizing the carotid arteries with 3D TOF MRA, we found that the frequency of flow void artifacts near the stenoses was 16%. Flow voids are often considered to represent severe (70–99%) stenosis on the basis of technical explanations. In the present clinical study, the positive predictive value of a flow void artifact for presence of severe (70–99%) stenosis was 84.3% according to the criterion standard of DSA. The median percentage of stenosis was 80%; stenoses ranged from 36% to 100%. In this clinical study, flow void artifacts represented severe stenosis in most of the arteries.
According to results of randomized trials, patients with a symptomatic severe (70–99%) stenosis of the ICA benefit from carotid endarterectomy. Patients with a 50–69% stenosis have a smaller benefit, and the publication of more determinants for selecting the appropriate subpopulation for surgical treatment is expected in the near future (3). The diagnosis of 70–99% stenosis remains crucial in the workup of patients with carotid artery disease. DSA has traditionally been the criterion standard for estimating the degree of stenosis in the ICA on basis of previous trial results. Because of the relative high risk of complications with DSA, noninvasive techniques such as MRA and DUS are increasingly used. A commonly applied technique is TOF MRA. If DSA is to be replaced, patients have to be selected for surgery on basis of MRA findings, most likely obtained after screening with DUS. The occurrence of flow related artifacts is one of the drawbacks of the TOF technique. However, the correct interpretation of the results is also important, with view on the decision to perform carotid endarterectomy. In the current study, patients whose DUS results suggested carotid artery stenosis were examined with TOF MRA. Flow void artifacts appeared in 16% of the observed arteries, which we think is relatively often. Although this population was selected because the patients were screened with DUS before undergoing DSA and MRA, it is exactly the group in whom MRA would be performed if DSA is replaced. In a noninvasive strategy, MRA is still likely to be preceded by DUS. Therefore, a flow-void frequency of 16% with MRA is a realistic number for clinical practice.
New developments, such as contrast-enhanced MRA, might decrease the problem of flow-related artifacts in the near future (13–15). The frequency of voids is expected to be lower, but the problem of artifacts has not yet been resolved with the introduction of this technique. Furthermore, the exact causes of artifacts with this technique remain unknown. Contrast-enhanced MRA has not yet been validated in large series by using DSA as the reference test. However, this technique shows promising first results in the imaging of carotid arteries, and its widespread assessment is expected in the near future.
Our data confirm the assumption that a flow void at TOF MRA represents a severe stenosis. In 84% of the voids that appeared in our study, the criterion-standard DSA results revealed a 70–99% stenosis. The voids ranged from 36% to 100%. In 14% of the cases (14 arteries), the stenosis was less than 70%. However, 10.8% of this group (11 arteries) was in the 50–69% category. The remaining two represented occlusion (100% stenosis) according to the DSA results. The DSA images that did not show severe stenosis seemed to be of lower quality, compared with the images that did show severe stenosis. Although this difference was not significant, we believe that finding this strengthens our conclusion that a flow void can be considered a severe stenosis in the workup of a patient before possible carotid endarterectomy. In all 16 cases in which a flow void on MRA images did not represent a severe (70–99%) stenosis on DSA images, a senior radiologist (W.P.Th.M.M.) and a senior vascular surgeon (B.C.E.). retrospectively re-evaluated the DUS and DSA results. In seven examinations, this nonblinded re-evaluation revealed the inferior quality of the DSA images, which made adequate measurement of the stenosis difficult. In these cases, the readers could not decide on carotid endarterectomy on basis of the presented DSA findings alone. In four cases, the stenosis or atherosclerotic disease seemed worse than that indicated by the stenosis measurement according to the protocol, and together with the DUS results, the readers tended to advise carotid endarterectomy. In five cases, the reviewers made no comment about the DSA or DUS examinations. In our series, the total number of cases involving a flow void that had available criterion-standard images was relatively large. In most cases in which MRA showed a flow void and in which DSA had not shown severe stenosis, the quality of the reference image was inferior or the stenosis seemed worse than that indicated by only the measurement of the degree of stenosis. The belief that the DSA images from the group with stenosis of less than 70% had shortcomings in establishing the severity of the disease was confirmed by the fact that DUS did show severe stenosis (PSV > 270 cm/s) in 10 of the 14 arteries.
