Abstract
Most children diagnosed with asthma have respiratory symptoms such as cough, dyspnoea and wheezing, which are also important markers of overall respiratory function. A decade of genome-wide association studies (GWAS) have investigated genetic susceptibility to asthma itself, but few have focused on important respiratory symptoms that characterise childhood asthma.
Using whole-genome sequencing (WGS) data for 894 asthmatic trios from a Costa Rican cohort, we performed family-based association tests (FBATs) to assess the association between genetic variants and multiple asthma-relevant respiratory phenotypes: cough, phlegm, wheezing, exertional dyspnoea and exertional chest tightness. We tested whether genome-wide significant associations were replicated in two additional studies: 1) 286 asthmatic trios from the Childhood Asthma Management Program (CAMP), and 2) 2691 African American current or former smokers from the COPDGene study.
In the 894 Costa Rican trios, we identified a genome-wide significant association (p=2.16×10−9) between exertional dyspnoea and the single nucleotide polymorphism (SNP) rs10165869, located on chromosome 2q37.3, that was replicated in the CAMP cohort (p=0.023) with the same direction of association (combined p=3.28×10−10). This association was not found in the African American participants from COPDGene. We also found suggestive evidence for an association between SNP rs10165869 and the atypical chemokine receptor 3 (ACKR3).
Our finding encourages the secondary association analysis of a wider range of phenotypes that characterise respiratory symptoms in other airway diseases/studies.
Keywords: childhood asthma, whole-genome sequencing, family-based genome-wide association study, exertional dyspnea, ACKR3
Tweetable Abstract
WGS data from a family-based association study suggest that the replicated SNP variant rs10165869 is associated with exertional dyspnoea, likely through the expression of ACKR3 https://bit.ly/3a5ddBd
INTRODUCTION
Asthma is the most common chronic respiratory disease of childhood and is characterised by inflammation of the airways, leading to airflow obstruction, and increased airway responsiveness that is generally caused by innate and adaptive immune responses to inhaled irritants and/or allergens [1]. The respiratory symptoms of an individual diagnosed with asthma consist of cough, dyspnoea, phlegm, wheezing and chest tightness, all of which are important markers of overall respiratory function and can signal asthma exacerbation [2].
Environmental exposures such as second-hand or personal exposure to tobacco smoke, viral respiratory infections, air pollutants and allergens can contribute to these respiratory symptoms, but do not account for all of them. Reporting or perception of respiratory symptoms differs among children with similar peak expiratory flow rate or forced expiratory volume in 1 s (FEV1), and such perceptual accuracy of lung function has been shown to further vary by ethnicity [3]. Moreover, spirometry is usually measured at rest and therefore does not explain exertional symptoms (e.g. dyspnoea) due to expiratory flow limitation and dynamic hyperinflation in response to exercise [4]. These data suggest that genetic variants may influence respiratory symptoms in children and that the magnitude of the effect of such variants may differ across ethnic groups.
To date, genome-wide association studies (GWAS) investigating genetic susceptibility to asthma have been limited to asthma affection status, lung function measures, asthma severity and response to asthma medications [5, 6]. Thus, GWAS of the respiratory symptoms are rare, particularly in children. While there have been some studies of mucus hypersecretion in adult smokers and of respiratory symptoms in general population-based cohorts, no such studies have been conducted in individuals with asthma [7, 8].
In this study, we performed a genome-wide association analysis using whole-genome sequencing (WGS) data for five major respiratory symptoms in a family-based study of childhood asthma in Costa Rica. We then attempted to replicate our findings in a WGS family-based multi-ethnic study of childhood asthma in North America. Because childhood asthma has been associated with the development of chronic obstructive pulmonary disease (COPD), and the prevalence of childhood asthma is increased in African Americans [9], we further attempted to replicate our findings among African Americans in the COPDGene study.
