Abstract
Nanomaterial-based delivery vehicles such as lipid-based, polymer-based, inorganics-based, and bio-inspired vehicles often carry distinct and attractive advantages in the development of therapeutic cancer vaccines. Based on various delivery vehicles, specifically designed nanomaterials-based vaccines are highly advantageous in boosting therapeutic and prophylactic antitumor immunities. Specifically, therapeutic vaccines featuring unique properties have made major contributions to the enhancement of antigen immunogenicity, encapsulation efficiency, biocompatibility, and stability, as well as promoting antigen cross-presentation and specific CD8+ T cell responses. However, for clinical applications, tumor-associated antigen-derived vaccines could be an obstacle, involving immune tolerance and deficiency of tumor specificities, in achieving maximum therapeutic indices. However, when using bioinformatics predictions with emerging innovations of in silico tools, neoantigen-based therapeutic vaccines might become potent personalized vaccines for tumor treatments. In this review, we summarize the development of preclinical therapeutic cancer vaccines and the advancements of nanomaterial-based delivery vehicles for cancer immunotherapies, which provide the basis for a personalized vaccine delivery platform. Moreover, we review the existing challenges and future perspectives of nanomaterial-based personalized vaccines for novel tumor immunotherapies.
Keywords: Nanomaterial-based delivery vehicles, bioinformatic prediction, neoantigen, personalized vaccines, tumor immunotherapy
Introduction
As a potential immunotherapeutic approach, cancer vaccines are attractive as enhancements for tumor therapy. Dating back to 1891, William Coley discovered a mixed bacterial vaccine, also named Coley’s toxin, which resulted in inconsistent therapeutic effects when treating cancer patients1. With the advent of immunotherapy, bacillus Calmette-Guérin was first used by Morales for the treatment of noninvasive bladder cancer in 19762. Early studies showed the capability of bacterial pathogens to induce nonspecific immune responses, but attempts to treat cancer with effective vaccines was not successful because of the inability to recognize tumor antigens. In parallel with the use of bacterial vaccines, dendritic cells (DCs) were discovered in 19733; vaccination showed their antigen-presenting potential in achieving a powerful stimulation to the immune system. Subsequently, the first human cancer antigen, melanoma-associated antigen 1, was recognized in 19914, and genetically modified whole cell tumor vaccines with the secretion of granulocyte-macrophage colony-stimulating factor were shown to elicit a specific CD8+ T cell response in 19935. The next major advance, involving the DC-based prostate cancer vaccine-Sipuleucel-T, was approved by the U.S. Food and Drug Administration (FDA) in 20106. Therapeutic cancer vaccines targeting tumor-associated antigens (TAAs) or tumor-specific antigens (TSAs) have been tested in numerous clinical studies over the past decade7–9. For example, IMA9010 and KRM2011, 2 multi-peptide vaccines consisting of TAAs, were tested in a phase III trial in 2012 and a phase I trial in 2015, respectively. More recently, cancer neoantigen-based vaccines have augmented the antitumor immune response, such as the DC-based vaccine presenting neoantigens for the treatment of human melanomas in 201512, RNA-based and multipeptide-based neoantigen vaccines for melanoma in 201713,14, and the phase Ib study of neoantigen vaccines designed for glioblastoma in 201915. However, the present clinical results have shown challenges in predicting tumor neoantigens because of complex technologies, time-consuming processes, and high expenses. Personalized cancer vaccines, although in the beginning stages, have nonetheless used the repertoire of the immune system to develop preclinical anticancer activities16.
Anticancer immunity consists of 3 main phenotypes: immune-desert, immune-excluded, and inflamed phenotypes17, which are subject to several key steps, including the release of cancer-cell antigens, antigen presentation, CD8+ T cell priming and activation, trafficking and infiltration of CD8+ T cells into tumors, and ultimately recognizing and killing cancer cells using CD8+ T cells. With the underlying obstructions of the immune system to eradicate cancer, each step can be targeted to address these challenges using multiple therapeutic cancer vaccines, which contribute to triggering the antigen-specific CD8+ T cell response18. Likewise, the current advancement of therapeutic cancer vaccines can target prior steps of tumor antigen processing and presentation, and can also increase the potency of efficient CD8+ T cells against cancer, so neoantigen-based vaccines will especially become a vital addition to facilitate immune evasion in complex heterogeneous tumor microenvironments (TMEs)19.
However, new and frequent mutations and genetic instabilities result in a negative impact on the identification of corresponding neoantigens20, even with high variability in a relapsing tumor, which in turn, demonstrates the challenge in developing personal and effective cancer vaccines21,22. Although personalized cancer vaccines are difficult to develop, they increase the magnitude of adaptive immune efficiencies and vaccine immunogenicities23. To overcome the limited pool of immune cells defending tumor invasion, qualitatively new approaches to screen individual-specific immunogens and efficiently deliver these specific antigens to antigen-presenting cells (APCs)24 are urgently needed to induce powerful immunities to resist tumor growth. In this regard, the sequence of neoantigenic peptides can be predicted by whole-exome sequencing technology and by prediction algorithms for antigen affinities with major histocompatibility complex (MHC) class I molecules25,26. In this case, neoantigen-based cancer vaccines are specifically recognized as foreign by activating immune cells, which cannot be excluded and eliminated rapidly in such immunotolerant TMEs27. Importantly, delivery vehicles have been widely utilized for cancer vaccines over the past 30 years, because they improve the stability of antigens, the intensities of immune responses, and the safety of vaccines28. In this regard, nanomaterial-based delivery systems designed for neoantigen vaccines offer key design advantages such as controlling the loading and release kinetics of antigens, and protecting the immune cargos from degradation before the generation of antigen-specific immune responses29. Consequently, the construction of potent immunomodulatory cancer vaccines accelerated by various vehicles and modern prediction techniques have resulted in unprecedented specificities. In this review, we summarize and clarify different HLA-presented tumor antigens, and also review the use of distinctive delivery vehicles associated with potential benefits and disadvantages, to guide the advanced development of nanomaterials-based strategies for therapeutic cancer vaccines.
Tumor antigens
The nomenclature of antigens presented on tumor cells has a long history. To optimize these antigens generally involves HLA ligands, which might be qualified as tumor-associated to be expressed on general tumor cells or on remaining tumor-specific cells30. Because tumor-specific neoantigens are usually rejected by malignant cells, they are specifically designed for cancer vaccines31.
TAAs
TAAs are autologous proteins that are aberrantly expressed on malignant cells, and may display mutations, misfoldings, and degradation or proteolytical cleavages32. Because TAAs can be recognized and subsequently trigger immune responses to attack tumor cells by activating lymphocytes, TAA-targeted cancer vaccines have attracted considerable interest in the field of vaccine design33. Typical classes of TAAs include overexpressed antigens in cancer cells16,34, such as human epidermal growth factor receptor 2 (HER2), mucin-1 protein (MUC1), survivin and human telomerase reverse transcriptase (hTERT) plus cancer/germline antigens in germline cells, such as New York esophageal cancer antigen-1 (NY-ESO-1), the melanoma-associated antigen (MAGE) family and X antigen family member-1b (XAGE-1b), lineage-specific antigens in specific cells such as tyrosinase, glycoprotein 100 (gp100), melanoma antigen recognized by T cell-1 (MART-1), and prostate-specific antigen (PSA). For TAA-directed therapeutic cancer vaccines, salient clinical trials have been conducted in recent years (Table 1). Unfortunately, TAA-derived cancer vaccines were disappointing in clinical trials because they lacked specificity towards tumor cells, and to some extent, induced immunological tolerance35. Moreover, TAAs faced the challenge of low affinity to CD8+ T cells when there was insufficient expression below the threshold of naive T cell recognition36. TAA-directed cancer vaccines therefore need to be developed to precisely conform to the natural recognition and subsequent presentation to effector T cells.
Table 1.
Clinical trials of TAA-directed cancer vaccines
| TAAs | Disease | Vaccine intervention | Trial number | Phase | Status | Locations |
|---|---|---|---|---|---|---|
| HER2 | Breast cancer | AdHER2/neu dendritic cell vaccine | NCT01730118 | Phase 1 | Completed | National Institutes of Health Clinical Center |
| MUC1 | Lung carcinoma | MUC1 Peptide-Poly-ICLC Vaccine | NCT03300817 | Phase 1 | Recruiting | Mayo Clinic in Rochester/University of Pittsburgh Cancer Institute |
| Survivin | Recurrent epithelial ovarian cancer | DC-006 vaccine | NCT01334047 | Phase 1/2 | Terminated | Oslo University Hospital-Norwegian Radium Hospital |
| hTERT | Metastatic prostate cancer | hTERT mRNA DC | NCT01153113 | Phase 1/2 | Withdrawn | University of Florida |
| NY-ESO-1 | Prostate cancer/bladder cancer/non-small cell lung cancer | NY-ESO-1 plasmid DNA Cancer Vaccine | NCT00199849 | Phase 1 | Completed | New York Presbyterian Hospital/UT MD Anderson Cancer Center Houston |
| MAGE-A3 | Squamous cell carcinoma of the head and neck | HPV-16 vaccine/MAGE-A3 | NCT00257738 | Phase 1 | Completed | University of Maryland School of Medicine Baltimore |
| Tyrosinase | Melanoma | Montanide ISA 51 | NCT00184067 | Phase 2 | Terminated | USC/Norris Comprehensive Cancer Center |
| gp100 | Melanoma | gp100 human melanoma peptide vaccine | NCT00001439 | Phase 1 | Completed | National Cancer Institute |
| MART-1 | Malignant melanoma | CYT004-MelQbG10 | NCT00306566 | Phase 1/2 | Completed | Centre Pluridisciplinaire d’Oncologie & LICR |
The identification of TAAs basically focuses on comparison of transcriptomes of normal and malignant tissues, to examine abnormally expressed gene products, with HLA-ligands detected and matched using tandem mass spectrometry (MS/MS)37. These methods of next-generation sequencing provide comprehensive sequences and datasets, confident detection of mutations and gene transcription, and high throughput, as well as validation of HLA-ligands with sequence-identical synthetic peptides38. However, there are limitations of next-generation sequencing, such as less correlated HLA presentation, uncertain transcript dynamics, and false positive results39. MS/MS also has many disadvantages involving limited HLA-ligand coverage, biased sampling and detection, and low throughput with high effort and input40.
Although targeting TAAs has had some early clinical trials, to date, the vaccines lacked potency because of issues with their central or peripheral tolerance mechanisms35. In addition, although TAA analyses using sequencing and MS/MS techniques showed successful identification, the results were not supported by adequate clinical data41. Thus, more attention should be directed towards developing tumor-specific and potent therapeutic vaccines to favor effective immune responses.
TSAs
TSAs arise as a result of somatic mutations that are cancer-specific, and which are important for use in cancer vaccines. Given that these antigens are not affected by immune tolerance, therapeutic vaccines targeting TSAs have become potent weapons against malignant tumor by activating the specific immune system42. Compared to TAAs, neoantigens or TSAs are very tumor-specific with diverse mutations and are especially suitable for personalized therapeutic vaccines43. Recent studies have shown that neoantigen-based vaccines are capable of generating neoantigen-specific CD4+ and CD8+ T cell responses, helping to develop neoantigen-dominated immunotherapies44. Furthermore, the prevalence of neoantigens has led to clinical trials, where the mutational burden decreased for individual cancer treatments45. The elementary features of neoantigen-targeted vaccines induced adaptive immune responses including23 (1) CD8+ T cell-directed specific killing of tumor cells, (2) reinforced and permanent immune memory, and (3) minor immunological tolerance and autoimmunity. Fundamentally, neoantigen vaccines can boost the activity of CD8+ and CD4+ T cells by including the respective MHC-I-binding and MHC-II-binding neoepitopes46. Alternatively, the mixed neoantigen vaccines optimally contain MHC-I-binding and MHC-II-binding neoepitopes to stimulate activation of both CD8+ and CD4+ T cells. However, neoantigen-targeted vaccines face challenges involving the identification of neoantigens47. From hundreds of mutations, neoantigens only account for a fraction of mutations that can bind with MHC molecules with high affinity, and consequently only this small percentage of neoantigens can be presented to CD8+ T cells48. When considering incomplete translation of mutated regions, limited regions correlating with neoepitopes can be encoded49. Moreover, through comprehensive whole-exome or transcriptome sequencing techniques, the subject sequences encoding HLA-binding neoantigen peptides can display false-positive results50. Thus, MS/MS can be used as an effective tool to detect HLA-restricted peptides and provide evidence of sequencing results to confirm the ability of neoantigen presentations51. Recently, new MHC-binding predictions based on MS data supported the identification and selection of candidate neoantigens that had the potential to be used in personalized vaccines52,53.
