Abstract
Asthma is one of the most common underlying diseases in women of reproductive age that can lead to potentially serious medical problems during pregnancy and lactation. A group of key stakeholders across multiple relevant disciplines was invited to take part in an effort to prioritize, strategize, and mobilize action steps to fill important gaps in knowledge regarding asthma medication safety in pregnancy and lactation. The stakeholders identified substantial gaps in the literature on the safety of asthma medications used during pregnancy and lactation and prioritized strategies to fill those gaps. Short-term action steps included linking data from existing complementary study designs (US and international claims data, single drug pregnancy registries, case-control studies, and coordinated systematic data systems). Long-term action steps included creating an asthma disease registry, incorporating the disease registry into electronic health record systems, and coordinating care across disciplines. The stakeholders also prioritized establishing new infrastructures/collaborations to perform research in pregnant and lactating women and to include patient perspectives throughout the process. To address the evidence gaps, and aid in populating product labels with data that inform clinical decision making, the consortium developed a plan to systematically obtain necessary data in the most efficient and timely manner.
Keywords: Asthma medication, lactation, pregnancy
Asthma affects 3% to 10% of women of reproductive age in the United States and is one of the most common underlying health conditions that can complicate pregnancy and lactation.1 In addition, there are substantial inequities in the burden of asthma in pregnancy by race and ethnic group.2 Asthma is associated with increased risk of maternal morbidity and perinatal complications including spontaneous abortion, gestational diabetes, hypertensive disorders of pregnancy, preterm delivery, fetal growth restriction, antepartum and postpartum bleeding, and congenital anomalies.3–5 The mechanisms underlying these are likely multifactorial, but an important element appears to be asthma control. Unfortunately, asthma medication nonadherence is common in pregnancy and has implications for disease activity.6–10 Many women report that concerns about medication safety are reasons for discontinuing appropriate therapies.11 The maternal need for medication is also frequently cited as a reason for early termination of breast-feeding, based on lack of data confirming safety for the infant.12
In 2015, the US Food and Drug Administration (FDA) introduced the Pregnancy and Lactation Labeling Rule, a new system that removed the pregnancy letter ratings (A, B, C, D, X) from all prescribing information for drugs approved after June 30, 2001, replacing the letters with a narrative summary of animal and human gestational safety data and clinical considerations. The intent is to provide the prescriber and patient with important safety and risk information about the use of a prescription product during pregnancy and lactation. More than 1500 drug labelings have been converted to the Pregnancy and Lactation Labeling Rule format since 2015. However, there has been a growing awareness that many prescription products lack good quality clinical pregnancy and lactation safety information, including most asthma medications. The revised prescribing information highlights a critical need for high-quality human safety data to inform the use of asthma medications during pregnancy and lactation.
WORKSHOP STRUCTURE AND OBJECTIVES
In November 2019, the National Heart, Lung, and Blood Institute and the Office of Research on Women’s Health in the Office of the Director, of the National Institutes of Health, and the US Food and Drug Administration Office of Women’s Health hosted a workshop titled “The Safety of Asthma Medications during Pregnancy and Lactation: Research Priorities and Methodology.” A group of key stakeholders across multiple relevant disciplines was invited to take part in an effort to prioritize, strategize, and mobilize action steps on gaps in knowledge regarding asthma medication safety in pregnancy and lactation. Stakeholder representatives included academic researchers, obstetric/maternal-fetal medicine specialists, regulatory and other federal agencies, the pharmaceutical industry, clinicians, patient advocacy groups, and patients. The conference was developed in response to recommendations of the Department of Health and Human Service’s Task Force on Research Specific to Pregnant Women and Lactating Women pursuant to the 21st Century Cures Act. The workshop proceedings are summarized in this article.
WORKSHOP PROCEEDINGS
Substantial gaps in the literature on the safety of asthma medications used during pregnancy and lactation were identified and strategies prioritized to fill those gaps. Recommended short-term actions include linking data from existing complementary study designs (US and international claims data, single drug pregnancy registries, case-control studies, and coordinated systematic data systems). Proposed long-term actions include creating an asthma disease registry, incorporating the disease registry into electronic health record systems, and coordinating care across disciplines. The stakeholders also prioritized establishing new infrastructures and collaborations to increase research in pregnant and lactating women and to include patient perspectives throughout the process.
Existing safety data and gaps
Recommended pharmacologic management of asthma during pregnancy follows a stepwise approach (Table I), based on the determination of asthma control. Medication is typically stepped up for uncontrolled asthma, after issues such as avoidance of environmental triggers, inhaler technique, and medication adherence are optimized. The available safety data for asthma medications in pregnancy are generally reassuring for several older and commonly used asthma medications, such as inhaled corticosteroids and short-acting beta agonists (Table II).
TABLE I.
Steps in asthma therapy during pregnancy
| Step | Preferred controller medication | Alternative controller medication |
|---|---|---|
| 1 | None | — |
| 2 | Low-dose ICS | LTRA, theophylline, or cromolyn |
| 3 | Medium-dose ICS or low-dose ICS plus LABA | Low-dose ICS plus LTRA or theophylline |
| 4 | Medium-dose ICS plus LABA | Medium-dose ICS plus either LTRA or theophylline |
| 5 | High-dose ICS plus LABA | Medium-dose ICS plus LABA plus tiotropium; consider adding omalizumab for patients with allergy or adding other asthma biologics (anti–IL-5, anti–IL-5Rα, anti–IL-4Rα) for appropriate candidates |
| 6 | High-dose ICS plus LABA plus oral prednisone | Consider adding omalizumab for patients with allergy or adding other asthma biologics (anti–IL-5, anti–IL-5Rα, anti–IL-4Rα) for appropriate candidates |
ICS, Inhaled corticosteroid; LABA, long-acting β-agonist; LTRA, leukotriene receptor antagonist.
There are no randomized clinical trials of asthma biologics that intentionally included pregnant women.
Data modified from Schatz and Dombrowski.13
TABLE II.
