Abstract
Objective:
In this post-hoc analysis, we evaluated anlotinib treatment-induced hypertension as a potential predictive factor of efficacy in esophageal squamous cell carcinoma (ESCC) patients.
Methods:
A total of 109 patients enrolled in the anlotinib group in a phase 2 trial were included. The tumor response was assessed by computed tomography at week 3, week 6, and then every 6 weeks until progressive disease was observed. The primary endpoint of the study was progression free survival (PFS). The secondary endpoints included overall survival (OS) and objective response rate (ORR).
Results:
In all patients, the median PFS was 3.02 months [95% confidence interval (CI): 2.63–3.65 months] and the OS was 6.11 months (95% CI: 4.40–7.79 months). The ORR was 7.34% (95% CI: 3.22%–13.95%). A total of 59 (54%) patients were diagnosed with treatment-induced hypertension (Group A), and the remaining patients (n = 50, 46%) were in Group B. Baseline prognostic factors were similar between the 2 groups. Patients in Group A had a longer PFS and OS and higher ORR. When stratifying patients using a previously known history of hypertension, treatment-induced hypertension was a predictor only for patients without previous hypertension, who had longer PFS [hazard ratio (HR): 0.40, 95% CI: 0.24–0.68] and OS (HR: 0.37, 95% CI: 0.21–0.67).
Conclusions:
We showed, for the first time, a correlation between treatment-induced hypertension and better prognoses in recurrent or metastatic ESCC patients treated with anlotinib, without a previously known history of hypertension. Treatment-induced hypertension may be a simple and low cost predictor for anlotinib antitumor efficacy in these patients, which may also reflect the intended target inhibition.
Keywords: Esophageal squamous cell carcinoma (ESCC), anlotinib, treatment-induced hypertension, prognostic predictor, antiangiogenesis
Introduction
Esophageal cancer is the seventh most common malignancy, the sixth leading cause of cancer-related deaths worldwide, and the fourth leading cause of cancer-related mortality in China1,2. Esophageal squamous cell carcinoma (ESCC) is the most common subtype with a poor 5-year survival rate of only about 15%–25% in China3. Over past decades, recurrent or metastatic ESCC has been managed mainly using platinum plus paclitaxel or fluorouracil-based chemotherapy4,5. However, the long-term survival remains poor and needs to be improved.
Anlotinib is an oral small molecule tyrosine kinase inhibitor (TKI) targeting the vascular endothelial growth factor receptor (VEGFR) 1/2/3, fibroblast growth factor receptor 1–4, platelet-derived growth factor receptor (PDGFR) α/β, Ret, and c-Kit6. A phase III trial has shown that anlotinib improved the progression-free survival (PFS) and overall survival (OS) of patients with advanced non-small cell lung cancer (NSCLC)7. In ESCC, expression of VEGF is associated with angiogenesis, tumorigenesis, and progressive disease. Various co-expression patterns of VEGFR1/2/3 and PDGFR α/β at the transcriptional level were observed in ESCC8. Multitarget TKIs may therefore have anti-tumor efficacy in ESCC. In a previous multicenter, randomized, double-blind, placebo-controlled phase II trial (registration number: NCT02649361), anlotinib significantly improved the median PFS when compared with the placebo, in patients with recurrent or metastatic ESCC [3.02 vs. 1.41 months, hazard ratio (HR): 0.46, 95% confidence interval (CI): 0.32–0.66, P < 0.0001]9.
Treatment-induced hypertension has been proposed as a potential predictive factor of the clinical efficacy of antiangiogenic agents. Many retrospective analyses showed a correlation between treatment-related hypertension and better clinical outcomes in patients treated with anti-angiogenic agents with various malignancies, including NSCLC, gastrointestinal stromal tumor, renal cell carcinoma, colorectal carcinoma, and differentiated thyroid cancer10–14. In patients with different types of malignancies who received anlotinib, treatment-induced hypertension was also an independent predictive factor for better prognoses15–18. Currently, there has been no similar study in ESCC patients receiving anlotinib or any other antiangiogenic agents. In this post-hoc analysis, we showed that treatment-induced hypertension was a predictive factor for the efficacy of anlotinib treatment in recurrent or metastatic ESCC patients.