METHODS
GWAS Subjects
Subject recruitment and the study protocol for the Genetic Epidemiology of Asthma in Costa Rica Study (GACRS) have been described in detail previously [10, 11]. In brief, GACRS is a family-based study of children with asthma. All participants were 6–14 years old and had asthma diagnosed by a physician and at least two respiratory symptoms (wheezing, cough or dyspnoea) or a history of asthma attacks in the previous year [12]. All participants also had at least six great-grandparents born in the Central Valley of Costa Rica, to ensure their descent from a founder population predominantly composed of Spaniards and Amerindians. The population is considered to be a semi-genetic isolate with Spanish and Amerindian ancestry owing to topographical separation from the coasts and countries to the north and south by mountain ranges.
We first attempted to replicate our findings in asthmatic offspring trios from the Childhood Asthma Management Program (CAMP). CAMP was a multicentre clinical trial designed to determine the long-term effects of three inhaled treatments for childhood asthma [13]. All of the participants had to have increased airway responsiveness (a provocative concentration causing a 20% fall in FEV1 ⩽12.5 mg·mL−1 of methacholine) and at least one of the following for at least 6 months in the previous year: respiratory symptoms at least twice per week, use of an inhaled bronchodilator at least twice per week or daily medication use for asthma. Children with severe asthma or other chronic medical conditions were excluded. Compared with GACRS, more participants in CAMP had moderate persistent asthma. We also attempted to replicate our results in African American current or former adult smokers in COPDGene. Subject recruitment and the study protocol for COPDGene have been described in detail previously [14].
Written parental consent and/or the subject’s assent were obtained for each study protocol and ancillary genetic testing. Study protocols were approved by local Institutional Review Boards at each recruitment site for both studies, and by the Institutional Review Board of Brigham and Women’s Hospital.
WGS data
WGS data for GACRS, CAMP and COPDGene were generated as part of the National Heart, Lung, and Blood Institute (NHLBI) Trans-Omics for Precision Medicine (TOPMed) programme [15]. Further information can be found in the supplementary material. After confirming pedigree information and removing duplicates based on genome-wide identity-by-descent estimates, generated by kinship-based inference for GWAS (KING) [16], WGS data for 894 GACRS trios (a total of 2682 subjects), 286 CAMP trios (216 European American, 28 African American, 19 Latinx, 23 other, with a total of 858 subjects) and 2691 COPDGene African American subjects were obtained. For variant quality control, single nucleotide polymorphisms (SNPs) with Mendelian errors (>3), genotype missing rate (⩾2%), minor allele frequency (<1%) or deviations from Hardy-Weinberg proportions (p<10−8) were removed.
Respiratory Symptoms
According to the standard practice in epidemiology studies of children between the ages of 6 and 14, questionnaires were used to obtain data on respiratory symptoms in GACRS, including usual cough, usual phlegm, wheezing without cold, exertional dyspnoea and exertional chest tightness. The questionnaires were administered at the same time as spirometry measurement or collection of blood samples. For each respiratory symptom, the child or her/his parent (usually the mother) had to answer “Yes” to the following questions: “Does the child usually have cough when he/she does not have a cold or a flu?”, “Is the child usually congested or does he/she bring up phlegm when he/she does not have a cold or a flu?”, “Has the child’s chest ever sounded wheezy or whistling when he/she did not have a cold or a flu?”, “Has there ever been an occasion in the child’s life when he/she had an attack of shortness of breath when hurrying on the level or walking up a slight hill?” and “Has there ever been an occasion in the child’s life when he/she had tightness in his/her chest when hurrying on the level or walking up a slight hill?”
Statistical analysis
Demographic characteristics and clinical features between groups depending on specific respiratory symptoms were compared using t-tests or Chi-squared tests, as appropriate. Association analysis in GACRS was conducted using the family-based association test (FBAT) software (version 2.04) with an additive genetic model and regression-based phenotype adjustment for age, sex, body mass index (BMI), lung function (FEV1/forced vital capacity (FVC)), log10(total IgE) and use of systemic oral corticosteroids in the previous year (as a proxy for asthma severity) [17]. For the top variants showing genome-wide significance, we also performed haplotype-based analysis using the same adjustment in GACRS. Because ~60% and 80% of the asthmatic subjects reported each respiratory symptom, the phenotype information for respiratory symptoms is similar to asthma affection status. To distinguish our associations from asthma-related associations, we investigated the transmission pattern in symptom-specific subgroups separately. We also analysed the association p-value for asthma affection status of the top significant variant. In an attempt to replicate the genome-wide significant hits, we performed FBAT analysis for respiratory symptoms based on the CAMP trios at 2-year follow-up using the same covariate adjustment of the phenotypes.
