Abstract
Objective:
Mucin 1 (MUC1/EMA) and sialyl Lewis X (sLex) indicate polarity reversal in invasive micropapillary carcinoma (IMPC). The purpose of this study was to evaluate the expression of MUC1/EMA and sLex and to assess their diagnostic and prognostic value in patients with IMPC.
Methods:
The expression of sLex and MUC1/EMA in 100 patients with IMPC and a control group of 89 patients with invasive ductal carcinoma not otherwise specified (IDC-NOS) were analyzed with IHC. Fresh tumor tissues were collected from patients with IMPC or IDC-NOS for primary culture and immunofluorescence analysis.
Results:
The rate of nodal metastasis was higher in patients with IMPC than those with IDC-NOS, and IMPC cells tended to express more sLex and MUC1/EMA in the cytomembranes (the stroma-facing surfaces of the micropapillary clusters) than IDC-NOS cells. In IMPC, high cytomembrane expression of sLex, but not MUC1/EMA, indicated poor prognosis. In addition, among the 100 patients with IMPC, 10 patients had sLex+/EMA– expression patterns, and 8 patients had sLex–/EMA+ expression patterns. The primary IMPC cells were suspended, non-adherent tumor cell clusters, whereas the primary IDC cells were adherent tumor cells. Immunofluorescence analysis showed that MUC1/EMA and sLex were co-expressed on the cytomembranes in IMPC cell clusters and in the cytoplasm in IDC-NOS cells.
Conclusions:
sLex can be used as a prognostic indicator and can be combined with MUC1/EMA as a complementary diagnostic indicator to avoid missed IMPC diagnosis.
Keywords: Invasive micropapillary carcinoma, polarity reversal, diagnostic indicator, EMA, sLex
Introduction
Invasive micropapillary carcinoma (IMPC) is a type of breast cancer accounting for approximately 7% of all breast cancer cases1. IMPC can be characterized by the morphotype of mulberry-like cell clusters, which lack central vascular bundles and are surrounded by a clear interstitial space2. IMPC can be recognized by the typical “inside-out” growth pattern, which indicates the “polarity reversal” of IMPC cells3. Cancer cells with polarity reversal show high metastatic potential and can be recognized by the presence of an inside-out expression pattern through MUC1/EMA or sLex staining4–7.
MUC1, also known as EMA8,9, is a high molecular weight (> 400,000) type I transmembrane glycoprotein mainly distributed in glandular epithelial cells. MUC1/EMA with high glycosylation (glycosylation > 50%) is composed of core peptides and sugar chains, most of which are attached through O-linked glycosylation. The core peptides of MUC1/EMA contain intracellular, transmembrane, and extracellular regions.
The carbohydrate ligand sialyl Lewis X (sLex) is an adhesion molecule expressed on the surfaces of human leukocytes and various cancer cells. It is the most important ligand for selectin, particularly E-selectin, which is expressed on the surfaces of endothelial cells10. sLex and MUC1/EMA are associated with the reversal of cell polarity, which enhances the metastatic potential of breast cancer, particularly lymph node metastasis of IMPC7,11.
Although few studies have proposed a relationship between sLex and breast cancer prognosis12–14, no detailed analysis has been performed on the expression of sLex in the invasive micropapillary structure and its prognostic value for IMPC. Several studies have shown that MUC1/EMA can be a carrier of sLex, and sLex in turn is an epitope of MUC1/EMA15,16. Therefore, this study evaluated the distribution of sLex and MUC1/EMA expression in IMPC and analyzed their prognostic value.
Materials and methods
Case selection
One hundred cases of breast IMPC diagnosed in the Department of Breast Pathology of Tianjin Medical University Cancer Institute and Hospital between January 2007 and December 2008 were selected, and contiguous slices were made. Eighty-nine cases of breast IDC-NOS diagnosed in the same period were randomly selected as a control group. Of the 100 cases of breast IMPC, 94 cases were of mixed type, and 6 cases were of pure type. Mixed type IMPC comprises both IMPC and IDC-NOS components. The median age of patients in the IMPC group at diagnosis was 52 years (range 28–89), and the median age of patients in the IDC-NOS group was 50 years (range 28–80). The follow-up time for the 2 groups was 1–100 months (median follow-up time of 63 months). A total of 3 IMPC and 3 IDC-NOS fresh tumor tissues were collected for primary culture. The study was approved by the Ethics Committee of Tianjin Medical University Cancer Institute and Hospital, and informed consent was obtained from all patients.
Immunohistochemistry
Four-micrometer serial whole-tissue sections were cut from the archived formalin-fixed, paraffin-embedded tissue blocks, dewaxed, and subsequently rehydrated with xylene and graded alcohol washes. Antigen retrieval (sLex) was performed in a pressure cooker in citrate buffer (pH 6.0) for 2 min 30 s, and EMA was performed in EDTA (pH 9.5). The sections were treated with 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity and then incubated with normal goat serum for 10 min to eliminate nonspecific background staining. Thereafter, primary antibodies to sLex (BD Biosciences, #551344, monoclonal, 1:150 dilution, CA, USA) or EMA (ZSGB-bio, #ZM-0095, monoclonal, Beijing, China) were incubated with the samples at 4 °C overnight. Antigen was sequentially detected with secondary biotin-labeled antibody and peroxidase-conjugated streptavidin. The chromogen was 3,3-diaminobenzidine. The sections were counterstained with hematoxylin.
Immunohistochemistry scoring
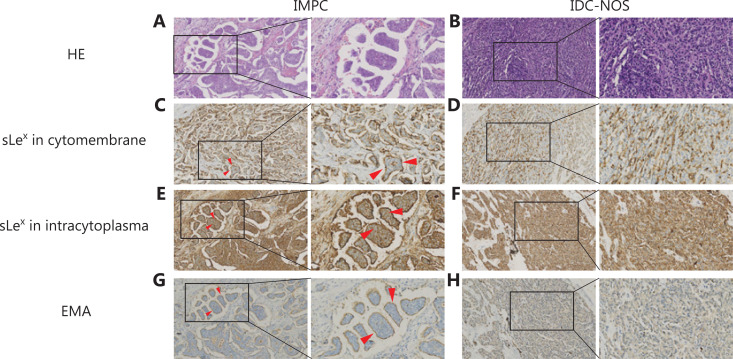
MUC1/EMA and sLex expression was localized to the cytomembrane (the stroma-facing surfaces of the cell clusters) or within the cytoplasm (Figure 1). Only IMPC component staining in mixed type IMPC was recorded. For the IDC-NOS group, we also recorded the staining of the cytomembrane or cytoplasm.
Figure 1.
Immunohistochemical expression of sLex and MUC1/EMA in breast IMPC and IDC-NOS. (A) IMPC consists of tumor cell clusters of epithelial cells surrounded by gaps [hematoxylin and eosin (HE) staining]. (B) Representative microphotographs of IDC-NOS (HE). Immunohistochemistry confirming the expression of sLex on the cytomembranes in IMPC cell clusters (C) and IDC-NOS cells (D). The expression of sLex within the cytoplasm in IMPC cell clusters (E) and IDC-NOS cells (F). EMA immunostaining showing the typical polarity reversal growth pattern of IMPC (G) and the normal pattern in IDC-NOS tumor cells (H) (200×, 400×, indicated by the red arrow).
