Abstract
Summary: Two patients with acute thiamine deficiency were examined with thalamic single-voxel proton MR spectroscopy. T2-weighted images exhibited increased signal intensity. N-acetylaspartate (NAA)/creatine (Cr) ratios were low without detectable lactate. Owing to substantially decreased choline (Cho) T2, the Cho/Cr ratio was not decreased. After thiamine therapy, the NAA/Cr ratio increased, paralleling clinical improvement and reduction in the areas of signal-intensity changes.
Deficiency of thiamine (vitamin B1) causes selective brain damage in animals and humans. This condition is reversed with the intravenous supplementation of this coenzyme (1). Proton MR spectroscopy is a powerful noninvasive tool in the biochemical characterizing of metabolic brain diseases in vivo. MR spectroscopy has been applied to investigations of thiamine deficiency in rats (2–4). We performed single-voxel MR spectroscopy in two patients with neurologic symptoms due to acute thiamine deficiency, or Wernicke encephalopathy, before and after therapy.
Case Reports
Case 1
A 53-year-old man underwent gastrectomy because of cancer. After 1 month of parenteral nutrition, he developed mental confusion. Ten days later, cranial T2-weighted MR imaging demonstrated symmetric areas of increased signal intensity in the medial thalami (Fig 1). The areas exhibited moderate enhancement after the intravenous injection of paramagnetic contrast material. To avoid the interference of contrast enhancement on the proton spectrum (5), MR spectroscopy was performed the next day. Two weeks after intravenous supplementation with thiamine (100 mg daily), the confusion subsided, and the MR images demonstrated partial regression in the areas with signal-intensity changes in the thalami. Post-treatment MR spectroscopy was performed in the same session with MR imaging.
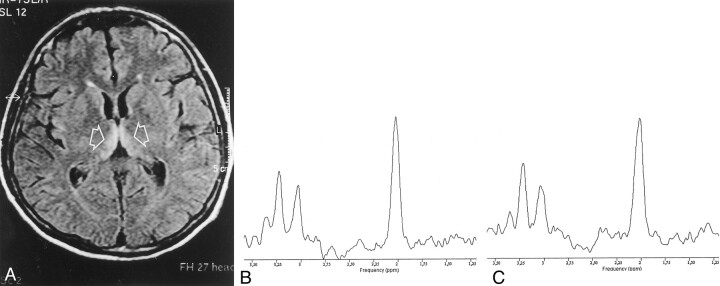
Fig 1.
Case 1.
A, Initial T2-weighted fluid-attenuated inversion-recovery MR image (TR/TI/TE, 4000/2000/100; echo train length, 12) shows areas of T2 prolongation in the medial thalami (arrows).
B and C, Proton MR spectra obtained with the PRESS sequence (TR/TE, 2000/272) at presentation (B) and after 2 weeks after the intravenous administration of thiamine (C). Note the relative increase of Cho peak in C and the lack of lactate in both spectra.
Case 2
A 61-year-old man underwent pancreaticoduodenectomy because of pancreatic cancer. After 42 days of parenteral nutrition, he developed rapidly progressive mental confusion and gait ataxia. Three days after this onset, cranial T2-weighted MR images showed areas of increased signal intensity in the inferior colliculi, periaqueductal gray matter, medial thalami, and mammillary bodies. The areas did not enhance after the intravenous administration of paramagnetic contrast material. MR spectroscopy was performed the next day when the MR imaging results were unchanged. Twelve days after intravenous supplementation with thiamine (100 mg daily), the patient’s symptoms had improved considerably, and the MR images showed normal signal intensity in the medial thalami, mammillary bodies, and midbrain. Post-treatment MR spectroscopy was performed in the same session with MR imaging.
MR Spectroscopy
MR spectroscopy was performed with a 1.5-T system (Gyroscan NT ACS; Philips, Best, the Netherlands) by using a standard quadrature head coil.