In the COPDGene cohort, EPACTS (https://genome.sph.umich.edu/wiki/EPACTS) was used after adjusting for age, sex, Global Lung Function Initiative-calculated spirometric z scores [18], pack-years of smoking and top six principal components adjusting for population structure. We explored the link between the genetic variants and potential candidate genes using Open Targets Genetics (https://genetics.opentargets.org) and the cis-expression quantitative trait loci (eQTL) in lung tissue based on the GTEx release version 8 database (www.gtexportal.org/home). Topologically associated domains in lung tissue were also checked in a three-dimensional genome browser (Hi-C Unifying Genomic Interrogator, https://yunliweb.its.unc.edu/hugin). R statistical software (www.R-project.org) was used to evaluate these tests.
RESULTS
Descriptive characteristics of children with asthma
The characteristics of the 894 GACRS children with asthma are summarised in table 1. The median age of study participants was 9.1 years with a sex ratio of 1:0.7 (male:female) and a median age of asthma onset of 2.0 years (range=0–12 years). Most children (83.6%) had at least one positive skin test to allergens and their median FEV1 % pred was 97.5%. In CAMP, study participants had a median age of 8.8 years with a sex ratio of 1:0.5 (male:female) and a median age of asthma onset of 2.0 years (range=0–11 years) (table 1).
TABLE 1.
Demographic characteristics and clinical features of GACRS and CAMP subjects
| GACRS Study | CAMP study | p-value | |
|---|---|---|---|
| Subjects n | 894 | 286 | |
| Age years | 9.28 ± 1.86 | 8.90 ± 2.16 | 7.59×10−3 |
| Male sex | 528 (59.06) | 193 (67.48) | 0.0134 |
| BMI kg/m2 | 18.45 ± 3.92 | 18.24 ± 3.28 | 0.357 |
| Spirometric measures | |||
| FEV1 L | 1.80 ± 0.52 | 1.65 ± 0.47 | 1.03×10−5 |
| FEV1 % pred | 98.87 ± 16.84 | 92.82 ± 13.40 | 1.17×10−9 |
| FEV1/FVC % | 84.36 ± 7.79 | 79.69 ± 8.07 | 2.20×10−16 |
| FEV1/FVC % pred | 94.87 ± 8.70 | 90.36 ± 8.94 | 5.36×10−13 |
| Bronchodilator Response as % of baseline FEV1 | 5.58 ± 10.20 | 10.86 ± 9.49 | 7.50×10−15 |
| Log10 dose-response slope from saline | 1.15 ± 0.54 | 0.97 ± 0.47 | 1.92×10−7 |
| Blood tests | |||
| Total serum IgE IU·mL−1 | 725.26 ± 898.96 | 444.56 ± 797.47 | 7.89×10−7 |
| Eosinophil, count·mm−2 | 553.84 ± 403.93 | 511.96 ± 425.43 | 0.148 |
| Positive skin tests to allergens | 3.08 ± 1.82 | 5.56 ± 4.20 | 2.20×10−16 |
Data are presented as mean±SD or n (%), unless otherwise indicated. GACRS: Genetic Epidemiology of Asthma in Costa Rica Study; CAMP: Childhood Asthma Management Program; BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
Table 2 shows the main characteristics of participants in GACRS according to the presence of each respiratory symptom. Subjects with cough or phlegm were slightly shorter than those without these symptoms, and children with exertional dyspnoea or chest tightness had a higher BMI than those without such symptoms. As expected, airway hyperresponsiveness and bronchodilator response were more common in children with wheezing, exertional dyspnoea or chest tightness than in those without these symptoms. Children with wheezing had a higher rate of positive skin prick tests than those without wheezing, and children with phlegm showed slightly higher eosinophil counts than those without phlegm. Subjects with each respiratory symptom were more likely to receive systemic corticosteroid medication compared to those without symptoms.