SLex immunostaining was assessed through light microscopy by 2 independent experienced pathologists (Li Fu and Fangfang Liu). Both pathologists reevaluated the staining and reached a conclusion by consensus. The antibody staining patterns were scored and calculated as the average of I × P, where I is the intensity of staining (0, 1, 2, or 3), and P is the percentage of positive tumor cells (0%–100%). The intensity of staining was scored as no staining (0), low intensity (1), moderate intensity (2), or high intensity (3). The sLex expression was categorized as negative when the score was below 2.5 or as high when the score was above 6017.
MUC1/EMA immunoreactivity was evaluated in a semiquantitative manner18. Staining intensity was classified as negative (0), low (1), moderate (2), or high (3). The percentage of positive cells was graded as follows: 0: no positive cells; 1: positive staining in less than 5% of cells; 2: positive staining in 5%–30% of cells; 3: positive staining in 30%–60% of cells; and 4: positive staining in more than 60% of cells. A semiquantitative scoring system was applied by multiplying the intensity and percentage of MUC1/EMA-positive cells. Total scores were grouped as follows: 0 (negative reaction); 1 to 6 (weak reaction); and 7 to 12 (strong reaction). A score of 0 to 6 was regarded as low expression, and a score greater than 6 was regarded as high expression.
Primary tumor cell culture
IMPC and IDC-NOS tissue samples were collected for primary cell culture. The samples were washed twice in normal saline and cut into small pieces (< 1 mm). Then 1.5 mL cell dispersing enzyme EZ solution was added, and samples were digested at 37 °C for 2 h on an oscillator. After the digestion was completed (i.e., many cells were observed to be separated from the tissue under a microscope), the cells were filtered with a 308 µM nylon net. The cell precipitates were re-suspended in 3 mL modified medium, then transferred to a 6 cm dish. The primary IMPC and IDC-NOS tumor cells were cultured in DMEM/F12 medium, which was supplemented with 5% horse serum, 10 µg/mL insulin, 20 ng/mL Maxime EGF, 0.5 µg/mL hydrocortisone, 100 ng/mL cholera toxin, 10 µM RhoA kinase inhibitor (Y - 27632), 4 mM L-glutamine, 1 mM pyruvate, 0.05% bovine pituitary extract, and 1% penicillin-streptomycin. Then the cells were cultured in an incubator at 37 °C, with 5% CO2.
3D culture and immunofluorescence staining of primary tumor cells
The primary IMPC and IDC-NOS cell suspensions were mixed with collagen A, B, and C (Nitta Gelatin, Osaka, Japan) at 8:1:1 on ice, and then 30 µL drops were deposited on a glass cover slide coated with fibronectin, which was placed on a 6-well plate (Corning, NY, USA). After culture in a 37 °C, 5% CO2 incubator for 30 min, the collagen droplets were solidified, and 2 mL medium was added for long-term culture. The IMPC and IDC-NOS cells were then fixed in collagen droplets with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100. Antibodies to sLex (BD Biosciences, #551344, monoclonal, 1:150 dilution, CA, USA) or EMA (ZSGB-bio, #ZM-0095, monoclonal, Beijing, China) were then incubated with the samples at 4 °C overnight. Secondary antibody was incubated at room temperature for 1 h. DAPI (Solarbio, Beijing, China) was used to stain the nuclei, and images were obtained through confocal microscopy.
Statistical analysis
Statistical analyses were performed in SPSS Statistics 23.0 (IBM Corporation, Chicago, IL, USA). A Spearman rank correlation test was performed to assess the relationships among MUC1/EMA and sLex expression and clinicopathological characteristics. The Kaplan-Meier method was used to construct survival curves (disease-free survival, DFS; overall survival, OS), which were compared with the log-rank test. The Cox proportional hazard model was used for univariate and multivariate analyses. A P-value < 0.05 was considered statistically significant.
Results
Clinicopathological characteristics
Compared with patients with IDC-NOS, patients with IMPC had a significantly higher frequency of lymph node metastasis (77.0% vs. 51.7%; P < 0.001) and lymphovascular invasion (LVI, 28.0% vs. 3.4%; P < 0.001), and a higher risk of local recurrence and metastasis (38.0% vs. 16.3%; P < 0.001). There was no significant difference in patient age, tumor size, distribution of histological grades, ER status, PR status, or HER-2 status between the IMPC and IDC-NOS groups (P > 0.05) (Table 1).
Table 1.
Clinicopathological characteristics of patients with IMPC and IDC-NOS
| Characteristics | IMPC n (%) | IDC-NOS n (%) | P |
|---|---|---|---|
| Age | 0.111 | ||
| ≤ 52 | 39 (39.0) | 45 (50.6) | |
| > 52 | 61 (61.0) | 44 (49.4) | |
| Tumor size | 0.182 | ||
| T1–T2 | 86 (86.0) | 82 (92.1) | |
| T3–T4 | 14 (14.0) | 7 (7.9) | |
| Histological grade | 0.897 | ||
| I | 13 (13.0) | 6 (6.7) | |
| II | 64 (64.0) | 69 (77.5) | |
| III | 23 (23.0) | 14 (15.7) | |
| ER | 0.418 | ||
| Negative | 38 (38.0) | 39 (43.8) | |
| Positive | 62 (62.0) | 50 (56.2) | |
| PR | 0.607 | ||
| Negative | 39 (39.0) | 38 (42.7) | |
| Positive | 61 (61.0) | 51 (57.3) | |
| *HER2 | 0.414 | ||
| Negative | 66 (66.0) | *53 (60.2) | |
| Positive | 34 (34.0) | *35 (39.8) | |
| Lymphovascular invasion | < 0.001* | ||
| Negative | 72 (72.0) | 86 (96.6) | |
| Positive | 28 (28.0) | 3 (3.4) | |
| pTNM | < 0.001* | ||
| I | 4 (4.0) | 14 (15.7) | |
| II | 57 (57.0) | 61 (68.6) | |
| III | 39 (39.0) | 14 (15.7) | |
| Lymph node metastasis | < 0.001* | ||
| Negative | 23 (23.0) | 43 (48.3) | |
| Positive | 77 (77.0) | 46 (51.7) | |
| sLex in cytomembrane | < 0.001* | ||
| Low | 60 (60.0) | 82 (92.1) | |
| High | 40 (40.0) | 7 (7.9) | |
| sLex in cytoplasm | < 0.001* | ||
| Low | 67 (67.0) | 83 (93.3) | |
| High | 33 (33.0) | 6 (6.7) | |
| EMA in cytomembrane | < 0.001* | ||
| Low | 62 (62.0) | 88 (98.8) | |
| High | 38 (38.0) | 1 (1.1) | |
| EMA in cytoplasm | < 0.001* | ||
| Low | 87 (87.0) | 89 (100.0) | |
| High | 13 (13.0) | 0 (0) | |
| Recurrence or metastasis | 0.002* | ||
| No | 62 (62.0) | 76 (85.4) | |
| Yes | 38 (38.0) | 13 (14.6) | |
IMPC, invasive micropapillary carcinoma; IDC-NOS, invasive ductal carcinoma, non-special type; sLex, sialylated Lewis glycoprotein X; EMA, epithelial membrane antigen. P < 0.05, calculated with chi-square test. *There is a IDC-NOS case lacking information about HER2.