The voxel of interest was a 2 × 2 × 2-cm cube centered in the right thalamus in case 1 and a 2 × 2 × 5-cm parallelepiped covering the medial thalami and encompassing the third ventricle in case 2. After automatic shimming and gradient tuning, a water suppression with water-selective excitation pulse was interactively optimized on the display console. A point-resolved proton spectroscopy sequence (PRESS) was used to acquire the proton spectrum with a TR of 2000 milliseconds and with 128 measurements. Because the spectra were obtained in areas of prolonged T2, that is, in areas of increased signal intensity on T2-weighted images, four TEs (80, 136, 272, and 500 milliseconds) were used to compute the T2 of the metabolites in case 1. In case 2, the PRESS sequence was performed with a TE of 272 milliseconds. With a matrix of 256 × 128, each spectrum acquisition time was 4.24 minutes. Postprocessing involved the following steps: zero filling, Gaussian filtering, exponential multiplication, Fourier transformation, and manual phase correction.
Spectral Analysis
The areas of the peaks at 2.07, 3.00, and 3.20 ppm corresponded to N-acetylaspartate (NAA), creatine (Cr), and choline (Cho). The ratios of NAA/Cr, Cho/Cr, and NAA/Cho were calculated in the 272-millisecond spectra by using the software provided by the manufacturer. The ratios were compared with reference values obtained in 10 healthy subjects.
In case 1, the T2 values were determined by fitting a two-parameter (amplitude S0 and decay constant 1/T2) mono-exponential equation to the data by using a linear least-squares algorithm, as follows: S(τ) = S0e–TE/T2.
Reference normal values of NAA, Cr, and Cho T2 and of T2-corrected NAA/Cr and Cho/Cr ratios for the same acquisition technique and site were available in five healthy subjects.
Results
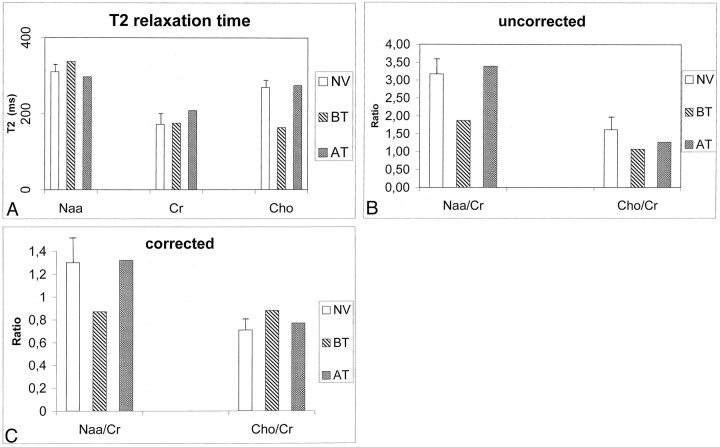
Initial 1H MR spectra with a TE of 272 millisecond (Fig 1) showed a low NAA/Cr ration (case 1, 1.80; case 2, 1 .86) and a low Cho/Cr ratio (case 1, 1 .06; case 2, 1.23), as compared with the control values (NAA/Cr ratio, 3.17 ± 0.44; Cho/Cr ratio, 1.61 ± 0.32) (Fig 2), without detectable lactate. Computation of metabolite T2 valued demonstrated a marked reduction in Cho T2 (163 milliseconds; normal value, 269 ms ± 18) whereas minor changes were noted in NAA T2 (337 milliseconds; normal value, 310 ms ± 19) and Cr T2 (175 ms; normal value, 172 ms ± 27) (Fig 2). After correction of the metabolite ratios for the T2 effects, the NAA/Cr ratio remained low (0.87; normal value, 1.30 ± 0.22), whereas the Cho/Cr ratio was slightly increased (0.88; normal value, 0.71 ± 0.09) (Fig 2).
Fig 2.
Comparison of results in healthy volunteers and in case 1. AT indicates after treatment; BT, before treatment; and NV, healthy volunteers. The error bars indicate the standard deviation of the control values.
A, Metabolite T2 in the thalamus in five healthy volunteers and in the patient in case 1 before and after treatment with thiamine.