Human pregnancy summary safety data for selected asthma medications
| Medication | Major birth defects | Other birth outcomes | Evidence gaps and recommendations | Lactation |
|---|---|---|---|---|
| Short-acting beta agonists (any, primarily albuterol) | No increase in major birth defects over expected among 1090 albuterol-exposed pregnancies in a claims database.14 No increase in major birth defects in 1753 albuterol-exposed pregnancies compared with other asthmatic pregnancies.15 | No increase in preterm delivery, low birth weight, or small-for- gestational-age infants in 1828 pregnancies exposed to short-acting beta agonists compared with other asthmatic pregnancies15 | First patented in 1972, albuterol is one of the most commonly used asthma medications. Despite this, there is still a lack of evidence regarding its safety when used during pregnancy. There have been reports of associations with specific congenital defects. These observations may be a result of uncontrolled confounding by indication | No published data. Poor bioavailability and low serum levels expected to produce low levels in milk |
| Modest increased risk in isolated cleft lip or cleft palate (odds ratios from 1.65 to 1.79) in albuterol-exposed pregnancies in case-control study of 2711 cases of oral clefts and 6482 controls.16 | ||||
| Several additional studies have suggested modest increased risks (odds ratios, <3) for specific birth defects such as any cardiac or gastroschisis, esophageal atresia, or omphalocele17–19 | ||||
| Any inhaled corticosteroid (ICS) including beclomethasone, budesonide, flunisolide, fluticasone, triamcinolone | No increased risk for major birth defects in 396 exposed compared with the general population.20 A meta-analysis of studies of inhaled steroids did not find increased risk of major birth defects overall21 |
No increased risks for preterm delivery, low birth weight, or pregnancy-induced hypertension in 396 exposed or in metaanalysis.22,23 Higher doses of ICSs may be associated with an increased risk of low birth weight, preterm delivery, and small-for- gestational-age infants21 | Budesonide and fluticasone may be preferred if starting ICS during pregnancy. Other ICSs may be continued in patients who were well controlled by these agents before pregnancy, especially if it is thought that changing formulations may jeopardize asthma control. Adverse outcomes associated with higher doses of ICSs need further study because confounding by severity may explain this association | |
| Budesonide | No increased risk for major birth defects overall or oral clefts among 2014 exposed in population-based Scandinavian register24 | No increased risks for preterm birth, reduced birth weight or length, or stillbirths in 2968 exposed in population-based Scandinavian register25 | Small amounts excreted in 8 women with asthma using inhaled budesonide26 | |
| Fluticasone | No increased risk of major congenital malformations overall in a cohort study of 1602 mother-infant pairs exposed to fluticasone compared with 3678 exposed to other ICSs, stratified by severity27 | No increased risk of low birth weight, preterm birth, or small-for- gestational-age infants in retrospective database study of infants of 3190 mothers exposed to fluticasone compared with 608 mothers exposed to budesonide28 | No published data. Poor bioavailability and low serum levels expected to produce low levels in milk | |
| Long-acting beta agonists (LABAs) | No evidence of increased risk in major birth defects in 65 salmeterol-exposed pregnancies.29 In an analysis of a database, increased risks for major cardiac and major l to produce low levels in milk pairs expo- trimester exposure in 165 pregnancies.30 However, in a later study from the same database, 841 pregnancies exposed to LABAs with low- or medium-dose ICSs showed no increased risk of major birth defects overall compared with pregnancies exposed to medium- to high-dose ICSs alone31 | No difference in low birth weight, preterm birth, or small-for-gestational-age infants was noted in infants of mothers exposed to salmeterol vs formoterol in a retrospective database study28 |
Limited observational data are available regarding the safety of LABA use during pregnancy. The benefits of the use of LABA appear to outweigh the risks as long as they are used concurrently with ICSs | Salmeterol: No published data. Poor bioavailability and low serum levels expected to produce low levels in milk |
| Montelukast/leukotriene receptor antagonist (LTRA) | No increased risk of major birth defects overall in 74 and 180 exposed pregnancies.32,33 No increased risk in major birth defects overall or specific birth defects in 1164 exposed pregnancies in claims study.34 No increased risk in major birth defects in 1827 exposed pregnancies in Danish register study35 |
No increased risk for reduced birth weight or shortened gestational age in 180 exposed when compared with other asthmatic patients.32 No increased risk for preterm delivery, low birth weight, or preeclampsia in 1827 exposed compared with other treated asthmatic 35 patients |
Data on the use of LTRA during human pregnancy are limited | Very low levels in breast milk of 7 women given 10 mg dose—0.68% of weight-adjusted maternal dose36 |
| Systemic corticosteroids | Meta-analysis of cohort studies showed no overall increased risk of major birth defects in pooled 535 exposed pregnancies; meta-analysis of 4 case-control studies showed an increased risk of ~3-fold for oral clefts.37 However, most recent and largest case-control study from US National Birth Defects Prevention Study showed no increased risk for oral clefts with first-trimester systemic steroid use for any indication in 2372 cases and 5922 controls38 | Preterm delivery, low birth weight or reduced birth weight, preeclampsia, and gestational diabetes have all been reported to occur more frequently in women treated with systemic steroids in pregnancy; however, studies that attempted to control for underlying maternal disease and disease activity typically find the associated risks for these outcomes reduced or eliminated39 | Adverse outcomes seen may be a result of severe asthma or the medication itself. These outcomes would be outweighed by the potential risks of a severe asthma exacerbation, which include maternal or fetal mortality. Oral corticosteroids are recommended when indicated for the management of severe asthma during pregnancy | Prednisone: Amounts very low in breast milk; no adverse effects noted in breast-fed infants.40,41 High doses may cause temporary loss of milk supply42,43 |
| Tiotropium | No published human data | No published data. Poor bioavailability and low serum levels expected to produce low levels in milk | ||
| Biologics | Continue biologics in patients who are responding to them before pregnancy. Consider starting biologics during pregnancy in women who (1) are candidates for the therapy, and (2) who have severe asthma and are at risk of asthma exacerbations or oral corticosteroid use. Data are needed | |||
| Omalizumab/anti-IgE | No increased risk compared with general population for major birth defects overall in 169 exposed pregnancies enrolled in a registry.44 Also when compared with a disease-matched unexposed cohort45 | The rates of prematurity (<37 wk’ gestation) and small for gestational age were not unlike those seen in other studies of severe pregnant asthmatic patients | Continue omalizumab in patients who are responding to it before pregnancy. Consider starting omalizumab during pregnancy in patients who (1) are candidates for this therapy and (2) have severe asthma and are at risk of asthma exacerbations or oral corticosteroid use. Consider omalizumab over other biologics in women who are candidates for more than 1 biologic due to some available human data. More data are needed |
No published data. Large protein is likely destroyed in infant gastrointestinal tract |
| Mepolizumab/anti–IL-5 | No published human data/Pregnancy registry through mothertobaby.org | No published data. Large protein is likely destroyed in infant gastrointestinal tract | ||
| Reslizumab/anti–IL-5 | No published human data | No published data. Large protein is likely destroyed in infant gastrointestinal tract | ||
| Benralizumab/anti–IL-5 receptor | No published human data/Pregnancy registry through mothertobaby.org ongoing | No published data. Large protein is likely destroyed in infant gastrointestinal tract | ||
| Dupilmuab/anti–IL-4 receptor | No published human data/Pregnancy registry through mothertobaby.org ongoing | No published data. Large protein is likely destroyed in infant gastrointestinal tract |
Adapted from Namazy et al.46
However, many older and most newer medications, such as asthma biologics, have limited epidemiologic studies on human pregnancy available. Despite lack of such data, current recommendations are to continue biologics during pregnancy, especially if a woman has shown a significant response to treatment before pregnancy.47 In addition, to minimize known maternal and fetal risks for poorly controlled asthma in pregnancy, providers may consider starting a biologic in a pregnant woman with severe asthma at risk for asthma exacerbations or need for oral corticosteroids.
Breast-feeding provides numerous health benefits for the mother and infant and is the recommended primary source of nutrition throughout the first 6 months of life and to be continued throughout the first year of life with complementary foods. However, it is estimated that 50% of postpartum women require the use of 1 or more prescription medication, including those to treat asthma.48 Multiple factors should be considered when determining drug compatibility with breast-feeding including chemical properties, dose/exposure/toxicity relationship, chronicity of exposure, health status and developmental stage of the infant, pharmacogenomics, and health status of the mother. Resources used to inform compatible medication use in the mother while breast-feeding include LactMed, Breastfeeding Handbook for Physicians, Hale and Rowe’s Medication and Mother’s Milk, Briggs and Freeman’s Drugs in Pregnancy and Lactation, and the FDA-approved labeling. However, the utility of these resources to inform medication safety during lactation for any indication, including asthma, is limited by the lack of informative data. As shown in Table II with data drawn from LactMed, for most asthma medications used during lactation, there are no published human data.49
Study methodologies and sources of data
Existing pregnancy registries.
Single drug registries are designed to capture data on exposed pregnancies and outcomes for new or existing medications, and have been a commonly used method for detection of early pregnancy safety signals for several decades. The typical design of a pregnancy registry is a convenience sample of pregnant women who have had exposure to the medication of interest and who provide informed consent to participate in the registry. Follow-up data collection is carried out to determine rates of major congenital malformations overall, and to capture information on preterm delivery, infant birth size, and pregnancy losses. Some registries use an external comparator group, and others are compared with an internal unexposed group. Advantages of pregnancy registries are that they can be initiated as soon as a new drug is marketed, can provide early signal detection for any unusual pattern of birth defects or other adverse pregnancy outcomes, and can capture data on a range of adverse outcomes. In addition, pregnancy registries are uniquely well positioned to capture information on lactation and the use of medications continued into the postpartum period. In many registries, the mother herself reports on medications, because medications recorded in medical records may not always reflect actual usage.50 In addition, the mother is the best source of information on relevant covariates such as folic acid supplementation, and tobacco and alcohol use. Limitations of pregnancy registries include that they rely on a volunteer sample, which may introduce selection bias, require informed consent, may not be representative of all pregnant women exposed to the drug, and are typically limited by small samples sizes. As a result, pregnancy registries are usually underpowered to examine risks for specific birth defects unless the magnitude of the risk is very large, and registries may take more than 10 years to accrue even modest sample sizes (Table III).