To increase prediction accuracy, neoantigen prediction algorithms and some recent MHC binding prediction tools have exploited MS training data sets, such as NetMHCpan4 (MHC Class I) and MixMHC2pred (MHC Class II), which accelerated prescreening of MHC binding neoantigens during the prevaccination period54. Regularly updated predictors have been developed to use individually calculated decision thresholds and predictive methods to increase prediction specificities and sensitivities52,55. Based on common predictive methods among artificial neural networks (ANN), clustering and linear regression (LR) and ANN-based pan-specific algorithms are the optimal predictors, especially NetMHCpan-4.1 (http://www.cbs.dtu.dk/services/NetMHCpan-4.1/) and NetMHCIIpan-4.0 (http://www.cbs.dtu.dk/services/NetMHCIIpan-4.0/), which outperformed respective high-binding efficiencies between predicted antigens and MHC molecules, because of the availability of MS-eluted MHC ligand data56. In general, the current neoantigen prediction workflow of neoantigen-based therapeutic cancer vaccines shown in Figure 1 shares 6 main modules33,57: (1) next-generation sequencing of tumor tissue and HLA haplotype acquisition, (2) candidate neoantigen prediction using predictors, (3) neoantigen peptides validation by MS, ELISpot, and fluorescent antibody-labeled MHC tetramers, (4) neoantigen selection with priority, (5) vaccine formulation with putative neoantigens, and (6) preclinical implementation and evaluation of neoantigen-based vaccines. However, personal neoantigen-derived vaccines are limited to eliciting specific CD8+ T cell responses because of the lack of tumor-infiltrating lymphocytes into tumor tissues, and the deficient combination of preselected neoepitopes using in silico MHC-I and MHC-II binders58–60.
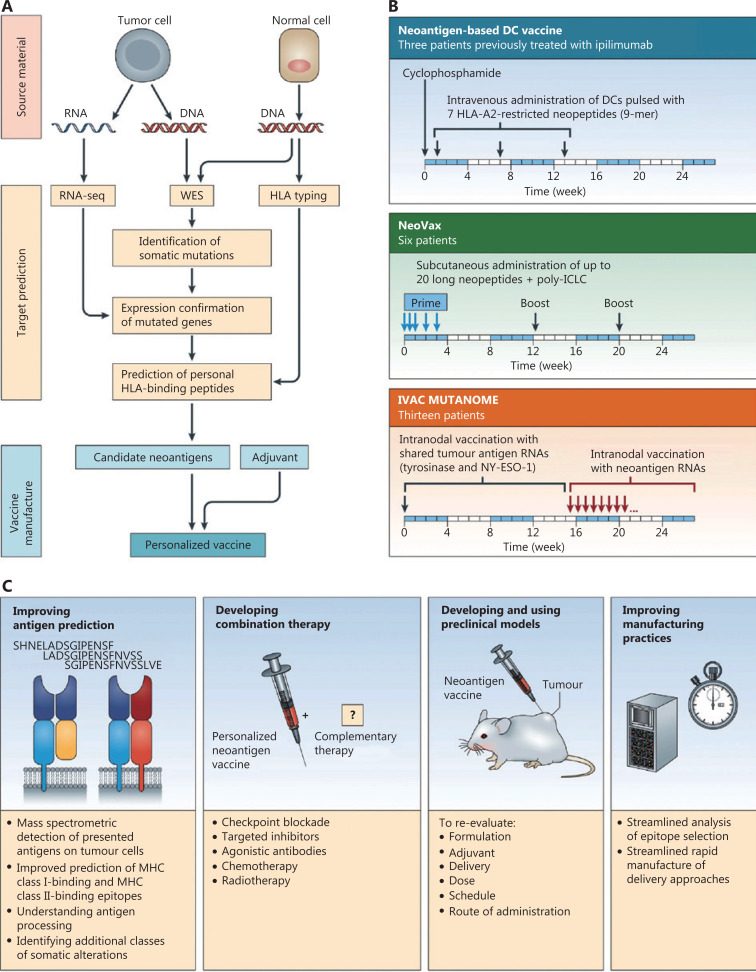
Figure 1.
Overview of neoantigen-based therapeutic cancer vaccines by Hu et al.33 (A) Neoantigen prediction and vaccine manufacture. (B) Clinical trials of neoantigen-based personalized vaccines. (C) Approaches of enhanced neoantigen-based vaccines.
Because the current clinical administration of neoantigens involves mixing with adjuvants or cytokines, neoantigens fail to be presented efficiently because of their rapid release. Further complementary vaccine delivery vehicles should be rationally designed to boost effective antigen delivery and immunostimulation.
Nanomaterial-based delivery vehicles
The TAAs and TSAs for vaccines have shown versatile use in the defense against diverse cancers. Antigen-based cancer vaccines, especially TSA-derived vaccines, involve potential therapeutic strategies with higher immunogenicities16. Free antigens are characterized by poor stability, easy degradation, limited immunogenicity, and deficient targeted delivery to APCs. However, current vaccine administration protocols based on delivery systems have increased rapidly to solve these problems61. Nanomaterial-based delivery vehicles provide a facile target for a multi-faceted portfolio, including improving the encapsulation efficiency and stability, enhancing immunogenicity, targeting APCs to promote antigen cross-presentation, and co-delivery of the antigen and immunostimulatory adjuvant or nucleic acid with higher transfection efficiency62,63.
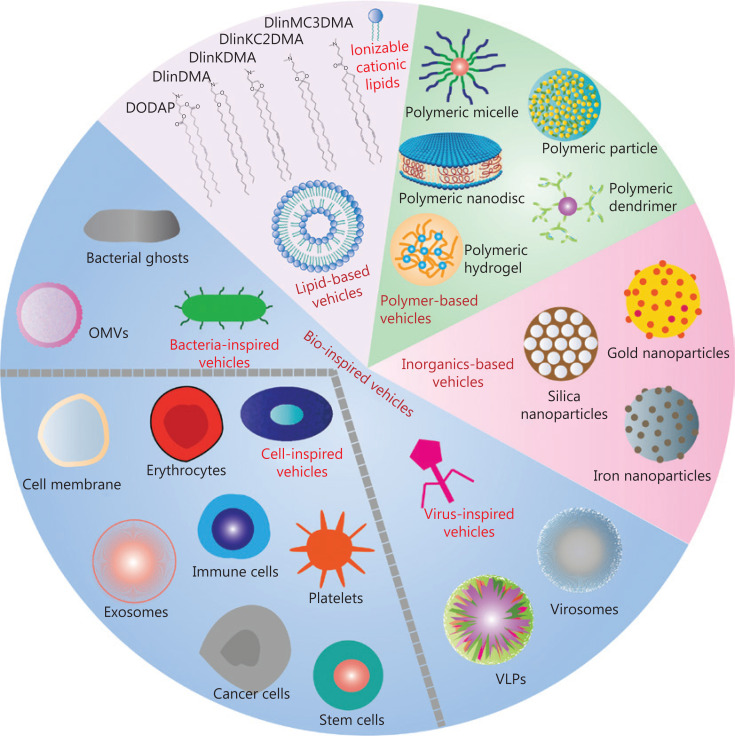
Current potent vaccine platforms evaluated in immunotherapy trials are emerging as a paradigm for TAAs and TSAs delivery64. As shown in Figure 2, nanomaterials-based delivery vehicles are classified into 2 categories, synthetic delivery vehicles (lipid-based vehicles, polymer-based vehicles, and inorganic-based vehicles) and bio-inspired vehicles. These delivery vehicles can be used for vaccine design as described below.
Figure 2.
Schematic illustration of nanomaterials-based vaccine delivery vehicles. Different synthetic delivery vehicles such as lipid-based vehicles, polymer-based vehicles, inorganics-based vehicles, and bio-inspired vehicles are used in vaccine construction.
Lipid-based vehicles
Lipid-based nanoparticles (LNPs) are promising candidates for vaccine delivery; liposomes are vehicles for clinical applications, which have gained popularity in the design of therapeutic cancer vaccines65. With unique spherical vesicles and similarities to the cell membranes, liposomes can encapsulate antigens to make them more stable than free antigens66, can easily fuse with cell membranes and preferentially present antigens to APCs, and can enhance immunogenicity and concomitantly trigger adaptive immunity. The first use of liposomes as vaccine delivery systems was approved by Allison and Gregoriadis in 1974, and demonstrated that liposomes served as safe and effective adjuvants in human vaccination protocols67. Since that time, liposome use for immunotherapies has become popular, with numerous liposome-based vaccine systems frequently submitted to the FDA for clinical trials68,69. In general, liposome-encapsulated antigens can induce both humoral and cellular-mediated immunities, and can protect the antigen from degradation and clearance by the immune system. The delivery vesicle allows antigens to sterically encapsulate into the hydrophilic center or embed into the lipophilic surface. For example, Shahum and Fortin70 encapsulated antigens in the internal liposomal aqueous phase or linked them to the liposomal surface. In addition, the use of cationic lipids, neutral lipids, anionic lipids, and PEG-lipids in liposomes has specific structure-functional properties71. The advantages of ionizable cationic lipid components (e.g., DODAP, DlinDMA, DlinKDMA, DLinKC2DMA, and DLinMC2DMA72,73) are the key elements used to deliver nucleic acid vaccines and bind them to cell membranes for persistent uptake by APCs74. Diverse modified cationic lipids elicite greater CD8+ and CD4+ T cell responses, inducing respective Th-1-based and Th-2-based immune responses, respectively75. Neutral lipids are usually used as helper lipids to improve the transfection efficiency of DNA or mRNA vaccines, such as the packaging of 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and cholesterol to formulate the stable core-shell structure of LNP, which enhanced antigen presentation capabilities76. When compared to cationic lipids, anionic lipids are less toxic in vivo, with better control, offering a sustained and potent immune response77. PEG-modified LNP provides a biocompatible platform for gene transfer, which typically increases the circulation time to extend the stability and effect of vaccines in vivo78,79. Liposome-based vaccines are also stable and are modified to induce lysosomal escape and antigen cross-presentation80. Of particular interest is the use of liposome targeted deliveries with specific ligands or by targeting molecules to the relevant receptors on APCs. For further laboratory-scale and clinical-scale applications of liposomes, the NanoAssembler platform and the NanoAssembler Scale-up platform have been used by Precision NanoSystems (Vancover, Canada) using microfluidic technologies81–83.
In addition to liposomes, more advanced LNPs have been developed for vaccine construction, because of lipid-induced higher bioavailability, higher safety, and controlled antigen release. Typical lipids that can self-assemble into LNPs include phosphatidylcholines (DMPC, DPPC, DSPC, DOPC, POPC, and SOPC), phosphatidylglycerols (PG, DMPG, DPPG, DSPG, and DOPG), and other amphiphilic compounds such as phosphatidylethanolamine, phosphatidylserine, and sphingomyelin66. Based on a library of lipid-like compounds, the Massachusetts Institute of Technology and Alnylam Pharmaceuticals (Cambridge, MA, USA)84 found that efficacious materials such as C12-200 achieved persistent gene silencing and multi-targeted approaches. Kim and colleagues85 formulated a small lipid nanoparticle-based nanovaccine platform to display the tumor antigen, ovalbumin (OVA), and combined it with immune checkpoint blockade therapy, which led to an effective antitumor effect. Lipid-assisted nanoparticles also improve the antigen load by using a double emulsion method, and subsequently this method enhances intracellular delivery of antigens to activate immunological responses86. To further develop lymph node-targeted delivery of vaccines that result in higher humoral and cellular immune responses, Yu et al.87 used melittin-lipid nanoparticles to confirm the ideal LN-target for cancer immunotherapy.
