Abstract
Multidomain bromodomain-containing proteins regulate gene expression via chromatin binding, interactions with the transcriptional machinery, and by recruiting enzymatic activity. Selective inhibition of members of the bromodomain and extra-terminal (BET) family is important to understand their role in disease and gene regulation, although due to the similar binding sites of BET bromodomains, selective inhibitor discovery has been challenging. To support the bromodomain inhibitor discovery process, here we report the first application of protein-observed fluorine (PrOF) NMR to the tandem bromodomains of BRD4 and BRDT to quantify the selectivity of their interactions with acetylated histones as well as small molecules. We further determine the selectivity profile of a new class of ligands, 1,4-acylthiazepanes, and find them to have ≥3–10-fold selectivity for the C-terminal bromodomain of both BRD4 and BRDT. Given the speed and lower protein concentration required over traditional protein-observed NMR methods, we envision that these fluorinated tandem proteins may find use in fragment screening and evaluating nucleosome and transcription factor interactions.
Graphical Abstract

Epigenetic regulation of gene expression is controlled through the alteration of the chromatin fiber and by the recruitment of downstream effector proteins and transcription factors.1 One of the well-studied mechanisms for epigenetic gene regulation is through dynamic post-translational modifications (PTMs) of histone proteins.2 Lysine acetylation is a PTM generally associated with an open chromatin state and transcriptional activation leading to gene expression.3 Bromodomains are ~110 amino acid structural motifs that recognize specific protein sequences containing acetylated lysines.4,5 Many bromodomain modules are found within multidomain proteins that can both interact with chromatin and recruit enzymatic activity.6 Examples include tandem bromodomains, plant homeodomain fingers, chromodomains, Pro-Trp-Trp-Pro domains, bromo-adjacent homology domains, and histone acetyl transferase domains. The presence of multiple domains within a single protein leads to context-dependent and selective functions via multivalent protein–protein and protein–nucleic-acid interactions along with enzymatic function.7 Thus, multidomain epigenetic proteins through a variety of interaction interfaces provide an enhanced level of control for regulating gene expression.
Of the 61 human bromodomains present in 46 nuclear and cytoplasmic bromodomain-containing proteins, the bromodomain and extra-terminal (BET) domain family has been studied in the most detail.8 Each protein in this family, BRD2, BRD3, BRD4, and the testis-specific BRDT, minimally consists of two tandem N-terminal bromodomains (BD1 and BD2) and an extra-terminal domain.9 The BET proteins have been shown to bind multiple acetylated lysines on histone tails and acetylated regions of various transcription factors.10–12 In some cases, preferred binding interactions have been determined for a single bromodomain, whereas in others, both domains play a synergistic role.13 The differing selectivity observed for BET bromodomain ligand interactions points to the importance of studying the biological roles of individual bromodomains within BET family members.
Given their significant role in disease, including cancer,14 cardiovascular,15 neurological,16,17 and inflammatory diseases, 18 BET bromodomains are a well-studied drug target class. First-generation inhibitors, such as (+)-JQ119 and iBET15120 retain high affinities for both the BD1 and BD2 domains of BET proteins. Due to their pan-inhibition of BET bromodomains, it is difficult to segregate the roles of individual BET proteins from each other. However, recent reports of potent and selective pan-BET BD1 and pan-BET BD2 inhibitors have been reported including ABBV-744,21 GSK778, and GSK046.22 Early preclinical results demonstrate different phenotypic responses from domain-selective BET inhibitors and reduced toxicity when using pan-BET BD2 selective inhibitors. Currently, few selective inhibitors exist for a single BET bromodomain, with the exception of recent reports on BRD4–BD1 selective molecules.23–25 Isoform and domain-specific inhibitors will help to dissect the individual roles of each BET bromodomain, and thus new tools are required to discover them.
Out of the many biophysical assays for bromodomain inhibitor drug discovery, structural methods based on nuclear magnetic resonance spectroscopy (NMR), including two-dimensional protein-observed 1H–15N heteronuclear single quantum coherence (HSQC) and 1H–13C heteronuclear multiple quantum coherence (HMQC) NMR, have been instrumental in establishing the characteristic bromodomain fold4 and elucidating binding-site interactions. These experiments have mostly been used on isolated domains,26,27 with a few examples of tandem BET proteins.28–31 However, these methods are often expensive due to the need for isotopic labeling and are material-intensive, typically requiring 70–500 μM protein for each experiment. These experiments can be time-consuming when applied to study 40–50 kDa proteins. For example, while investigating interactions between tandem bromodomains of BRDT and acetylated nucleosomes via 1H–15N HSQC NMR, Miller et al. reported an experiment time of 20 h.30
Here, we use an alternative approach, based on protein-observed fluorine (PrOF) NMR, that we previously used for studying bromodomain–ligand interactions.32–34 19F is the second most sensitive stable NMR active nucleus (83% as sensitive as 1H), is 100% isotopically abundant, and is hyper-responsive to changes in its environment as evidenced by its ~400 ppm chemical shift range.35–38 PrOF NMR employing fluorinated aromatic amino acids was initially described using the individual bromodomains of BRD4, BRDT, and BPTF for detecting interactions with synthetic ligands. Due to the speed and quantitative nature of the experiment, PrOF NMR has subsequently been used for fragment screening in the discovery of bromodomain inhibitors.39 A dual-protein PrOF NMR screen was also reported in which two bromodomains were screened together in the same NMR tube against a library of synthetic ligands.40,41 These experiments provided selectivity information at the onset of the inhibitor discovery process.42
Inspired by the advantages of the dual-protein screen and the challenge of designing domain-selective inhibitors for BET proteins, here we show the first application of PrOF NMR with multidomain proteins using the tandem bromodomains of BET family members, BRD4 and BRDT. We first verify a lack of significant structural and functional perturbations from fluorine incorporation. Subsequently, we demonstrate the application of PrOF NMR of a dual-domain protein to simultaneously quantify the affinity and selectivity of the weak interactions of BET proteins with acetylated histone H4 and H3 peptides. Using small molecules, we apply PrOF NMR to the tandem bromodomains of BRD4 (BRD4-T) for acquiring qualitative 19F NMR signatures of high-affinity domain-selective molecules. Finally, we demonstrate a pilot screen of a recently reported new class of 3D fragments, 1,4-acylthiazepanes43 against fluorinated BRD4-T, and report a previously unidentified selectivity for BET BD2 domains. We cross-validate these findings using fluorescence polarization (FP). These results suggest that PrOF NMR studies using multidomain BET proteins offer a unique tool for quantitative selectivity analysis of the native transient protein–protein interactions formed with each bromodomain, as well as a screening approach to identify domain-selective inhibitors.
RESULTS AND DISCUSSION
Structure and Functional Analysis of Fluorinated Tandem BRD4 and BRDT Bromodomains.
Proteins are labeled with fluorine via metabolic incorporation of fluorinated aromatic amino acids such as 5-fluorotryptophan (5FW) and 3-fluorotyrosine (3FY). Aromatic amino acids are generally less abundant in the protein but are enriched at protein–protein interaction interfaces.44,45 This is particularly the case for bromodomains. Incorporation of a fluorinated precursor of tryptophan, 5-fluoroindole, during the protein expression results in sequence-selective labeling of all tryptophans in BRD4-T and BRDT-T (Figure 1A). In BRD4-T, W75, W81, and W120 are present in the first bromodomain (BRD4–BD1), and W374 is in the second domain (BRD4–BD2). W81 and W374 are the residues in the “WPF shelf” of the binding sites. Similarly, in BRDT-T, W44 and W50 are in the first domain (BRDT–BD1) and W293 is present in the binding site of the second domain (BRDT–BD2). W50 and W293 are located in the WPF shelves. 19F resonances from the labeled tryptophans are well-resolved in the PrOF NMR spectrum of the fluorinated tandem bromodomains (Figure 1A).
Figure 1.
Protein expression and sequence assignments of 5FW BRD4-T and 5FW BRDT-T. (A) Schematic of the BRD4 Tandem (BRD4-T) and BRDT Tandem (BRDT-T) domain architecture. Also shown is the sequence-selective labeling of the tandem proteins with 5-fluorotryptophan to produce well-resolved 1D-19F NMR spectra. (B) 19F resonances from 5FW BRD4-T and (C) 5FW BRDT-T were assigned by overlaying the 19F NMR spectra of the tandem and the isolated fluorinated domains. W81 and W374 and W50 and W293 are the WPF shelf tryptophans in BD1 and BD2 of BRD4-T and BRDT-T, colored red and blue, respectively, and changes in the chemical shift (Δδ) are indicated in purple.