TABLE III.
Complementary strengths and weaknesses of the 3 study arms of VAMPSS
| Characteristic | Strengths | Potential weaknesses |
|---|---|---|
| Study design | ||
| Cohort | • Prospective • Multiple outcomes |
• Limited statistical power for specific birth defects • More costly |
| Case-control | • Multiple exposures • Statistical power for specific birth defects • Less costly |
• Retrospective • Limited statistical power for infrequent exposures |
| Database | • Prospective • Population-based • Multiple exposures • Multiple outcomes • Statistical power for groups of defects possible • May be least costly |
|
| Medication exposure capture | ||
| Cohort | • Data captured on medication as actually taken • Data captured on OTC medications (including vitamins) • Data captured on borrowed medication |
|
| Case-control | • Data captured on medication as actually taken • Data captured on OTC medications (including vitamins) • Data captured on borrowed medication |
• Retrospective exposure information |
| Database | • Medication prescribed may not be taken • Timing of exposure estimated when gestational age is not captured |
|
| Bias and confounding | ||
| Cohort | • Outcomes confirmed by interview and medical records • Data available on confounders, eg, alcohol, tobacco, and folic acid use |
• Potential volunteer bias |
| Case-control | • Outcomes confirmed by interview and medical records • Data available on confounders, eg, alcohol, tobacco, and folic acid use |
• Potential volunteer bias • Potential recall bias • Potential biased control selection |
| Database | • No selection bias • Diagnoses in pregnancy losses rarely captured |
• Data not available for some key confounders, eg body mass index |
OTC, Over-the-counter.
Databases.
In the postmarketing setting, population-based automated health care databases, including national registries (eg, Nordic registers), administrative claims databases (eg, Medicaid), and electronic health record databases (eg, Clinical Practice Research Datalink), are standard sources of information for drug safety studies.51–55 They provide prospectively collected data for large populations, and the clinical care represented in these databases reflects the real world.56 Although the cost and time of working with health care data sets can be high, this approach is usually less costly than collection of data directly from women and providers for a specific study (Table III).
Some automated health care databases have substantial limitations, for example, incomplete information on birth weight, gestational age, maternal smoking, or use of nonprescription drugs.57,58 When the specific drug of interest is used by a small fraction of pregnant women, as new medications often are, even these large cohorts are constrained in their number of exposed subjects. Multisite collaborations such as the Medication Exposure in Pregnancy Risk Evaluation Program,59 now part of the Sentinel network,55 or the International Pregnancy Safety Study60 consortium can offer larger sample sizes. For asthma in particular, one limitation of databases is the underrecording of mild asthma because patients with no clinical encounters or prescriptions for asthma will not be identified as subjects with asthma. However, claims-based asthma definitions validated by chart review have had high specificity and a positive predictive value of around 95%.59,61 Although ascertainment of outcomes from coded claims can also lead to misclassification (eg, ruleout diagnosis codes may generate false positives), the most common pregnancy outcomes of interest are also typically identified through validated algorithms with moderate or higher positive predictive value.62,63 For example, Andrade et al59 reported a positive predictive value of 71% for congenital heart defects and 87% for preterm birth. Most concerning is that drug utilization is based on filled prescriptions, which does not guarantee that the medication was actually taken, therefore potentially overestimating and misclassifying exposure. Moreover, treatments used may be missed if prescriptions were filled before the exposure window (eg, an old prescription still being used). The observed irregular prescription dispensing patterns in database studies support the difficulties of assessing asthma treatment use based on pharmacy claims.61,64
Case-control studies.
Population-based case-control studies can make a unique contribution to understanding the safety or risk of asthma medication use in pregnancy by providing an efficient study design to detect increases in specific serious birth defects. Since 1998, the US Centers for Disease Control and Prevention has funded and coordinated 2 major case-control studies of birth defects: the National Birth Defects Prevention Study, which was succeeded by the Birth Defects Study to Evaluate Pregnancy Exposures.65–67 Because individual types of birth defects are relatively rare, this study design is most effective when implemented in multiple sites and for multiple years. These case-control studies are built on a foundation of population-based birth defects surveillance, meaning that they represent all residents of a defined geographic region. They include major birth defects of unknown etiology, with a goal of identifying modifiable risk factors, and have also been extended to include all stillbirths in some sites. These case-control studies use maternal interviews to assess exposures including dates of exposure relative to pregnancy timing and include an assessment of use of asthma medications. A major strength of this approach is that it relies on maternal report of actual exposure and does not rely on prescribing or dispensing records, and the maternal interview collects important data on additional factors such as maternal smoking, substance use, travel history, and illnesses (Table III). Limitations of case-control studies include that participants selected from a pool of eligible cases and controls must volunteer to enroll, require informed consent, may not be representative of all pregnant women exposed to the drug, are challenged by control selection and recall biases, which can misclassify exposure status, and assess a limited number of outcomes. Moreover, they are usually underpowered to examine risks for infrequently used drugs unless the magnitude of the risk is very large.
Surveillance systems.
The Vaccines and Medications in Pregnancy Surveillance System (VAMPSS) was initiated in 2009 to be a national systematic postmarketing surveillance system combining multiple study designs. The goals were to (1) identify as early as possible the circumstances in which a drug or immunization causes harm and (2) to provide reassuring data for those drugs and immunizations (likely the majority) that are safe during pregnancy. The VAMPSS is coordinated by the American Academy of Allergy, Asthma, and Immunology and includes 3 research arms and an independent Advisory Committee.68–71
The 3 complementary VAMPSS research arms represent examples of each of the approaches described above. One arm is a prospective cohort single drug registry design conducted under the MotherToBaby Pregnancy Studies program at the University of California San Diego. This arm provides information on multiple outcomes, including spontaneous abortion, preterm delivery, pre- and postnatal growth deficiency, and birth defects overall. The second is a database arm using Medicaid and commercial claims databases. Outcomes assessed in this arm include preterm delivery, prenatal growth deficiency, and specific congenital malformation groups. The Pregnancy Research Team at Harvard University conducts this study component. The third research arm is the Birth Defects Study, a case-control birth defects surveillance design conducted by the Slone Epidemiology Center at Boston University, which assesses specific congenital malformations and exposure prevalence. Although the Birth Defects Study does not have information regarding drugs marketed after November 2015, the VAMPSS will be collaborating with the 2 Centers for Disease Control and Prevention case-control surveillance studies, which are described above, and all 3 arms have a focus on the safety of asthma medications in pregnancy. This approach takes advantages of the strengths of each design, and one design’s strengths compensates at least to some degree for another design’s limitations (Table III).
Regulatory perspective
Since the implementation of Pregnancy and Lactation Labeling Rule, FDA has sought and received input on improving the communication of information under the rule. This lack of clinical safety information is due in part to the long-standing, standard practice of excluding pregnant and lactating women from clinical trials. Systematic exclusion of pregnant and lactating women from trials due to concern for fetal safety has prevented the earlier collection of needed safety information.72,73 FDA has been working to increase the appropriate enrollment of pregnant patients in clinical trials, and published a draft guidance in 2018 titled “Pregnant Women: Scientific and Ethical Considerations for Inclusion in Clinical Trials.”74
FDA has relied primarily on collection of human safety data on a prescription product’s use during pregnancy after the drug has been approved. After the medication is marketed, case reports help to generate hypotheses about a potential increased risk for malformations or other adverse pregnancy outcomes. However, these reports are generally inadequate by themselves to support labeling or pregnancy risk information for prescribers because they do not allow for estimation of the incidence of the finding, and they tend to be biased toward reporting of adverse outcomes. Under the Food and Drug Administration Amendments Act (2007), FDA may require companies to conduct postapproval studies, such as pregnancy safety studies.56 In 2019, the FDA published the Postapproval Pregnancy Safety Studies Guidance, which discusses 3 approaches to postmarketing data collection in pregnant women: pharmacovigilance, pregnancy registries, and studies using electronic health care data, each of which can provide important information for product labeling.75 In each of these approaches, there are asthma-specific challenges in producing data appropriate for the labeling. These include attention to appropriate measurement of asthma severity and symptom control in women who are treated with the medication as well as valid comparator groups.