The recent use of LNPs as carriers involves their application in lipid-complexed mRNA vaccines, which increase uptake, extend half-life, and favor efficient delivery of mRNA13,88. To address insufficient intracellular mRNA expression and deficient presentation to DCs, a variety of LNP-assisted mRNA vaccines have been designed to deliver mRNA and activate a systemic immune response, such as heterocyclic LNPs to activate immunity via the mRNA-mediated STING pathway89, mRNA loaded into cationic DOTAP nanoparticles to enhance transfection efficiency90, and a nucleoside-modified mRNA-LNP vaccine to efficiently provide potent immune responses91. The optimal targets of mRNA vaccination strategies involve efficient antigen delivery into the DC cytosol, as DCs are related to both innate and adaptive immunities. Coolen et al.92 modulated the DC-elicited immune response with a lipid-based mRNA delivery system by activating pattern recognition receptors. Another promising approach is remodeling the TME by lipid-complexed mRNA vaccines resulting in the effective delivery of mRNA-encoding immunomodulatory genes. For example, Li93 evaluated self-replicating IL-12 RNAs encapsulated in LNPs, which induced long-lasting immune memory, remodeled the TME, and thus primed antitumor immunity. Beyond vaccines against cancer, LNP-encapsulated mRNA vaccines have emerged as an alternative and promising candidate to combat COVID-19 infections94. Furthermore, most of these mRNA-based vaccine candidates are currently being tested in clinical trials95,96. In conclusion, lipid-based delivery systems validate potential insights toward a comprehensive and productive vector for enhanced vaccine strategies, which may offer effective immune protection against severe diseases such as cancers, viral infections, and pathogen invasions.
Polymer-based vehicles
Polymer-based vehicles are attracting increasing attention in vaccine development, including antigen encapsulation and protection, conjugations and modifications, and controlled release of cargo with slow degradation29,97. Two different types of polymer-based vehicles are used including natural polymer-based and synthetic polymer-based vehicles. Natural polymer-based vehicles are comprised of protein/peptide and glycan nano/microcarriers. In combination with Imiquimod, nab-paclitaxel as an U.S. FDA-approved drug was tested for the treatment of breast cancer cutaneous metastases in a Phase II study98. Pancreatic cancer patients treated with nab-paclitaxel plus gemcitabine had a longer survival, which resulted in an improvement in the treatment of this disease99. Based on these results, other protein- and peptide-based vehicles such as albumin and self-assembled polypeptide-formulated nanoparticles/micelles can hopefully be utilized for vaccine development. Like natural protein/peptide polymers, chitosan is an amino-polysaccharide derivative of chitin100, and cyclodextrins as a family of cyclic oligosaccharides101 have attracted interest as a vaccine delivery system, which is nontoxic, consistent with their histocompatibility and biodegradability. Chitosan-based glycan nanoparticles assistant antigens in passing through the epithelial tissue barrier, to prolong the retention time in immunological cells or organs to enhance immunostimulation. Antigens encapsulated into chitosan nanoparticles significantly enhanced the expressions of co-stimulatory signal molecules and pro-inflammatory factors when presented to APCs. Although chitosan has significant advantages as a vaccine delivery system, its low solubility and reticuloendothelial system clearance result in minimal accumulation in tumors. To address this challenge, glycol chitosan nanoparticles provide stimulus-responsive strategies to reject tumors and improve cancer nanomedicine102. Cyclodextrin is another popular glycan polymer and 1 of the immunogenic enhancers used in vaccine carrier development. Based on a α-cyclodextrin-fabricated gel system, Qin et al.101 developed a versatile gel system for combinatorial tumor therapy, which promoted antigen uptake and DC maturation, and inhibited tumor growth and metastasis. In addition, cyclodextrin carriers promoted MHC I and MHC II binding to enhance the respective proliferation of antigen-specific CD8+ T cells and CD4+ T cells. For LN-targeted delivery, sulfobutylether-β-cyclodextrin and mannosylated N,N,N-trimethylchitosan DNA vaccines have been developed to enhance DC-targeting and reduce immunosuppression for an efficient antitumor immune response103. Collectively, natural polymer-based vehicles are considered as ideal carriers because of their biodegradability, histocompatibility, and mucosal adhesion, but their uneven particle size and weak specificity need to be further refined to facilitate the design of more effective vaccines.
Synthetic polymer-based vehicles are currently widely used to develop numerous vaccines in clinical trials. Owing to their chemical versatility involving covalent or noncovalent binding, synthetic polymer-based vehicles are a potential tool for vaccine delivery. The most extensively used synthetic biodegradable polymers include poly(lactic-co-glycolic acid) (PLGA), polycaprolactone, polylactic acid, polyglycolic acid, polyvinyl alcohol, polyhydroxybutyric acid, polyethyleneimine, and acrylic polymers104–108. An example of an U.S. FDA approved polymer is PLGA, which has excellent biocompatibility and biodegradability, sustained and controlled release of antigens, and the ability to be modified to provide specific functions like lysosomal escape109. Other synthetic polymers such as polyethylenimine (PEI), a cationic polymer, has been exploited to synthesize linear and branched polymers with different molecular weights110. With a positive charge to link nucleic acids through electrostatic interactions, PEI-based nanoparticles are extensively used in gene delivery111. Various types of artificial polymers that bind to heparan proteoglycan on the surface of APCs are internalized by cell endocytosis112, and the nanoparticles then disintegrate using the proton sponge effect in the acidic environment of lysosomes, thus facilitating cytoplasmic release of loaded substances inside nanoparticles113. Polymethylmethacrylate, poly (ethylacrylic acid), poly (propylacrylic acid), and tert-butyl acrylate in acrylic polymers also have several advantages, including a strong immunological adjuvant effect, simple preparation, safety, and biocompatibility114,115. Moreover, synthetic polymer-based carriers are usually composed of polymeric micro/nanoparticles, polymeric micelles, dendrimers, nanodiscs, and hydrogels116–118. Polymeric micro/nanoparticles are micro/nano-sized colloidal particles that assemble hydrophilic and hydrophobic molecules, stably delivering antigens with good efficacy and with easy entrapment and adsorption119. Polymeric micelles are self-assembled spherical structures generated by amphiphilic block copolymers inside aqueous media120, which carry a hydrophobic core shell and a hydrophilic outer shell with sizes ranging from 10–100 nm121. The internal hydrophobic and surface hydrophilic components of micelles are able to load bioactive molecules with diverse solubility properties. Dendrimers are highly branched spherical carriers consisting of 3 components: a central core, dendritic monomers, and peripheral functional groups122. Similar to polymeric micelles, dendrimers are comprised of a hydrophobic core and a hydrophilic surface resulting from polymerization of branching modules123. Because of their hyperbranched structures, dendrimers have the characteristics of low viscosity, macromolecular size, and the ability to be chemically modified by covalent or noncovalent binding124,125. These synthetic polymer-based vaccines can stimulate and regulate the immune system, and then inflame the tumor microenvironment, and are conducive to inducing systemic antitumor immunity.
Ma et al.126 engineered several homogeneous chitosan, PLGA, and PLA-based chassis particles, which displayed antigens on macro/nanoparticles, and which supported excellent antigen delivery and functioned as superior immunostimulants127. To further improve immune recognition, Xia et al.128 constructed a deformable engineered vaccine using PLGA nanoparticles instead of surfactants, where antigens were assembled on a dynamic flexible emulsion chassis to increase the preventive and therapeutic effects. Compared with bare polymer-based carriers, biodegradable composite materials are being used to engineer vaccines with responsive features, such as autofluorescence, pH sensitivity, and other general physical and chemical properties. PEI-based nanovaccines can be successfully used as powerful tools for visual vaccine delivery and enhanced immunotherapy with autofluorescence129. Many pH sensitive polymers (such as PDEA, PEPA, PDPA, PDBA, PC6A, and PC7A) conjugated with antigens, adjuvants, and small molecule agonists, facilitate pH-triggered payload release in acidic tissues, to increase the efficacies of immunological therapies130. For better physicochemical properties, PEGylated polymeric nanoparticles and micelles are useful because of their narrow size distribution, homogeneous particle formation, and particular geometry. These nanoparticles co-assemble with subunit antigens to improve the stability of antigens and enhance the cooperativity of material components for specific vaccine performance131. In addition, functionalized polymeric vehicles provide new strategies for understanding the metabolism of improved immune effectiveness by virtue of their novel properties with distinctive modifications. Liu and co-workers132 recently fabricated F-PEI-based OVA nanoparticles featuring a cross-presentation ability resulting from cytosolic transportation of antigens within dendritic cells, indicating that their fluoropolymers facilitated endosomal escape. For biomaterials-based regulation of signaling pathways, Langer et al.133 developed PLGA microparticles encapsulated with a STING agonist to boost the systemic antitumor immune response. Notably, DC-targeted and CD8+ T cell-targeted nanovectors contributed to the development of vaccine development and immunization. In a study, Conniot et al.134 synthesized mannose-grafted polymeric nanoparticles to target delivery to DCs and subsequent T-cell priming, which potentiated the antitumor immune response. Considering that DCs are used to maximize CD8+ T cell activation, synthesized polymers based on artificial APCs aim to directly stimulate CD8+ T cells, to trigger stronger T cell proliferation, and to subsequently kill tumor cells135. These multifunctional polymeric vehicles therefore hold great promise for vaccine delivery and cancer immunotherapy.
Inorganic-based vehicles
Vaccines with inorganic-based vehicles support biochemical elaboration elements for superior behavior in cancer immunotherapy. Examples of inorganic-based vehicles include silica, gold, iron oxide, silver, carbon, graphene, selenium, copper oxide, zinc oxide nanoparticles, metal-organic-frameworks (MOFs), and quantum dots64,136. Multiple inorganics fabricated vaccines are more preferable in inducing persistent immunostimulatory effects with good in vivo stabilities, which are comparable to organic materials137. In general, inorganic-based delivery strategies should be used to ensure the enrichment and retention time of antigens in lymphatic circulation, where immune effectiveness is induced and then increased. Diverse representative inorganic nanoparticles are discussed below.
Silica nanoparticles widely studied in cancer therapy are predominantly synthetic amorphous silica based nanoparticles, specifically mesoporous silica nanoparticles (MSNs), which have the advantageous properties of a porous structure, high surface area, tunable surface functionality, and high loading efficiency138. MSNs are smart antigen delivery systems that rely on the aforementioned superior properties, which have resulted in their use as vectors139. MSNs possess qualities that improve the therapeutic effects, such as protection of vaccine cargo, on-demand release of antigen, antigen targeting transportation, and tumor penetration. In addition, MSNs possess adjuvant behaviors due to the properties of morphology, size, and modified groups140. Thus far, a myriad of functionalized silica nanoparticles, acting as adjuvants or carriers, have been used for therapeutic cancer vaccines141,142. For example, various types of hollow MSNs have been considered as promising vaccine adjuvants to direct humoral and cellular immune responses and subsequently deprive tumor cells during tumor challenge143. To induce greater immune responses, Al-OH-rich silicate adjuvants formulated with 6-coordinate Al-OH groups, using coordination chemistry, induced an effective immunity, which provided new therapeutic options capable of commercial adjuvant development144. As vaccine carriers, metal-organic-framework (MOF)-gated MSNs145 and mesoporous silica-coated upconversion nanoparticles146 accommodated large amounts of antigens using their large pore, niche, and void space, providing the expertise to forgo conventional silica nanoparticle applications. Despite MSNs use as widespread vehicles for cancer vaccinations, mesoporous silica rods (MSRs) recruited APCs in local macroporous scaffolds to induce robust immune activation and antigen-specific adaptive immunity147. MSR-MSNs dual-scale vaccines consist of MSNs and MSRs148, both of which are internalized by DCs and recruit DCs by virtue of their larger space. This process reverses the immunosuppressive pathway and educates immune cells to combat cancer. Moreover, silica hybrid vaccines such as PEI embedded in MSRs significantly facilitate DC maturation and maximize CD8+ T cell stimulation, in contrast to MSR vaccines139. As a consequence of the synthetic diversity of silica materials, silica-based vaccines combined with other therapies (e.g., chemotherapy, photothermal therapy, and photodynamic therapy)147 overcome the side effects and suppressive efficacies present in tumor pathophysiological processes.