Resonance assignments were supported by overlaying the 19F NMR spectra of the tandem domains with those of the 5FW-labeled isolated domains (Figure 1B and C). W75, W81, and W374 19F resonances in 5FW BRD4-T are aligned with the resonances in the isolated domains. However, W120 in 5FW BRD4-T moves downfield by 0.42 ppm. W120 in BRD4-T is present opposite the acetyllysine-binding site in BD1 but close to the 180-residue-long, flexible, and unstructured region connecting the individual domains. We attribute this difference in the chemical environment to the downfield shift of the W120 19F resonance in the tandem bromodomain, rather than a significant structural perturbation. The PrOF NMR spectrum of 5FW BRDT-T was overlaid with only 5FW BRDT–BD1. Here, both W44 and W50 in 5FW BRDT-T are in similar positions but move downfield by 0.24 and 0.11 ppm, respectively. Assignments were subsequently confirmed through ligand binding analyses. The similarities in spectra between the tandem and the isolated domains support minimal effects on the overall protein structure.
To further investigate the structure of the tandem proteins, we determined the longitudinal T1 and transverse T2 relaxation values of the 19F resonances and compared them with the values for the isolated domains (Table S3). T1 and T2 relaxation values of the 19F nuclei are dependent on the molecular weight of the protein and are expected to decrease for the higher molecular weight (~50 kDa) tandem proteins as compared to the individual domains (~18 kDa).38 However, we found that the relaxation values were not drastically different between the tandem proteins (T1, 690–1180 ms; T2, 5–13 ms) and the individual domains (T1, 610–1100 ms; T2, 7–16 ms, Table S3). This indicates that the bromodomains in the tandem proteins behave more as isolated domains and do not exhibit any domain–domain interactions or dimerization that has been observed for other BET proteins.46,47 Overall, well-resolved resonances in the PrOF NMR spectra indicate that our 5FW-labeled tandem proteins are properly folded with the two domains behaving similarly to the two isolated domains.
To further probe any potential structural and functional perturbations from fluorine incorporation before using the fluorinated tandem bromodomains for PrOF NMR experiments, we conducted circular dichroism (CD) and FP direct binding experiments (Figure 2).
Figure 2.
Circular dichroism and fluorescence polarization experiments to assess structural and functional effects from fluorine incorporation. (A) Tm and Kd values of unlabeled and fluorinated tandem proteins as determined by CD and FP direct binding experiments. (B) Fluorescence polarization direct binding isotherms of unlabeled and fluorinated BRD4-T. X-axis scale is the logarithm of total bromodomain concentration, which is twice the tandem protein concentration.
Far UV CD spectral comparison of the unlabeled and fluorine-labeled tandem proteins indicated an α-helical secondary structure. However, thermal melt curves of both unlabeled and fluorinated BRD4-T showed a biphasic plot with the final melt phase not saturating at higher temperatures (Figure S1A). Inspection of the thermal melt curves led to an estimated lower thermal melting temperature (Tm) of 5FW BRD4-T relative to the unlabeled protein. However, due to the incomplete saturation, the data for the fluorinated protein could not be fit to yield a final Tm value. Prior analysis with the BRD4–BD1 also showed a 2 °C destabilization upon fluorination.33 The current origins of biphasic behavior are unclear. Thermal melt profiles of the BRDT-T proteins did not show any biphasic behavior; rather a sigmoidal two-state melting was seen and the unlabeled and fluorinated proteins had similar Tm values (Figures S1B and S2A).
Given the effects of fluorination on the thermal stability of BRD4-T, direct binding experiments using FP were also performed to determine Kd values for binding to (+)-JQ1 labeled with fluorescein (Figures 2 and S2). For both BRD4-T and BRDT-T proteins, the affinities of fluorinated protein for (+)-JQ1 were within 2-fold of those of the unlabeled proteins. Despite the unclear CD thermal melt curves of the BRD4-T protein, our FP analysis indicated only modest structural and minimal functional perturbation after 19F incorporation, thus making our 5FW-labeled proteins suitable for ligand binding PrOF NMR experiments.
Evaluation of Histone Peptide Interactions with Fluorinated Tandem Bromodomains.
Acetylated histone peptides typically form weak interactions with bromodomains with affinities in the low millimolar to high micromolar range thus making PrOF NMR a useful tool to quantify these weak interactions. Histone peptide interactions with BET proteins have been elucidated utilizing SPOT peptide microarrays,8 isothermal titration calorimetry (ITC),30,48 Western blotting,8,49 FP,50 surface plasmon resonance,51 and NMR.52–54 BET proteins have a modest affinity for monoacetylated histones, but the affinity increases remarkably for multiple acetylated marks within a stretch of histone tail. This increased affinity is either attributed to the bidentate acetyl-lysine recognition in a single hydrophobic binding pocket, protein dimerization, or an avidity effect.8,55
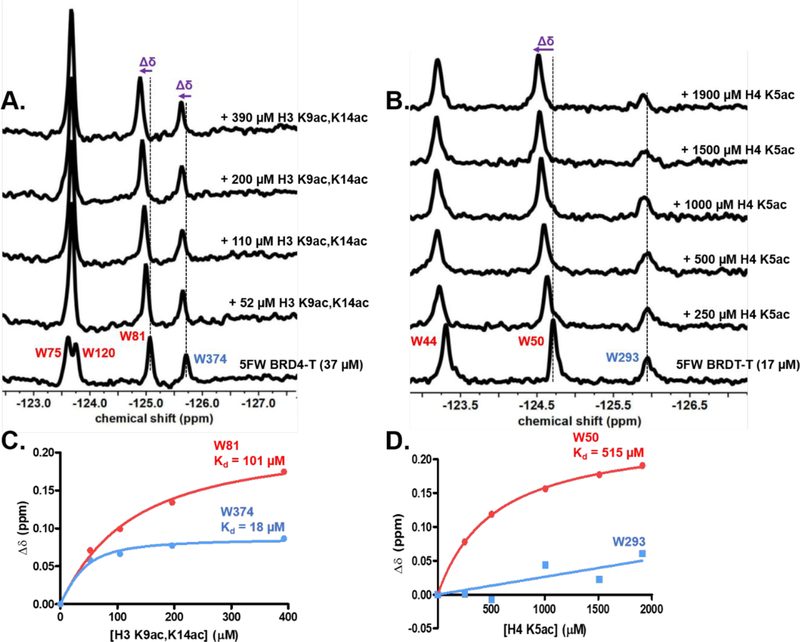
Most of these peptide-binding biophysical studies have been performed with individual N- or C-terminal bromodomains. Here, we investigate the interactions between the histone peptides and BRD4 and BRDT tandem proteins using our PrOF NMR methods. As representative examples, we chose to test H4 K5ac and H3 K9ac,K14ac against 5FW BRDT-T and 5FW BRD4-T, respectively. These peptides represent the N-terminal 21 amino acids of H4 and H3 with an additional N-terminal tyrosine residue acting as a chromophore for accurate concentration determination. In the prior SPOT array experiments, H4 K5ac was found to bind to BRDT–BD1, and H3 K9ac,K14ac showed a selectivity preference for BRD4–BD2 over BRD4–BD1 through qualitative binding experiments with individual domains.8 We sought to use our tandem bromodomains to quantify the selectivity in a single experiment.
Figure 3A shows the results of PrOF NMR titration experiments of H3 K9ac,K14ac with 5FW BRD4-T. Stacked 19F NMR spectra show that the binding of the H3 K9ac,K14ac peptide leads to chemical shift perturbations in the fast chemical exchange regime on the NMR time scale with a dose-dependent change in the chemical shift (Δδ) of W81 and W374. Plotting the Δδ of the WPF shelf resonances of BD1 (W81) and BD2 (W374), using a one-site binding model, yielded a Kd of 101 μM and 18 μM, respectively (Figure 3C).
Figure 3.