In addition to these FDA guidances related to pregnancy, in 2019, FDA published a guidance titled “Clinical Lactation Studies: Considerations for Study Design.” This guidance provides new recommendations related to the conduct and analysis of clinical lactation studies.76 These efforts are part of FDA’s overall efforts in addressing the need for data collection in pregnant and lactating women.
Pharmaceutical company perspective
When developing a new medicine, pharmaceutical companies, in conjunction with regulators, use nonhuman data (eg, animal and reproductive toxicology studies) to evaluate any potential reproductive effects of a medication. These studies may not predict human outcomes and may be challenging for a clinician to use to inform patient care. The first human exposure data in pregnancy may come from clinical trials for a new medication. However, pregnant women have been typically excluded from trials, and trials are usually not designed to study pregnancy outcomes. Therefore, the numbers of exposed pregnancies are small, and important relevant confounding information is not always collected.
After marketing, the pharmaceutical company conducts pharmacovigilance, which includes spontaneous reporting and can include monitoring for exposure counts from health care databases to determine the prevalence of the new medication’s use among pregnant women. These data can inform the feasibility of conducting postmarketing safety studies, when deemed necessary, using 1 or more of the various approaches described above. If postmarketing studies are conducted, they need to be well designed to achieve adequate sample size, evaluate a range of adverse outcomes including nonlive births, incorporate systematic and validated collection of exposure and outcomes, include an appropriate comparison group, be representative of a generalizable population, and not be limited by self-referral bias or losses to follow-up. Such studies, especially those for asthma medications, should also have the ability to assess confounding by indication, disease severity, dose and duration, and other factors and comorbidities that could be related to the outcomes. These strategies were supported by the stakeholders. Well-conducted and well-designed postmarketing studies can provide robust effect estimates of an association between medication and adverse pregnancy outcomes, which may be informative for the pharmaceutical company’s pregnancy labeling.
Addressing evidence gaps
Among the challenges of single drug pregnancy registries described above are the difficulties in raising and maintaining awareness among clinicians and patients about the existence of a registry for a specific medication. In the case of asthma, several distinct registries, even if they meet sample size goals, are inefficient and unlikely to be individually adequate to provide definitive evidence of risk or safety. A multiproduct, disease-based approach can help overcome this limitation.
An example of a successful multiproduct registry is the Antiretroviral Pregnancy Registry (APR). The APR is a voluntary, international, prospective exposure-registration cohort study designed to assist clinicians and patients in weighing potential risks and benefits of HIV treatment during pregnancy. Its objectives are to provide any early warning signals of major teratogenicity, to estimate prevalence of major birth defects and compare to prevalence in the general population, and to supplement preclinical, clinical, and epidemiological study data. The APR was established in 1989 and has been used to address FDA postmarketing commitments or requirements for the 28 sponsoring manufacturers. Currently, the APR monitors prenatal exposures to 164 drugs used for HIV treatment and prevention. The APR has outcomes of more than 20,000 prospective enrollments from 70 countries (78% are from the United States).
The APR uses multiple levels of analysis: overall, for each drug class, and at the individual drug level. Comparisons are made internally based on timing of exposure and externally to 2 background reference groups. The APR uses a Scientific Advisory Committee that reviews the data and forms an independent consensus statement.77
Despite their complexity, multiproduct, disease-based registries such as the APR have distinct advantages, as shown in Fig 1, including efficiency, interpretation, and enhanced/facilitated recruitment. Also shown in Figure 1 are some challenges. In addition, as with any study sample, those pregnancies included in a disease-based registry may not represent the entire range of exposed women. As a recent example, a signal of concern for a antiretroviral drug, dolutegravir, was identified in a Botswana sample but the same signal was not evident in the APR.79 However, the return on investment for evaluating safety of multiple products used for the same or similar indications compares favorably to other alternative approaches and would be amenable to a disease state such as asthma in pregnancy and lactation.
FIG 1.
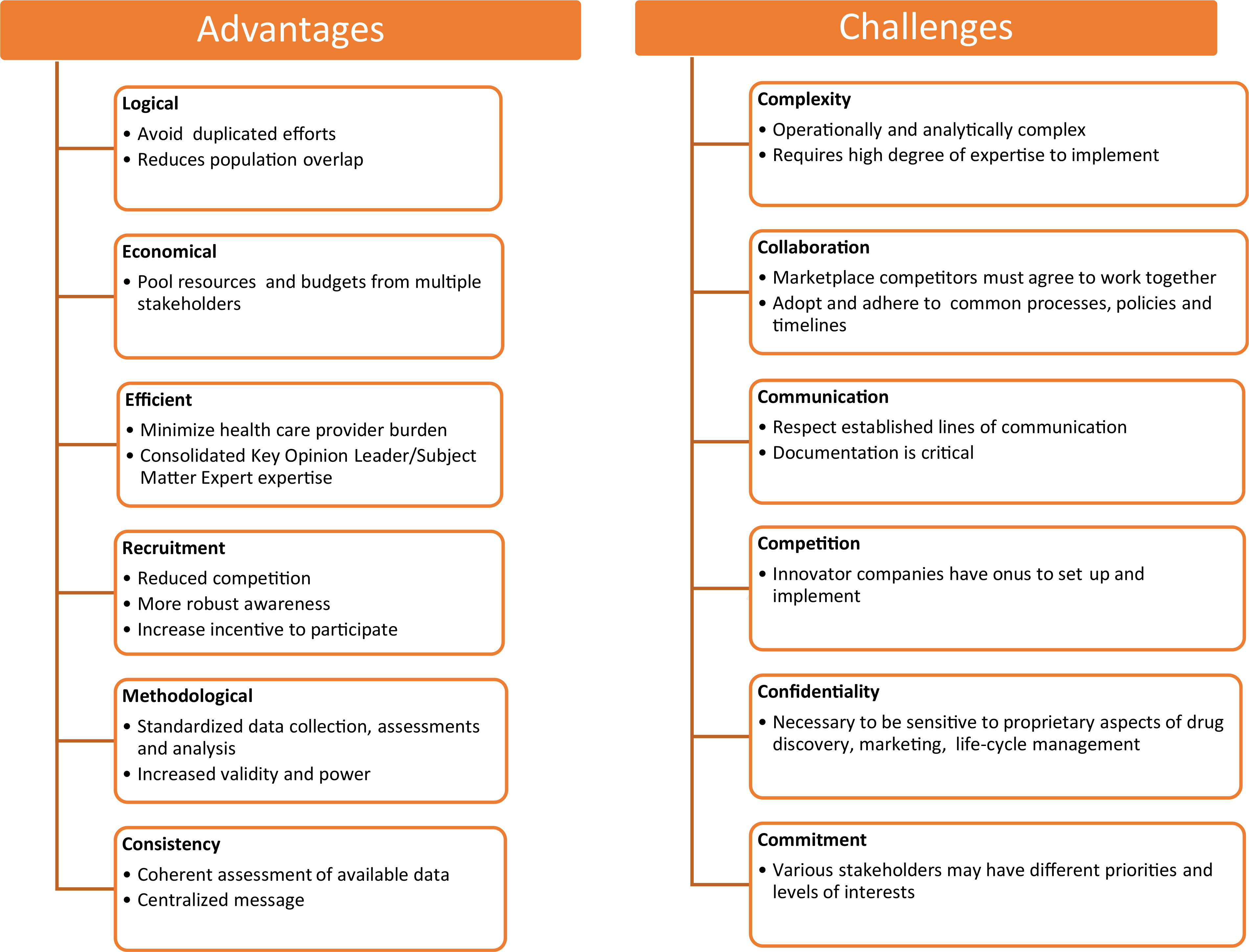
Advantages and challenges of conducting multiproduct, disease-based registries.78
Research networks
The Maternal Fetal Medicine Unit (MFMU) network was established in 1986 and has been continuously funded by the Eunice Kennedy Shriver National Institute of Child Health and Development to facilitate well-designed clinical trials in maternal fetal medicine and obstetrics. Over the years, the MFMU network has completed more than 50 studies (more than 30 randomized trials) that have provided an evidence base for obstetric practice. These have included studies specific to maternal conditions such as asthma, diabetes, and thyroid disorders; preterm birth studies aimed at improving outcome, prediction, and prevention; labor management, including assessment of adjuncts to fetal heart rate monitoring and assessment of vaginal birth after cesarean; and studies of fetal growth, stuck twins, and delivery timing.