Gold nanoparticles (AuNPs) can serve as an effective antigen delivery system, and can also be used for cancer irradiation therapy; they possess the unique combinatorial therapy of photothermal therapy and immunotherapy. Their inert properties assist them in providing a reliable platform for antigen transportation and medical imaging, as well as providing optical and thermal properties as thermal transducers for near-infrared (NIR)-irradiation tumor therapy149. In a previous study, light-activatable polybubbles engineered in this technology were used for antigen delivery vehicles in NIR-responsive cancer treatments150. With an extremely small size of 4.5 nm, AuNPs function as vehicles and even adjuvants by activating NLRP3 inflammasomes to induce OVA-specific antibody secretion151. The size distribution of AuNPs-based vaccine are therefore used, with different modifications, over a wide range of responses. Zhou et al.152 evaluated different-sized AuNPs associated with OVA, and found that 15–80 nm sized vaccines were capable of improving DC homing and inducing CD8+ T cell activation. However, the systemic toxicity and decreased specificity of AuNPs limited them in clinical studies. Based on these deficiencies, available surface-modified vaccine delivery vehicles such as PEG-grafted vaccines are the most common in the field of immune engineering. In these studies, PEG increased the stability and safety of AuNPs and enabled tumor accumulation using chemical bonding methodology153. Additionally, as imaging agents, AuNPs offer attractive opportunities for medical imaging, including positron emission tomography, magnetic resonance imaging (MRI), and computed tomography (CT), facilitating real-time visualization in tumor diagnoses and treatments154,155.
Because of their promising magnetic properties, iron nanoparticles or super paramagnetic iron oxide nanoparticles have also been used as excellent candidates for biomedical diagnoses and imaging. In addition to this property, hyperthermia-based vaccination is also currently used because of the thermal properties156. In the field of multifunctional vaccine construction, iron nanoparticles are used as antigen carriers, imaging agents, and heat generation agents with extensive applications in cancer immunotherapy. The clinical administration of iron nanoparticles involves NanoTherm, approved by the U.S. FDA in 2011, which dictates their biological applications; they are accompanied by excellent safety and nontoxicity157. Iron nanoparticles accompanied by antigens are therefore synergistic for the treatment of tumors. For early prediction of individual vaccine treatments, iron oxide components are used for DC homing and tracking using MRI. In this study, multifunctional RNA-loaded magnetic liposomes provide a simple procedure to predict the antitumor response158, which functions as an early visual tool for vaccine evaluation. With regards to the superior photothermal conversion effect, Guo et al.159 fabricated magnetic-responsive nanoagents for precise image-guided immunotherapy, using tumor ablation to release tumor-associated antigens, combined with an immunoadjuvant to elicit robust immunity against tumor growth. Except for the abovementioned autologous vaccine-like nanoagents, the FeO-OVA vaccine efficiently inhibited tumor growth and metastasis, and showed that the FeO-based nanovaccine had better clinical prospects. More importantly, iron oxide nanoparticles were encapsulated in micelles and combined with an immune checkpoint inhibitor such as programmed death-ligand 1 (PD-L1)160, which showed the promising result of enhanced immunotherapy during tumor challenge with a high level of effective memory T cells.
Bio-inspired vehicles
Synthetic delivery vehicles are often excluded by in vivo defense mechanisms, weakening the efficacy and safety of vaccine administration, and thus they encounter obstacles for clinical applications. Unlike synthetic delivery vehicles, bio-inspired vehicles increase their potential to associate with physiological elements because they resemble biological systems. They carry the capacities of large drug-loading, antigenicity, adjuvant activity, considerable safety, and targeted delivery161. It has been predicted that bio-inspired delivery vehicles will provide a novel opportunity to revolutionize vaccine delivery systems, as well as integrate synthetic materials and bio-inspired agents (Figure 3)162,163. Notably, 3 kinds of bio-inspired vehicles, including bacteria-inspired, mammalian cell-inspired, and virus-inspired delivery systems, are used for vaccine-based tumor immunotherapy and are described herein in detail.
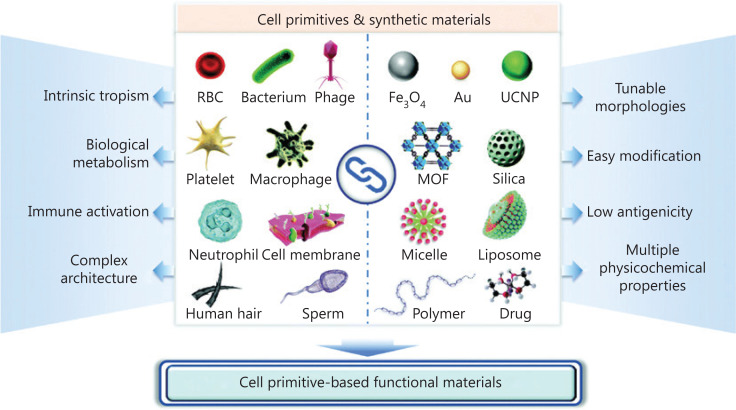
Figure 3.
Schematic of the combination of cell primitives and synthetic materials with their functions by Luo et al.163
Bacteria-inspired delivery vehicles such as bacterial ghosts and outer membrane vesicles (OMVs) are predominantly used for therapeutic vaccines164. These antigen-loaded vehicles induce safe and potent immune activation, when compared with living bacteria, because of their attenuated pathogenicity and altered immunogenicity. These promising vaccine delivery systems can act as vaccine delivery vehicles, but can also act as adjuvants by virtue of preserved intrinsic immunomodulators such as lipopolysaccharides (LPS) and peptidoglycan163,165. Bacterial ghosts derived from Gram-negative bacteria with simple and high dose production procedures feature empty bacterial shells and show structural stability at room temperature. In addition, bacterial ghosts have the safety profile of not being living, but preserve the antigenic units for further vaccine construction163. Numerous studies have shown that DNA-loaded bacterial ghosts were effectively internalized and presented to DCs, and amplified direct immune effectiveness in cancer therapy166. With bacterial ghost-based DNA delivery vectors, Kudela et al.167 used this system to optimize drug delivery and select specific formulations of bacterial ghosts for use in clinical studies. In a similar manner, OMVs are another example of bacteria-inspired delivery vehicles used in advanced vaccine delivery. OMVs are also made from Gram-negative bacteria with certain pathogen-associated molecular patterns, such as LPS, outer membrane proteins, and lipoproteins. Heterogeneous antigens are capable of being presented on the surface of the OMVs through the expression of surface-exposed lipoproteins (e.g., OspA and ClyA)168. Beyond the antigen presentation by DCs in modulating the immune response, OMVs can regulate TME reprogramming without side effects169. The superior usefulness of OMVs can be used to carry various cargos, such as target antibodies, small interfering RNAs, peptide antigens, and nanoparticles170. Altogether, bacteria-inspired delivery vehicles are a promising approach in vaccine development involving antigen delivery and preclinical applications.
Cell-inspired delivery vehicles imitate natural properties of primitive cells, which are used in drug delivery systems with their natural biology of living cells. Based on novel developed nanotechnology, smart cells, especially mammalian cells or cell membranes, integrated with functional nanoparticles, have shown excellent prospects in the field of vaccine fabrication171. The main types of cell-inspired delivery vehicles used for tumor immunotherapy are mammalian cell-derived vehicles (e.g., erythrocytes, platelets, immune cells, and stem cells), exosomes, and cell membranes172,173. Erythrocytes function as a unique ‘‘don’t eat me’’ signal to hinder phagocytosis and capture certain pathogens to present to APCs. In a recent study, erythrocytes were used to deliver vaccine nanoparticles without additional adjuvants, achieving antigen specific cellular and humoral immune responses174. In a similar manner, erythrocyte membranes showed the same features when used to coat antigen-based nanoparticles, where the surface antigens circulated for longer times to elicit a stronger immunity175. Platelets and platelet membranes have the advantage of long circulation, damage of immune evasion, self-aggregation, and adhesion at tumor sites for the coating of vaccine nanoparticles176. Immune cell-based vaccine vehicles play an important role within a group of immune cells for protecting the host against tumor cells. The types of immune cells include macrophages, neutrophils, DCs, natural killer cells, T cells, and B cells. Because they may trigger undesired immune responses, the immune cells used in vaccine-based tumor immunotherapy are screened. For example, a biomimetic DC-derived nanovaccine fabricated with PLGA nanoparticles by an extrusion method delayed tumor growth and reduced tumor metastasis177. In addition, PEI-modified macrophage cell membrane-coated PLGA nanoparticles delivering OVA showed superior therapeutic efficacy with antitumor immune responses178. Although stem cells are difficult to collect and maintain their integrity, stem cell-based vaccines are a hopeful therapeutic strategies to treat tumors179. Exosome-based bio-inspired vaccine delivery cargoes induced systemic antitumor immunity and long-term immunological memory180. In particular, exosomes originating from DCs have the ability to induce antigen-specific T-cell responses for promising vaccine immunization. Additionally, cell membrane-based vaccination promotes immune responses by taking advantage of inherent functions from parent cells175,181. In this application, cancer cell membrane-wrapped nanoparticles provided excellent insight into the vaccine delivery and potent immunotherapy182. Collectively, such cell-inspired vaccine delivery vehicles, especially with hybrid nanoparticles, generate mimetic vaccine advancement for fighting cancer.
Virus-inspired delivery vehicles are used for vaccine delivery because of their self-replication ability, to transfer specific genes and immunologically escape to target transportation. By combining viral vectors with nanoparticle release, the limitation of nucleic acid cargos could be overcome to increase their versatile applications172. Viral vectors such as adenoviruses carry magnetic iron oxide nanoparticles for gene delivery and visual monitoring183. However, shortcomings involving safety concerns and restricted loading capacity need to be further addressed. To benefit from viruses and to avoid these concerns, additional vectors such as virus-like particles (VLPs) and virosomes have been introduced184. As highly self-assembled particles, VLPs mimic viral capsids and remain as empty shells to preserve antigenicity. They are easy to scale-up at a low cost, and possess natural tropism and targeting capacity with the aid of distinct modifications, which can load antigens for therapeutic vaccine delivery. In addition, the stable architecture of VLPs makes them safer in complex immune systems. Ong et al.185 suggested various VLP-based cancer vaccines to be used for the treatment of pancreatic cancer, prostate cancer, breast cancer, and skin cancer, facilitating VLPs as optimal therapeutic vaccine candidates for clinical applications. Similar to VLPs, virosomes also contain a hollow compartment, which allows them to be reconstituted with diverse cargoes. Without the genetic component of viruses, virosomes are commonly produced by influenza virus with low toxicity and empty envelope glycoproteins186. These glycoproteins exert adjuvant activity and have the potential of endosomal escape when used for vaccine delivery, showing the substantial advantage of virosomes as delivery vehicles162. Because of endosomal escape, virosomes enable DCs to present antigens on the major MHC I molecules to elicit effective cellular immune responses and induce durable tumor suppression. In a previous study, virosomes increased cross-presentation of the encoded immunogen and facilitated DNA vaccine efficacy to an encoded toxic protein, to induce cell death187. Thus, both VLPs and virosomes are attractive delivery systems for cancer treatment, representing an important approach for bio-inspired vaccine strategy.
Current disadvantages and future strategies for further improvement of nanomaterial-based delivery vehicles
Although the use of nanomaterial-based delivery vehicles can facilitate antigen delivery for antitumor therapy with an emphasis on intensified vaccine construction, their clinical translation is difficult because of safety and validity limitations. Regarding synthetic delivery vehicles, lipid-based vehicles present disadvantages, including limited encapsulation efficiency and non-ideal biodistribution, which enhance the aggregation of the liver and spleen cells29. Polymer-based vehicles can be limited by toxicity, biodegradability, and impaired immune responses because of chemical modulations64. Inorganic-based vehicles increase the risk of solubility and in vivo toxicity caused by acute toxic effects136. In addition, these synthetic delivery vehicles also limit the fabrication process of composite materials, and adjuvants are needed to improve their use in vaccine immunogenicity. Furthermore, the associated constraints of bio-inspired vehicles include low antigen loading efficiency, superfluous immunogenicity, unpredictable side effects, and even cytotoxicity with susceptible action mechanisms and pharmacokinetic properties163,166. Overall, the disadvantages of nanomaterials-based delivery vehicles face the aforementioned obstacles, which involve physical and biological barriers leading to a decrease of clinical vaccine applications, as well as a variety of administration routes that possibly increase the targeting efficiency. This is especially true for nanomaterial-based delivery vehicles, which reflect the tailoring design of these delivery systems to minimize existing risks.