IHC expression of sLex and MUC1/EMA in IMPC and IDC
The immunohistochemical results showed that MUC1/EMA and sLex were mainly expressed on the cytomembrane in IMPC (Figure 1G and 1C). The expression of MUC1/EMA and sLex in IDC-NOS was mainly within the cytoplasm (Figure 1H and 1F). Both cytomembrane and intracytoplasmic expression of sLex or MUC1/EMA was much more frequently detected in IMPC sections than in IDC-NOS sections (P < 0.001, P < 0.001 for cytomembrane expression and P < 0.001, P < 0.001 for intracytoplasmic expression) (Table 3). However, some IMPC cases showed either negative MUC1/EMA or sLex expression on the cytomembrane, including 10 cases displaying sLex+/EMA– expression and 8 cases displaying sLex–/EMA+ expression (Table 2). The high expression of sLex within the cytoplasm in patients with IMPC was associated with high histological grade and ER expression (P < 0.001, P = 0.041, P = 0.048), and patients with IMPC with high expression of sLex, either on the cytomembrane or in the cytoplasm, appeared to have a higher frequency of tumor recurrence and metastasis (P < 0.001), whereas patients with high expression of MUC1/EMA did not. In addition, patients with IMPC with high expression of MUC1/EMA on the cytomembrane were mostly older than 52 years and had pTNM stage I or II disease (P = 0.004; P = 0.029). Patients with HER2 positive expression tended to express more MUC1/EMA within the cytoplasm in IMPC tumor cells than those with negative HER2 expression (P = 0.025) (Table 3).
Table 3.
EMA and sLex expression in IMPC and their correlation with clinicopathologic parameters
| Characteristics | sLex in cytomembrane |
sLex in cytoplasm |
EMA in cytomembrane |
EMA in cytoplasm |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (%) | High (%) | P | Low (%) | High (%) | P | Low (%) | High (%) | P | Low (%) | High (%) | P | |
| Age | 0.318 | 0.073 | 0.004* | 0.209 | ||||||||
| ≤ 52 | 21 (35.0) | 18 (45.0) | 22 (32.8) | 17 (51.5) | 31 (50.0) | 8 (21.1) | 36 (41.4) | 3 (23.1) | ||||
| > 52 | 39 (65.0) | 22 (55.0) | 45 (67.2) | 16 (48.5) | 31 (50.0) | 30 (78.9) | 51 (58.6) | 10 (76.9) | ||||
| Tumor size | 0.815 | 0.147 | 0.436 | 0.878 | ||||||||
| T1–T2 | 52 (86.7) | 34 (85.0) | 60 (89.6) | 26 (78.8) | 52 (83.9) | 34 (89.5) | 75 (86.2) | 11 (84.6) | ||||
| T3–T4 | 8 (13.3) | 6 (15.0) | 7 (10.4) | 7 (21.2) | 10 (16.1) | 4 (10.5) | 12 (13.8) | 2 (15.4) | ||||
| Histological grade | 0.170 | 0.041* | 0.782 | 0.177 | ||||||||
| I | 11 (18.3) | 2 (5.0) | 12 (17.9.) | 1 (3.0) | 7 (11.3) | 6 (15.8) | 13 (15.0) | 0 (0.0) | ||||
| II | 36 (60.0) | 28 (70.0) | 42 (62.7) | 22 (66.7) | 41 (66.1) | 23 (60.5) | 55 (63.2) | 9 (69.2) | ||||
| III | 13 (21.7) | 10 (25.0) | 13 (19.4) | 10 (30.3) | 14 (22.6) | 9 (23.7) | 19 (21.8) | 4 (30.8) | ||||
| ER | 0.181 | 0.048* | 0.146 | 0.567 | ||||||||
| Negative | 26 (43.3) | 12 (30.0) | 30 (44.8) | 8 (24. 2) | 27 (43.5) | 11 (28.9) | 34 (39.1) | 4 (30. 8) | ||||
| Positive | 34 (56.7) | 28 (70.0) | 37 (55.2) | 25 (75.8) | 35 (56.5) | 27 (71.1) | 53 (60.9) | 9 (69.2) | ||||
| PR | 0.134 | 0.213 | 0.620 | 0.966 | ||||||||
| Negative | 27 (45.0) | 12 (30.0) | 29 (43.3) | 10 (30.3) | 23 (37.1) | 16 (42.1) | 34 (39.1) | 5 (38.5) | ||||
| Positive | 33 (55.0) | 28 (70.0) | 38 (56.7) | 23 (69.7) | 39 (62.9) | 22 (57.9) | 53 (60.9) | 8 (61.5) | ||||
| HER2 | 0.864 | 0.091 | 0.368 | 0.025* | ||||||||
| Negative | 40 (66.7) | 26 (65.0) | 48 (71.6) | 18 (54.5) | 43 (69.4) | 23 (60.5) | 61 (70.1) | 5 (38.5) | ||||
| Positive | 20 (33.3) | 14 (35.0) | 19 (28.4) | 15 (45.5) | 19 (30.6) | 15 (39.5) | 26 (29.9) | 8 (61.5) | ||||
| Lymph node metastasis | 0.997 | 0.134 | 0.680 | 0.637 | ||||||||
| N0 | 23 (39.0) | 14 (35.0) | 26 (39.4) | 11 (33.3) | 23 (37.7) | 14 (36.8) | 32 (37.2) | 5 (38.5) | ||||
| N1 | 17 (28.8) | 17 (42.5) | 24 (36.4) | 10 (30.3) | 22 (36.1) | 12 (31.6) | 30 (34.9) | 4 (30.8) | ||||
| N2 | 12 (20.3) | 2 (5.0) | 10 (15.2) | 4 (12.1) | 8 (13.1) | 6 (15.8) | 10 (11.6) | 4 (30.8) | ||||
| N3 | 7 (11.9) | 7 (17.5) | 6 (9.0) | 8 (24.3) | 8 (13.1) | 6 (15.8) | 14 (16.3) | 0 (0.0) | ||||
| Lymphovascular invasion | 0.148 | 0.910 | 0.869 | 0.370 | ||||||||
| Negative | 40 (66.7) | 32 (80.0) | 48 (71.6) | 24 (72.7) | 45 (72.6) | 27 (71.1) | 64 (73.6) | 8 (61.5) | ||||
| Positive | 20 (33.3) | 8 (20.0) | 19 (28.4) | 9 (27.3) | 17 (27.4) | 11 (28.9) | 23 (26.4) | 5 (38.5) | ||||
| pTNM stage | 0.434 | 0.328 | 0.029* | 0.794 | ||||||||
| I | 4 (6.9) | 0 (0.0) | 4 (6.2) | 0 (0.0) | 1 (1.6) | 3 (8.1) | 4 (4.7) | 0 (0.0) | ||||
| II | 32 (55.2) | 24 (60.0) | 37 (56.9) | 19 (57.6) | 32 (52.5) | 24 (64.9) | 48 (56.5) | 8 (61.5) | ||||
| III | 22 (37.9) | 16 (40.0) | 24 (36.9) | 14 (42.4) | 28 (45.9) | 10 (27.0) | 33 (38.8) | 5 (38.5) | ||||
| Recurrence or metastasis | < 0.001* | < 0.001* | 0.563 | 0.720 | ||||||||
| No | 43 (79.6) | 14 (36.8) | 47 (78.3) | 10 (31.2) | 34 (59.6) | 23 (65.7) | 49 (61.2) | 8 (66.7) | ||||
| Yes | 11 (20.4) | 24 (63.2) | 13 (21.7) | 22 (68.8) | 23 (40.4) | 12 (34.3) | 31 (38.8) | 4 (33.3) | ||||
*P < 0.05 was considered statistically significant. ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal factor receptor 2; IMPC, invasive micropapillary carcinoma; IDC-NOS, invasive ductal carcinoma, non-special type; sLex, sialylated Lewis glycoprotein X; EMA, epithelial membrane antigen. P < 0.05, calculated with chi-square test.