B and C, NAA/Cr and Cho/Cr ratios in the healthy volunteers and in case 1 before and after treatment without (B) and with (C) correction for metabolite T2 changes.
After thiamine therapy, proton MR spectra with a TE of 272 milliseconds showed an increase in the NAA/Cr ratio (case 1, 3.39; case 2, 2.45) and in the Cho/Cr ratio (case 1, 1.26; case 2, 1.44), without detectable lactate (Figs 1, 2). The Cho T2 change was reversed (274 milliseconds), and the corrected NAA/Cr ratio of 1.32 and Cho/Cr ratio of 0.77 were within 1 standard deviation of the control values (Fig 2).
Discussion
Because the clinical features of acute thiamine deficiency in humans are nonspecific and possibly subtle, the MR imaging demonstration of a symmetric area of signal-intensity changes in the mesencephalic tegmentum, mammillary bodies, and medial thalami is fundamental for the diagnosis and treatment of Wernicke encephalopathy (6). Proton MR spectroscopy can provide information about the pathophysiology of thiamine deficiency in vivo (2–4). Experimental models of thiamine deficiency involve rats deprived of thiamine with a regimen of thiamine-deficient chow and intraperitoneal injections of the thiamine antagonist pyrithiamine (2–4).
Besides the possible differences in pathophysiology of thiamine deficiency in humans compared with that in rats, three factors must be considered in comparing our results with those obtained with MR spectroscopy in experimental models. First, in experimental conditions, early metabolic changes can be monitored by looking at the behavior of the animals (2–4), presumably before severe tissue damage develops. On the contrary, in humans, clinical symptoms presumably develop on a background of a severe metabolic failure, which has already caused tissue damage detectable at histopathologic examination. In other words, the stage at which MR spectroscopy can be performed is certainly and considerably earlier in experimental conditions. Second, because the damage in Wernicke encephalopathy is regionally selective (7), we placed our volumes of interest in the thalami, which are some of the regions with maximal tissue damage, as inferred from the neuropathologic descriptions (7) and the MR imaging findings in our two patients. In experimental models, MR spectroscopy was performed without any spatial reference to possible areas of signal-intensity changes on MR images. Finally, because changes in metabolite T2 can interfere with metabolite ratios (8), we measured metabolite T2 and corrected the metabolite ratios for T2 effects in one of our patients. In experimental studies of thiamine deficiency, T2 effects were not considered, and quantification of the metabolites was performed with other methods. The T2 of brain metabolites that we measured in the thalamus in our control subjects were similar to those reported with 1.5-T studies in the cerebral gray matter (9, 10).
The reversible decrease in the NAA/Cr ratio that we observed in the thalami of acute Wernicke encephalopathy, even after we corrected for T2 effects, was presumably due to edema (8). This finding is in line with the decrease in NAA that Rose et al (2) measured in rats.
Thiamine deficiency implies decreased activity of the thiamine-dependent enzyme α-ketoglutarate dehydrogenase that leads to a dysfunction of the tricarboxilic acid cycle and of pyruvate dehydrogenase, with lactate accumulation (1). Unexpectedly, lactate was not observed in our two patients. However, an increase in lactate levels was not mentioned in the experimental models studied by Lee et al (3, 4), and Rose et al (2) observed increased lactate levels in thiamine-deficient rats only after glucose loading. In our opinion, further studies are needed to establish if the lactate increase occurs and when it does so in thiamine-deficient brains examined in vivo.
Before correcting for T2 effects, we found a reduction in the Cho/Cr ratio in our two patients with acute Wernicke encephalopathy. Lee et al (3, 4) observed a reduction in the Cho/Cr ratio and in the Cho concentration in experimental animals. This finding is explained by the deficit of the thiamine-dependent enzymes transketolase, which leads to dysfunction of the pentose phosphate shunt with a reduction in glycerophosphocholine and phosphorylcholine levels (1). These substances are intermediates in phospholipid synthesis and degradation, and their reduction could impair the incorporation of lipids into the cell membranes, with a disruption of osmotic gradients across cell membranes and with the accumulation of intra- and extracellular fluid (11). Although one could expect that edema influences the T2 relaxation times of all metabolites (8), we observed a selective T2 shortening with Cho in our patient with acute Wernicke encephalopathy. When this T2 change was considered, no decrease in the Cho/Cr ratio in the acute phase of Wernicke encephalopathy was observed in one of our patients. We submit that the decrease in the Cho/Cr ratio, as determined by the metabolic derangement associated with thiamine deficiency, could have been counterbalanced by demyelination in our patient; this is an early histopathologic feature of Wernicke encephalopathy (12) and is associated with an increase in Cho/Cr and Cho concentrations in diseases such as multiple sclerosis (11).