Materials and methods
Patients and treatments
This was a post-hoc analysis from a randomized, double-blind, placebo-controlled, phase II trial, which was conducted in 13 hospitals in China (Trial Registration No. NCT02649361). Eligible patients were 18–75 years of age, and had histologically confirmed recurrent or metastatic ESCC (stage IV) with at least one measurable lesion. A total of 109 patients enrolled in the anlotinib group were included in this post-hoc analysis. Patients received oral anlotinib (12 mg per day) in 3-week cycles (2 weeks on and 1 week off). Scheduled visits and computed tomography scans were performed on weeks 3, 6, and then every 6 weeks until disease progression was observed. The tumor responses were assessed based on the Response Evaluation Criteria In Solid Tumors (RECIST), version 1.119. Safety data were documented during the treatment and first 30 days after the last administration of anlotinib. The investigators graded all adverse events according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE, version 4.0). All participants were followed-up every 2 months for survival status after the last administration of anlotinib.
The ethics committee at each study hospital approved the study protocol and all amendments (Approval No. 15-125/1052). The trial was conducted in accordance with Good Clinical Practice guidelines and the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients before enrollment.
Outcomes
The primary endpoint of the study was PFS, which was defined as the time from randomization to disease progression or death from any cause, whichever occurred first. The secondary endpoints were the OS (defined as the time from randomization to death from any cause) and ORR (the percentage of patients with a confirmed complete or partial response).
Treatment-induced hypertension
Blood pressure (BP) was measured during each visit. According to NCI-CTCAE, version 4.0, treatment-induced hypertension was defined as systolic BP ≥ 120 mmHg and/or diastolic BP ≥ 80 mmHg without a known history of hypertension, or patients who required intensification of medication due to worsening of previously known hypertension, at any time after day 1 of treatment.
Statistical analysis
The PFS and OS were estimated using the Kaplan-Meier method and compared between patients with (Group A) or without (Group B) treatment-induced hypertension using the log-rank test. Subgroup analysis was further conducted in patients with or without previous hypertension (history of hypertension disease). The ORR was compared between groups using Pearson’s chi-square or Fisher’s exact test, as appropriate. A two-tailed P < 0.05 was considered significant. SAS 9.2 software (SAS Institute, Cary, NC, USA) was used for statistical analyses.
Results
Between January 6, 2016 and May 22, 2018, a total of 109 patients were recruited to receive anlotinib. By the data cutoff date of July 22, 2018, the median treatment duration was 2.56 months (range: 0.50–20.86 months). The investigator-assessed median PFS was 3.02 months (95% CI: 2.63–3.65 months) and the OS was 6.11 months (95% CI: 4.40–7.79 months). The ORR was 7.34% (95% CI 3.22%–13.95%).