TABLE 2.
Demographic characteristics and clinical features for different groups according to respiratory symptoms among the asthmatic GACRS children
| Cough | Phlegm | Wheezing | Exertional Dyspnea | Exertional Chest tightness | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | p-value | No | Yes | p-value | No | Yes | p-value | No | Yes | p-value | No | Yes | p-value | |
| Subjects | 190 (21.25) | 704 (78.75) | 414 (46.31) | 479 (53.58) | 105 (11.74) | 789 (88.26) | 193 (21.59) | 701 (78.41) | 266 (29.75) | 624 (69.80) | |||||
| Age years | 9.49 ± 1.84 | 9.22 ± 1.86 | 0.0773 | 9.52 ± 1.87 | 9.07 ± (1.83) | 3.07×10−4 | 9.28 ± 1.84 | 9.28 ± 1.86 | 0.972 | 9.19 ± 1.94 | 9.30 ± 1.84 | 0.466 | 9.08 ± 1.77 | 9.35 ± 1.89 | 0.0428 |
| Male sex | 112 (58.95) | 416 (59.09) | 1 | 247 (59.66) | 281 (58.66) | 0.815 | 61 (58.10) | 467 (59.19) | 0.914 | 124 (64.25) | 404 (57.63) | 0.116 | 157 (59.02) | 369 (59.13) | 1 |
| Height m | 1.35 ± 0.12 | 1.32 ± (0.12) | 1.84×10−3 | 1.35 ± 0.12 | 1.32 ± 0.12 | 5.13×10−4 | 1.32 ± 0.12 | 1.33 ± 0.12 | 0.563 | 1.32 ± 0.13 | 1.33 ± 0.12 | 0.301 | 1.32 ± 0.12 | 1.33 ± 0.12 | 0.139 |
| BMI kg/m2 | 18.67 ± 4.28 | 18.39 ± 3.81 | 0.413 | 18.75 ± 4.08 | 18.20 ± 3.76 | 0.0347 | 18.34 ± 3.85 | 18.47 ± 3.93 | 0.756 | 17.86 ± 3.54 | 18.62 ± 4.00 | 0.0114 | 18.01 ± 3.98 | 18.63 ± 3.89 | 0.0335 |
| Number of older siblings | 1.33 ± 1.43 | 1.09 ± 1.27 | 0.0366 | 1.16 ± 1.28 | 1.12 ± 1.33 | 0.656 | 1.11 ± 1.35 | 1.14 ± 1.31 | 0.784 | 1.15 ± 1.27 | 1.14 ± 1.32 | 0.912 | 1.11 ± 1.30 | 1.15 ± 1.32 | 0.640 |
| Spirometric measures | |||||||||||||||
| FEV1/FVC % | 85.17 ± 7.53 | 84.14 ± 7.85 | 0.0967 | 84.56 ± 8.25 | 84.20 ± 7.37 | 0.495 | 84.80 ± 6.72 | 84.30 ± 7.92 | 0.492 | 84.91 ± 7.68 | 84.21 ± 7.82 | 0.266 | 84.89 ± 7.73 | 84.10 ± 7.81 | 0.161 |
| FEV1/FVC % pred | 95.84 ± 8.49 | 94.61 ± 8.74 | 0.0805 | 95.17 ± 9.29 | 94.64 ± 8.16 | 0.369 | 95.33 ± 7.72 | 94.81 ± 8.82 | 0.525 | 95.57 ± 8.51 | 94.68 ± 8.75 | 0.204 | 95.39 ± 8.60 | 94.60 ± 8.74 | 0.212 |
| Absolute response to bronchodilator mL | 95.33 ± 124.61 | 82.61 ± 139.98 | 0.230 | 83.28 ± 143.08 | 86.84 ± 131.34 | 0.703 | 63.52 ± 101.68 | 88.12 ± 140.53 | 0.0325 | 74.24 ± 125.28 | 88.41 ± 139.85 | 0.180 | 73.39 ± 133.75 | 90.39 ± 138.37 | 0.0897 |