The present results validate the finding of flow voids artifacts on MRA images in a clinical series. These data, however, remain insufficient for use in policy-making decisions. Over the last decade, many diagnostic studies have been conducted to compare MRA with DSA. However, a recent review of reports on this topic published between 1993 and 1998 criticized the design of the studies (28). Often, the study populations were small, or the diagnostic test results were collected retrospectively. Furthermore, from the limited published evidence available to date, the cost-effectiveness of carotid endarterectomy and of the preoperative investigations remains unclear (29). To be able to decide on carotid endarterectomy on the basis of a flow void artifact or on MRA findings in general, the accuracy of MRA must be assessed in a sufficiently large cohort. Furthermore, to make the right policy decisions and to provide a valid estimate of the accuracy of noninvasive testing, the cost-effectiveness of the techniques should be taken into account.
Conclusion
In our opinion, flow voids, which appear in 16% of TOF MRA images of the carotid arteries, must be assessed with care in the interpretation of TOF MRA results in clinical practice. In our patient series, flow void artifacts represented severe stenosis in most of the arteries. We believe that this finding is strengthened by the fact that the quality of the DSA images tended to be worse and that DUS showed severe stenosis in most of the arteries in the group in which DSA showed stenosis of less than 70%. In general, the assumption that a flow void on a 3D TOF MRA image represents severe stenosis is justified.
Acknowledgments
We wish to thank Aloys Wüst, Department of Radiology, University Medical Center Utrecht, the Netherlands for observing all of the diagnostic tests.
Footnotes
Partly supported by a grant (OG/030) from the Dutch Ministry of Health, Welfare and Sports.
References
- 1.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 1991;325:445–453 [DOI] [PubMed] [Google Scholar]
- 2.European Carotid Surgery Trialists’ Collaborative Group. MRC European Carotid Surgery Trial: interim results for symptomatic patients with severe (70–99%) or with mild (0–29%) carotid stenosis. Lancet 1991;337:1235–1243 [PubMed] [Google Scholar]
- 3.Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis: North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339:1415–1425 [DOI] [PubMed] [Google Scholar]
- 4.European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 1998;351:1379–1387 [PubMed] [Google Scholar]
- 5.Hankey GJ, Warlow CP, Molyneux AJ. Complications of cerebral angiography for patients with mild carotid territory ischaemia being considered for carotid endarterectomy. J Neurol Neurosurg Psychiatry 1990;53:542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies KN, Humphrey PR. Complications of cerebral angiography in patients with symptomatic carotid territory ischaemia screened by carotid ultrasound. J Neurol Neurosurg Psychiatry 1993;56:967–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston DC, Goldstein LB. Clinical carotid endarterectomy decision making: noninvasive vascular imaging versus angiography. Neurology 2001;56:1009–1015 [DOI] [PubMed] [Google Scholar]
- 8.Jackson MR, Chang AS, Robles HA, et al. Determination of 60% or greater carotid stenosis: a prospective comparison of magnetic resonance angiography and duplex ultrasound with conventional angiography. Ann Vasc Surg 1998;12:236–243 [DOI] [PubMed] [Google Scholar]
- 9.Kent KC, Kuntz KM, Patel MR, et al. Perioperative imaging strategies for carotid endarterectomy: an analysis of morbidity and cost-effectiveness in symptomatic patients. JAMA 1995;20;274:888–893 [DOI] [PubMed] [Google Scholar]
- 10.Kagawa R, Moritake K, Shima T, Okada Y. Validity of B-mode ultrasonographic findings in patients undergoing carotid endarterectomy in comparison with angiographic and clinicopathologic features. Stroke 1996;27:700–705 [DOI] [PubMed] [Google Scholar]