Table 2.
Crosstabulation of EMA and sLex expression in IMPC and their correlation
| Characteristics | EMA in cytomembrane |
EMA in cytoplasm |
||||
|---|---|---|---|---|---|---|
| Negative n (%) | Positive n (%) | P value | Negative n (%) | Positive n (%) | P value | |
| sLex in cytomembrane | 0.328 | |||||
| Negative | 0 (0.0) | 8 (8.9) | ||||
| Positive | 10 (100.0) | 82 (91.1) | ||||
| sLex in cytoplasm | 0.288 | |||||
| Negative | 6 (17.6) | 18 (27.3) | ||||
| Positive | 28 (82.4) | 48 (72.7) | ||||
IMPC, invasive micropapillary carcinoma; IDC-NOS, invasive ductal carcinoma, non-special type; sLex, sialylated Lewis glycoprotein X; EMA, epithelial membrane antigen. P < 0.05, calculated with chi-square test.
Expression of sLex and MUC1/EMA in IMPC and IDC primary cells
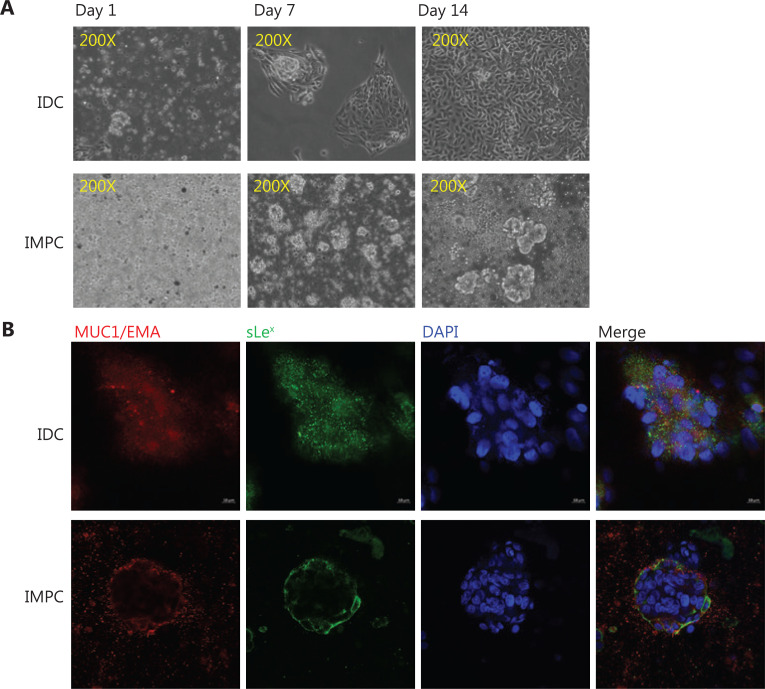
The primary IMPC cells were suspended, non-adherent tumor cell clusters, whereas the primary IDC cells were adherent tumor cells (Figure 2A). Immunofluorescence showed that MUC1/EMA and sLex were mainly expressed on the cytomembranes in IMPC cell clusters (Figure 2B), whereas the expression of MUC1/EMA and sLex in IDC-NOS cells was mainly within the cytoplasm (Figure 2B). Moreover, immunofluorescence indicated that MUC1/EMA and sLex were co-expressed on the cytomembranes in IMPC cell clusters (Figure 2B), in agreement with the results of IHC staining of MUC1/EMA and sLex.
Figure 2.
Immunofluorescence expression of sLex and MUC1/EMA in breast IMPC and IDC-NOS. (A) Cell morphology of primary cultured IMPC and IDC-NOS cells. Primary IMPC cells are suspended, non-adherent tumor cell clusters; primary IDC cells are adherent tumor cells (200×). (B) Immunofluorescence confirming the expression of MUC1/EMA and sLex on the cytomembranes in IMPC cell clusters and IDC-NOS cells (630×).
Prognostic value of sLex and MUC1/EMA expression in IMPC
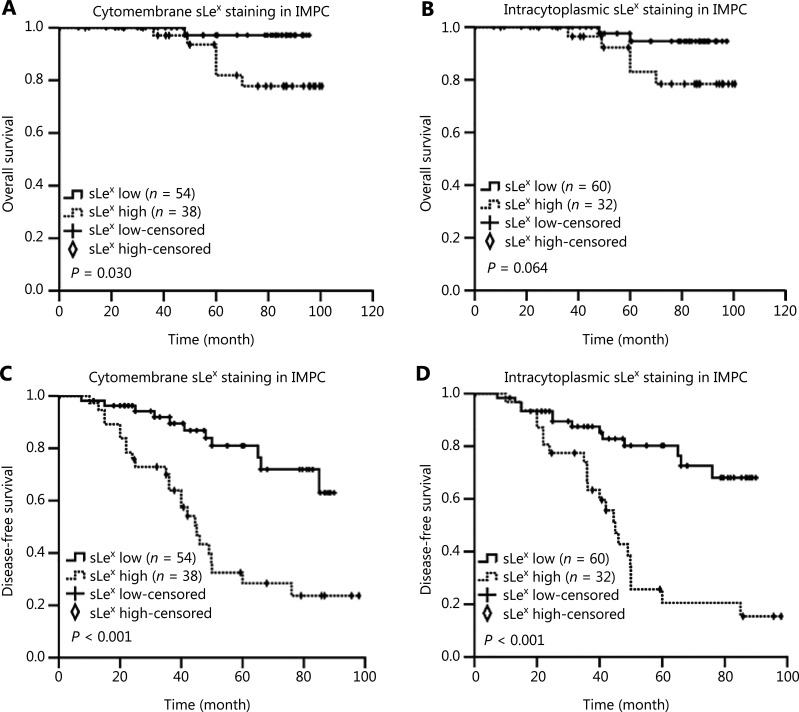
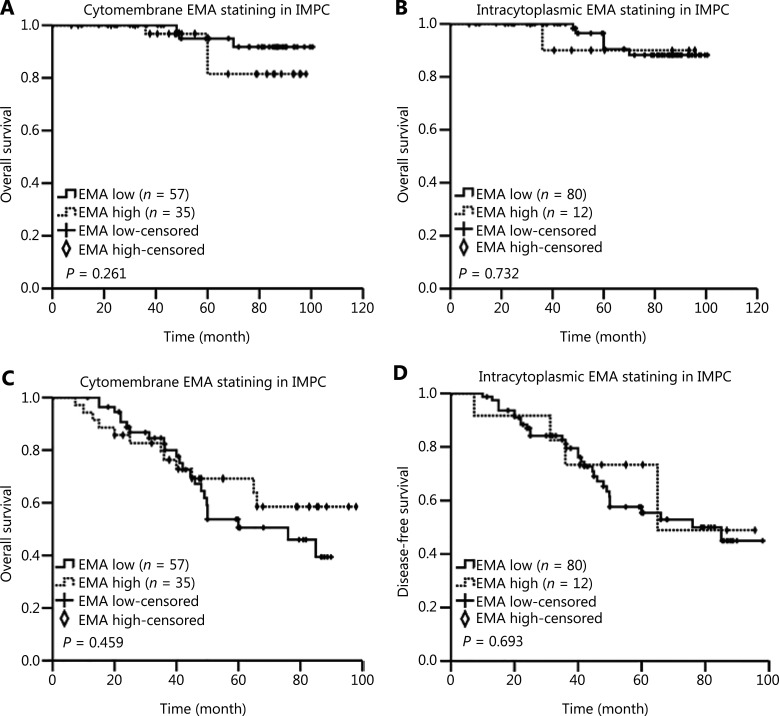
Kaplan-Meier survival curves revealed that the high expression of sLex on the cytomembranes in the IMPC tumor cell masses was associated with poor prognosis in terms of OS and DFS in patients with IMPC (P = 0.030 and P < 0.001, Figure 3A and 3C). High sLex expression within the cytoplasm in IMPC was indicative of relatively short DFS in patients with IMPC (P < 0.001, Figure 3D). Moreover, high expression of MUC1/EMA in all parts of the cell in the IMPC group had no significant association with OS and DFS (Figure 4A–4D). Univariate Cox proportional hazard model analysis confirmed that histological grade, lymph node metastasis, and high expression of sLex on the cytomembrane, but not in the cytoplasm, were prognostic factors for DFS in patients with IMPC (Table 4). Multivariate Cox regression analysis showed that lymph node metastasis and high expression of sLex on the cytomembrane were independent predictors of poor DFS in patients with IMPC (P = 0.014, HR = 1.486, 95%CI = 1.084–2.037; P = 0.025, HR = 3.099, 95%CI = 1.150–8.352) (Table 5).