A total of 59 (54%) patients were diagnosed with treatment-induced hypertension (Group A); the remaining patients (n = 50, 46%) were in Group B. There were no significant differences in most important baseline prognostic factors between Groups A and B (age, gender, ECOG performance status score, tumor differentiation, previous tumor surgery, previous chemotherapy, or previous hypertension; all, P > 0.05, Table 1). When stratifying patients according to previous hypertension, the baseline characteristics were also balanced (Supplementary Table S1; all, P > 0.05). The BP of all patients with treatment-induced hypertension was controllable by prescribing or adjusting antihypertensive medications.
Table 1.
Baseline characteristics
| Characteristics | Group A (n = 59) | Group B (n = 50) |
|---|---|---|
| Age (years) | ||
| ≥ 65 | 20 (33.9%) | 15 (30.0%) |
| < 65 | 39 (66.1%) | 35 (70.0%) |
| Gender | ||
| Male | 43 (72.9%) | 43 (86.0%) |
| Female | 16 (27.1%) | 7 (14.0%) |
| ECOG performance status score | ||
| 0 | 8 (13.6%) | 6 (12.0%) |
| 1 | 49 (83.1%) | 38 (76.0%) |
| 2 | 2 (3.4%) | 6 (12.0%) |
| Tumor differentiation | ||
| Undifferentiated or poorly differentiated | 16 (27.1%) | 18 (36.0%) |
| Moderately or well differentiated | 43 (72.9%) | 32 (64.0%) |
| Previous tumor surgery | ||
| Yes | 38 (64.4%) | 34 (68.0%) |
| No | 21 (35.6%) | 16 (32.0%) |
| Previous chemotherapy | ||
| One line | 19 (32.2%) | 20 (40.0%) |
| Two or more lines | 40 (67.8%) | 30 (60.0%) |
| Previous hypertension | ||
| Yes | 16 (27.1%) | 17 (34.0%) |
| No | 43 (72.9%) | 33 (66.0%) |
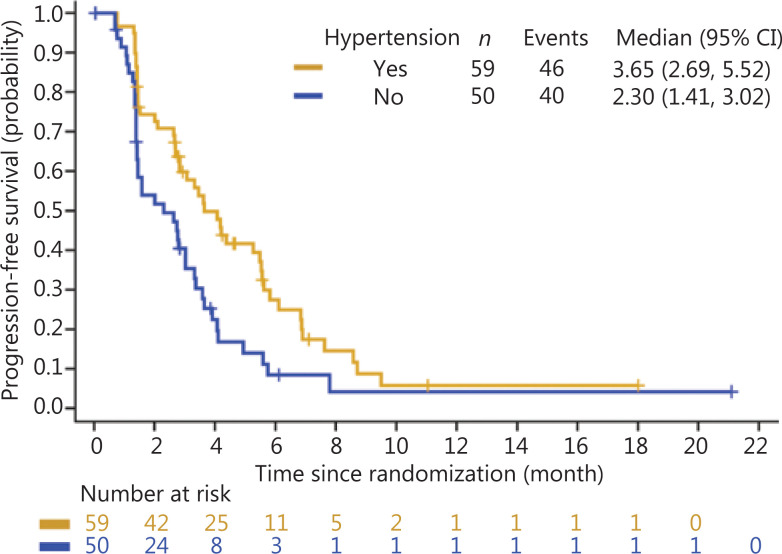
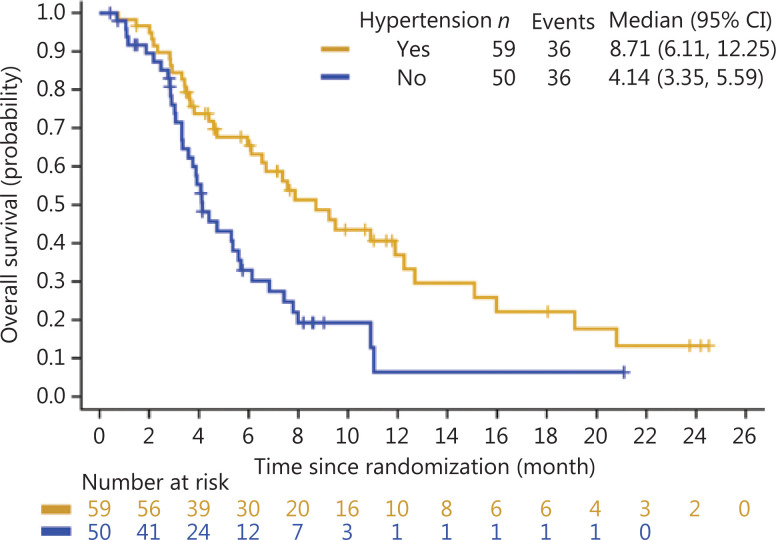
Overall, treatment-induced hypertension was associated with longer PFS and OS. The median PFS was 3.65 months in Group A (95% CI: 2.69–5.52 months) vs. 2.30 months in Group B (95% CI: 1.41–3.02 months) (HR: 0.56, 95% CI: 0.37–0.86) (Figure 1). The median OS was 8.71 months in Group A (95% CI: 6.11–12.25 months) and 4.14 months in Group B (95% CI: 3.35–5.59 months) (HR: 0.47, 95% CI: 0.29–0.76) (Figure 2). Treatment-induced hypertension was also associated with improved clinical outcomes. The ORR in Groups A and B were 10.20% and 4.00%, respectively (P = 0.048).
Figure 1.
Kaplan-Meier curve of progression-free survival of treatment-induced hypertension in the anlotinib group.
Figure 2.
Kaplan-Meier curve of the overall survival of treatment- induced hypertension in the anlotinib group.
However, when stratifying patients according to a history of hypertension disease, similar findings were only observed in patients without previous hypertension (Table 2). In this subgroup of patients, the median PFS was 4.17 months in Group A (95% CI: 2.63–5.55 months) vs. 1.41 months in Group B (95% CI: 1.35–2.76 months) (HR: 0.40, 95% CI: 0.24–0.68), the median OS was 8.71 months in Group A (95% CI: 5.95–15.08 months) and 4.07 months in Group B (95% CI: 3.32–5.59 months) (HR: 0.37, 95% CI: 0.21–0.67), the ORR in Groups A and B were 9.30% (95% CI: 2.59%–22.14%) and 3.03% (95% CI: 0.08%–15.76%), respectively, with P = 0.3806.
Table 2.