PrOF NMR experiments with acetylated histone peptides and fluorinated tandem bromodomains. Stacked 19F NMR spectra with an increasing concentration of (A) H3 K9ac,K14ac peptide with 37 μM 5FW BRD4-T and (B) H4 K5ac with 17 μM 5FW BRDT-T. Binding isotherm of (C) H3 K9ac,K14ac binding to 5FW BRD4-T and (D) H4 K5ac binding to 5FW BRDT-T. W81 and W374 and W50 and W293 are the WPF shelf tryptophans in BD1 and BD2 of BRD4-T and BRDT-T, colored red and blue, respectively. The change in the chemical shift (Δδ) is shown in purple.
The higher affinity for the C-terminal bromodomain is in line with the result obtained from the prior SPOT array experiments.8 We used a one-site binding model to quantify the affinities against the fluorinated dual-domain protein even though it had two distinct binding sites, given the possibility for the rapid equilibrium of the free ligand for accessing both binding sites. To support our hypothesis, we conducted two additional control experiments. We first measured the affinities of H3 K9ac,K14ac with the single domains, 5FW BRD4–BD1 (Figure S3A and B) and 5FW BRD4–BD2 (Figure S3C and D) leading to similar Kd values of 73 μM for BD1 and 11 μM for BD2. These values are less than 2-fold relative to affinities obtained from 5FW BRD4-T.
In a second experiment, we used fluorinated N140A and N433A mutants of BRD4-T for quantifying ligand binding by PrOF NMR. N140 in BRD4–BD1 and N433 in BRD4–BD2 form a critical hydrogen bond with the acetylated lysine.8 Mutation of these highly conserved asparagine residues to alanine significantly reduces affinity for histones. Adding an increasing concentration of H3 K9ac,K14ac peptide with N140A 5FW BRD4-T resulted in a dose-dependent Δδ of only W374 yielding a Kd of 25 μM, whereas W81 was not perturbed (Figure S4). In the reciprocal experiment, a PrOF NMR titration of H3 K9ac,K14ac with N433A 5FW BRD4–BD1 yielded a Kd of 114 μM based on perturbation of the W81 resonance (Figure S5). The Kd values obtained from the fluorinated domain mutants (N140A and N433A) of BRD4-T and isolated fluorinated domains were within 1–2 fold in comparison to those obtained from fluorinated BRD4-T. These results support our hypothesis of using the one-site binding model to simultaneously quantify affinities of a weak ligand in the high micromolar range for each bromodomain.
Extending our approach to another dual-domain BET protein, we next tested H4 K5ac (1–21) against 5FW BRDT-T. This monoacetylated histone peptide has been previously tested against BD1 of BRDT via the peptide SPOT array and by ITC (Kd not determined). Thus, we were interested in seeing if we could find the affinity of the monoacetylated peptide for the BRDT tandem bromodomains by PrOF NMR. Figure 3B shows the PrOF NMR titration of H4 K5ac with 5FW BRDT-T. Increasing the concentration of H4 K5ac resulted in a dose-dependent Δδ of W50 leading to a Kd of 515 μM (Figure 3D). A similar but higher Kd of 931 μM (Figure S6) was determined for the single BRDT–BD1 domain. For BD2 affinity, W293 broadened slightly but did not show a significant dose-dependent chemical shift perturbation up to a 1.9 mM peptide concentration, indicating BD1 selectivity for the H4 K5ac peptide.
As a final peptide binding experiment, we tested the higher affinity diacetylated peptide H4 K5ac,K8ac against both 5FW BRD4-T and 5FW BRDT-T. The BD1 W81 resonance in 5FW BRD4-T showed slow–intermediate exchange behavior in PrOF NMR titration consistent with a low micromolar affinity (Figure S7A and C). Alternatively, resonance perturbation of W374 was in the fast chemical exchange regime, and poor fitting of the binding isotherm data indicated a weaker BD2 interaction. This NMR behavior is consistent with the reported BRD4 affinity of this peptide with a Kd of 6.8 μM and 63 μM for BD1 and BD2, respectively.8 Similarly in the PrOF NMR titration of H4 K5ac,K8ac with 5FW BRDT-T, slow chemical exchange was observed for the W50 BD1 resonance, indicating higher affinity while fast chemical exchange and poor fitting of the binding isotherm data for the W293 resonance indicated a weaker affinity for BD2. We conclude from these experiments that testing the acetylated histone peptides against fluorinated dual-domain BET proteins by PrOF NMR allows one to determine the quantitative affinities of weak interactions for both the domains, whereas for high-affinity ligands, qualitative selectivity profiles can be assessed.
PrOF NMR to Investigate the Selectivity of Small Molecules for BET Proteins.
We next turned our attention to characterizing small molecule interactions with our tandem BET bromodomains. The development of new techniques to rapidly screen small molecules for evaluating their potency and selectivity can help address the challenge of developing isoform-selective inhibitors. As a proof-of-concept, we first tested three different molecules against 5FW BRD4-T: a modest BD2 selective inhibitor RVX-208,56 a BRD4–BD1 selective inhibitor, 1,25 and pan-BET inhibitor (+)-JQ1.19
RVX-208 is a BD2-selective inhibitor with a Kd of 135 nM for BRD4–BD2 and almost 9-fold selectivity over BRD4–BD1.56 Figure 4A shows the PrOF NMR titration of 5FW BRD4-T with RVX-208. At substoichiometric concentrations, the addition of RVX-208 leads to a broadening and shift of the W374 BD2 19F resonance. At 1.0 equivalent of RVX-208 relative to the total protein concentration, the W81 BD1 resonance is perturbed with a partial reduction in the W81 resonance intensity. The BD1 resonances W75 and W120 also begin to merge. This implies that at a 1:1 ratio of ligand to protein, RVX-208 only partially binds to BRD4–BD1 and has a higher preference for BRD4–BD2 as evident by the total disappearance of the unbound W374 19F resonance and appearance of a new ligand-bound W374 resonance at −123.4 ppm. As a control for our interpretations, we confirmed the assignment of W374 in the bound state and the response of W81 by performing the PrOF NMR titrations of RVX-208 with individual 5FW BRD4–BD2 and 5FW BRD4–BD1, respectively (Figure S8).
Figure 4.
PrOF NMR experiments of RVX-208, 1, and (+)-JQ1 with 5FW BRD4-T. Stacked 19F NMR spectra with an increasing concentration of (A) RVX-208 with 34 μM 5FW BRD4-T, (B) 1 with 28 μM 5FW BRD4-T, and (C) (+)-JQ1 with 34 μM 5FW BRD4-T. W81 and W374 are the WPF shelf tryptophans in BD1 and BD2 of BRD4-T, colored red and blue, respectively. 19F resonances for the ligand-bound protein are indicated with an asterisk.
Next, we tested the recently disclosed BRD4–BD1 selective inhibitor, 1, that has an IC50 of 310 nM for BRD4–BD1 and a >50-fold selectivity for BRD4–BD1 over BRD4–BD2.25 Figure 4B shows the PrOF NMR titrations of 5FW BRD4-T with 1. At 1.0 equivalent of ligand relative to the total protein concentration (28 μM of 1), the W374 BD2 resonance intensity is relatively unperturbed, whereas the W81 BD1 unbound protein resonance is no longer present and shows up as a shoulder to the W75 and W120 merged resonances. This implies that at stoichiometric concentrations of BRD4 and 1, binding almost exclusively favors BD1. Resonance assignments of W75 and W81 in the ligand-bound state were further verified by testing 1 against 5FW BRD4–BD1 (Figure S9A). With superstoichiometric concentrations of 1, the BD2 W374 19F resonance ultimately broadens and shifts to ‒125.7 ppm, consistent with a weaker binding ligand in the intermediate to fast chemical exchange regime on the NMR time scale. The broad bound state resonance was confirmed by testing 1 against 5FW BRD4–BD2 (Figure S9).
As a final test of nonselective high-affinity ligands, we titrated the pan-BET inhibitor (+)-JQ1 against 5FW BRD4-T (Figure 4C).19 In line with its submicromolar binding affinities for both bromodomains of BRD4, there was a similar drop of the intensities of the unbound resonances for W374 and W81 at 34 μM ligand concentration, 1.0 equivalent relative to the total protein concentration. At 2.0 equiv of (+)-JQ1, the unbound W374 and W81 resonances had completely disappeared, and the ligand-bound resonances appeared together at −123.6 ppm. The resonance assignments were verified by testing (+)-JQ1 against individual 5FW BRD4–BD1 and 5FW BRD4–BD2 (Figure S10).