| Dose-response slope to methacholine μmol | 28.94 ± 36.59 | 27.21 ± 40.58 | 0.603 | 28.10 ± 39.11 | 27.17 ± 40.37 | 0.747 | 22.23 ± 31.92 | 28.35 ± 40.74 | 0.0901 | 22.17 ± 33.16 | 29.03 ± 41.27 | 0.0267 | 21.44 ± 28.64 | 30.30 ± 43.54 | 9.08×10−4 |
| PD20 mg | 1.69 ± 2.21 | 1.84 ± 2.35 | 0.504 | 1.69 ± 2.36 | 1.91 ± 2.30 | 0.276 | 2.43 ± 2.86 | 1.71 ± 2.22 | 0.0379 | 2.20 ± 2.79 | 1.71 ± 2.18 | 0.0747 | 2.18 ± 2.63 | 1.67 ± 2.18 | 0.0298 |
| Blood tests | |||||||||||||||
| Total serum IgE IU·mL−1 | 731.46 ± 982.71 | 723.59 ± 875.80 | 0.921 | 728.68 ± (927.43) | 713.37 ± 853.38 | 0.799 | 583.52 ± 863.08 | 744.19 ± 902.50 | 0.0770 | 674.34 ± 793.46 | 739.24 ± 925.88 | 0.334 | 676.38 ± 797.89 | 749.62 ± 940.18 | 0.237 |
| Positive IgE to dust mite | 132 (69.47) | 531 (75.43) | 0.103 | 303 (73.19) | 359 (74.95) | 0.606 | 71 (67.62) | 592 (75.03) | 0.120 | 141 (73.06) | 522 (74.47) | 0.716 | 194 (72.93) | 467 (74.84) | 0.556 |
| Positive IgE to cockroach | 80 (42.11) | 307 (43.61) | 0.750 | 179 (43.24) | 207 (43.21) | 1 | 35 (33.33) | 355 (44.61) | 0.0350 | 81 (41.97) | 306 (43.65) | 0.714 | 104 (39.10) | 282 (45.19) | 0.100 |
| Positive IgE to Ascaris | 67 (35.26) | 267 (37.93) | 0.571 | 155 (37.44) | 178 (37.16) | 0.962 | 29 (27.62) | 305 (38.66) | 0.0343 | 62 (32.12) | 272 (38.80) | 0.111 | 88 (33.08) | 244 (39.10) | 0.105 |
| Eosinophil count·mm−2 | 503.54 ± 382.75 | 567.39 ± 408.66 | 0.0481 | 517.06 ± 374.41 | 582.96 ± 424.11 | 0.0151 | 495.11 ± 436.92 | 561.62 ± 399.01 | 0.147 | 529.81 ± 406.98 | 560.45 ± 403.14 | 0.361 | 537.91 ± 407.68 | 563.57 ± 402.16 | 0.394 |
| Positive skin tests to allergens | 3.01 ± 1.86 | 3.10 ± 1.81 | 0.554 | 3.12 ± 1.81 | 3.06 ± 1.83 | 0.623 | 2.57 ± 1.96 | 3.15 ± 1.79 | 5.40×10−3 | 3.15 ± 1.74 | 3.06 ± 1.84 | 0.531 | 3.18 ± 1.85 | 3.04 ± 1.80 | 0.335 |
| Systemic corticosteroid use last year | 135 (71.05) | 567 (80.54) | 6.41×10−3 | 308 (74.40) | 393 (82.05) | 7.08×10−3 | 67 (63.81) | 635 (80.48) | 1.56×10−4 | 137 (70.98) |
565 (80.60) |
5.42×10−3 | 193 (72.56) | 505 (80.93) | 7.13×10−3 |
Data are presented as mean±SD or n (%), unless otherwise indicated. GACRS: Genetic Epidemiology of Asthma in Costa Rica Study; BMI: body mass index; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; PD20: the cumulative dose of methacholine required to produce a 20% fall in FEV1 from the post saline FEV1.