Progression-free survival, overall survival, and the objective response rate according to previous hypertension
| Previous hypertension | Treatment-induced hypertension | n | Median (95% CI) | HRa/ORb (95% CI) |
|---|---|---|---|---|
| OSc | ||||
| Yes | Group A | 16 | 9.23 months (3.61, 12.25) | 0.74 (0.31, 1.77) |
| Group B | 17 | 5.36 months (3.02, 11.04) | ||
| No | Group A | 43 | 8.71 months (5.95, 15.08) | 0.37 (0.21, 0.67) |
| Group B | 33 | 4.07 months (3.32, 5.59) | ||
| PFSd | ||||
| Yes | Group A | 16 | 3.61 months (2.69, 6.83) | 0.86 (0.39, 1.92) |
| Group B | 17 | 3.02 months (2.00, 5.59) | ||
| No | Group A | 43 | 4.17 months (2.63, 5.55) | 0.40 (0.24, 0.68) |
| Group B | 33 | 1.41 months (1.35, 2.76) | ||
| ORRe | ||||
| Yes | Group A | 16 | 12.5% (1.55%, 38.35%) | 0.44 (0.04, 5.36) |
| Group B | 17 | 5.88% (0.15%, 28.69%) | ||
| No | Group A | 43 | 9.30% (2.59%, 22.14%) | 0.30 (0.03, 2.86) |
| Group B | 33 | 3.03% (0.08%, 15.76%) |
aHR, hazard ratio; bOR, odds ratio; cOS, overall survival; dPFS, progression-free survival; eORR, objective response rate.
In Group A, anlotinib-induced hypertension involved grades 1–3 according to NIC-CTCAE, version 4.0, in which the case numbers of each grade were 13, 29, and 17, respectively. There was no grade 4 or 5 hypertension. The percentage of dose reduction and discontinuation of treatment related to hypertension were both 3.39% (2/59).
Discussion
In this study, treatment-induced hypertension was correlated with longer PFS/OS and better ORR in recurrent or metastatic ESCC patients treated with anlotinib, who did not have a history of hypertension disease. Our results suggested that treatment-induced hypertension could be a potential predictor for anlotinib efficacy in these patients.
According to earlier findings, the development or worsening of hypertension was a predictive factor for responses to anlotinib and favorable outcomes in NSCLC15,16, soft tissue sarcoma17, and medullary thyroid carcinoma18 patients. In the present study, we found that anlotinib treatment-induced hypertension was also associated with significantly improved clinical outcomes in recurrent or metastatic ESCC. Patients with treatment-induced hypertension had a 1.35 and 4.57 months longer median PFS and OS, respectively, and 6.2% higher ORR. The improvements in PFS and OS in the overall population were significant with HRs of 0.56 and 0.47, respectively. However, when stratifying patients with/without previous hypertension, improvements were only observed in patients without previous hypertension (HR for PFS and OS: 0.40 and 0.37, respectively). It was unclear why treatment-induced hypertension failed to predict outcomes in patients with a previous history of hypertension; however, the relatively small case number (33 in total; 16 and 17 in Groups A and B, respectively) may not have been sufficient to demonstrate any meaningful difference. Nevertheless, these results suggested that treatment-induced hypertension could be a potential factor for predicting better prognosis in recurrent or metastatic ESCC patients without a known history of hypertension.
The underlying mechanism of the correlation between treatment-induced hypertension and better prognoses in patients treated with antiangiogenic therapy requires further study. Antagonism of the VEGF pathway has been shown to promote hypertension by decreasing nitric oxide production, which leads to vasculature constriction and reduction in sodium ion renal excretion. A recent study reported a correlation involving a significant reduction of capillary density, changes in vascular morphology, and significantly prolonged PFS in renal cell carcinoma patients treated with sunitinib20. The authors hypothesized that the more robust antiangiogenic effect may have been linked to the susceptibility of both normal blood vessels and tumor vessels, and may have led to both development of hypertension and enhanced clinical outcomes20. Other studies suggested that the susceptibility of antiangiogenic agent treatment-induced hypertension may be associated with certain single nucleotide polymorphisms (SNPs) in the genes of the VEGF pathway21–25. Some genotypes of these SNPs were also associated with prognosis in advanced breast cancer24 or colorectal cancer patients25 treated by bevacizumab, and in clear cell renal cell carcinoma patients treated by sunitinib23.