The strengths of using a network such as the MFMU for clinical research include the use of a common protocol, an independent data center, availability of large populations (>120,000 deliveries/y), long-term follow-up, nimbleness to address pressing issues (such as H1N1), and cost-effectiveness by leveraging the infrastructure to support numerous trials simultaneously. The MFMU network has been an important resource for the study of the safety of asthma medications in pregnancy. For example, 1 MFMU study provided safety information for short-acting beta agonists, inhaled corticosteroids, and theophylline in a cohort of 2123 pregnant asthmatic women.15
Similar to the MFMU, the Eunice Kennedy Shriver National Institute of Child Health and Development’s Pediatric Trials Network is sponsored by Eunice Kennedy Shriver National Institute of Child Health and Development and represents an alliance of clinical research sites cooperating in the design and conduct of pediatric clinical trials. The Pediatric Trials Network has recently applied its methodology to study drug exposure in lactating women and their breast-fed infants. The objective of the Commonly Used Drugs During Lactation and Infant Exposure (clinicaltrials.gov NCT03511118) trial is to characterize the pharmacokinetics of understudied, off-patent drugs administered to lactating women receiving these medications per standard of care as prescribed by their treating caregiver. To understand drug transfer into breast milk and subsequent infant exposure, biological samples are collected from lactating women (blood and expressed breast milk) and infants (blood). Ideally, all 3 matrices are provided for each mother-infant pair at multiple time points. However, to be enrolled in the study, a mother-infant pair only needs to provide 1 breast milk and 1 infant plasma sample. Convenience sampling techniques are used by collecting biological samples during routine lab draws at clinic visits and hospitalization whenever possible. During the first year, this trial enrolled more than 500 mother-infant pairs on 10 commonly used medications. As drug cohorts fill, new medications of interest will be added to the trial. The data collected through this initiative will provide valuable pharmacokinetics, dosing, and safety information that will be appropriate to include in the product labels to inform clinicians and patients.
Incorporating data into clinical guidelines
Clinical practice guidelines are critical to establishing evidence-based standards to inform decision making by patients, caregivers, clinicians, payers, policymakers, and other stakeholders. There is a scarcity of information in clinical practice guidelines for the management of asthma in individuals who are pregnant or lactating. To be useful and trustworthy, the development of guidelines should follow best practices and be developed in concert with relevant professional groups such as The American College of Obstetricians and Gynecologists, the American College of Physicians, and the American Thoracic Society. In 2011, the National Academy of Medicine (previously known as the Institute of Medicine) published a seminal report about 8 standards that today are considered the bedrock of “trustworthy guidelines”80: (1) establishing transparency in the methods; (2) managing conflicts of interest; (3) composition of guideline development groups; (4) systematic reviews to synthesize the evidence; (5) rating the strength of recommendations; (6) articulating the recommendations; (7) external review of draft guidelines; and (8) updating the guidelines as new evidence is identified.
The American Thoracic Society adheres to these standards in the development of its official guidelines, and starting in 2005, has been using the “Grading of Recommendations, Assessment, Development and Evaluations” (GRADE) framework for developing and presenting evidence summaries and transforming the evidence to clinical recommendations.81 GRADE recognizes the importance of all the available evidence, including the trade-offs inherent to relying on randomized clinical trials. GRADE encourages the integration of evidence from randomized clinical trials, observational study designs about treatment effects, and expert opinion. GRADE also takes into account patient values and preferences; balance between benefits and harms; burden of treatments on patients, providers, and society due to resource requirements; and feasibility, acceptability, and impact on equity. Recommendations using the GRADE framework are worded as “strong” in cases in which there is certainty that desirable effects of intervention substantially outweigh undesirable effects and that virtually all well-informed patients would want the intervention, or “weak” when there is uncertainty, and when most well-informed patients would want the intervention, but a substantial minority of patients may not. The standardization of wording to support clinical decision making will facilitate communication between decision makers. In addition, the GRADE approach to formulating recommendations highlights the importance of shared decision making, irrespective of the quality of evidence to support a course of action. Unfortunately, there are no GRADE-based guidelines available for the management of asthma and pregnancy, but it is hoped that such guidelines could be developed in the future on the basis of more robust data using methods described in this report.
Patient engagement
Diverse patient involvement is essential at every stage of research on the safety of asthma medications during pregnancy and lactation. Partnering with patient advocacy and other community groups at every step can engage potential study participants and ensure that the study design elements are responsive to patient concerns and are adequately representative of the population. A systematic review of strategies for disseminating recommendations or guidelines to patients found that diverse patient participation in the entire process is one of the most important keys to success.82 However, support and training for researchers and patients alike is necessary for that patient involvement to be successful.82,83 Diverse patient involvement in developing participant materials that use best practices in health literacy can help ensure successful patient engagement in research studies. The inclusion of trained and diverse patient advisors can increase participant’s comfort level with the research process as well as increase knowledge of their health condition. Participants in the PCORI-funded Training Patients with Asthma to Understand and Participate in Patient Centered Outcomes Research demonstrated a 10% increase in correct research-related knowledge and a 16% average increase in correct general asthma information.84 Patients may also benefit from peer support. The 2019 Perceptions and Insights study by the Center for Information and Study on Clinical Research Participation found that 75% of survey respondents (n = 12,451) overall indicated interest in discussing research participation with their peers in an online patient community.85
CONCLUSIONS
Asthma is one of the most common underlying medical conditions complicating pregnancy and lactation. As a result of this conference, a multistakeholder consortium on asthma medications in pregnancy and lactation has been developed. To address the evidence gaps and aid in populating product labeling with data that inform clinical decision making, the consortium has developed a plan to systematically obtain necessary data in the most efficient and timely manner. Existing data on the effects of asthma and asthma medications on pregnancy and the infant can be used to formulate current management guidelines. Therefore, the consortium recommends the development of multisociety guidelines with the support of the US federal government for the evaluation and management of asthma during pregnancy and lactation that adheres to the “trustworthy” standards developed by the National Academy of Medicine. Guidelines that use National Academy of Medicine standards would not only offer recommendations for patients, caregivers, and health care providers at the point of care but also systematically highlight specific evidence gaps that merit further research.
However, even with the development of management guidelines, many knowledge gaps remain regarding the safety of asthma medications during pregnancy and lactation, particularly for newer medications. A number of perspectives must be considered relative to obtaining and disseminating the needed information, including those of patients, clinicians, pharmaceutical companies, and regulators. Various study methodologies exist to study the safety of medications during pregnancy, each with strengths and weaknesses. An asthma disease-based registry approach along with the coordinated use of additional complementary methodologies would seem to be the most productive way forward. Ultimately, the collaboration of all these stakeholders using traditional and novel approaches to collection of safety data may help patients with asthma who need treatment during pregnancy and lactation.