GWAS of respiratory symptoms
Genome-wide FBAT results are displayed in quantile–quantile and Manhattan plots (figure 1). In this analysis, SNP rs10165869 (located on chromosome 2q37.3) was associated with exertional dyspnoea (p=2.16×10−9). This association remained significant even after a Bonferroni correction for the five phenotypes tested (i.e. p<1.0×10−8 or 5×10−8 divided by 5). SNPs near rs10165869, including rs6725280, rs1865671, rs7607911, rs30102 and rs10168628, had p-values that achieved genome-wide significance, ranging from 3.77×10−9 to 3.97×10−8 (figure 1d). A haplotype-based test using all six SNPs identified a haplotype that is significantly associated with exertional dyspnoea (p=1.30×10−8, supplementary table S1, supplementary figure S1) [19].
Figure 1.
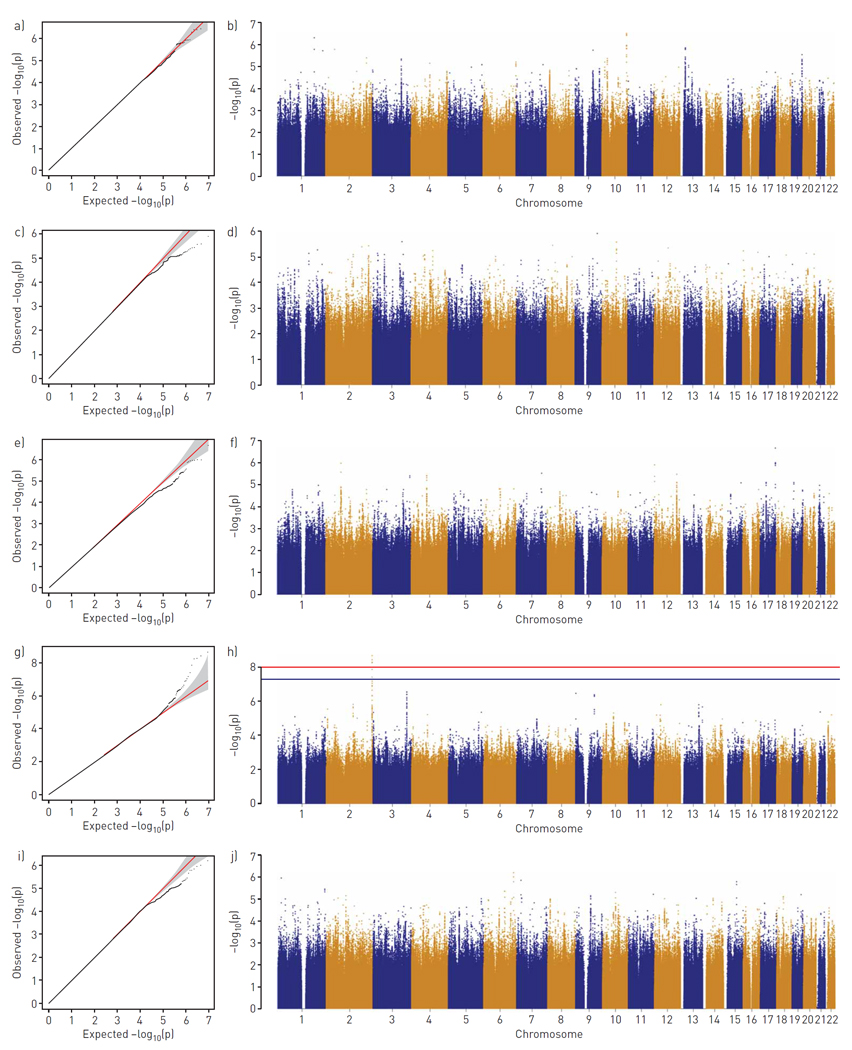
Genome-wide association study based on 894 asthmatic Genetic Epidemiology of Asthma in Costa Rica Study trios for respiratory symptoms. a, b) Cough; c, d) phlegm; e, f) wheezing without cold; g, h) exertional dyspnoea; i, j) exertional chest tightness. Data visualised as quantile–quantile plots (a, c, e, g, i) and Manhattan plots (b, d, f, h, j). The red line corresponds to a Bonferroni-corrected genome-wide significance threshold for a total of five phenotypes (p=1.0×10−8) and the blue line indicates the commonly used genome-wide significance level (p=5×10−8).