Figure 3.
Kaplan-Meier curves showing the prognostic value of sLex expression in patients with IMPC. Kaplan-Meier curve for (A) OS and (C) DFS, based on the cytomembrane expression of sLex. (B) OS and (D) DFS, based on the intracytoplasmic expression of sLex. P-values were calculated with the log-rank test. Eight patients were lost to follow-up.
Figure 4.
Kaplan-Meier curves showing the prognostic value of EMA expression in patients with IMPC. Kaplan-Meier curve for (A) OS and (C) DFS, based on the cytomembrane expression of EMA. (B) OS and (D) DFS, based on the intracytoplasmic expression of EMA. P-values were calculated with the log-rank test. Eight patients were lost to follow-up.
Table 4.
Univariate analysis of patients with breast IMPC
| Factors | DFS |
OS |
||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Age (≤ 52 vs. > 52) | 0.717 | 0.361–1.422 | 0.341 | 0.708 | 0.158–3.166 | 0.652 |
| Tumor size | ||||||
| (T1–T2 vs. T3–T4) | 1.557 | 0.705–3.436 | 0.273 | 0.770 | 0.093–6.400 | 0.809 |
| Histological grade | ||||||
| (I vs. II vs. III) | 1.947 | 1.086–3.489 | 0.025* | 0.376 | 0.107–1.318 | 0.126 |
| ER (negative vs. positive) | 1.567 | 0.752–3.264 | 0.231 | 3.489 | 0.420–29.010 | 0.248 |
| PR (negative vs. positive) | 0.859 | 0.439–1.681 | 0.657 | 0.759 | 0.170–3.392 | 0.718 |
| HER2 (negative vs. positive) | 1.100 | 0.562–2.152 | 0.781 | 0.642 | 0.124–3.314 | 0.597 |
| sLex in cytomembrane | 4.137 | 2.019–8.478 | < 0.001* | 7.247 | 0.872–60.200 | 0.067 |
| sLex in cytoplasm | 4.207 | 2.101–8.422 | < 0.001* | 4.114 | 0.798–21.211 | 0.091 |
| EMA in cytomembrane | 0.769 | 0.382–1.548 | 0.462 | 2.278 | 0.512–10.252 | 0.278 |
| EMA in cytoplasm | 0.812 | 0.286–2.302 | 0.695 | 1.442 | 0.173–12.011 | 0.735 |
| Chemotherapy | 0.993 | 0.300–3.286 | 0.991 | 0.445 | 0.052–3.813 | 0.460 |
| Radiotherapy | 2.016 | 0.987–4.117 | 0.054 | 4.277 | 0.783–23.376 | 0.094 |
| Endocrine therapy | 0.658 | 0.295–1.466 | 0.306 | 0.836 | 0.153–4.579 | 0.837 |
| Lymph nodes | ||||||
| (N0 vs. N1 vs. N2 vs. N3) | 1.480 | 1.106–1.981 | 0.008* | 1.756 | 0.917–3.362 | 0.090 |
*P < 0.05 was considered statistically significant. DFS, disease-free survival; OS, overall survival; ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal factor receptor 2; IMPC, invasive micropapillary carcinoma; IDC-NOS, invasive ductal carcinoma, non-special type; sLex, sialylated Lewis glycoprotein X; EMA, epithelial membrane antigen. P < 0.05, calculated with the log-rank test.
Table 5.
Multivariate analysis of patients with breast IMPC
| Factors | DFS |
OS |
||||
|---|---|---|---|---|---|---|
| HR | 95%CI | P | HR | 95%CI | P | |
| Age (≤ 52 vs. > 52) | 0.762 | 0.370–1.569 | 0.461 | 0.798 | 0.163–3.910 | 0.781 |
| Tumor size | ||||||
| (T1–T2 vs. T3–T4) | 1.007 | 0.423–2.400 | 0.987 | 0.977 | 0.103–9.321 | 0.984 |
| Histological grade | ||||||
| (I vs. II vs. III) | 1.492 | 0.790–2.821 | 0.218 | 0.305 | 0.071–1.318 | 0.112 |
| sLex in cytomembrane | 3.099 | 1.150–8.352 | 0.025* | 5.740 | 0.467–70.562 | 0.172 |
| sLex in cytoplasm | 1.526 | 0.569–4.092 | 0.401 | 1.324 | 0.138–12.657 | 0.808 |
| Lymph nodes | ||||||
| (N0 vs. N1 vs. N2 vs. N3) | 1.486 | 1.084–2.037 | 0.014* | 1.470 | 0.718–3.011 | 0.292 |
*P < 0.05 was considered statistically significant. DFS, disease-free survival; OS, overall survival; IMPC, invasive micropapillary carcinoma; IDC-NOS, invasive ductal carcinoma, non-special type; sLex, sialylated Lewis glycoprotein X; EMA, epithelial membrane antigen. P < 0.05, calculated with the log-rank test.