Acknowledgments
This study was supported by the National Institutes of Health (grant/award no. R13HL149440 to C.C.), the National Institutes of Health Office of Research on Women’s Health, and the US Food & Drug Administration Office of Women’s Health.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosure of potential conflict of interest: C. D. Chambers receives research funding from Amgen, Inc, AstraZeneca, Celgene, GlaxoSmithKline, Janssen Pharmaceuticals, Pfizer, Inc, Regeneron, Hoffman La-Roche-Genentech, Genzyme Sanofi-Aventis, Takeda Pharmaceutical Company Limited, Sanofi, UCB Pharma, USA, Sun Pharma Global FZE, and the Gerber Foundation. J. A. Krishnan received research funding from the National Institutes of Health, Patient Centered Outcomes Research Institute, Regeneron, Inogen, ResMed, and the Sergey Bring Family Foundation. J. D. Albano is an employee of Syneos Health, Morrisville, NC, and owns company stock. L. Alba and M. Carver are employed by the Asthma and Allergy Foundation of America, a nonprofit patient organization, which has received grants for research and unbranded initiatives from Amgen, Amphastar, AstraZeneca, Boehinger Ingelheim, Genentech/Roche, GlaxoSmithKline, Mylan, Novartis, Regeneron, Sanofi Genzyme, and Teva. L. Alba has also received honoraria from serving on the AstraZeneca patient partnership program. S. Hernandez-Diaz reports being an investigator on grants to her institution from GlaxoSmithKline and Takeda for unrelated studies; personal fees from UCB and Roche outside the submitted work; and having served as an epidemiologist with the North America AED pregnancy registry, which is funded by multiple companies. L. S. Cohen receives research support from National Pregnancy Registry for Atypical Antipsychotics: Alkermes Biopharmaceuticals, AstraZeneca Pharmaceuticals, Forest/Actavis Pharmaceuticals, Ortho-McNeil Janssen Pharmaceuticals, Inc, Otsuka Pharmaceuticals, Sunovion Pharmaceuticals, Inc, Teva Pharmaceuticals, and Johnson and Johnson and receives other research support from Brain & Behavior Research Foundation, JayMac Pharmaceuticals, National Institute on Aging, National Institutes of Health, National Institute of Mental Health, SAGE Therapeutics, Takeda/Lundbeck Pharmaceuticals Advisory/Consulting: Alkermes Biopharmaceuticals (through MGH Clinical Trials Network Initiative), JDS Therapeutics LLC, and Praxis Precision Medicines, Inc (through MGH Clinical Trials Network Initiative). B. L. Jones receives authorship fees from The Merck Manuals (Merck & Co) and grant funding support from National Institutes of Health (grant nos. 1 R01 HD100545-01 and 1R01MD015409-01). K. E. Wurst is employed by and holds stock/shares in GlaxoSmithKline. L. Yao is employed by the US Food and Drug Administration. J. A. Namazy has received funding as a speaker for GlaxoSmithKline. M. Schatz receives research grant support paid to his institution from ALK, Merck, and Teva and receives honoraria from UpToDate and a stipend from the American Academy of Allergy, Asthma, and Immunology for his role as Editor-in-Chief of the Journal of Allergy and Clinical Immunology: In Practice. The rest of the authors declare that they have no relevant conflicts of interest.
Abbreviations used
- APR
Antiretroviral Pregnancy Registry
- FDA
Food and Drug Administration
- GRADE
Grading of Recommendations, Assessment, Development and Evaluations
- MFMU
Maternal Fetal Medicine Unit
- VAMPSS
Vaccines and Medications in Pregnancy Surveillance System
REFERENCES
- 1.National Center for Health Statistics. Summary Health Statistics Tables for U.S. Adults: National Health Interview Survey, 2018. 2018. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Current asthma prevalence by race and ethnicity (2016–2018).
- 3.Murphy VE, Namazy JA, Powell H, Schatz M, Chambers C, Attia J, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG 2011;118: 1314–23. [DOI] [PubMed] [Google Scholar]
- 4.Murphy VE, Wang G, Namazy JA, Powell H, Gibson PG, Chambers C, et al. The risk of congenital malformations, perinatal mortality and neonatal hospitalisation among pregnant women with asthma: a systematic review and meta-analysis. BJOG 2013;120:812–22. [DOI] [PubMed] [Google Scholar]
- 5.Wang G, Murphy VE, Namazy J, Powell H, Gibson PG, Chambers C, et al. The risk of maternal and placental complications in pregnant women with asthma: a systematic review and meta-analysis. J Matern-Fetal Neonat Med 2014;27: 934–42. [DOI] [PubMed] [Google Scholar]
- 6.Cydulka RK, Emerman CL, Schreiber D, Molander KH, Woodruff PG, Camargo CA Jr. Acute asthma among pregnant women presenting to the emergency department. Am J Respir Crit Care Med 1999;160:887–92. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Kim J, Park SY, Um HY, Kim K, Kim Y, et al. Effect of pregnancy in asthma on health care use and perinatal outcomes. J Allergy Clin Immunol 2015;136:1215–23.e1211–6. [DOI] [PubMed] [Google Scholar]
- 8.Matsui D Adherence with drug therapy in pregnancy. Obstetr Gynecol Int 2012; 2012:796590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy VE, Gibson P, Talbot PI, Clifton VL. Severe asthma exacerbations during pregnancy. Obstetr Gynecol 2005;106:1046–54. [DOI] [PubMed] [Google Scholar]
- 10.Murphy VE, Gibson PG, Talbot PI, Kessell CG, Clifton VL. Asthma self-management skills and the use of asthma education during pregnancy. Eur Respir J 2005;26:435–41. [DOI] [PubMed] [Google Scholar]
- 11.van Trigt AM, Waardenburg CM, Haaijer-Ruskamp FM, de Jong-van den Berg LT. Questions about drugs: how do pregnant women solve them? Pharm World Sci 1994;16:254–9. [DOI] [PubMed] [Google Scholar]
- 12.Odom EC, Li R, Scanlon KS, Perrine CG, Grummer-Strawn L. Reasons for earlier than desired cessation of breastfeeding. Pediatrics 2013;131:e726–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schatz M, Dombrowski MP. Asthma in pregnancy. N Engl J Med 2009;360:1862–9. [DOI] [PubMed] [Google Scholar]
- 14.Briggs GG, Freeman RK, Towers CV, Forinash AB. Drugs in pregnancy and lactation: a reference guide to fetal and neonatal risk. 11th ed. Philadelphia, Pa: Wolters Kluwer; 2017. [Google Scholar]
- 15.Schatz M, Dombrowski MP, Wise R, Momirova V, Landon M, Mabie W, et al. The relationship of asthma medication use to perinatal outcomes. J Allergy Clin Immunol 2004;113:1040–5. [DOI] [PubMed] [Google Scholar]
- 16.Munsie JW, Lin S, Browne ML, Campbell KA, Caton AR, Bell EM, et al. Maternal bronchodilator use and the risk of orofacial clefts. Hum Reprod 2011;26:3147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin S, Munsie JP, Herdt-Losavio ML, Druschel CM, Campbell K, Browne ML, et al. Maternal asthma medication use and the risk of selected birth defects. Pediatrics 2012;129:e317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin S, Munsie JP, Herdt-Losavio ML, Bell E, Druschel C, Romitti PA, et al. Maternal asthma medication use and the risk of gastroschisis. Am J Epidemiol 2008;168:73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garne E, Hansen AV, Morris J, Zaupper L, Addor MC, Barisic I, et al. Use of asthma medication during pregnancy and risk of specific congenital anomalies: a European case-malformed control study. J Allergy Clin Immunol 2015;136: 1496–502.e1497. [DOI] [PubMed] [Google Scholar]