To further improve nanomaterials-based delivery vehicles used as cancer vaccines, more promising designs have the potential to utilize excellent synthesis strategies, such as specific chemical and engineered modifications, bio-responsive moieties and architectures, and incorporation of targeting components188. For a notable subset of synthetic delivery vehicles, elaborate material conjugation will improve the physical and chemical features, as well as the immunoregulatory capacities, with remaining adjuvant properties to regulate immune responses and increase the intrinsic safety68,106. In particular, more attention should be directed to their beneficial effects, combined with biocompatible and specialized synthetic materials, such as some U.S. FDA approved polymers. Another subclass of vaccine delivery systems is commonly referred to as immunogenic delivery systems, involving bio-inspired vehicles, which can be formulated by genetic manipulation and purification processes to increase their positive antitumor immune responses172. Additionally, large-scale preparation enables convenient acquisition of nanomaterials-based delivery vehicles with simple and available preparation procedures, accelerating clinical applications and marketing. Notably, a great deal of cell primitive-based therapeutic delivery vehicles have been developed with increased bioengineering and bioconjugation technologies, which have many advantages, when compared with single delivery systems164.
Overall, there are no real insurmountable obstacles to overcome. As an innovative type of advanced vaccine carrier for tumor immunotherapy, hybrid delivery vehicles are currently undergoing testing in many preclinical studies.
Conclusions and perspectives
The use of nanomaterials-based vaccines has been characterized by a rapidly emerging approach in the advancement of therapeutic cancer vaccines. As discussed in this review, 2 types of tumor antigens and 4 types of nanomaterials-based delivery vehicles have achieved interesting fabrications of cancer vaccines, which is attributed to the unique properties of each profile. Identification of TAAs and TSAs creates a solid foundation for vaccine-based tumor immunotherapy, enabling these vaccines to induce antigen-specific immune responses. However, a multitude of predicted TSAs fails to exist in tumors, and worse still, most predicted neoepitopes are immune privileged when tested for CD4+ and CD8+ T cell activations. In addition, recent predictors have designed multiple algorithms for predicting MHC-I and MHC-II binders, which elicit CD8+ and CD4+ T-cell antitumor immunities, respectively. This suggests that the algorithms involving in silico tools of MHC-I and MHC-II binders play an important role in personalized immunotherapy to support antitumor immune responses. Although the profundity and accuracy of neoepitope prediction hinders the development of personalized vaccines, immunogenic neoantigens are urgently required for neoantigen-based individual treatments.
This review also suggested that hybrids of different vaccine delivery vehicles are critical for therapeutic purposes, presenting fascinating prospects for future personalized therapeutic vaccines. The functioning of these delivery vehicles, such as for real-time imaging modules, enhancing immunogenicity modules, targeting modules, and environmental responsiveness modules, represents this combinatorial modification, and provides an intriguing strategy for innovative and specific cancer treatments. Among many delivery vehicles, the combination of synthetic delivery vehicles and bio-inspired delivery vehicles has shown reasonable treatment efficacies, because they mimic natural cell characteristics, leading to prominent potentials of bio-friendly vaccine platforms in clinical reality. Nevertheless, this application is still in its preliminary stages, with challenges including coating efficiencies and cost-effectiveness considerations. It is therefore important for researchers to develop a better understanding of biological delivery mechanisms by ameliorating diverse preparation techniques. At the same time, based on computational algorithms, the innovation research of such integrated delivery vehicles should analyze in vivo systems to maximize long-term circulation and tumor site accumulation. However, this novel approach requires a large number of samples to verify these algorithms with annotated data. We therefore imagine building optimal delivery vehicles using biological interaction data and computational algorithms to guide future hybrid delivery systems. These delivery vehicles artificially control pharmacokinetic properties of these elaborate therapeutic vaccines with maximum acceptable delivery efficacies to target sites. While currently far from this goal, the physicochemical properties of delivery vehicles interacting with biological environments are being developed for delivery and vaccine design guidance at the sub-organ or subcellular levels.
The broader concept of antitumor immunity used for up-to-date studies will lead to the development of engineered vaccines as adaptive immunomodulators. Effective adaptive immunity has the potential to eliminate tumor cells within a limited time, but excessive inflammation will also promote the progression of tumor growth, owing to complex phenotypes and heterogeneous TMEs. Innate immune training emerges as a novel therapeutic focus, which can promote antitumor activity, eliciting a durable immune response. With the introduced mechanism of trained immunity, nanomaterials-based therapeutic vaccines may be beneficial for cancer treatment involving appropriate reprogramming. These considerations indicate a new design of cancer vaccines, which will accelerate the production of next-generation personalized vaccines to meet the needs of cancer therapy. However, it is essential to transform scientific advances into clinical applications using nanomaterials-based delivery platforms.
Footnotes
Conflict of interest statement No potential conflicts of interest are disclosed.
Grant support
This work was supported by the National Key R&D Program of China (Grant Nos. 2018YFA0208900 and 2018YFE0205300), the Beijing Natural Science Foundation of China (Grant No. Z200020), the Beijing Nova Program (Grant No. Z201100006820031), the Taishan Scholars Program of Shandong Province (Grant No. ts20190987), the National Natural Science Foundation of China (Grant Nos. 31800838, 31820103004, 31730032, and 31800799), the Key Research Project of Frontier Science of the Chinese Academy of Sciences (Grant No. QYZDJ-SSW-SLH022), the Innovation Research Group of National Natural Science Foundation (Grant No. 11621505), and the Hundred-Talent Program of the Chinese Academy of Sciences.
References
- 1.Starnes CO. Coley’s toxins in perspective. Nature. 1992;357:11–2. doi: 10.1038/357011a0. [DOI] [PubMed] [Google Scholar]
- 2.Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–3. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–62. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 5.Faries MB, Hsueh EC, Ye X, Hoban M, Morton DL. Effect of granulocyte/macrophage colony-stimulating factor on vaccination with an allogeneic whole-cell melanoma vaccine. Clin Cancer Res. 2009;15:7029–35. doi: 10.1158/1078-0432.CCR-09-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 7.Kulkarni P, Shiraishi T, Rajagopalan K, Kim R, Mooney SM, Getzenberg RH. Cancer/testis antigens and urological malignancies. Nat Rev Urol. 2012;9:386–96. doi: 10.1038/nrurol.2012.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delamarre L, Mellman I, Yadav M. Cancer immunotherapy. Neo approaches to cancer vaccines. Science. 2015;348:760–1. doi: 10.1126/science.aab3465. [DOI] [PubMed] [Google Scholar]
- 9.Tanyi JL, Bobisse S, Ophir E, Tuyaerts S, Roberti A, Genolet R, et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci Transl Med. 2018;10:eaao5931. doi: 10.1126/scitranslmed.aao5931. [DOI] [PubMed] [Google Scholar]
- 10.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–61. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi M, Arai G, Matsumoto K, Naito S, Moriya F, Suekane S, et al. Phase I trial of a cancer vaccine consisting of 20 mixed peptides in patients with castration-resistant prostate cancer: dose-related immune boosting and suppression. Cancer Immunol Immunother. 2015;64:493–505. doi: 10.1007/s00262-015-1660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803–8. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547:222–6. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 14.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. 2017;547:217–21. doi: 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keskin DB, Anandappa AJ, Sun J, Tirosh I, Mathewson ND, Li S, et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature. 2019;565:234–9. doi: 10.1038/s41586-018-0792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4:7. doi: 10.1038/s41541-019-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin JD, Cabral H, Stylianopoulos T, Jain RK. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol. 2020;17:251–66. doi: 10.1038/s41571-019-0308-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–68. doi: 10.1038/s41577-020-0306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phuengkham H, Song C, Lim YT. A designer scaffold with immune nanoconverters for reverting immunosuppression and enhancing immune checkpoint blockade therapy. Adv Mater. 2019;31:e1903242. doi: 10.1002/adma.201903242. [DOI] [PubMed] [Google Scholar]
- 20.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–21. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laumont CM, Vincent K, Hesnard L, Audemard É, Bonneil É, Laverdure JP, et al. Noncoding regions are the main source of targetable tumor-specific antigens. Sci Transl Med. 2018;10:eaau5516. doi: 10.1126/scitranslmed.aau5516. [DOI] [PubMed] [Google Scholar]
- 22.Smart AC, Margolis CA, Pimentel H, He MX, Miao D, Adeegbe D, et al. Intron retention is a source of neoepitopes in cancer. Nat Biotechnol. 2018;36:1056–8. doi: 10.1038/nbt.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto TN, Kishton RJ, Restifo NP. Developing neoantigen-targeted T cell-based treatments for solid tumors. Nat Med. 2019;25:1488–99. doi: 10.1038/s41591-019-0596-y. [DOI] [PubMed] [Google Scholar]
- 24.Harari A, Graciotti M, Bassani-Sternberg M, Kandalaft LE. Antitumour dendritic cell vaccination in a priming and boosting approach. Nat Rev Drug Discov. 2020;19:635–52. doi: 10.1038/s41573-020-0074-8. [DOI] [PubMed] [Google Scholar]
- 25.Richters MM, Xia H, Campbell KM, Gillanders WE, Griffith OL, Griffith M. Best practices for bioinformatic characterization of neoantigens for clinical utility. Genome Med. 2019;11:56. doi: 10.1186/s13073-019-0666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yewdell JW, Dersh D, Fåhraeus R. Peptide channeling: the key to MHC class I immunosurveillance? Trends Cell Biol. 2019;29:929–39. doi: 10.1016/j.tcb.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Desrichard A, Snyder A, Chan TA. Cancer neoantigens and applications for immunotherapy. Clin Cancer Res. 2016;22:807–12. doi: 10.1158/1078-0432.CCR-14-3175. [DOI] [PubMed] [Google Scholar]
- 28.Aikins ME, Xu C, Moon JJ. Engineered nanoparticles for cancer vaccination and immunotherapy. Acc Chem Res. 2020;53:2094–105. doi: 10.1021/acs.accounts.0c00456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li WH, Li YM. Chemical strategies to boost cancer caccines. Chem Rev. 2020;120:11420–78. doi: 10.1021/acs.chemrev.9b00833. [DOI] [PubMed] [Google Scholar]
- 30.Roy S, Sethi TK, Taylor D, Kim YJ, Johnson DB. Breakthrough concepts in immune-oncology: cancer vaccines at the bedside. J Leukoc Biol. 2020;108:1455–89. doi: 10.1002/JLB.5BT0420-585RR. [DOI] [PubMed] [Google Scholar]