Our fluorinated tandem protein has reporter residues in the binding pocket of both domains. By monitoring the drop in the intensities of 19F resonances of both W81 and W374, we conclude from these small molecule studies using established high-affinity ligands that we can characterize the binding footprints and qualitatively assess their selectivity against both the domains of BRD4 in one PrOF NMR experiment.
Pilot Screening of 1,4-Thiazepane Ligands against BET Proteins.
As a final test case, we sought to explore if our PrOF NMR experiment with dual-domain proteins could be used to discover domain selectivity of fragment-like molecules. We recently reported an NMR fragment screen against BRD4–BD1 with 3D-enriched fragments and identified 1,4-acylthiazepanes as an underrepresented bromodomain inhibitor scaffold39 and subsequently reported a synthetic route to access 1,4-thiazepanes and 1,4-thiazepanones.43 Motivated by our efforts in assaying domain-selective ligands, we tested a representative 3D fragment, a 1,4-thiazepane with an ethyl carbamate, TH-1, against 5FW BRD4-T (R1 = thiophenyl, R2 = ethyl carbamate; Figures 5 and S11). As expected, the chemical shift of the BD1 W81 19F resonance changed in a dose-dependent manner signifying a fast chemical exchange consistent with its reported Kd of 290 μM.39 Unexpectedly, the W374 resonance showed a binding signature lying in the intermediate–slow exchange regime consistent with a higher affinity interaction with BRD4–BD2. With this interesting result regarding BD2 selectivity, we next performed a 12-compound thiazepane/thiazepanone pilot screen against 5FW BRD4-T to survey an initial structure–activity relationship.
Figure 5.
Pilot screen of 1,4-acylthiazepanes with 5FW BRD4-T. Stacked PrOF NMR spectra with 50 μM ligand added to 34 μM 5FW BRD4-T protein. Compounds in purple were followed up with full titrations. The core thiazepane scaffold with R1 and R2 substituents is shown at the top. Two thiazepanones were also included in this screen. W81 and W374 are the WPF shelf tryptophans in BD1 and BD2 of BRD4-T, colored red and blue, respectively. 19F resonances for the ligand-bound protein are indicated with an asterisk.
Figure 5 shows the PrOF NMR stacked spectra of the tested fragments using an initial concentration of 50 μM against 5FW BRD4-T. Our fragments had structural variations at the R1 position ranging from thiophenyl, p-chlorobenzyl, naphthyl, biphenyl, and (methylenedioxy)benzyl while keeping the methyl carbamate group (R2) constant. The R2 group was also varied to include both amides and carbamates. Two enantiomers of the methyl carbamate bearing the thiophene group were included, as we previously showed a preference for the S-enantiomer binding to BRD4. Two thiazepenones were also tested. As an initial assessment of the selectivity, given the higher affinity for BD2 in BRD4-T, the percentage drops in the intensity were measured for W81 and W374 resonances with respect to the 1% DMSO 19F NMR control spectrum (Table S4). Of the 12 fragments, TH-4 and TH-8–TH-13 showed significant resonance perturbation to W374 with reduced effects at W81.
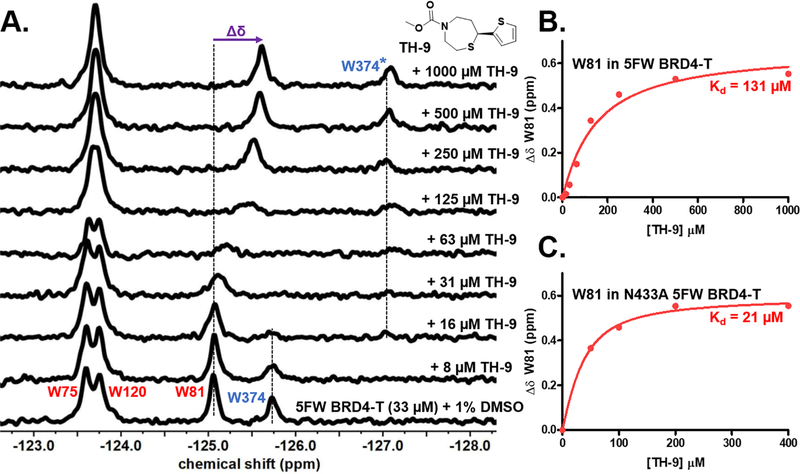
The thiazepane fragments leading to the largest perturbation of the BD2 resonance relative to BD1, TH-4, TH-9, and TH-11 were tested against 5FW BRD4-T for evaluating domain selectivity via a full PrOF NMR titration. Figure S12 shows the PrOF NMR titration of TH-11 with 5FW BRD4-T. At a concentration of 63 μM, the W374 19F resonance from the free protein had completely broadened into a baseline, and the bound W374 19F resonance appeared upfield whereas the W81 19F resonance was perturbed and shifted upfield only at higher ligand concentrations thus showing BD2 selectivity. Similar behavior was observed for TH-9 (Figure S17). TH-4, containing an electrophilic acrylamide group, minimally perturbed W81 19F resonance while the W374 19F resonance was significantly broadened and completely disappeared into the baseline by 250 μM (Figure S13). Olp et al. recently reported a covalent cysteine-reactive fragment with an acrylate warhead to selectively react with the C356 of BRD4–BD2.57 On the basis of this report, we evaluated the possibility of covalent labeling, but we did not see any adduct mass in the LC-MS traces of the fragment (200 μM) with either BRD4-T or BRD4–BD2 (data not shown), indicating noncovalent binding. Similar selectivity profiles were observed with PrOF NMR titrations with 5FW BRDT-T for TH-4, TH-9, and TH-11 (Figure S14–16).
Although the BD2 resonance perturbations were in intermediate chemical exchange, we sought to quantify the BD1 binding affinities by PrOF NMR, using TH-9. In the case of BRDT-T, plotting Δδ of W50 against TH-9 total ligand concentration yielded a Kd of 85 μM, which is close to the literature Kd value of 83 μM as measured by PrOF NMR with 5FW BRDT–BD1 (Figure S16). TH-9 was reported previously to bind to 5FW BRD4–BD1 with a Kd of 20 μM.39 Figure 6 shows the PrOF NMR titration of TH-9 with 5FW BRD4-T. Plotting Δδ of W81 against TH-9 gives a Kd of 131 μM that is 6.6-fold above the reported Kd (Figure 6B). However, below 31 μM TH-9, the Δδ values are small (0.01–0.06 ppm) before more significant resonance broadening is observed above the total protein concentration (Figure S17A). We hypothesized that the small perturbations are due to the tight-binding ligand being sequestered by BD2. Thus, to account for the ligand depletion caused by the BD2 in BRD4-T, we subtracted the protein concentration for quantifying the chemical shift change data. Figure S17B shows the new binding isotherm constructed with adjusted ligand concentrations. The 54 μM Kd obtained is 2.7-fold that of the literature Kd of 20 μM.
Figure 6.
PrOF NMR experiments with TH-9 binding to 5FW BRD4-T. (A) Stacked 19F NMR spectra with an increasing concentration of TH-9 with 33 μM 5FW BRD4-T. (B) Binding isotherm obtained by plotting the Δδ of W81 in 5FW BRD4-T against the total ligand concentration. (C) Binding isotherm obtained by plotting the Δδ of W81 in N433A 5FW BRD4-T against the ligand concentration. W81 and W374 are the WPF shelf tryptophans in BD1 and BD2 of BRD4-T, colored red and blue, respectively. The change in the chemical shift (Δδ) is shown in purple.
Thus, by accounting for ligand depletion caused by the sequestering effect of BD2 for slow-exchange high-affinity ligands, we can approximate an apparent Kd in the fluorinated dual-domain protein. We believe that the sequestering effect is dominant for BRD4-T as compared to BRDT-T because of the higher affinity of the fragment for BRD4 (Table 1). As another control to mimic the isolated domains, we tested TH-9 against the fluorinated N433A mutant of BRD4-T (Figures 6C and S18). In the PrOF NMR titration, we obtained a Kd of 21 μM for BRD4–BD1 within the error of the literature Kd value.
Table 1.