Discussion
IMPC was listed in the 2003 WHO Histological Classification of Breast Tumors as a subtype of invasive carcinoma19. This tumor can be identified by the characteristic feature of an “inside-out” growth pattern, suggesting a reversal in cell polarity. IMPC cancer cells with reversed polarity have more aggressive biological behaviors, particularly a striking propensity for lymphatic invasion and nodal metastasis, regardless of the proportion of their IMPC component20–25. However, the underlying mechanism remains unclear. The metastasis of breast cancers is highly dependent on the interaction of adhesion molecules in cell-cell and/or cell-matrix contexts, and alterations in glycosylation patterns on the cancer cell surface can drive cancer metastasis though ligand-receptor-mediated interactions26.
Acs3 has proposed that the expression of MUC1/EMA on the periphery of IMPC tumor cell clusters may contribute to tumor progression and lymphatic metastasis. However, our findings suggest that the high expression of MUC1/EMA on the cell membrane and within the cytoplasm is not associated with prognosis in patients with IMPC or IDC-NOS. This finding may be because MUC1 is mainly involved in the transcriptional activation of several oncogenes, despite MUC1/EMA’s high molecular weight and multiple functions driving breast cancer progression27–29. It cannot be used as a dedicated ligand by vascular and lymphatic endothelial cells to promote the metastasis of cancer cells.
sLex, also known as CD15s, is a sialylated tetrasaccharide structure displayed at the cell surface, on both glycoproteins and glycolipids. sLex is present on the surfaces of leukocytes, and it plays an important role in cell-cell interaction. sLex has been proposed to participate in regulating the adhesion between cancer cells and endothelial cells. As the most important glycan ligand for E-selectin (which is expressed on the surfaces of endothelial cells)10,30–32, sLex is present on the surfaces of many types of cancer cells, including breast IMPC cells7,12–16, and it plays an important role in the extravasation of cancer cells from the blood or lymph vessels, thereby promoting the migration of cancer cells to distant organs. Miyara has demonstrated that CD15s is highly specific for activated, terminally differentiated, and most suppressive FOXP3high effector Treg cells, thus suggesting that cancer cells can mimic the behavior of immune cells to escape immunological surveillance and establish new metastatic foci33. Therefore, overexpression of sLex has frequently been reported to correspond with poorer outcomes and malignancy recurrence10,11,34. In addition, glycosyltransferases, which produce sLex exclusively by fucosylation of sialylated LacNAc in humans, have been reported to increase EMT and migration ability in breast and hepatic cancers35–37.
In this study, we indeed found that both cytomembrane and intracytoplasmic expression of sLex were much more frequently detected in IMPC than in IDC-NOS, and the high expression of sLex on the cell membrane and within the cytoplasm was identified as a factor indicating poor prognosis and DFS, but not OS, in patients with IMPC; however, patients with IMPC with high expression of sLex showed a relatively shorter OS time. We attributed the lack of significance in our results to the small sample size studied. Moreover, univariate and multivariate Cox regression analyses showed that high expression of sLex on the cytomembrane was an independent predictor of poor DFS in patients with IMPC, but the expression of sLex in patients with IDC-NOS was not associated with DFS or OS; this finding may be explained by the observation that cells overexpressing sLex in IDC-NOS did not exhibit polarity reversal.
MUC1/EMA has long been used as an auxiliary marker for the clinical diagnosis of IMPC38–41. We found that some IMPC cases showed negative MUC1/EMA or sLex expression on the cytomembrane, including 10 cases with sLex+/EMA– expression and 8 cases with sLex–/EMA+ expression, thus indicating that a combined pathological diagnosis of IMPC with MUC1/EMA and sLex assessment is needed to avoid missed diagnosis. In contrast to sLex, the expression of MUC1/EMA on the cytomembrane or in the cytoplasm, was not associated with patient prognosis in IMPC. We speculate that MUC1/EMA may not be the only glycoprotein on the cytomembranes in IMPC clusters that can be modified by sLex. MUC1/EMA is the major carrier of sLex in serous borderline ovarian cancer, adenocarcinoma, and micropapillary bladder urothelial carcinoma, whereas sLex is an epitope of MUC1/EMA15,16. Our IHC results suggest that the role of sLex in promoting IMPC cluster transfer to distant metastatic sites is not completely dependent on MUC1/EMA—an aspect that might mainly be due to the interaction of adhesion molecules on endothelial cells. sLex, which is overexpressed on the cytomembranes in IMPC cell clusters, interacts with E-selectin (which is highly expressed on the surfaces of lymphatic endothelial cells); this mechanism may be partially responsible for the lymphatic invasion and lymph node metastasis of IMPC cell clusters. In addition, the sugar chain of sLex contains large amounts of sialic acid, which makes cell surfaces negatively charged. The fucose portion of sLex specifically binds the C-type lectin-like region of E-selectin, both of which are negatively charged and form strong ionic bonds mediated by calcium ions, thus helping cancer cells pass through the basement membranes of vascular or lymphatic endothelial cells, and promoting tumor cell invasion and metastasis42–45. However, in the immunological microenvironment of IMPC, sLex may evade the cytotoxicity of immune cells mainly through its interaction with NK cells. The sialic acid component of sLex can directly mask tumor antigens, thus decreasing the sensitivity of tumors to NK cells46–48. These reasons may explain why sLex promotes tumor cell metastasis.
Our results showed that the overexpression of sLex on the cytomembranes in cell clusters was an independent negative prognostic factor in patients with IMPC but not those with IDC-NOS. This result differed from Sozzani’s findings that sLex expression is not a prognostic factor in patients with breast cancer49. This may be the reason that sLex is closely associated with the polarity reversal pattern in patients with IMPC, but Sozzani found that sLex expression was not a prognostic factor in patients with breast cancer49, we thought the explanation was that IMPC (a special type of breast cancer) had a special structure (polarity reversal pattern) which IDC-NOS (ordinary breast cancer) had not, and sLex was closely associated with the polarity reversal pattern in patients with IMPC.
In contrast to the use of MUC1/EMA for tissue diagnosis, the use of sLex, which has a lower molecular weight and is of prognostic value, can enable more convenient serum-based diagnosis and may serve as an index for IMPC glycopeptide monitoring, thus potentially improving the sensitivity and specificity of diagnostic targets and providing strong support for glycopeptide detection. In addition, the metastasis of IMPC clusters may be prevented by blocking the interaction between E-selectin and sLex or by decreasing the expression of E-selectin, thus providing a potential target for targeted IMPC therapy. Therefore, sLex may be an important supplementary diagnostic marker of IMPC, and the overexpression of sLex on the cytomembranes in cell clusters may serve as an independent factor indicating poor prognosis in patients with IMPC. Therapies targeting sLex or the interaction of sLex and E-selectin may provide benefits for patients with IMPC in the future.
Because we were unable to find a suitable cell model to represent IMPC cells to perform a series of experiments, we attempted to establish a research model of IMPC through primary culture. Unfortunately, the primary tumor cells, particularly IMPC cell clusters, were difficult to culture, because the primary tumor cell clusters scarcely proliferated. Constructing a suitable cell model to study the mechanisms of IMPC clusters will be the primary task in our future research.
Footnotes
Conflict of interest statement No potential conflicts of interest are disclosed.
Grant support
This work was supported by funding from the National Natural Science Foundation of China (Grant Nos. 81672637 and 81872164).