- 31.Smith CC, Selitsky SR, Chai S, Armistead PM, Vincent BG, Serody JS. Alternative tumour-specific antigens. Nat Rev Cancer. 2019;19:465–78. doi: 10.1038/s41568-019-0162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Servin-Blanco R, Zamora-Alvarado R, Gevorkian G, Manoutcharian K. Antigenic variability: obstacles on the road to vaccines against traditionally difficult targets. Hum Vaccin Immunother. 2016;12:2640–8. doi: 10.1080/21645515.2016.1191718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18:168–82. doi: 10.1038/nri.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melero I, Gaudernack G, Gerritsen W, Huber C, Parmiani G, Scholl S, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11:509–24. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 35.Gross DA, Graff-Dubois S, Opolon P, Cornet S, Alves P, Bennaceur-Griscelli A, et al. High vaccination efficiency of low-affinity epitopes in antitumor immunotherapy. J Clin Invest. 2004;113:425–33. doi: 10.1172/JCI19418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalaora S, Wolf Y, Feferman T, Barnea E, Greenstein E, Reshef D, et al. Combined analysis of antigen presentation and T-cell recognition reveals restricted immune responses in melanoma. Cancer Discov. 2018;8:1366–75. doi: 10.1158/2159-8290.CD-17-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bassani-Sternberg M, Pletscher-Frankild S, Jensen LJ, Mann M. Mass spectrometry of human leukocyte antigen class I peptidomes reveals strong effects of protein abundance and turnover on antigen presentation. Mol Cell Proteomics. 2015;14:658–73. doi: 10.1074/mcp.M114.042812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515:572–6. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 39.Haen SP, Löffler MW, Rammensee HG, Brossart P. Towards new horizons: characterization, classification and implications of the tumour antigenic repertoire. Nat Rev Clin Oncol. 2020;17:595–610. doi: 10.1038/s41571-020-0387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adhikari AN, Gallagher RC, Wang Y, Currier RJ, Amatuni G, Bassaganyas L, et al. The role of exome sequencing in newborn screening for inborn errors of metabolism. Nat Med. 2020;26:1392–7. doi: 10.1038/s41591-020-0966-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bibli SI, Hu J, Looso M, Weigert A, Ratiu C, Wittig J, et al. Mapping the endothelial cell S-sulfhydrome highlights the crucial role of integrin sulfhydration in vascular function. Circulation. 2021;143:935–48. doi: 10.1161/CIRCULATIONAHA.120.051877. [DOI] [PubMed] [Google Scholar]
- 42.Liu Z, Li JP, Chen M, Wu M, Shi Y, Li W, et al. Detecting tumor antigen-specific T cells via interaction-dependent fucosyl-biotinylation. Cell. 2020;183:1117–33 e19. doi: 10.1016/j.cell.2020.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kishton RJ, Lynn RC, Restifo NP. Strength in numbers: identifying neoantigen targets for cancer immunotherapy. Cell. 2020;183:591–3. doi: 10.1016/j.cell.2020.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Trinh A, Polyak K. Tumor neoantigens: when too much of a good thing is bad. Cancer Cell. 2019;36:466–7. doi: 10.1016/j.ccell.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Hilf N, Kuttruff-Coqui S, Frenzel K, Bukur V, Stevanović S, Gouttefangeas C, et al. Actively personalized vaccination trial for newly diagnosed glioblastoma. Nature. 2019;565:240–5. doi: 10.1038/s41586-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 46.Tran E, Turcotte S, Gros A, Robbins PF, Lu YC, Dudley ME, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344:641–5. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wells DK, van Buuren MM, Dang KK, Hubbard-Lucey VM, Sheehan KCF, Campbell KM, et al. Key parameters of tumor epitope immunogenicity revealed through a consortium approach improve neoantigen prediction. Cell. 2020;183:818–34 e13. doi: 10.1016/j.cell.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roudko V, Bozkus CC, Orfanelli T, McClain CB, Carr C, O’Donnell T, et al. Shared immunogenic poly-epitope frameshift mutations in microsatellite unstable tumors. Cell. 2020;183:1634–49 e17. doi: 10.1016/j.cell.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pettitt SJ, Frankum JR, Punta M, Lise S, Alexander J, Chen Y, et al. Clinical BRCA1/2 reversion analysis identifies hotspot mutations and predicted neoantigens associated with therapy resistance. Cancer Discov. 2020;10:1475–88. doi: 10.1158/2159-8290.CD-19-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van den Eynden J, Jimenez-Sanchez A, Miller ML, Larsson E. Lack of detectable neoantigen depletion signals in the untreated cancer genome. Nat Genet. 2019;51:1741–8. doi: 10.1038/s41588-019-0532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bassani-Sternberg M, Braunlein E, Klar R, Engleitner T, Sinitcyn P, Audehm S, et al. Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat Commun. 2016;7:13404. doi: 10.1038/ncomms13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonsack M, Hoppe S, Winter J, Tichy D, Zeller C, Kupper MD, et al. Performance evaluation of MHC class-I binding prediction tools based on an experimentally validated MHC-peptide binding data set. Cancer Immunol Res. 2019;7:719–36. doi: 10.1158/2326-6066.CIR-18-0584. [DOI] [PubMed] [Google Scholar]
- 53.Hundal J, Kiwala S, McMichael J, Miller CA, Xia H, Wollam AT, et al. pVACtools: a computational toolkit to identify and visualize cancer neoantigens. Cancer Immunol Res. 2020;8:409–20. doi: 10.1158/2326-6066.CIR-19-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reynisson B, Alvarez B, Paul S, Peters B, Nielsen M. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020;48:W449–54. doi: 10.1093/nar/gkaa379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen B, Khodadoust MS, Olsson N, Wagar LE, Fast E, Liu CL, et al. Predicting HLA class II antigen presentation through integrated deep learning. Nat Biotechnol. 2019;37:1332–43. doi: 10.1038/s41587-019-0280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nielsen M, Lundegaard C, Blicher T, Lamberth K, Harndahl M, Justesen S, et al. NetMHCpan, a method for quantitative predictions of peptide binding to any HLA-A and -B locus protein of known sequence. PLoS One. 2007;2:e796. doi: 10.1371/journal.pone.0000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shemesh CS, Hsu JC, Hosseini I, Shen BQ, Rotte A, Twomey P, et al. Personalized cancer vaccines: clinical landscape, challenges, and opportunities. Mol Ther. 2021;29:555–70. doi: 10.1016/j.ymthe.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Axelrod ML, Cook RS, Johnson DB, Balko JM. Biological consequences of MHC-II expression by tumor cells in cancer. Clin Cancer Res. 2019;25:2392–402. doi: 10.1158/1078-0432.CCR-18-3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alspach E, Lussier D, Miceli A, Kizhvatov I, DuPage M, Luoma A, et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature. 2019;574:696–701. doi: 10.1038/s41586-019-1671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marty Pyke R, Thompson WK, Salem RM, Font-Burgada J, Zanetti M, Carter H. Evolutionary pressure against MHC class II binding cancer mutations. Cell. 2018;175:1991. doi: 10.1016/j.cell.2018.11.050. [DOI] [PubMed] [Google Scholar]
- 61.Anandappa AJ, Wu CJ, Ott PA. Directing traffic: how to effectively drive T cells into tumors. Cancer Discov. 2020;10:185–97. doi: 10.1158/2159-8290.CD-19-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H, Sobral MC, Zhang DKY, Cartwright AN, Li AW, Dellacherie MO, et al. Metabolic labeling and targeted modulation of dendritic cells. Nat Mater. 2020;19:1244–52. doi: 10.1038/s41563-020-0680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu S, Jiang Q, Zhao X, Zhao R, Wang Y, Wang Y, et al. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat Mater. 2020;20:421–30. doi: 10.1038/s41563-020-0793-6. [DOI] [PubMed] [Google Scholar]
- 64.Bourquin J, Milosevic A, Hauser D, Lehner R, Blank F, Petri-Fink A, et al. Biodistribution, clearance, and long-term fate of clinically relevant nanomaterials. Adv Mater. 2018;30:e1704307. doi: 10.1002/adma.201704307. [DOI] [PubMed] [Google Scholar]
- 65.Rietwyk S, Peer D. Next-generation lipids in RNA interference therapeutics. ACS Nano. 2017;11:7572–86. doi: 10.1021/acsnano.7b04734. [DOI] [PubMed] [Google Scholar]
- 66.Rideau E, Dimova R, Schwille P, Wurm FR, Landfester K. Liposomes and polymersomes: a comparative review towards cell mimicking. Chem Soc Rev. 2018;47:8572–610. doi: 10.1039/c8cs00162f. [DOI] [PubMed] [Google Scholar]
- 67.Allison AC, Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974;252:252–2. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- 68.Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release. 2015;200:138–57. doi: 10.1016/j.jconrel.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 69.Butts C, Murray N, Maksymiuk A, Goss G, Marshall E, Soulieres D, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005;23:6674–81. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 70.Shahum E, Therien HM. Immunopotentiation of the humoral response by liposomes: encapsulation versus covalent linkage. Immunology. 1988;65:315–7. [PMC free article] [PubMed] [Google Scholar]
- 71.Noble GT, Stefanick JF, Ashley JD, Kiziltepe T, Bilgicer B. Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol. 2014;32:32–45. doi: 10.1016/j.tibtech.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 72.Jayaraman M, Ansell SM, Mui BL, Tam YK, Chen J, Du X, et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew Chem Int Ed Engl. 2012;51:8529–33. doi: 10.1002/anie.201203263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cullis PR, Hope MJ. Lipid nanoparticle systems for enabling gene therapies. Mol Ther. 2017;25:1467–75. doi: 10.1016/j.ymthe.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Newcomb CJ, Sur S, Ortony JH, Lee OS, Matson JB, Boekhoven J, et al. Cell death versus cell survival instructed by supramolecular cohesion of nanostructures. Nat Commun. 2014;5:3321. doi: 10.1038/ncomms4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu D, Hu C, Fan F, Qin Y, Huang C, Zhang Z, et al. Co-delivery of antigen and dual agonists by programmed mannose-targeted cationic lipid-hybrid polymersomes for enhanced vaccination. Biomaterials. 2019;206:25–40. doi: 10.1016/j.biomaterials.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 76.Marty R, N’Soukpoe-Kossi CN, Charbonneau D, Weinert CM, Kreplak L, Tajmir-Riahi HA. Structural analysis of DNA complexation with cationic lipids. Nucleic Acids Res. 2009;37:849–57. doi: 10.1093/nar/gkn1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Courant T, Bayon E, Reynaud-Dougier HL, Villiers C, Menneteau M, Marche PN, et al. Tailoring nanostructured lipid carriers for the delivery of protein antigens: Physicochemical properties versus immunogenicity studies. Biomaterials. 2017;136:29–42. doi: 10.1016/j.biomaterials.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 78.Sekiya T, Yamagishi J, Gray JHV, Whitney PG, Martinelli A, Zeng W, et al. PEGylation of a TLR2-agonist-based vaccine delivery system improves antigen trafficking and the magnitude of ensuing antibody and CD8(+) T cell responses. Biomaterials. 2017;137:61–72. doi: 10.1016/j.biomaterials.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 79.Warriner HE, Idziak SH, Slack NL, Davidson P, Safinya CR. Lamellar biogels: fluid-membrane-based hydrogels containing polymer lipids. Science. 1996;271:969–73. doi: 10.1126/science.271.5251.969. [DOI] [PubMed] [Google Scholar]
- 80.Uchida T, Taneichi M. Clinical application of surface-linked liposomal antigens. Mini Rev Med Chem. 2008;8:184–92. doi: 10.2174/138955708783498140. [DOI] [PubMed] [Google Scholar]
- 81.Chen D, Love KT, Chen Y, Eltoukhy AA, Kastrup C, Sahay G, et al. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J Am Chem Soc. 2012;134:6948–51. doi: 10.1021/ja301621z. [DOI] [PubMed] [Google Scholar]
- 82.Stroock AD, Dertinger SK, Ajdari A, Mezic I, Stone HA, Whitesides GM. Chaotic mixer for microchannels. Science. 2002;295:647–51. doi: 10.1126/science.1066238. [DOI] [PubMed] [Google Scholar]
- 83.Grimaldi N, Andrade F, Segovia N, Ferrer-Tasies L, Sala S, Veciana J, et al. Lipid-based nanovesicles for nanomedicine. Chem Soc Rev. 2016;45:6520–45. doi: 10.1039/c6cs00409a. [DOI] [PubMed] [Google Scholar]