IC50, Percentage Inhibition, Kd (μM), and Ki (μM) of TH-9 and TH-11 against Bromodomains of BRD4 and BRDT Determined by Fluorescence Polarization and PrOF NMR
| BRD4–BD1 |
BRD4–BD2 |
||||
|---|---|---|---|---|---|
| fragment | FP percent inhibition at 100 μM (%) | FP Kia (μM) | PrOF NMR Kd (μM) | FP IC50 (μM) | FP Kib (μM) |
| TH-9 | 50 | 26 | 21 | 13 | 2.7 ± 0.6 |
| TH-11 | 50 | 26 | N.D. | 18 | 3.0 ± 0.2 |
| BRDT–BD1 | BRDT–BD2 | ||||
| TH-9 | 35 | >26 | 83 | 34 | 7.8 ± 0.3 |
| TH-11 | 33 | >26 | N.D. | 50 | 8.8 ± 1.2 |
For incomplete inhibition, Ki values were only estimated for values leading to at least 50% inhibition.
Data represent the mean and SEM of three to four independent experiments, each consisting of two technical replicates.
The lead fragments tested so far via PrOF NMR exhibit slow–intermediate chemical exchange rates, thus preventing determination of affinity values for BD2 and the selectivity over BD1. This prompted us to explore an orthogonal fluorescence polarization competition assay for which we could also confirm that the lead fragments could compete with a tracer molecule known to bind to the histone binding site.58 Using the assay, we determined inhibitory potencies (IC50s) and inhibitor dissociation constants (Kis) for TH-9 and TH-11 for BD1 and BD2 of both BRD4 and BRD-T (Table 1 and Figure S19). In the case of TH-9, the Ki values for BRD4–BD2 and BRDT–BD2 were 2.7 μM and 7.8 μM, consistent with their NMR behavior in the intermediate chemical exchange regime. Given the low molecular weight of TH-9, this leads to a high ligand efficiency of 0.47 and 0.44. Similar affinities were determined for TH-11 of 3.0 μM and 8.8 μM, respectively. In terms of BD1 selectivity, the IC50 values for TH-9 and TH-11 against BD1 of BRD4 and BRDT were above or at 100 μM, the highest concentration tested which leads to a lower limit of selectivity. For TH-9, we estimate a selectivity of 9.6-fold for BD2 over BD1 of BRD4, and it is ≥3.3-fold selective for BD2 of BRDT over BD1. Similarly, TH-11 binds to BRD4–BD2 8.7-fold better than to BRD4–BD1 and shows ≥2.9-fold selectivity for BRDT–BD2 over BD1. The affinity of TH-9 for BRD4–BD1 determined by PrOF NMR was 21 μM. This affinity is near the sensitivity limit of 26 μM for the FP assay. A Kd of 83 μM was also obtained for BRDT–BD1, which is consistent with a ~10-fold selectivity for BD2. Together, these studies support a highly ligand efficient thiazepane scaffold with BET BD2 selectivity.
In this study, we demonstrate the first application of PrOF NMR to the dual-bromodomain-containing BET proteins, BRD4 and BRDT. With PrOF NMR, we can rapidly acquire a well-resolved 1D 19F NMR spectrum in under 20 min at a low protein concentration (20–40 μM) and can show the simultaneous engagement of both the bromodomains. Using the fluorinated dual-domain protein, we can obtain both qualitative and quantitative information about the native interactions of acetylated histone peptides with the tandem bromodomains of BRD4 and BRDT, which have been primarily studied using isolated domains. Taking advantage of the dual fluorinated reporter residues in the acetyllysine binding pockets of the tandem bromodomains, we characterized their interactions with the domain-selective small molecules to see if we could spectroscopically observe the reported selectivity by differential engagement of either “WPF shelf” tryptophan from BD1 or BD2. Finally, we utilized PrOF NMR with dual-domain proteins as a ligand discovery tool and evaluated the structure–activity relationship of a new class of ligands, 1,4-acylthiazepanes. Using PrOF NMR and fluorescence polarization, we characterized the affinity and selectivity profile of a few fragments for the BET bromodomains of BRD4 and BRDT and found these ligand efficient fragments to have 3–10-fold better selectivity for the C-terminal bromodomain of both the BET proteins tested. These results further motivate the continued exploration of 3D fragments for developing selective inhibitors. Future studies aim to elucidate the structural basis of the observed selectivity along with an expanded scope of analogs to increase the potency. Finally, we envision in the future that fluorinated tandem proteins may find use in investigating native interactions with the components of transcriptional machinery and chromatin.
METHODS
Unlabeled and Fluorinated Protein Expression.
The pNIC28-Bsa4 plasmid containing the bromodomain of the first bromodomain of BRD4 (Addgene plasmid #38943; http://n2t.net/addgene:38943; RRID: Addgene_38943) and BRDT (Addgene plasmid #38898; http://n2t.net/addgene:38898; RRID: Addgene_38898) were a kind gift from Nicola Burgess-Brown. The pET-28a (+) plasmid containing the second bromodomain of BRD4 (Residues 333–460) was purchased from GenScript. The pET-28b(+) plasmid (Kanamycin resistant) containing the tandem bromodomains of BRD4 (38–460) and the pET-15b plasmid (Ampicillin resistant) containing the tandem domains of BRDT (2–416) were a kind gift from Prof. Brian Smith (Medical College of Wisconsin). The procedure for fluorinated protein expression by Gee et al. was followed.28 The E. coli strain BL21 Star (DE3) was transformed with the plasmid containing the desired protein gene and plated onto an agar plate containing the appropriate antibiotics. The plate was incubated overnight at 37 °C. A 5 mL LB culture containing antibiotics was inoculated using a single colony from this plate and grown overnight at 37 °C and shaking at 215 rpm. The primary culture was used to inoculate 1 L of LB media containing chloramphenicol (35 mg/L) and kanamycin (100 mg/L) or ampicillin (100 mg/L). This secondary culture was grown at 37 °C at 215 rpm until the optical density at 600 nm had reached 0.6–0.8. At this point, for unlabeled protein expression, an equilibration time of 30 min at 20 °C and 215 rpm was followed by the addition of 1 mM IPTG to induce protein expression. For 5-fluorotryptophan (5FW) labeling, the cells were pelleted by centrifugation and resuspended in 1 L of defined media, and 5-fluoroindole (80 mg) dissolved in dimethyl sulfoxide (DMSO, 200 μL) was added. After a recovery time of 90 min at 37 °C and 215 rpm, followed by a 30 min cooling to the induction temperature of 20 °C, the culture was induced with 1 mM IPTG and allowed to shake for 16–20 h. Cells were pelleted by centrifugation at 8000g and stored at –80 °C until purification.
Protein-Observed Fluorine NMR (PrOF NMR).
Experiments were run on either a Bruker Avance III HD 500 with a 5 mm Prodigy TCI inverse cryoprobe or a Bruker 600-MHz Avance NEO (6002), equipped with a 5 mm triple resonance cryoprobe. 5FW-labeled bromodomains were diluted in 50 mM HEPES, 100 mM NaCl, and a pH = 7.4 buffer by the addition of D2O and 0.1% TFA to final concentrations of 4% and 0.4%, respectively. Two one-dimensional 19F NMR spectra were taken of the control protein sample at an O1P of −75 ppm, NS = 16, D1 = 1, and AQ = 0.5 (TFA Reference set to ‒75.25 ppm) and an O1P of ‒125 ppm, NS = 1000–3000, D1 = 0.6, and AQ = 0.05 s (protein resonances). Peptide stock solutions of 15–35 mM prepared in Milli-Q water or ligand stock solutions of 10–100 mM prepared in d6-DMSO were titrated into bromodomain protein solutions (25–35 μM). The change in chemical shift of the protein resonance (Δδobs) was plotted as a function of ligand concentration to generate binding isotherms using eq 1 where Δδmax is the maximum change in fluorine chemical shift, [L] is the concentration of the ligand, and [P] is the concentration of the protein, and [PL] is the concentration of bound complex. All titrations were performed in a single replicate unless otherwise specified.
| (1) |
Peptide Synthesis.