Treatment-induced hypertension with anlotinib ranged from 39% to 68% in other carcinomas, such as NSCLC16, soft tissue sarcoma17, medullary thyroid carcinoma18, small-cell lung carcinomaa26, and renal cell carcinoma27,28. Our results are consistent with previous findings (54%). In the present study, anlotinib-induced hypertension may be successfully controlled in most cases without a reduction in anlotinib dosing or interruptions in treatment. Similar findings have been reported with other inhibitors of the VEGF signaling pathway in other tumor types, such as the treatment of sunitinib in advanced renal cell carcinoma29.
Our study was limited by its retrospective nature, so the findings need to be validated in further prospective studies. Moreover, this study included Chinese patients only. Whether these results could be reproduced in other patient populations is still unclear.
Conclusions
We had shown, for the first time, a correlation between treatment-induced hypertension and better prognoses in recurrent or metastatic ESCC patients treated with anlotinib, who did not have a previously known history of hypertension. Treatment-induced hypertension may be a simple and low cost predictor for anlotinib antitumor efficacy in these patients, which may also reflect the intended target inhibition.
Supporting Information
Acknowledgements
We could like to thank all patients and their families who participated in this study. We also thank all investigators and staff in each center. The study was funded by the Chia Tai Tianqing Pharmaceutical Group Co, Ltd.
Footnotes
Conflict of interest statement Jing Huang is a member of the consultant/advisory board for Merck and Jiangsu Hengrui. All the remaining authors have declared no conflicts of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu L, et al. Report of incidence and mortality in China cancer registries, 2009. Chin J Cancer Res. 2013;25:10–21. doi: 10.3978/j.issn.1000-9604.2012.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ajani JA, D’Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:855–83. doi: 10.6004/jnccn.2019.0033. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi K, Ando N, Watanabe H, Ide H, Nagai K, Aoyama N, et al. Phase II evaluation of protracted infusion of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG) Trial (JCOG9407) Jpn J Clin Oncol. 2001;31:419–23. doi: 10.1093/jjco/hye090. [DOI] [PubMed] [Google Scholar]
- 5.Huang J, Zhou Y, Zhang H, Qu T, Mao Y, Zhu H, et al. A phase II study of biweekly paclitaxel and cisplatin chemotherapy for recurrent or metastatic esophageal squamous cell carcinoma: ERCC1 expression predicts response to chemotherapy. Med Oncol. 2013;30:343. doi: 10.1007/s12032-012-0343-4. [DOI] [PubMed] [Google Scholar]
- 6.Syed YY. Anlotinib: first global approval. Drugs. 2018;78:1057–62. doi: 10.1007/s40265-018-0939-x. [DOI] [PubMed] [Google Scholar]
- 7.Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol. 2018;4:1569–75. doi: 10.1001/jamaoncol.2018.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kashyap MK, Abdel-Rahman O. Expression, regulation and targeting of receptor tyrosine kinases in esophageal squamous cell carcinoma. Mol Cancer. 2018;17:54. doi: 10.1186/s12943-018-0790-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Xiao J, Fang W, Lu P, Fan Q, Shu Y, et al. Anlotinib in chemotherapy-refractory metastatic esophageal squamous cell carcinoma (ESCC): a randomized, double-blind, multicenter phase II trial. J Clin Oncol. 2019;37(4 Suppl):S95. [Google Scholar]
- 10.George S, Reichardt P, Lechner T, Li S, Cohen DP, Demetri GD. Hypertension as a potential biomarker of efficacy in patients with gastrointestinal stromal tumor treated with sunitinib. Ann Oncol. 2012;23:3180–7. doi: 10.1093/annonc/mds179. [DOI] [PubMed] [Google Scholar]
- 11.Bono P, Rautiola J, Utriainen T, Joensuu H. Hypertension as predictor of sunitinib treatment outcome in metastatic renal cell carcinoma. Acta Oncol. 2011;50:569–73. doi: 10.3109/0284186X.2010.543696. [DOI] [PubMed] [Google Scholar]
- 12.Dahlberg SE, Sandler AB, Brahmer JR, Schiller JH, Johnson DH. Clinical course of advanced non-small-cell lung cancer patients experiencing hypertension during treatment with bevacizumab in combination with carboplatin and paclitaxel on ECOG 4599. J Clin Oncol. 2010;28:949–54. doi: 10.1200/JCO.2009.25.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scartozzi M, Galizia E, Chiorrini S, Giampieri R, Berardi R, Pierantoni C, et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20:227–30. doi: 10.1093/annonc/mdn637. [DOI] [PubMed] [Google Scholar]