- 84.Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci U S A. 2010;107:1864–9. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim Y, Kang S, Shin H, Kim T, Yu B, Kim J, et al. Sequential and timely combination of a cancer nanovaccine with immune checkpoint blockade effectively inhibits tumor growth and relapse. Angew Chem Int Ed Engl. 2020;59:14628–38. doi: 10.1002/anie.202006117. [DOI] [PubMed] [Google Scholar]
- 86.Fan Y, Stronsky SM, Xu Y, Steffens JT, van Tongeren SA, Erwin A, et al. Multilamellar vaccine particle elicits potent immune activation with protein antigens and protects mice against Ebola virus infection. ACS Nano. 2019;13:11087–96. doi: 10.1021/acsnano.9b03660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu X, Dai Y, Zhao Y, Qi S, Liu L, Lu L, et al. Melittin-lipid nanoparticles target to lymph nodes and elicit a systemic anti-tumor immune response. Nat Commun. 2020;11:1110. doi: 10.1038/s41467-020-14906-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 89.Miao L, Li L, Huang Y, Delcassian D, Chahal J, Han J, et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat Biotechnol. 2019;37:1174–85. doi: 10.1038/s41587-019-0247-3. [DOI] [PubMed] [Google Scholar]
- 90.Sayour EJ, Grippin A, De Leon G, Stover B, Rahman M, Karachi A, et al. Personalized tumor RNA loaded lipid-nanoparticles prime the systemic and intratumoral milieu for response to cancer immunotherapy. Nano Lett. 2018;18:6195–206. doi: 10.1021/acs.nanolett.8b02179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pardi N, Hogan MJ, Naradikian MS, Parkhouse K, Cain DW, Jones L, et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018;215:1571–88. doi: 10.1084/jem.20171450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coolen AL, Lacroix C, Mercier-Gouy P, Delaune E, Monge C, Exposito JY, et al. Poly(lactic acid) nanoparticles and cell-penetrating peptide potentiate mRNA-based vaccine expression in dendritic cells triggering their activation. Biomaterials. 2019;195:23–37. doi: 10.1016/j.biomaterials.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 93.Li Y, Su Z, Zhao W, Zhang X, Momin N, Zhang C, et al. Multifunctional oncolytic nanoparticles deliver self-replicating IL-12 RNA to eliminate established tumors and prime systemic immunity. Nature Cancer. 2020;1:882–93. doi: 10.1038/s43018-020-0095-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walsh EE, Frenck RW, Jr., Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–50. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sahin U, Karikó K, Türeci Ö. mRNA-based therapeutics – developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–80. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 96.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–79. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bookstaver ML, Tsai SJ, Bromberg JS, Jewell CM. Improving vaccine and immunotherapy design using biomaterials. Trends Immunol. 2018;39:135–50. doi: 10.1016/j.it.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Salazar LG, Lu H, Reichow JL, Childs JS, Coveler AL, Higgins DM, et al. Topical imiquimod plus nab-paclitaxel for breast cancer cutaneous metastases: a phase 2 clinical trial. JAMA Oncol. 2017;3:969–73. doi: 10.1001/jamaoncol.2016.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garrido-Laguna I, Hidalgo M. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12:319–34. doi: 10.1038/nrclinonc.2015.53. [DOI] [PubMed] [Google Scholar]
- 100.Mohan K, Ganesan AR, Muralisankar T, Jayakumar R, Sathishkumar P, Uthayakumar V, et al. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci Technol. 2020;105:17–42. doi: 10.1016/j.tifs.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qin L, Cao J, Shao K, Tong F, Yang Z, Lei T, et al. A tumor-to-lymph procedure navigated versatile gel system for combinatorial therapy against tumor recurrence and metastasis. Sci Adv. 2020;6:eabb3116. doi: 10.1126/sciadv.abb3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ryu JH, Yoon HY, Sun IC, Kwon IC, Kim K. Tumor-targeting glycol chitosan nanoparticles for cancer heterogeneity. Adv Mater. 2020;32:e2002197. doi: 10.1002/adma.202002197. [DOI] [PubMed] [Google Scholar]
- 103.Xu J, Xu B, Tao J, Yang Y, Hu Y, Huang Y. Microneedle-assisted, DC-targeted codelivery of pTRP-2 and adjuvant of paclitaxel for transcutaneous immunotherapy. Small. 2017;13:1700666. doi: 10.1002/smll.201700666. [DOI] [PubMed] [Google Scholar]
- 104.Saini P, Arora M, Kumar M. Poly(lactic acid) blends in biomedical applications. Adv Drug Deliv Rev. 2016;107:47–59. doi: 10.1016/j.addr.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 105.Lu L, Yaszemski MJ, Mikos AG. Retinal pigment epithelium engineering using synthetic biodegradable polymers. Biomaterials. 2001;22:3345–55. doi: 10.1016/s0142-9612(01)00172-7. [DOI] [PubMed] [Google Scholar]
- 106.Saito N, Murakami N, Takahashi J, Horiuchi H, Ota H, Kato H, et al. Synthetic biodegradable polymers as drug delivery systems for bone morphogenetic proteins. Adv Drug Deliv Rev. 2005;57:1037–48. doi: 10.1016/j.addr.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 107.Duncan R, Vicent MJ. Polymer therapeutics-prospects for 21st century: the end of the beginning. Adv Drug Deliv Rev. 2013;65:60–70. doi: 10.1016/j.addr.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 108.Xie X, Hu Y, Ye T, Chen Y, Zhou L, Li F, et al. Therapeutic vaccination against leukaemia via the sustained release of co-encapsulated anti-PD-1 and a leukaemia-associated antigen. Nat Biomed Eng. 2020 doi: 10.1038/s41551-020-00624-6. [DOI] [PubMed] [Google Scholar]
- 109.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 110.Roy S, Zhu D, Parak WJ, Feliu N. Lysosomal proton buffering of poly(ethylenimine) measured in situ by fluorescent pH-sensor microcapsules. ACS Nano. 2020;14:8012–23. doi: 10.1021/acsnano.9b10219. [DOI] [PubMed] [Google Scholar]
- 111.Wei X, Shao B, He Z, Ye T, Luo M, Sang Y, et al. Cationic nanocarriers induce cell necrosis through impairment of Na(+)/K(+)-ATPase and cause subsequent inflammatory response. Cell Res. 2015;25:237–53. doi: 10.1038/cr.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gong N, Zhang Y, Teng X, Wang Y, Huo S, Qing G, et al. Proton-driven transformable nanovaccine for cancer immunotherapy. Nat Nanotechnol. 2020;15:1053–64. doi: 10.1038/s41565-020-00782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu G, Lynn GM, Jacobson O, Chen K, Liu Y, Zhang H, et al. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat Commun. 2017;8:1954. doi: 10.1038/s41467-017-02191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paolillo G, Astarita T. Perspective camera model with refraction correction for optical velocimetry measurements in complex geometries. IEEE Trans Pattern Anal Mach Intell. 2020 doi: 10.1109/TPAMI.2020.3046467. [DOI] [PubMed] [Google Scholar]
- 115.Murthy N, Robichaud JR, Tirrell DA, Stayton PS, Hoffman AS. The design and synthesis of polymers for eukaryotic membrane disruption. J Control Release. 1999;61:137–43. doi: 10.1016/s0168-3659(99)00114-5. [DOI] [PubMed] [Google Scholar]
- 116.Kompella UB, Amrite AC, Pacha Ravi R, Durazo SA. Nanomedicines for back of the eye drug delivery, gene delivery, and imaging. Prog Retin Eye Res. 2013;36:172–98. doi: 10.1016/j.preteyeres.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2017;16:489–96. doi: 10.1038/nmat4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shah NJ, Najibi AJ, Shih TY, Mao AS, Sharda A, Scadden DT, et al. A biomaterial-based vaccine eliciting durable tumour-specific responses against acute myeloid leukaemia. Nat Biomed Eng. 2020;4:40–51. doi: 10.1038/s41551-019-0503-3. [DOI] [PubMed] [Google Scholar]
- 119.Min Y, Roche KC, Tian S, Eblan MJ, McKinnon KP, Caster JM, et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol. 2017;12:877–82. doi: 10.1038/nnano.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lu Y, Lin J, Wang L, Zhang L, Cai C. Self-assembly of copolymer micelles: higher-level assembly for constructing hierarchical structure. Chem Rev. 2020;120:4111–40. doi: 10.1021/acs.chemrev.9b00774. [DOI] [PubMed] [Google Scholar]
- 121.Mei L, Rao J, Liu Y, Li M, Zhang Z, He Q. Effective treatment of the primary tumor and lymph node metastasis by polymeric micelles with variable particle sizes. J Control Release. 2018;292:67–77. doi: 10.1016/j.jconrel.2018.04.053. [DOI] [PubMed] [Google Scholar]
- 122.Sapra R, Verma RP, Maurya GP, Dhawan S, Babu J, Haridas V. Designer peptide and protein dendrimers: a cross-sectional analysis. Chem Rev. 2019;119:11391–441. doi: 10.1021/acs.chemrev.9b00153. [DOI] [PubMed] [Google Scholar]
- 123.Mignani S, Rodrigues J, Tomas H, Zablocka M, Shi X, Caminade AM, et al. Dendrimers in combination with natural products and analogues as anti-cancer agents. Chem Soc Rev. 2018;47:514–32. doi: 10.1039/c7cs00550d. [DOI] [PubMed] [Google Scholar]
- 124.Tian WD, Ma YQ. Theoretical and computational studies of dendrimers as delivery vectors. Chem Soc Rev. 2013;42:705–27. doi: 10.1039/c2cs35306g. [DOI] [PubMed] [Google Scholar]
- 125.Caminade AM, Yan D, Smith DK. Dendrimers and hyperbranched polymers. Chem Soc Rev. 2015;44:3870–3. doi: 10.1039/c5cs90049b. [DOI] [PubMed] [Google Scholar]
- 126.Xi X, Ye T, Wang S, Na X, Wang J, Qing S, et al. Self-healing microcapsules synergetically modulate immunization microenvironments for potent cancer vaccination. Sci Adv. 2020;6:eaay7735. doi: 10.1126/sciadv.aay7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pan C, Wu J, Qing S, Zhang X, Zhang L, Yue H, et al. Biosynthesis of self-assembled proteinaceous nanoparticles for vaccination. Adv Mater. 2020;32:e2002940. doi: 10.1002/adma.202002940. [DOI] [PubMed] [Google Scholar]
- 128.Xia Y, Wu J, Wei W, Du Y, Wan T, Ma X, et al. Exploiting the pliability and lateral mobility of Pickering emulsion for enhanced vaccination. Nat Mater. 2018;17:187–94. doi: 10.1038/nmat5057. [DOI] [PubMed] [Google Scholar]
- 129.Dong X, Liang J, Yang A, Qian Z, Kong D, Lv F. Fluorescence imaging guided CpG nanoparticles-loaded IR820-hydrogel for synergistic photothermal immunotherapy. Biomaterials. 2019;209:111–25. doi: 10.1016/j.biomaterials.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 130.Li S, Bennett ZT, Sumer BD, Gao J. Nano-immune-engineering approaches to advance cancer immunotherapy: lessons from ultra-pH-sensitive nanoparticles. Acc Chem Res. 2020;53:2546–57. doi: 10.1021/acs.accounts.0c00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yi X, Xu M, Zhou H, Xiong S, Qian R, Chai Z, et al. Ultrasmall hyperbranched semiconducting polymer nanoparticles with different radioisotopes labeling for cancer theranostics. ACS Nano. 2018;12:9142–51. doi: 10.1021/acsnano.8b03514. [DOI] [PubMed] [Google Scholar]
- 132.Xu J, Lv J, Zhuang Q, Yang Z, Cao Z, Xu L, et al. A general strategy towards personalized nanovaccines based on fluoropolymers for post-surgical cancer immunotherapy. Nat Nanotechnol. 2020;15:1043–52. doi: 10.1038/s41565-020-00781-4. [DOI] [PubMed] [Google Scholar]
- 133.Lu X, Miao L, Gao W, Chen Z, McHugh KJ, Sun Y, et al. Engineered PLGA microparticles for long-term, pulsatile release of STING agonist for cancer immunotherapy. Sci Transl Med. 2020;12:eaaz6606. doi: 10.1126/scitranslmed.aaz6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Conniot J, Scomparin A, Peres C, Yeini E, Pozzi S, Matos AI, et al. Immunization with mannosylated nanovaccines and inhibition of the immune-suppressing microenvironment sensitizes melanoma to immune checkpoint modulators. Nat Nanotechnol. 2019;14:891–901. doi: 10.1038/s41565-019-0512-0. [DOI] [PubMed] [Google Scholar]
- 135.Wang C, Sun W, Ye Y, Bomba HN, Gu Z. Bioengineering of artificial antigen presenting cells and lymphoid organs. Theranostics. 2017;7:3504–16. doi: 10.7150/thno.19017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mohammadpour R, Dobrovolskaia MA, Cheney DL, Greish KF, Ghandehari H. Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv Drug Deliv Rev. 2019;144:112–32. doi: 10.1016/j.addr.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Luther DC, Huang R, Jeon T, Zhang X, Lee YW, Nagaraj H, et al. Delivery of drugs, proteins, and nucleic acids using inorganic nanoparticles. Adv Drug Deliv Rev. 2020;156:188–213. doi: 10.1016/j.addr.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Croissant JG, Butler KS, Zink JI, Brinker CJ. Synthetic amorphous silica nanoparticles: toxicity, biomedical and environmental implications. Nat Rev Mater. 2020;5:886–909. [Google Scholar]