- 20.Namazy J, Schatz M, Long L, Lipkowitz MA, Lillie MA, Voss M, et al. Use of inhaled steroids by pregnant asthmatic women does not reduce intrauterine growth. J Allergy Clin Immunol 2004;113:427–32. [DOI] [PubMed] [Google Scholar]
- 21.Rahimi R, Nikfar S, Abdollahi M. Meta-analysis finds use of inhaled corticosteroids during pregnancy safe: a systematic meta-analysis review. Hum Exp Toxicol 2006;25:447–52. [DOI] [PubMed] [Google Scholar]
- 22.Sawicki E, Stewart K, Wong S, Paul E, Leung L, George J. Management of asthma by pregnant women attending an Australian maternity hospital. Austr N Z J Obstetr Gynaecol 2012;52:183–8. [DOI] [PubMed] [Google Scholar]
- 23.Rejnö G, Lundholm C, Gong T, Larsson K, Saltvedt S, Almqvist C. Asthma during pregnancy in a population-based study–pregnancy complications and adverse perinatal outcomes. PloS One 2014;9:e104755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Källén B, Rydhstroem H, Aberg A. Congenital malformations after the use of inhaled budesonide in early pregnancy. Obstetr Gynecol 1999;93:392–5. [PubMed] [Google Scholar]
- 25.Norjavaara E, de Verdier MG. Normal pregnancy outcomes in a population-based study including 2,968 pregnant women exposed to budesonide. J Allergy Clin Immunol 2003;111:736–42. [DOI] [PubMed] [Google Scholar]
- 26.Fält A, Bengtsson T, Kennedy BM, Gyllenberg A, Lindberg B, Thorsoon L, et al. Exposure of infants to budesonide through breast milk of asthmatic mothers. J Allergy Clin Immunol 2007;120:798–802. [DOI] [PubMed] [Google Scholar]
- 27.Charlton RA, Snowball JM, Nightingale AL, Davis KJ. Safety of fluticasone propionate prescribed for asthma during pregnancy: a UK population-based cohort study. J Allergy Clin Immunol Pract 2015;3:772–9.e773. [DOI] [PubMed] [Google Scholar]
- 28.Cossette B, Beauchesne MF, Forget A, Lemiere C, Larivee P, Rey E, et al. Relative perinatal safety of salmeterol vs formoterol and fluticasone vs budesonide use during pregnancy. Ann Allergy Asthma Immunol 2014;112:459–64. [DOI] [PubMed] [Google Scholar]
- 29.Wilton LV, Pearce GL, Martin RM, Mackay FJ, Mann RD. The outcomes of pregnancy in women exposed to newly marketed drugs in general practice in England. Br J Obstetr Gynaecol 1998;105:882–9. [DOI] [PubMed] [Google Scholar]
- 30.Eltonsy S, Forget A, Blais L. Beta2-agonists use during pregnancy and the risk of congenital malformations. Birth Def Res A Clin Mol Teratol 2011;91:937–47. [DOI] [PubMed] [Google Scholar]
- 31.Eltonsy S, Forget A, Beauchesne MF, Blais L. Risk of congenital malformations for asthmatic pregnant women using a long-acting β2-agonist and inhaled corticosteroid combination versus higher-dose inhaled corticosteroid monotherapy. J Allergy Clin Immunol 2015;135:123–30. [DOI] [PubMed] [Google Scholar]
- 32.Sarkar M, Koren G, Kalra S, Ying A, Smorlesi C, De Santis M, et al. Montelukast use during pregnancy: a multicentre, prospective, comparative study of infant outcomes. Eur J Clin Pharmacol 2009;65:1259–64. [DOI] [PubMed] [Google Scholar]
- 33.Bakhireva LN, Jones KL, Schatz M, Klonoff-Cohen HS, Johnson D, Slymen DJ, et al. Safety of leukotriene receptor antagonists in pregnancy. J Allergy Clin Immunol 2007;119:618–25. [DOI] [PubMed] [Google Scholar]
- 34.Nelsen LM, Shields KE, Cunningham ML, Stoler JM, Bamshad MJ, Eng PM, et al. Congenital malformations among infants born to women receiving montelukast, inhaled corticosteroids, and other asthma medications. J Allergy Clin Immunol 2012;129:251–4.e251–6. [DOI] [PubMed] [Google Scholar]
- 35.Cavero-Carbonell C, Vinkel-Hansen A, Rabanque-Hernández MJ, Martos C, Garne E. Fetal exposure to montelukast and congenital anomalies: a population based study in Denmark. Birth Def Res 2017;109:452–9. [DOI] [PubMed] [Google Scholar]
- 36.Datta P, Rewers-Felkins K, Baker T, Hale TW. Transfer of montelukast into human milk during lactation. Breastfeed Med 2017;12:54–7. [DOI] [PubMed] [Google Scholar]
- 37.Park-Wyllie L, Mazzotta P, Pastuszak A, Moretti ME, Beique L, Hunnisett L, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology 2000;62:385–92. [DOI] [PubMed] [Google Scholar]
- 38.Skuladottir H, Wilcox AJ, Ma C, Lammer EJ, Rasmussen SA, Werler MM, et al. Corticosteroid use and risk of orofacial clefts. Birth Def Res A Clin Mol Teratol 2014;100:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bandoli G, Palmsten K, Forbess Smith CJ, Chambers CD. A review of systemic corticosteroid use in pregnancy and the risk of select pregnancy and birth outcomes. Rheum Dis Clin North America 2017;43:489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katz FH, Duncan BR. Letter: entry of prednisone into human milk. N Engl J Med 1975;293:1154. [DOI] [PubMed] [Google Scholar]
- 41.Constantinescu S, Pai A, Coscia LA, Davison JM, Moritz MJ, Armenti VT. Breastfeeding after transplantation. Best Pract Res 2014;28:1163–73. [DOI] [PubMed] [Google Scholar]
- 42.McGuire E Sudden loss of milk supply following high-dose triamcinolone (Kenacort) injection. Breastfeed Rev 2012;20:32–4. [PubMed] [Google Scholar]
- 43.Babwah TJ, Nunes P, Maharaj RG. An unexpected temporary suppression of lactation after a local corticosteroid injection for tenosynovitis. Eur J Gen Pract 2013; 19:248–50. [DOI] [PubMed] [Google Scholar]
- 44.Namazy J, Cabana MD, Scheuerle AE, Thorp JM, Chen H, Carrigan G, et al. The Xolair Pregnancy Registry (EXPECT): the safety of omalizumab use during pregnancy. J Allergy Clin Immunol 2015;135:407–12. [DOI] [PubMed] [Google Scholar]
- 45.Namazy JA, Blais L, Andrews EB, Scheuerle AE, Cabana MD, Thorp JM, et al. Pregnancy outcomes in the omalizumab pregnancy registry and a disease-matched comparator cohort. J Allergy Clin Immunol 2020;145:528–36.e521. [DOI] [PubMed] [Google Scholar]
- 46.Namazy JA, Schatz M, Litonjua AA. Severe asthma in pregnancy: special considerations. In: Difficult to treat asthma; 2000. pp. 243–64. 10.1007/978-3-030-20812-7_13. [DOI] [Google Scholar]
- 47.Pfaller B, Yepes-Nuñez JJ, Agache I, Akdis CA, Alsalamah M, Bavbek S, et al. Biologicals in atopic disease in pregnancy: an EAACI position paper. Allergy 2021;76:71–89. [DOI] [PubMed] [Google Scholar]
- 48.Saha MR, Ryan K, Amir LH. Postpartum women’s use of medicines and breastfeeding practices: a systematic review. Int Breastfeed J 2015;10:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Byrne JJ, Spong CY. “Is it safe?” The many unanswered questions about medications and breast-deeding. N Engl J Med 2019;380:1296–7. [DOI] [PubMed] [Google Scholar]
- 50.Palmsten K, Bandoli G, Vazquez-Benitez G, Xi M, Johnson DL, Xu R, et al. Oral corticosteroid use during pregnancy and risk of preterm birth. Rheumatology (Oxford) 2020;59:1262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Charlton RA, Cunnington MC, de Vries CS, Weil JG. Data resources for investigating drug exposure during pregnancy and associated outcomes: the General Practice Research Database (GPRD) as an alternative to pregnancy registries. Drug Saf 2008;31:39–51. [DOI] [PubMed] [Google Scholar]