Conclusion
Our observations indicate that, when proton spectra are obtained in the thalami of patients with acute Wernicke encephalopathy, only a thiamine-reversible decrease of NAA/Cr is observed. Future studies of thiamine deficiency in animals, and, with hope, in humans, should evaluate possible T2 changes of metabolites.
References
- 1.Hazell AS, Todd KG, Butterworth RF. Mechanisms of neuronal cell death in Wernicke’s encephalopathy. Metab Brain Dis 1998;13:97–122 [DOI] [PubMed] [Google Scholar]
- 2.Rose SE, Nixon PF, Zelaya OF, et al. Application of high field localized in vivo 1H MRS to study biochemical changes in the thiamine deficient rat brain under glucose load. NMR Biomed 1993;6:324–328 [DOI] [PubMed] [Google Scholar]
- 3.Lee H, Tarter J, Holburn GE, Price RR, Weinstein DD, Martin PR. In vivo localized proton NMR spectroscopy of thiamine-deficient rat brain. Magn Reson Med 1995;34:313–318 [DOI] [PubMed] [Google Scholar]
- 4.Lee H, Holburn GE, Price RR. In vivo and in vitro proton NMR spectroscopic studies of thiamine-deficient rat brain. J Magn Reson Imaging 2001;13:163–166 [DOI] [PubMed] [Google Scholar]
- 5.Sijens PE, van den Bent MJ, Nowak PJ, van Dijk P, Oudkerk M. 1H chemical shift imaging reveals loss of brain tumor choline signal after administration of Gd-contrast. Magn. Reson Med 1997;37:222–225 [DOI] [PubMed] [Google Scholar]
- 6.Gallucci M, Bozzao A, Splendiani A, Masciocchi C, Passariello R. Wernicke encephalopathy. MR findings in five patients. AJNR Am J Neuroradiol 1990;11:887–892 [PMC free article] [PubMed] [Google Scholar]
- 7.Torvik A. Two types of brain lesions in Wernicke’s encephalopathy. Neuropathol Appl Neurobiol 1985;11:179–190 [DOI] [PubMed] [Google Scholar]
- 8.Kamada K, Houkin K, Hida K, et al. Localized proton spectroscopy of focal brain pathology in humans: significant effects of edema on spin-spin relaxation time. Magn. Res Med 1994;31:537–540 [DOI] [PubMed] [Google Scholar]
- 9.Christiansen P, Toft P, Larsson HBW, Stubgaard M, Henriksen O. The concentration of N-acetyl aspartate, creatine + phosphocreatine, and choline in different parts of the brain in adulthood and senium. Magn Reson Imaging 1993;11:799–806 [DOI] [PubMed] [Google Scholar]
- 10.Longo R, Bampo A, Vidimari R, Magnaldi S, Giorgini A. Absolute quantitation of brain 1H nuclear magnetic resonance spectra: comparison of different approaches. Invest Radiol 1995;30:199–203 [DOI] [PubMed] [Google Scholar]
- 11.Harper C, Butterworth R. Nutritional and metabolic disorders. In: Graham DI, Lantos PL, eds. Greenfield’s Neuropathology. 6th ed. Vol 2. London: Arnold;1997. :601–652
- 12.Miller BL. A review of chemical issues in 1H-NMR spectroscopy: N-acetyl-L aspartate, creatine and choline. NMR Biomed 1991;4:47–52 [DOI] [PubMed] [Google Scholar]