- 14.Wirth LJ, Tahara M, Robinson B, Francis S, Brose MS, Habra MA, et al. Treatment-emergent hypertension and efficacy in the phase 3 Study of (E7080) lenvatinib in differentiated cancer of the thyroid (SELECT) Cancer. 2018;124:2365–72. doi: 10.1002/cncr.31344. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Zhao Y, Wang Q, Zhang L, Shi J, Wang Z, et al. Prognostic factors of refractory NSCLC patients receiving anlotinib hydrochloride as the third- or further-line treatment. Cancer Biol Med. 2018;15:443–51. doi: 10.20892/j.issn.2095-3941.2018.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang ZH, Han B, Li K, Cheng Y, Liu J, Guo J, et al. Clinical outcomes of patients with or without common AEs in anlotinib cohort: Subgroup analysis of the ALTER0303 trial. J Clin Oncol. 2019;37:e20507 [Google Scholar]
- 17.Fang Z, Chi Y, Yao Y, Wang S, Huang G, Cai Q, et al. Evaluation of hypertension and hand-foot syndrome as markers of anlotinib efficacy in advanced soft tissue sarcoma. Ann Oncol. 2018;29:viii587. [Google Scholar]
- 18.Chi Y, Gao M, Tang P, Zheng X, Xu Z, Li D, et al. Exploration of associations between adverse drug reactions and clinical outcomes in anlotinib treatment against medullary thyroid carcinoma (MTC): a subgroup analysis based on the ALTER01031 trial. J Clin Oncol. 2020;38:e18518 [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Rini BI, Cohen DP, Lu DR, Chen I, Hariharan S, Gore ME, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103:763–73. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eechoute K, van der Veldt AA, Oosting S, Kappers MH, Wessels JA, Gelderblom H, et al. Polymorphisms in endothelial nitric oxide synthase (eNOS) and vascular endothelial growth factor (VEGF) predict sunitinib-induced hypertension. Clin Pharmacol Ther. 2012;92:503–10. doi: 10.1038/clpt.2012.136. [DOI] [PubMed] [Google Scholar]
- 22.Jain L, Sissung TM, Danesi R, Kohn EC, Dahut WL, Kummar S, et al. Hypertension and hand-foot skin reactions related to VEGFR2 genotype and improved clinical outcome following bevacizumab and sorafenib. J Exp Clin Cancer Res. 2010;29:95. doi: 10.1186/1756-9966-29-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JJ, Vaziri SA, Rini BI, Elson P, Garcia JA, Wirka R, et al. Association of VEGF and VEGFR2 single nucleotide polymorphisms with hypertension and clinical outcome in metastatic clear cell renal cell carcinoma patients treated with sunitinib. Cancer. 2012;118:1946–54. doi: 10.1002/cncr.26491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider BP, Wang M, Radovich M, Sledge GW, Badve S, Thor A, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–8. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sibertin-Blanc C, Mancini J, Fabre A, Lagarde A, Del Grande J, Levy N, et al. Vascular Endothelial Growth Factor A c.*237C>T polymorphism is associated with bevacizumab efficacy and related hypertension in metastatic colorectal cancer. Dig Liver Dis. 2015;47:331–7. doi: 10.1016/j.dld.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Cheng Y, Wang Q, Li K, Shi J, Wu L, Han B, et al. Anlotinib as third-line or further-line treatment in relapsed SCLC: A multicentre, randomized, double-blinded phase 2 trial. J Thorac Oncol. 2018;13:S351–2. [Google Scholar]
- 27.Zhou AP, Bai Y, Song Y, Luo H, Ren XB, Wang X, et al. Anlotinib Versus sunitinib as first-line treatment for metastatic renal cell carcinoma: a randomized phase II clinical trial. Oncologist. 2019;24:e702–8. doi: 10.1634/theoncologist.2018-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma J, Song Y, Shou J, Bai Y, Li H, Xie X, et al. Anlotinib for patients with metastatic renal cell carcinoma previously treated with one vascular endothelial growth factor receptor-tyrosine kinase inhibitor: a phase 2 trial. Front Oncol. 2020;10:664. doi: 10.3389/fonc.2020.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bamias A, Lainakis G, Manios E, Koroboki E, Gyftaki R, Zakopoulos N, et al. Diagnosis and management of hypertension in advanced renal cell carcinoma: prospective evaluation of an algorithm in patients treated with sunitinib. J Chemother. 2009;21:347–50. doi: 10.1179/joc.2009.21.3.347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.