- 52.Palmsten K, Huybrechts KF, Mogun H, Kowal MK, Williams PL, Michels KB, et al. Harnessing the Medicaid Analytic eXtract (MAX) to evaluate medications in pregnancy: design considerations. PloS One 2013;8:e67405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cea-Soriano L, García Rodríguez LA, Fernández Cantero O, Hernández-Díaz S. Challenges of using primary care electronic medical records in the UK to study medications in pregnancy. Pharmacoepidemiol Drug Saf 2013;22:977–85. [DOI] [PubMed] [Google Scholar]
- 54.Hornbrook MC, Whitlock EP, Berg CJ, Callaghan WM, Bachman DJ, Gold R, et al. Development of an algorithm to identify pregnancy episodes in an integrated health care delivery system. Health Serv Res 2007;42:908–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andrade SE, Toh S, Houstoun M, Mott K, Pitts M, Kieswetter C, et al. Surveillance of medication use during pregnancy in the Mini-Sentinel Program. Matern Child Health J 2016;20:895–903. [DOI] [PubMed] [Google Scholar]
- 56.Piper JM, Ray WA, Griffin MR, Fought R, Daughtery JR, Mitchel E Jr. Methodological issues in evaluating expanded Medicaid coverage for pregnant women. Am J Epidemiol 1990;132:561–71. [DOI] [PubMed] [Google Scholar]
- 57.Margulis AV, Setoguchi S, Mittleman MA, Glynn RJ, Dormuth CR, Hernández-Díaz S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiol Drug Saf 2013;22:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Margulis AV, Palmsten K, Andrade SE, Charlton RA, Hardy JR, Cooper WO, et al. Beginning and duration of pregnancy in automated health care databases: review of estimation methods and validation results. Pharmacoepidemiol Drug Saf 2015;24:335–42. [DOI] [PubMed] [Google Scholar]
- 59.Andrade SE, Scott PE, Davis RL, Li DK, Getahun D, Cheetham TC, et al. Validity of health plan and birth certificate data for pregnancy research. Pharmacoepidemiol Drug Saf 2013;22:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huybrechts KF, Bröms G, Christensen LB, Einarsdottir K, Engeland A, Furu K, et al. Association between methylphenidate and amphetamine use in pregnancy and risk of congenital malformations: a cohort study from the International Pregnancy Safety Study Consortium. JAMA Psychiatry 2018;75:167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Enriquez R, Wu P, Griffin MR, Gebretsadik T, Shintani A, Mitchel E, et al. Cessation of asthma medication in early pregnancy. Am J Obstetr Gynecol 2006;195:149–53. [DOI] [PubMed] [Google Scholar]
- 62.Cooper WO, Hernandez-Diaz S, Gideon P, Dyer SM, Hall K, Dudley J, et al. Positive predictive value of computerized records for major congenital malformations. Pharmacoepidemiol Drug Saf 2008;17:455–60. [DOI] [PubMed] [Google Scholar]
- 63.Palmsten K, Huybrechts KF, Kowal MK, Mogun H, Hernández-Díaz S. Validity of maternal and infant outcomes within nationwide Medicaid data. Pharmacoepidemiol Drug Saf 2014;23:646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cohen JM, Bateman BT, Huybrechts KF, Mogun H, Yland J, Schatz M, et al. Poorly controlled asthma during pregnancy remains common in the United States. J Allergy Clin Immunol Pract 2019;7:2672–80.e2610. [DOI] [PubMed] [Google Scholar]
- 65.Yoon PW, Rasmussen SA, Lynberg MC, Moore CA, Anderka M, Carmichael SL, et al. The National Birth Defects Prevention Study. Public Health Rep 2001;116: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reefhuis J, Gilboa SM, Anderka M, Browne ML, Feldkamp ML, Hobbs CA, et al. The National Birth Defects Prevention Study: a review of the methods. Birth Defects Res A Clin Mol Teratol 2015;103:656–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tinker SC, Carmichael SL, Anderka M, Browne ML, Conway KM, Meyer RE, et al. Next steps for birth defects research and prevention: the birth defects study to evaluate pregnancy exposures (BD-STEPS). Birth Defects Res A Clin Mol Teratol 2015;103:733–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schatz M, Chambers CD, Jones KL, Louik C, Mitchell AA. Safety of influenza immunizations and treatment during pregnancy: the Vaccines and Medications in Pregnancy Surveillance System. Am J Obstetr Gynecol 2011;204:S64–8. [DOI] [PubMed] [Google Scholar]
- 69.Chambers CD, Johnson D, Xu R, Luo Y, Jones KL. Oseltamivir use in pregnancy: risk of birth defects, preterm delivery, and small for gestational age infants. Birth Defects Res 2019;111:1487–93. [DOI] [PubMed] [Google Scholar]
- 70.Louik C, Kerr S, Van Bennekom CM, Chambers C, Jones KL, Schatz M, et al. Safety of the 2011–12, 2012–13, and 2013–14 seasonal influenza vaccines in pregnancy: preterm delivery and specific malformations, a study from the case-control arm of VAMPSS. Vaccine 2016;34:4450–9. [DOI] [PubMed] [Google Scholar]
- 71.Van Bennekom CM, Kerr SM, Mitchell AA. Oseltamivir exposure in pregnancy and the risk of specific birth defects. Birth Defects Res 2019;111:1479–86. [DOI] [PubMed] [Google Scholar]
- 72.Gomes MF, de la Fuente-Núñez V, Saxena A, Kuesel AC. Protected to death: systematic exclusion of pregnant women from Ebola virus disease trials. Reprod Health 2017;14:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwartz DA. Clinical trials and administration of Zika virus vaccine in pregnant women: lessons (that should have been) learned from excluding immunization with the Ebola vaccine during pregnancy and lactation. Vaccines 2018;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Administration USFD. Pregnant women: scientific and ethical considerations for inclusion in clinical trials.
- 75.Postapproval pregnancy safety studies guidance for industry. 2019.
- 76.Clinical lactation studies: considerations for study design guidance for industry. 2019.
- 77.Vannappagari V, Thorne C. Pregnancy and neonatal outcomes following prenatal exposure to dolutegravir. J Acquir Immune Defic Syndr 2019;81:371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Committee APRS. Antiretroviral Pregnancy Registry Interim Report for 1 January 1989 through 31 January 2020. Wilmington, NC: Registry Coordinating Center; 2020. [Google Scholar]
- 79.Zash R, Holmes L, Diseko M, Jacobson DL, Brummel S, Mayondi G, et al. Neuraltube defects and antiretroviral treatment regimens in Botswana. N Engl J Med 2019;381:827–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Graham R, Mancher M, Miller Wolman D, Greenfield S, Steinberg E, editors. Clinical practice guidelines we can trust. Washington, DC: National Academies Press; 2011; Copyright 2011 by the National Academy of Sciences. All rights reserved. [PubMed] [Google Scholar]
- 81.Schünemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, Ernst A, et al. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. Am J Respir Crit Care Med 2006; 174:605–14. [DOI] [PubMed] [Google Scholar]
- 82.Schipper K, Bakker M, De Wit M, Ket JC, Abma TA. Strategies for disseminating recommendations or guidelines to patients: a systematic review. Implement Sci 2016;11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.The patient voice in value: The National Health Council Patient-Centered Value Model Rubric. 2016.
- 84.Asthma and Allergy Foundation of America. 2017. Asthma and Allergy Foundation of America PCORI trainings: summary of findings from participant survey.
- 85.The 2019 Perceptions and Insights Study by the Center for Information and Study on Clinical Research Participation (CISCRP). 2019. Available at: https://www.ciscrp.org/wp-content/uploads/2019/12/Deciding-to-Participate-04DEC-1.pdf.