Peptides were synthesized using standard N-9-fluorenylmethoxycarbonyl (Fmoc) solid-phase synthesis methods on NovaSyn TGR resin (Novabiochem, 0.25 mmol/g) using a Liberty Blue automated microwave synthesizer (CEM) and N,N′-diisopropylcarbodiimide (DIC) and Oxyma for amino acid activation. All peptides were cleaved from the solid support in a mixture of 95:2.5:2.5 trifluoroacetic acid (TFA)/triisopropylsilane/water for 2–5 h followed by evaporation of the solvent under a nitrogen stream. The crude peptides were precipitated into cold diethyl ether and purified by reverse phase HPLC on a C-18 column using 0.1% TFA water and CH3CN as solvents (0–50% CH3CN gradient over 60 min). Peptide molecular weight was confirmed using an Ab-Sciex 5800 matrix-assisted laser desorption ionization (MALDI) time-of-flight mass spectrometer. Peptide theoretical and observed masses are listed in Table S2.
Fluorescence Polarization.
Fluorescence-polarization experiments were carried out in 50 mM HEPES, 100 mM NaCl, and 4 mM CHAPS (pH 7.4) in 384-well plates (Corning 4511). Stock solutions (25 μM) of a fluorescent tracer, fluorescein-labeled (+)-JQ159 in DMSO, were diluted to 15 nM for these experiments. Plates were read on a Tecan Infinity 500 with an excitation wavelength at 485 nm and emission at 535 nm. For direct-binding experiments, the protein was serially diluted across the plate, and the resulting polarization values were fit using eq 2 in GraphPad Prism, where b and c are the maximum and minimum polarization values, respectively, a is the concentration of fluorescent tracer, x is the concentration of the protein, and y is the observed polarization value.
| (2) |
Circular Dichroism.
To check the secondary structural content, far-UV CD spectra (200–260 nm) of unlabeled and labeled proteins were collected using a Peltier equipped temperature controlled Jasco J-815 spectropolarimeter at 25 °C. For all measurements, 5–10 μM of protein in 5 mM phosphate buffer at pH 7.4 containing 10 mM NaCl and a 1 mm cuvette path-length were used. Spectral data were collected at a scan rate of 50 nm/min with averaging of five spectra. Processed data were baseline corrected against the spectra taken with buffer alone. Thermal stabilities of labeled and unlabeled proteins were measured by the change in ellipticity at 222 nm with the increase in temperature from 20 to 100 °C at a scan rate of 60°/h. The midpoint of transition was calculated by a sigmoidal fit to determine the Tm.
Site-Directed Mutagenesis.
Site-directed mutagenesis of N140A and N433A on BRD4-T was conducted using previously reported transfer-PCR procedures in 50 μL reaction mixtures.60 Reaction mixtures contained Phusion High-Fidelity Master Mix (NEB), template DNA, and 50 nM concentrations of each of the primers: the T7-forward primer (5′-TAATACGACTCACTATAGGG-3′) and the reverse-complement primer (N140A: 5′-GTCATCTCCAGGCTTCGCGTAGATGTAACAATT-3′; N433A : CTCATGGTCAGGAGGCGCGTACTTATAGCAGTT-3′). Successful mutagenesis was confirmed by DNA sequencing.
Supplementary Material
Acknowledgments
Funding
Financial support for this project is acknowledged from the Masonic Cancer Center at the University of Minnesota and the NICHD (HHSN27520130001 and 1 U54 HD09354). A.D. was supported by the Chemical Biology Interface Training Grant 5T32GM132029-01.
ABBREVIATIONS
- PTM
post-translation modification
- PHD
plant homeodomain
- PWWP
pro-trp-trp-pro
- BAH
bromo adjacent homology
- BET
bromo- and extra-terminal domain
- HSQC
heteronuclear single quantum coherence spectroscopy
- HMQC
heteronuclear multiple quantum coherence spectroscopy
- PrOF NMR
protein-observed fluorine NMR
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschembio.0c00720.
Supplemental protein expression methods, analytical data for compound characterization, NMR, and FP analyses (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acschembio.0c00720
Contributor Information
Prakriti Kalra, Department of Chemistry, University of Minnesota, Minneapolis, Minnesota 55455, United States.
Logan McGraw, Department of Chemistry, University of Minnesota, Minneapolis, Minnesota 55455, United States.
Jennifer R. Kimbrough, Department of Chemistry, University of Minnesota, Minneapolis, Minnesota 55455, United States.
Anil K. Pandey, Department of Chemistry, University of Minnesota, Minneapolis, Minnesota 55455, United States
Jonathan Solberg, Institute for Therapeutics Discovery and Development, Department of Medicinal Chemistry, University of Minnesota, Minneapolis, Minnesota 55414, United States.
Huarui Cui, Department of Chemistry, University of Minnesota, Minneapolis, Minnesota 55455, United States.
Anand Divakaran, Department of Medicinal Chemistry, University of Minnesota, Minneapolis, Minnesota 55455, United States.
Kristen John, Institute for Therapeutics Discovery and Development, Department of Medicinal Chemistry, University of Minnesota, Minneapolis, Minnesota 55414, United States.
Jon E. Hawkinson, Institute for Therapeutics Discovery and Development, Department of Medicinal Chemistry and Department of Medicinal Chemistry, University of Minnesota, Minneapolis, Minnesota 55414, United States.
William C. K. Pomerantz, Department of Chemistry and Department of Medicinal Chemistry, University of Minnesota, Minneapolis, Minnesota 55455, United States.
REFERENCES
- (1).Jenuwein T, and Allis CD (2001) Science 293 (5532), 1074–1080. [DOI] [PubMed] [Google Scholar]
- (2).Strahl BD, and Allis CD (2000) The Language of Covalent Histone Modifications. Nature 403 (6765), 41–45. [DOI] [PubMed] [Google Scholar]
- (3).Allis CD, and Jenuwein T (2016) The Molecular Hallmarks of Epigenetic Control. Nat. Rev. Genet. 17 (8), 487–500. [DOI] [PubMed] [Google Scholar]
- (4).Dhalluin C, Carlson JE, Zeng L, He C, Aggarwal AK, Zhou M-M, and Zhou M-M (1999) Structure and Ligand of a Histone Acetyltransferase Bromodomain. Nature 399, 491–496. [DOI] [PubMed] [Google Scholar]
- (5).Owen DJ (2000) The Structural Basis for the Recognition of Acetylated Histone H4 by the Bromodomain of Histone Acetyltransferase Gcn5p. EMBO J. 19 (22), 6141–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Muller S, Filippakopoulos P, and Knapp S (2011) Bromodomains as Therapeutic Targets. Expert Rev. Mol. Med. 13, e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Fujisawa T, and Filippakopoulos P (2017) Functions of Bromodomain-Containing Proteins and Their Roles in Homeostasis and Cancer. Nat. Rev. Mol. Cell Biol 18 (4), 246–262. [DOI] [PubMed] [Google Scholar]
- (8).Filippakopoulos P, Picaud S, Mangos M, Keates T, Lambert JP, Barsyte-Lovejoy D, Felletar I, Volkmer R, Müller S, Pawson T, Gingras AC, Arrowsmith CH, and Knapp S (2012) Histone Recognition and Large-Scale Structural Analysis of the Human Bromodomain Family. Cell 149 (1), 214–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Wang C, and Filippakopoulos P (2015) Beating the Odds: BETs in Disease. Trends Biochem. Sci 40 (8), 468–479. [DOI] [PubMed] [Google Scholar]