References
- 1.Li W, Han Y, Wang C, Guo X, Shen B, Liu F, et al. Precise pathologic diagnosis and individualized treatment improve the outcomes of invasive micropapillary carcinoma of the breast: a 12-year prospective clinical study. Mod Pathol. 2018;31:956–64. doi: 10.1038/s41379-018-0024-8. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y, Liu B, Zhang X, Fu L. Invasive micropapillary carcinoma of the breast: an update. Arch Pathol Lab Med. 2016;140:799–805. doi: 10.5858/arpa.2016-0040-RA. [DOI] [PubMed] [Google Scholar]
- 3.Acs G, Esposito NN, Rakosy Z, Laronga C, Zhang PJ. Invasive ductal carcinomas of the breast showing partial reversed cell polarity are associated with lymphatic tumor spread and may represent part of a spectrum of invasive micropapillary carcinoma. Am J Surg Pathol. 2010;34:1637–46. doi: 10.1097/PAS.0b013e3181f5539c. [DOI] [PubMed] [Google Scholar]
- 4.Nassar H, Wallis T, Andea A, Dey J, Adsay V, Visscher D. Clinicopathologic analysis of invasive micropapillary differentiation in breast carcinoma. Mod Pathol. 2001;14:836–41. doi: 10.1038/modpathol.3880399. [DOI] [PubMed] [Google Scholar]
- 5.Nassar H, Pansare V, Zhang H, Che M, Sakr W, Ali-Fehmi R, et al. Pathogenesis of invasive micropapillary carcinoma: role of MUC1 glycoprotein. Mod Pathol. 2004;17:1045–50. doi: 10.1038/modpathol.3800166. [DOI] [PubMed] [Google Scholar]
- 6.Nassar H. Carcinomas with micropapillary morphology: clinical significance and current concepts. Adv Anat Pathol. 2004;11:297–303. doi: 10.1097/01.pap.0000138142.26882.fe. [DOI] [PubMed] [Google Scholar]
- 7.Wei J, Cui L, Liu F, Fan Y, Lang R, Gu F, et al. E-selectin and Sialyl Lewis X expression is associated with lymph node metastasis of invasive micropapillary carcinoma of the breast. Int J Surg Pathol. 2010;18:193–200. doi: 10.1177/1066896908320832. [DOI] [PubMed] [Google Scholar]
- 8.Apostolopoulos V, Stojanovska L, Gargosky SE. MUC1 (CD227): a multi-tasked molecule. Cell Mol Life Sci. 2015;72:4475–500. doi: 10.1007/s00018-015-2014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creaney J, Segal A, Sterrett G, Platten MA, Baker E, Murch AR, et al. Overexpression and altered glycosylation of MUC1 in malignant mesothelioma. Br J Cancer. 2008;98:1562–9. doi: 10.1038/sj.bjc.6604340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell. 2000;103:467–79. doi: 10.1016/s0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- 11.Jeschke U, Mylonas I, Shabani N, Kunert-Keil C, Schindlbeck C, Gerber B, et al. Expression of sialyl lewis X, sialyl Lewis A, E-cadherin and cathepsin-D in human breast cancer: immunohistochemical analysis in mammary carcinoma in situ, invasive carcinomas and their lymph node metastasis. Anticancer Res. 2005;25:1615–22. [PubMed] [Google Scholar]
- 12.Nakagoe T, Fukushima K, Itoyanagi N, Ikuta Y, Oka T, Nagayasu T, et al. Expression of ABH/Lewis-related antigens as prognostic factors in patients with breast cancer. J Cancer Res Clin Oncol. 2002;128:257–64. doi: 10.1007/s00432-002-0334-5. [DOI] [PubMed] [Google Scholar]
- 13.Narita T, Funahashi H, Satoh Y, Watanabe T, Sakamoto J, Takagi H. Association of expression of blood group-related carbohydrate antigens with prognosis in breast cancer. Cancer. 1993;71:3044–53. doi: 10.1002/1097-0142(19930515)71:10<3044::aid-cncr2820711026>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 14.Nakagoe T, Fukushima K, Tuji T, Sawai T, Nanashima A, Yamaguchi H, et al. Immunohistochemical expression of ABH/Lewis-related antigens in primary breast carcinomas and metastatic lymph node lesions. Cancer Detect Prev. 1998;22:499–505. doi: 10.1046/j.1525-1500.1998.00045.x. [DOI] [PubMed] [Google Scholar]
- 15.Ricardo S, Marcos-Silva L, Valente C, Coelho R, Gomes R, David L. Mucins MUC16 and MUC1 are major carriers of SLe(a) and SLe(x) in borderline and malignant serous ovarian tumors. Virchows Arch. 2016;468:715–22. doi: 10.1007/s00428-016-1929-6. [DOI] [PubMed] [Google Scholar]
- 16.Shinagawa T, Hoshino H, Taga M, Sakai Y, Imamura Y, Yokoyama O, et al. Clinicopathological implications to micropapillary bladder urothelial carcinoma of the presence of sialyl Lewis X-decorated mucin 1 in stroma-facing membranes. Urol Oncol. 2017;35:606.e17–e23. doi: 10.1016/j.urolonc.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Julien S, Ivetic A, Grigoriadis A, QiZe D, Burford B, Sproviero D, et al. Selectin ligand sialyl-Lewis x antigen drives metastasis of hormone-dependent breast cancers. Cancer Res. 2011;71:7683–93. doi: 10.1158/0008-5472.CAN-11-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zwenger A, Rabassa M, Demichelis S, Grossman G, Segal-Eiras A, Croce MV. High expression of sLex associated with poor survival in Argentinian colorectal cancer patients. Int J Biol Markers. 2014;29:e30–9. doi: 10.5301/jbm.5000060. [DOI] [PubMed] [Google Scholar]
- 19.Tavassoli FA, Devilee P. Pathol Genet. Lyon: IARC; 2003. Tumours of the breast and female genital organs. WHO classification of tumours.p. 10 [Google Scholar]
- 20.Yun SU, Choi BB, Shu KS, Kim SM, Seo YD, Lee JS, et al. Imaging findings of invasive micropapillary carcinoma of the breast. J Breast Cancer. 2012;15:57–64. doi: 10.4048/jbc.2012.15.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo X, Chen L, Lang R, Fan Y, Fu L. Relationship between lymph node metastasis and pathologic features of invasive micropapillary carcinoma of breast. Zhonghua Bing Li Xue Za Zhi. 2006;35:8–12. [PubMed] [Google Scholar]
- 22.Guo X, Chen L, Lang R, Fan Y, Zhang X, Fu L. Invasive micropapillary carcinoma of the breast: association of pathologic features with lymph node metastasis. Am J Clin Pathol. 2006;126:740–6. doi: 10.1309/AXYY-4AJT-MNW6-FRMW. [DOI] [PubMed] [Google Scholar]
- 23.Chen L, Fan Y, Lang R, Guo X, Sun Y, Fu L. Diagnosis and prognosis study of breast carcinoma with micropapillary component. Zhonghua Bing Li Xue Za Zhi. 2007;36:228–32. [PubMed] [Google Scholar]
- 24.Chen L, Fan Y, Lang R, Guo X, Sun Y, Cui L, et al. Breast carcinoma with micropapillary features: clinicopathologic study and long-term follow-up of 100 cases. Int J Surg Pathol. 2008;16:155–63. doi: 10.1177/1066896907307047. [DOI] [PubMed] [Google Scholar]
- 25.Chen HL, Ding A. Comparison of invasive micropapillary and triple negative invasive ductal carcinoma of the breast. Breast. 2015;24:723–31. doi: 10.1016/j.breast.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Magdolen V, Krüger A, Sato S, Nagel J, Sperl S, Reuning U, et al. Inhibition of the tumor-associated urokinase-type plasminogen activation system: effects of high-level synthesis of soluble urokinase receptor in ovarian and breast cancer cells in vitro and in vivo. Recent Results Cancer Res. 2003;162:43–63. doi: 10.1007/978-3-642-59349-9_4. [DOI] [PubMed] [Google Scholar]