- 11.Blakeley DD, Oddone EZ, Hasselblad V, Simel DL, Matchar DB. Noninvasive carotid artery testing: a meta-analytic review. Ann Intern Med 1995;122:360–367 [DOI] [PubMed] [Google Scholar]
- 12.Westwood ME, Kelly S, Berry E, et al. Use of magnetic resonance angiography to select candidates with recently symptomatic carotid stenosis for surgery: systematic review. Br Med J 2002;324:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leclerc X, Gauvrit JY, Nicol L, Pruvo JP. Contrast-enhanced MR angiography of the craniocervical vessels: a review. Neuroradiology 1999;41:867–874 [DOI] [PubMed] [Google Scholar]
- 14.Thanh Phan, Huston J, Bernstein MA, Riederer SJ, Brown RD. Contrast-enhanced magnetic resonance angiography of the cervical vessels: experience with 422 patients. Stroke 2001;32 ,2282–2286 [DOI] [PubMed] [Google Scholar]
- 15.Remonda L, Senn P, Barth A, Arold M, Lövblad KO, Schroth G. Contrast-enhanced 3D MR Angiography of the carotid artery: comparison with conventional digital subtraction angiography. AJNR Am J Neurorad 2002;23:213–219 [PMC free article] [PubMed] [Google Scholar]
- 16.Hoogeveen RM, Bakker CJ, Viergever MA. Phase-derivative analysis in MR angiography: reduced Venc dependency and improved vessel wall detection in laminar and disturbed flow. J Magn Reson Imaging 1997;7:321–330 [DOI] [PubMed] [Google Scholar]
- 17.Serfaty JM, Chirossel P, Chevallier JM, Ecochard R, Froment JC, Douek PC. Accuracy of three-dimensional gadolinium-enhanced MR angiography in the assessment of extracranial carotid artery disease. AJR Am J Roentgenol 2000;175:455–463 [DOI] [PubMed] [Google Scholar]
- 18.Binaghi S, Maeder P, Uske A, Meuwly JY, Devuyst G, Meuli RA. Three-dimensional computed tomography angiography and magnetic resonance angiography of carotid bifurcation stenosis. Eur Neurol 2001;46:25–34 [DOI] [PubMed] [Google Scholar]
- 19.Randoux B, Marro B, Koskas F, et al. Carotid artery stenosis: prospective comparison of CT, three-dimensional gadolinium-enhanced MR, and conventional angiography. Radiology 2001;220:179–185 [DOI] [PubMed] [Google Scholar]
- 20.Back MR, Wilson JS, Rushing G, et al. Magnetic resonance angiography is an accurate imaging adjunct to duplex ultrasound scan in patient selection for carotid endarterectomy. J Vasc Surg 2000;32:429–438 [DOI] [PubMed] [Google Scholar]
- 21.Huston J, Nichols DA, Luetmer PH, et al. MR angiographic and sonographic indications for endarterectomy. AJNR Am J Neuroradiol 1998;19:309–315 [PMC free article] [PubMed] [Google Scholar]
- 22.Nederkoorn PJ, Mali WPThM, Eikelboom BC, et al. Preoperative diagnosis of carotid artery stenosis. Stroke 2002;33:2003–2008 [DOI] [PubMed] [Google Scholar]
- 23.Mustert BR, Williams DM, Prince MR. In vitro model of arterial stenosis: correlation of MR signal dephasing and trans-stenotic pressure gradients. Magn Reson Imaging 1998;16:301–310 [DOI] [PubMed] [Google Scholar]
- 24.Gatenby JC, McCauley TR, Gore JC. Mechanisms of signal loss in magnetic resonance imaging of stenoses. Med Phys 1993;20:1049–1057 [DOI] [PubMed] [Google Scholar]
- 25.Oshinski JN, Ku DN, Pettigrew RI. Turbulent fluctuation velocity: the most significant determinant of signal loss in stenotic vessels. Magn Reson Med 1995;33:193–199 [DOI] [PubMed] [Google Scholar]
- 26.Hunink MG, Polak JF, Barlan MM, O’Leary DH. Detection and quantification of carotid artery stenosis: efficacy of various Doppler velocity parameters. AJR Am J Roentgenol 1993;160:619–625 [DOI] [PubMed] [Google Scholar]
- 27.Elgersma OE, van Leersum M, Buijs PC, et al. Changes over time in optimal duplex threshold for the identification of patients eligible for carotid endarterectomy. Stroke 1998;29:2352–2356 [DOI] [PubMed] [Google Scholar]
- 28.Rothwell PM, Pendlebury ST, Wardlaw J, Warlow CP. Critical appraisal of the design and reporting of studies of imaging and measurement of carotid stenosis. Stroke 2000;31:1444–1450 [DOI] [PubMed] [Google Scholar]
- 29.Benade MM, Warlow CP. Costs and benefits of carotid endarterectomy and associated preoperative arterial imaging: a systematic review of health economic literature. Stroke 2002;33:629–638 [DOI] [PubMed] [Google Scholar]