- (10).Huang B, Yang X-D, Zhou M-M, Ozato K, and Chen L-F (2009) Brd4 Coactivates Transcriptional Activation of NF- B via Specific Binding to Acetylated RelA. Mol. Cell. Biol. 29 (5), 1375–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Zou Z, Huang B, Wu X, Zhang H, Qi J, Bradner J, Nair S, and Chen LF (2014) Brd4Maintains Constitutively Active NF-ΚB in Cancer Cells by Binding to Acetylated RelA. Oncogene 33 (18), 2395–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Shi J, Wang Y, Zeng L, Wu Y, Deng J, Zhang Q, Lin Y, Li J, Kang T, Tao M, Rusinova E, Zhang G, Wang C, Zhu H, Yao J, Zeng YX, Evers BM, Zhou MM, and Zhou BP (2014) Disrupting the Interaction of BRD4 with Diacetylated Twist Suppresses Tumorigenesis in Basal-like Breast Cancer. Cancer Cell 25 (2), 210–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Asangani IA, Dommeti VL, Wang X, Malik R, Cieslik M, Yang R, Escara-Wilke J, Wilder-Romans K, Dhanireddy S, Engelke C, Iyer MK, Jing X, Wu YM, Cao X, Qin ZS, Wang S, Feng FY, and Chinnaiyan AM (2014) Therapeutic Targeting of BET Bromodomain Proteins in Castration-Resistant Prostate Cancer. Nature 510 (7504), 278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Arrowsmith CH, Bountra C, Fish PV, Lee K, and Schapira M (2012) Epigenetic Protein Families: A New Frontier for Drug Discovery. Nat. Rev. Drug Discovery 11 (5), 384–400. [DOI] [PubMed] [Google Scholar]
- (15).Duan Q, McMahon S, Anand P, Shah H, Thomas S, Salunga HT, Huang Y, Zhang R, Sahadevan A, Lemieux ME, Brown JD, Srivastava D, Bradner JE, McKinsey TA, and Haldar SM (2017) BET Bromodomain Inhibition Suppresses Innate Inflammatory and Profibrotic Transcriptional Networks in Heart Failure. Sci. Transl. Med. 9 (390), 23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Jakovcevski M, and Akbarian S (2012) Epigenetic Mechanisms in Neurological Disease. Nat. Med. 18 (8), 1194–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Benito E, Ramachandran B, Schroeder H, Schmidt G, Urbanke H, Burkhardt S, Capece V, Dean C, and Fischer A (2017) The BET/BRD Inhibitor JQ1 Improves Brain Plasticity in WT and APP Mice. Transl. Psychiatry 7 (9), e1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Belkina AC, Nikolajczyk BS, and Denis GV (2013) BET Protein Function Is Required for Inflammation: Brd2 Genetic Disruption and BET Inhibitor JQ1 Impair Mouse Macrophage Inflammatory Responses. J. Immunol. 190 (7), 3670–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, Mckeown MR, Wang Y, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La Thangue N, French CA, Wiest O, Kung AL, Knapp S, and Bradner JE (2010) Selective Inhibition of BET Bromodomains. Nature 468 (7327), 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung C. w., Chandwani R, Marazzi I, Wilson P, Coste H, White J, Kirilovsky J, Rice CM, Lora JM, Prinjha RK, Lee K, and Tarakhovsky A (2010) Suppression of Inflammation by a Synthetic Histone Mimic. Nature 468, 1119–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Faivre EJ, McDaniel KF, Albert DH, Mantena SR, Plotnik JP, Wilcox D, Zhang L, Bui MH, Sheppard GS, Wang L, Sehgal V, Lin X, Huang X, Lu X, Uziel T, Hessler P, Lam LT, Bellin RJ, Mehta G, Fidanze S, Pratt JK, Liu D, Hasvold LA, Sun C, Panchal SC, Nicolette JJ, Fossey SL, Park CH, Longenecker K, Bigelow L, Torrent M, Rosenberg SH, Kati WM, and Shen Y (2020) Selective Inhibition of the BD2 Bromodomain of BET Proteins in Prostate Cancer. Nature 578 (7794), 306–310. [DOI] [PubMed] [Google Scholar]
- (22).Gilan O, Rioja I, Knezevic K, Bell MJ, Yeung MM, Harker NR, Lam EYN, Chung C, Bamborough P, Petretich M, Urh M, Atkinson SJ, Bassil AK, Roberts EJ, Vassiliadis D, Burr ML, Preston AGS, Wellaway C, Werner T, Gray JR, Michon AM, Gobbetti T, Kumar V, Soden PE, Haynes A, Vappiani J, Tough DF, Taylor S, Dawson SJ, Bantscheff M, Lindon M, Drewes G, Demont EH, Daniels DL, Grandi P, Prinjha RK, and Dawson MA (2020) Selective Targeting of BD1 and BD2 of the BET Proteins in Cancer and Immunoinflammation. Science (Washington, DC, U. S.) 368 (6489), 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Liu Z, Chen H, Wang P, Li Y, Wold EA, Leonard PG, Joseph S, Brasier AR, Tian B, and Zhou J (2020) Discovery of Orally Bioavailable Chromone Derivatives as Potent and Selective BRD4 Inhibitors: Scaffold Hopping, Optimization, and Pharmacological Evaluation. J. Med. Chem 63 (10), 5242–5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Raux B, Voitovich Y, Derviaux C, Lugari A, Rebuffet E, Milhas S, Priet S, Roux T, Trinquet E, Guillemot JC, Knapp S, Brunel JM, Fedorov AY, Collette Y, Roche P, Betzi S, Combes S, and Morelli X (2016) Exploring Selective Inhibition of the First Bromodomain of the Human Bromodomain and Extra-Terminal Domain (BET) Proteins. J. Med. Chem 59 (4), 1634–1641. [DOI] [PubMed] [Google Scholar]
- (25).Pomerantz WCK, Cui H, Divakaran A, Pandey AK, Johnson JA, Zahid H, Hoell ZJ, Ellingson MO, Shi K, Aihara H, and Harki DA Selective N-terminal BET bromodomain inhibitors by targeting non-conserved residues and structured water displacement. Angew. Chem., Int. Ed. 2020. [Accepted], DOI: 10.1002/anie.202008625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Harner MJ, Chauder BA, Phan J, and Fesik SW (2014) Fragment-Based Screening of the Bromodomain of ATAD2. J. Med. Chem 57 (22), 9687–9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Yu JL, Chen TT, Zhou C, Lian FL, Tang XL, Wen Y, Shen JK, Xu YC, Xiong B, and Zhang NX (2016) NMR-Based Platform for Fragment-Based Lead Discovery Used in Screening BRD4-Targeted Compounds. Acta Pharmacol. Sin. 37 (7), 984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Waring MJ, Chen H, Rabow AA, Walker G, Bobby R, Boiko S, Bradbury RH, Callis R, Clark E, Dale I, Daniels DL, Dulak A, Flavell L, Holdgate G, Jowitt TA, Kikhney A, McAlister M, Méndez J, Ogg D, Patel J, Petteruti P, Robb GR, Robers MB, Saif S, Stratton N, Svergun DI, Wang W, Whittaker D, Wilson DM, and Yao Y (2016) Potent and Selective Bivalent Inhibitors of BET Bromodomains. Nat. Chem. Biol 12 (12), 1097–1104. [DOI] [PubMed] [Google Scholar]
- (29).Ren C, Zhang G, Han F, Fu S, Cao Y, Zhang F, Zhang Q, Meslamani J, Xu Y, Ji D, Cao L, Zhou Q, Cheung K. lung, Sharma R, Babault N, Yi Z, Zhang W, Walsh MJ, Zeng L, and Zhou MM(2018) Spatially Constrained Tandem Bromodomain Inhibition Bolsters Sustained Repression of BRD4 Transcriptional Activity for TNBC Cell Growth. Proc. Natl. Acad. Sci. U. S. A. 115 (31), 7949–7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Miller TCR, Simon B, Rybin V, Grötsch H, Curtet S, Khochbin S, Carlomagno T, and Müller CW (2016) A Bromodomain-DNA Interaction Facilitates Acetylation-Dependent Bivalent Nucleosome Recognition by the BET Protein BRDT. Nat. Commun 7, 13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Williams FP, Milbradt AG, Embrey KJ, and Bobby R (2016) Segmental Isotope Labelling of an Individual Bromodomain of a Tandem Domain BRD4 Using Sortase A. PLoS One 11 (4), e0154607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Divakaran A, Kirberger SE, and Pomerantz WCK (2019) SAR by (Protein-Observed) 19F NMR. Acc. Chem. Res 52 (12), 3407–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Mishra NK, Urick AK, Ember SWJ, Schonbrunn E, and Pomerantz WC (2014) Fluorinated Aromatic Amino Acids Are Sensitive 19F NMR Probes for Bromodomain-Ligand Interactions. ACS Chem. Biol . 9, 2755–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Gee CT, Arntson KE, Urick AK, Mishra NK, Hawk LML, Wisniewski AJ, and Pomerantz WCK (2016) Protein-Observed 19F-NMR for Fragment Screening, Affinity Quantification and Druggability Assessment. Nat. Protoc 11 (8), 1414–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Kitevski-LeBlanc JL, and Prosser RS (2012) Current Applications Of19F NMR to Studies of Protein Structure and Dynamics. Prog. Nucl. Magn. Reson. Spectrosc. 62, 1–33. [DOI] [PubMed] [Google Scholar]