- 139.Li AW, Sobral MC, Badrinath S, Choi Y, Graveline A, Stafford AG, et al. A facile approach to enhance antigen response for personalized cancer vaccination. Nat Mater. 2018;17:528–34. doi: 10.1038/s41563-018-0028-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang X, Li X, Ito A, Yoshiyuki K, Sogo Y, Watanabe Y, et al. Hollow structure improved anti-cancer immunity of mesoporous silica nanospheres in vivo. Small. 2016;12:3510–5. doi: 10.1002/smll.201600677. [DOI] [PubMed] [Google Scholar]
- 141.Liu Q, Zhou Y, Li M, Zhao L, Ren J, Li D, et al. Polyethylenimine hybrid thin-shell hollow mesoporous silica nanoparticles as vaccine self-adjuvants for cancer immunotherapy. ACS Appl Mater Interfaces. 2019;11:47798–809. doi: 10.1021/acsami.9b19446. [DOI] [PubMed] [Google Scholar]
- 142.Yang Y, Lu Y, Abbaraju PL, Zhang J, Zhang M, Xiang G, et al. Multi-shelled dendritic mesoporous organosilica hollow spheres: roles of composition and architecture in cancer immunotherapy. Angew Chem Int Ed Engl. 2017;56:8446–50. doi: 10.1002/anie.201701550. [DOI] [PubMed] [Google Scholar]
- 143.Vallet-Regi M, Colilla M, Izquierdo-Barba I, Manzano M. Mesoporous silica nanoparticles for drug delivery: current insights. Molecules. 2017;23:47. doi: 10.3390/molecules23010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang Y, Tang J, Song H, Yang Y, Gu Z, Fu J, et al. Dendritic mesoporous silica nanoparticle adjuvants modified with binuclear aluminum complex: coordination chemistry dictates adjuvanticity. Angew Chem Int Ed Engl. 2020;59:19610–7. doi: 10.1002/anie.202006861. [DOI] [PubMed] [Google Scholar]
- 145.Li X, Wang X, Ito A, Tsuji NM. A nanoscale metal organic frameworks-based vaccine synergises with PD-1 blockade to potentiate anti-tumour immunity. Nat Commun. 2020;11:3858. doi: 10.1038/s41467-020-17637-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ding B, Shao S, Yu C, Teng B, Wang M, Cheng Z, et al. Large-pore mesoporous-silica-coated upconversion nanoparticles as multifunctional immunoadjuvants with ultrahigh photosensitizer and antigen loading efficiency for improved cancer photodynamic immunotherapy. Adv Mater. 2018;30:e1802479. doi: 10.1002/adma.201802479. [DOI] [PubMed] [Google Scholar]
- 147.Nguyen TL, Choi Y, Kim J. Mesoporous silica as a versatile platform for cancer immunotherapy. Adv Mater. 2019;31:e1803953. doi: 10.1002/adma.201803953. [DOI] [PubMed] [Google Scholar]
- 148.Nguyen TL, Cha BG, Choi Y, Im J, Kim J. Injectable dual-scale mesoporous silica cancer vaccine enabling efficient delivery of antigen/adjuvant-loaded nanoparticles to dendritic cells recruited in local macroporous scaffold. Biomaterials. 2020;239:119859. doi: 10.1016/j.biomaterials.2020.119859. [DOI] [PubMed] [Google Scholar]
- 149.Hess KL, Medintz IL, Jewell CM. Designing inorganic nanomaterials for vaccines and immunotherapies. Nano Today. 2019;27:73–98. doi: 10.1016/j.nantod.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Arun Kumar S, Good J, Hendrix D, Yoo E, Kim D, Deo KA, et al. Nanoengineered light-activatable polybubbles for on-demand therapeutic delivery. Adv Funct Mater. 2020;30:2003579. doi: 10.1002/adfm.202003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhu M, Du L, Zhao R, Wang HY, Zhao Y, Nie G, et al. Cell-penetrating nanoparticles activate the inflammasome to enhance antibody production by targeting microtubule-associated protein 1-light chain 3 for degradation. ACS Nano. 2020;14:3703–17. doi: 10.1021/acsnano.0c00962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhou Q, Zhang Y, Du J, Li Y, Zhou Y, Fu Q, et al. Different-sized gold nanoparticle activator/antigen increases dendritic cells accumulation in liver-draining lymph nodes and CD8+ T cell responses. ACS Nano. 2016;10:2678–92. doi: 10.1021/acsnano.5b07716. [DOI] [PubMed] [Google Scholar]
- 153.Wang Y, Quinsaat JEQ, Ono T, Maeki M, Tokeshi M, Isono T, et al. Enhanced dispersion stability of gold nanoparticles by the physisorption of cyclic poly(ethylene glycol) Nat Commun. 2020;11:6089. doi: 10.1038/s41467-020-19947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mao W, Lee S, Shin J, Yoo H. Surface-initiated atom transfer polymerized anionic corona on gold nanoparticles for anti-cancer therapy. Pharmaceutics. 2020;12:261. doi: 10.3390/pharmaceutics12030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Goddard ZR, Marin MJ, Russell DA, Searcey M. Active targeting of gold nanoparticles as cancer therapeutics. Chem Soc Rev. 2020;49:8774–89. doi: 10.1039/d0cs01121e. [DOI] [PubMed] [Google Scholar]
- 156.Kobayashi T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol J. 2011;6:1342–7. doi: 10.1002/biot.201100045. [DOI] [PubMed] [Google Scholar]
- 157.Rivera Gil P, Hühn D, del Mercato LL, Sasse D, Parak WJ. Nanopharmacy: inorganic nanoscale devices as vectors and active compounds. Pharmacol Res. 2010;62:115–25. doi: 10.1016/j.phrs.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 158.Grippin AJ, Wummer B, Wildes T, Dyson K, Trivedi V, Yang C, et al. Dendritic cell-activating magnetic nanoparticles enable early prediction of antitumor response with magnetic resonance imaging. ACS Nano. 2019;13:13884–98. doi: 10.1021/acsnano.9b05037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guo Y, Ran Y, Wang Z, Cheng J, Cao Y, Yang C, et al. Magnetic-responsive and targeted cancer nanotheranostics by PA/MR bimodal imaging-guided photothermally triggered immunotherapy. Biomaterials. 2019;219:119370. doi: 10.1016/j.biomaterials.2019.119370. [DOI] [PubMed] [Google Scholar]
- 160.Traini G, Ruiz-de-Angulo A, Blanco-Canosa JB, Zamacola Bascaran K, Molinaro A, Silipo A, et al. Cancer immunotherapy of TLR4 agonist-antigen constructs enhanced with pathogen-mimicking magnetite nanoparticles and checkpoint blockade of PD-L1. Small. 2019;15:e1803993. doi: 10.1002/smll.201803993. [DOI] [PubMed] [Google Scholar]
- 161.Kroll AV, Jiang Y, Zhou J, Holay M, Fang RH, Zhang L. Biomimetic nanoparticle vaccines for cancer therapy. Adv Biosyst. 2019;3:e1800219. doi: 10.1002/adbi.201800219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10:521–35. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 163.Luo GF, Chen WH, Zeng X, Zhang XZ. Cell primitive-based biomimetic functional materials for enhanced cancer therapy. Chem Soc Rev. 2021;50:945–85. doi: 10.1039/d0cs00152j. [DOI] [PubMed] [Google Scholar]
- 164.Li M, Zhou H, Jiang W, Yang C, Miao H, Wang Y. Nanovaccines integrating endogenous antigens and pathogenic adjuvants elicit potent antitumor immunity. Nano Today. 2020;35:101007 [Google Scholar]
- 165.Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol. 2015;13:605–19. doi: 10.1038/nrmicro3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ngandeu Neubi GM, Opoku-Damoah Y, Gu X, Han Y, Zhou J, Ding Y. Bio-inspired drug delivery systems: an emerging platform for targeted cancer therapy. Biomater Sci. 2018;6:958–73. doi: 10.1039/C8BM00175H. [DOI] [PubMed] [Google Scholar]
- 167.Kudela P, Koller VJ, Lubitz W. Bacterial ghosts (BGs) – advanced antigen and drug delivery system. Vaccine. 2010;28:5760–7. doi: 10.1016/j.vaccine.2010.06.087. [DOI] [PubMed] [Google Scholar]
- 168.Gerritzen MJH, Martens DE, Wijffels RH, van der Pol L, Stork M. Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol Adv. 2017;35:565–74. doi: 10.1016/j.biotechadv.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 169.Qing S, Lyu C, Zhu L, Pan C, Wang S, Li F, et al. Biomineralized bacterial outer membrane vesicles potentiate safe and efficient tumor microenvironment reprogramming for anticancer therapy. Adv Mater. 2020;32:e2002085. doi: 10.1002/adma.202002085. [DOI] [PubMed] [Google Scholar]
- 170.Holay M, Guo Z, Pihl J, Heo J, Park JH, Fang RH, et al. Bacteria-inspired nanomedicine. ACS Applied Bio Materials. 2020 doi: 10.1021/acsabm.0c01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Angsantikul P, Thamphiwatana S, Gao W, Zhang L. Cell membrane-coated nanoparticles as an emerging antibacterial vaccine platform. Vaccines. 2015;3:814–28. doi: 10.3390/vaccines3040814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Parodi A, Molinaro R, Sushnitha M, Evangelopoulos M, Martinez JO, Arrighetti N, et al. Bio-inspired engineering of cell- and virus-like nanoparticles for drug delivery. Biomaterials. 2017;147:155–68. doi: 10.1016/j.biomaterials.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 173.Zhou J, Kroll AV, Holay M, Fang RH, Zhang L. Biomimetic nanotechnology toward personalized vaccines. Adv Mater. 2020;32:e1901255. doi: 10.1002/adma.201901255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ukidve A, Zhao Z, Fehnel A, Krishnan V, Pan DC, Gao Y, et al. Erythrocyte-driven immunization via biomimicry of their natural antigen-presenting function. Proc Natl Acad Sci U S A. 2020;117:17727–36. doi: 10.1073/pnas.2002880117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Liu Y, Luo JS, Chen XJ, Liu W, Chen TK. Cell membrane coating technology: a promising strategy for biomedical applications. Nanomicro Lett. 2019;11:100. doi: 10.1007/s40820-019-0330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hu QY, Bomba HN, Gu Z. Engineering platelet-mimicking drug delivery vehicles. Front Chem Sci Eng. 2017;11:624–32. [Google Scholar]
- 177.Cheng S, Xu C, Jin Y, Li Y, Zhong C, Ma J, et al. Artificial mini dendritic cells boost T cell-based immunotherapy for ovarian cancer. Adv Sci (Weinh) 2020;7:1903301. doi: 10.1002/advs.201903301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang Z, Li D, Li X, Guo Z, Liu Y, Ma X, et al. PEI-modified macrophage cell membrane-coated PLGA nanoparticles encapsulating Dendrobium polysaccharides as a vaccine delivery system for ovalbumin to improve immune responses. Int J Biol Macromol. 2020;165:239–48. doi: 10.1016/j.ijbiomac.2020.09.187. [DOI] [PubMed] [Google Scholar]
- 179.Wang L, Pegram MD, Wu JC. Induced pluripotent stem cells as a novel cancer vaccine. Expert Opin Biol Ther. 2019;19:1191–7. doi: 10.1080/14712598.2019.1650909. [DOI] [PubMed] [Google Scholar]
- 180.Zhang M, Zang X, Wang M, Li Z, Qiao M, Hu H, et al. Exosome-based nanocarriers as bio-inspired and versatile vehicles for drug delivery: recent advances and challenges. J Mater Chem B. 2019;7:2421–33. doi: 10.1039/c9tb00170k. [DOI] [PubMed] [Google Scholar]
- 181.Fang RH, Jiang Y, Fang JC, Zhang L. Cell membrane-derived nanomaterials for biomedical applications. Biomaterials. 2017;128:69–83. doi: 10.1016/j.biomaterials.2017.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Harris JC, Scully MA, Day ES. Cancer cell membrane-coated nanoparticles for cancer management. Cancers. 2019;11:1836. doi: 10.3390/cancers11121836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Huh YM, Lee ES, Lee JH, Jun Yw, Kim PH, Yun CO, et al. Hybrid nanoparticles for magnetic resonance imaging of target-specific viral gene delivery. Adv Mater. 2007;19:3109–12. [Google Scholar]
- 184.Roldao A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert Rev Vaccines. 2010;9:1149–76. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 185.Ong HK, Tan WS, Ho KL. Virus like particles as a platform for cancer vaccine development. PeerJ. 2017;5:e4053. doi: 10.7717/peerj.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hodgson CP, Solaiman F. Virosomes: cationic liposomes enhance retroviral transduction. Nat Biotechnol. 1996;14:339–42. doi: 10.1038/nbt0396-339. [DOI] [PubMed] [Google Scholar]
- 187.Gargett T, Grubor-Bauk B, Miller D, Garrod T, Yu S, Wesselingh S, et al. Increase in DNA vaccine efficacy by virosome delivery and co-expression of a cytolytic protein. Clin Transl Immunology. 2014;3:e18. doi: 10.1038/cti.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20:101–24. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]