- 27.Brayman M, Thathiah A, Carson DD. MUC1: a multifunctional cell surface component of reproductive tissue epithelia. Reprod Biol Endocrinol. 2004;2:4. doi: 10.1186/1477-7827-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez-Vilar J, Hill RL. The structure and assembly of secreted mucins. J Biol Chem. 1999;274:31751–4. doi: 10.1074/jbc.274.45.31751. [DOI] [PubMed] [Google Scholar]
- 29.Pisarev VM, Kinarsky L, Caffrey T, Hanisch FG, Sanderson S, Hollingsworth MA, et al. T cells recognize PD(N/T)R motif common in a variable number of tandem repeat and degenerate repeat sequences of MUC1. Int Immunopharmacol. 2005;5:315–30. doi: 10.1016/j.intimp.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 30.St Hill CA. Interactions between endothelial selectins and cancer cells regulate metastasis. Front Biosci (Landmark Ed) 2011;16:3233–51. doi: 10.2741/3909. [DOI] [PubMed] [Google Scholar]
- 31.Jin F, Wang F. The physiological and pathological roles and applications of sialyl Lewis x, a common carbohydrate ligand of the three selectins. Glycoconj J. 2020;37:277–91. doi: 10.1007/s10719-020-09912-4. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira IG, Carrascal M, Mineiro AG, Bugalho A, Borralho P, Silva Z, et al. Carcinoembryonic antigen is a sialyl Lewis x/a carrier and an E‑selectin ligand in non‑small cell lung cancer. Int J Oncol. 2019;55:1033–48. doi: 10.3892/ijo.2019.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyara M, Chader D, Sage E, Sugiyama D, Nishikawa H, Bouvry D, et al. Sialyl Lewis x (CD15s) identifies highly differentiated and most suppressive FOXP3high regulatory T cells in humans. Proc Natl Acad Sci USA. 2015;112:7225–30. doi: 10.1073/pnas.1508224112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshida S, Yoshida K, Jaroensong T, Lee SJ, Kamida A, Saeki K, et al. Aberrant expression of sLex and sLea as candidate prognostic factors for feline mammary gland tumour. J Feline Med Surg. 2014;16:257–64. doi: 10.1177/1098612X13503826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhat R, Belardi B, Mori H, Kuo P, Tam A, Hines WC, et al. Nuclear repartitioning of galectin-1 by an extracellular glycan switch regulates mammary morphogenesis. Proc Natl Acad Sci USA. 2016;113:E4820–7. doi: 10.1073/pnas.1609135113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choo M, Tan HL, Ding V, Castangia R, Belgacem O, Liau B, et al. Characterization of H type 1 and type 1 N-acetyllactosamine glycan epitopes on ovarian cancer specifically recognized by the anti-glycan monoclonal antibody mAb-A4. J Biol Chem. 2017;292:6163–76. doi: 10.1074/jbc.M116.768887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandrasekaran EV, Xue J, Neelamegham S, Matta KL. The pattern of glycosyl- and sulfotransferase activities in cancer cell lines: a predictor of individual cancer-associated distinct carbohydrate structures for the structural identification of signature glycans. Carbohydr Res. 2006;341:983–94. doi: 10.1016/j.carres.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Adsay NV, Merati K, Basturk O, Iacobuzio-Donahue C, Levi E, Cheng JD, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839–48. doi: 10.1097/00000478-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Ueda M, Miura Y, Kunihiro O, Ishikawa T, Ichikawa Y, Endo I, et al. MUC1 overexpression is the most reliable marker of invasive carcinoma in intraductal papillary-mucinous tumor (IPMT) Hepatogastroenterology. 2005;52:398–403. [PubMed] [Google Scholar]
- 40.Guo Z, Yang Z, Li D, Tang J, Xu J, Shen H, et al. Colorectal cancer with invasive micropapillary components (IMPCs) shows high lymph node metastasis and a poor prognosis: a retrospective clinical study. Medicine (Baltimore) 2020;99:e20238. doi: 10.1097/MD.0000000000020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Y, Kaimakliotis HZ, Williamson SR, Koch MO, Huang K, Barboza MP, et al. Micropapillary urothelial carcinoma of urinary bladder displays immunophenotypic features of luminal and p53-like subtypes and is not a variant of adenocarcinoma. Urol Oncol. 2020;38:449–58. doi: 10.1016/j.urolonc.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Barra PA, Ribeiro AJ, Ramos MJ, Jimenez VA, Alderete JB, Fernandes PA. Binding free energy calculations on E-selectin complexes with sLe(x) oligosaccharide analogs. Chem Biol Drug Des. 2017;89:114–23. doi: 10.1111/cbdd.12837. [DOI] [PubMed] [Google Scholar]
- 43.Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci. 2004;95:377–84. doi: 10.1111/j.1349-7006.2004.tb03219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Häuselmann I, Roblek M, Protsyuk D, Huck V, Knopfova L, Grässle S, et al. Monocyte induction of E-selectin-mediated endothelial activation releases VE-cadherin junctions to promote tumor cell extravasation in the metastasis cascade. Cancer Res. 2016;76:5302–12. doi: 10.1158/0008-5472.CAN-16-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill CA, Baharo-Hassan D, Farooqui M. C2-O-sLeX glycoproteins are E-selectin ligands that regulate invasion of human colon and hepatic carcinoma cells. PLoS One. 2011;6:e16281. doi: 10.1371/journal.pone.0016281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumoto N, Ribaudo RK, Abastado JP, Margulies DH, Yokoyama WM. The lectin-like NK cell receptor Ly-49A recognizes a carbohydrate-independent epitope on its MHC class I ligand. Immunity. 1998;8:245–54. doi: 10.1016/s1074-7613(00)80476-8. [DOI] [PubMed] [Google Scholar]
- 47.Higai K, Ichikawa A, Matsumoto K. Binding of sialyl Lewis X antigen to lectin-like receptors on NK cells induces cytotoxicity and tyrosine phosphorylation of a 17-kDa protein. Biochim Biophys Acta. 2006;1760:1355–63. doi: 10.1016/j.bbagen.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 48.Ohyama C, Kanto S, Kato K, Nakano O, Arai Y, Kato T, et al. Natural killer cells attack tumor cells expressing high levels of sialyl Lewis x oligosaccharides. Proc Natl Acad Sci USA. 2002;99:13789–94. doi: 10.1073/pnas.212456599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sozzani P, Arisio R, Porpiglia M, Benedetto C. Is Sialyl Lewis x antigen expression a prognostic factor in patients with breast cancer? Int J Surg Pathol. 2008;16:365–74. doi: 10.1177/1066896908324668. [DOI] [PubMed] [Google Scholar]