- (36).Sharaf NG, and Gronenborn AM (2015) 19F-Modified Proteins and 19F-Containing Ligands as Tools in Solution NMR Studies of Protein Interactions. Methods Enzymol. 565, 67–95. [DOI] [PubMed] [Google Scholar]
- (37).Sykes BD, and Hull WE (1978) Fluorine Nuclear Magnetic Resonance Studies of Proteins. Methods Enzymol. 49, 270–295. [DOI] [PubMed] [Google Scholar]
- (38).Gerig JT (1994) Fluorine NMR of Proteins. Prog. Nucl. Magn. Reson. Spectrosc. 26, 293–370. [Google Scholar]
- (39).Johnson JA, Nicolaou CA, Kirberger SE, Pandey AK, Hu H, and Pomerantz WCK (2019) Evaluating the Advantages of Using 3D-Enriched Fragments for Targeting BET Bromodomains. ACS Med. Chem. Lett. 10 (12), 1648–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Urick AK, Hawk LML, Cassel MK, Mishra NK, Liu S, Adhikari N, Zhang W, Dos Santos CO, Hall JL, and Pomerantz WCK (2015) Dual Screening of BPTF and Brd4 Using Protein-Observed Fluorine NMR Uncovers New Bromodomain Probe Molecules. ACS Chem. Biol. 10 (10), 2246–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Johnson JA, Olson NM, Tooker MJ, Bur SK, and Pomerantz WCK (2020) Workflow to Screen Fragment Cocktails against Multiple Proteins: A Case Study Using Bromodomains. Molecules 25, 3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Pomerantz WCK, Johnson JA, and Ycas PD Applied Biophysics for Bromodomain Drug Discovery; Wiley, 2018; pp 1–45. [Google Scholar]
- (43).Pandey AK, Kirberger SE, Johnson JA, Kimbrough JR, Partridge DKD, and Pomerantz WCK (2020) Efficient Synthesis of 1,4-Thiazepanones and 1,4-Thiazepanes as 3d Fragments for Screening Libraries. Org. Lett 22 (10), 3946–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Clackson T, and Wells J (1995) Hot Spot of Binding Energy in a Hormone-Receptor Interface Author (s): Tim Clackson and James A. Wells Published by: American Association for the Advancement of Science Stable URL: Https://Www.Jstor.Org/Stable/2886248 JSTOR Is a Not-for-Profit Serv. Science (Washington, DC, U. S.) 267 (5196), 383–386. [DOI] [PubMed] [Google Scholar]
- (45).Moreira IS, Martins JM, Ramos RM, Fernandes PA, and Ramos MJ (2013) Understanding the Importance of the Aromatic Amino-Acid Residues as Hot-Spots. Biochim. Biophys. Acta, Proteins Proteomics 1834 (1), 404–414. [DOI] [PubMed] [Google Scholar]
- (46).Nakamura Y, Umehara T, Nakano K, Jang MK, Shirouzu M, Morita S, Uda-Tochio H, Hamana H, Terada T, Adachi N, Matsumoto T, Tanaka A, Horikoshi M, Ozato K, Padmanabhan B, and Yokoyama S (2006) Crystal Structure of the Human BRD2 Bromodomain: Insights into Dimerization and Recognition of Acetylated Histone H4. J. Biol. Chem 282 (6), 4193–4201. [DOI] [PubMed] [Google Scholar]
- (47).Garcia-Gutierrez P, Mundi M, and Garcia-Dominguez M (2012) Association of Bromodomain BET Proteins with Chromatin Requires Dimerization through the Conserved Motif B. J. Cell Sci. 125 (15), 3671–3680. [DOI] [PubMed] [Google Scholar]
- (48).Morinière J, Rousseaux S, Steuerwald U, Soler-López M, Curtet S, Vitte AL, Govin J, Gaucher J, Sadoul K, Hart DJ, Krijgsveld J, Khochbin S, Müller CW, and Petosa C (2009) Cooperative Binding of Two Acetylation Marks on a Histone Tail by a Single Bromodomain. Nature 461 (7264), 664–668. [DOI] [PubMed] [Google Scholar]
- (49).Dey A, Chitsaz F, Abbasi A, Misteli T, and Ozato K (2003) The Double Bromodomain Protein Brd4 Binds to Acetylated Chromatin during Interphase and Mitosis. Proc. Natl. Acad. Sci. U. S. A. 100 (15), 8758–8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Sudhamalla B, Dey D, Breski M, and Islam K (2017) Site-Specific Azide-Acetylysine Photochemistry on Epigenetic Readers for Interactome Profiling. Chem. Sci. 8, 4250–4256, DOI: 10.1039/c7sc00284j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Jung M, Philpott M, Müller S, Schulze J, Badock V, Eberspächer U, Moosmayer D, Bader B, Schmees N, Fernández-montalván A, and Haendler B (2014) Affinity Map of Bromodomain Protein 4 (BRD4) Interactions with the Histone H4 Tail and the Small Molecule Inhibitor JQ1. J. Biol. Chem. 289 (13), 9304–9319, DOI: 10.1074/jbc.M113.523019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Perell GT, Mishra NK, Sudhamalla B, Ycas PD, Islam K, and Pomerantz WCK (2017) Specific Acetylation Patterns of H2A.Z Form Transient Interactions with the BPTF Bromodomain. Biochemistry 56 (35), 4607–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Liu Y, Wang X, Zhang J, Huang H, Ding B, Wu J, and Shi Y (2008) Structural Basis and Binding Properties of the Second Bromodomain of Brd4 with Acetylated Histone Tails. Biochemistry 47 (24), 6403–6417. [DOI] [PubMed] [Google Scholar]
- (54).Olson NM, Kroc S, Johnson JA, Zahid H, Ycas PD, Chan A, Kimbrough JR, Kalra P, Schönbrunn E, and Pomerantz WCK (2020) NMR Analyses of Acetylated H2A.Z Isoforms Identify Differential Binding Interactions with the Bromodomain of the NURF Nucleosome Remodeling Complex. Biochemistry 59 (20), 1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Jacobson RH, Ladurner AG, King DS, and Tjian R (2000) Structure and Function of a Human TAF(II)250 Double Bromodomain Module. Science (Washington, DC, U. S.) 288 (5470), 1422–1425. [DOI] [PubMed] [Google Scholar]
- (56).Picaud S, Wells C, Felletar I, Brotherton D, Martin S, Savitsky P, Diez-Dacal B, Philpott M, Bountra C, Lingard H, Fedorov O, Muller S, Brennan PE, Knapp S, and Filippakopoulos P (2013) RVX-208, an Inhibitor of BET Transcriptional Regulators with Selectivity for the Second Bromodomain. Proc. Natl. Acad. Sci. U. S. A. 110 (49), 19754–19759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Olp MD, Sprague DJ, Goetz CJ, Kathman SG, Wynia-Smith SL, Shishodia S, Summers SB, Xu Z, Statsyuk AV, and Smith BC (2020) Covalent-Fragment Screening of BRD4 Identifies a Ligandable Site Orthogonal to the Acetyl-Lysine Binding Sites. ACS Chem. Biol. 15 (4), 1036–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Paulson CN, Guan X, Ayoub AM, Chan A, Karim RM, Pomerantz WCK, Schönbrunn E, Georg GI, and Hawkinson JE (2018) Design, Synthesis, and Characterization ofa Fluorescence Polarization Pan-BET Bromodomain Probe. ACS Med. Chem. Lett 9 (12), 1223–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Divakaran A, Talluri SK, Ayoub AM, Mishra N, Cui H, Widen JC, Berndt N, Zhu J-Y, Carlson AS, Topczewski JJ, Schönbrunn E, Harki DA, and Pomerantz WCK (2018) Molecular Basis for the N-Terminal Bromodomain and Extra Terminal (BET) Family Selectivity of a Dual Kinase-Bromodomain Inhibitor. J. Med. Chem 61 (20), 9316–9334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Erijman A, Dantes A, Bernheim R, Shifman JM, and Peleg Y (2011) Transfer-PCR (TPCR): A Highway for DNA Cloning and Protein Engineering. J. Struct. Biol 175 (2), 171–177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.