Using asthma affection status as the target phenotype, SNP rs10165869 was not significant (p=0.247). Subgroup analyses of the GACRS children either with or without exertional dyspnoea disclosed different transmission behaviour of the minor allele in each group (z=3.744 overtransmission, p=1.81×10−4, number of informative families=447 versus z= −4.704 undertransmission, p=2.55×10−6, number of informative families=120), which demonstrates the association between rs10165869 and exertional dyspnoea.
The SNP rs10165869 was replicated for exertional dyspnoea in the ethnically diverse CAMP study (p=0.023). The combined FBAT p-value was 3.28×10−10. The corresponding subgroup analysis in CAMP showed the same pattern as in GACRS (z=1.353, p=0.176, number of informative families=102 versus z= −1.844, p=0.065, number of informative families=71). A subgroup analysis by ethnicity was also performed (supplementary table S2). rs10165869 was marginally associated with exertional dyspnoea in the African American subjects from COPDGene cohort but with the opposite effect direction (p=0.070).
SNP rs10165869 and potential gene atypical chemokine receptor 3
According to the Open Targets Genetics database, the potential genes functionally implicated by rs10165869 are atypical chemokine receptor 3 (ACKR3), COPS8, IQCA1 and COL6A3, from highest to lowest. The Hi-C Unifying Genomic Interrogator also captured a significant association between ACKR3 and rs10165869 in lung tissue after Bonferroni correction (p<1.0×10−11, supplementary figure S2). According to the GTEx database, our top SNPs including rs10165869 are associated with an increased expression of ACKR3 in lung tissue (supplementary table S3).
Discussion
Based on our literature review, this is the first family-based association study of the five major respiratory symptoms in childhood asthma using WGS data. In this analysis, we identified rs10165869 as a novel SNP for exertional dyspnoea among Costa Rican children with asthma, with replication in the independent and ethnically diverse CAMP study. The dose-response slope to methacholine did not show any association in GACRS with the locus after the same adjustment (p=0.380), even though it was associated with both exertional phenotypes at baseline (table 2). Given that dyspnoea in childhood asthma is influenced by sex [20], we performed a sex interaction analysis on SNP rs10165869 using the unadjusted exertional dyspnoea phenotype but it was nonsignificant (p=0.379) [21].
ACKR3, also known as C-X-C chemokine receptor type 7 (CXCR7), is a G protein-coupled receptor (GPCR) for CXCL12, a chemokine that is involved in the inflammatory process regulating leukocyte extravasation into inflamed tissues [22, 23]. In asthmatic subjects, CXCL12 has been found in high concentrations in bronchoalveolar lavage fluid, correlated with circulating leukocytes, thus suggesting a role in airway inflammation and airway hyperresponsiveness [22]. CXCR4 had long been investigated as the only GPCR for CXCL12; however, it has now been shown that ACKR3 has ~10-fold higher binding affinity for CXCL12 [23]. In a murine model with the characteristic features of asthma, knockdown of ACKR3 by a lentiviral delivery system in the lung reduces mucus secretion, allergic airway inflammation, serum allergen-specific IgE production, T-cell cytokine production and airway hyperresponsiveness [24]. The predominant role of ACKR3 in the CXCL12/CXCR4/ACKR3 axis contributing to airway inflammation has also been shown in the pulmonary epithelium, polymorphonuclear neutrophils (PMN) and transepithelial PMN migration [25].
Aside from inflammatory conditions, CXCL12 and ACKR3 are also highly expressed under hypoxic conditions in pro-angiogenic environments such as various tumours, where CXCL12 enhances angiogenesis through ACKR3 activation [22, 26]. ACKR3 expression is more sensitive to hypoxia for up to 48 h compared to CXCR4, especially in lung endothelial cells (supplementary figure S3) [27]. Endothelial cell dysfunction in pathogenesis regeneration under alveolar hypoxia, similar to that found in lung disease, is chiefly mediated by ACKR3 [28]. The role of ACKR3 on pulmonary epithelial and endothelial cells in preclinical studies supports the idea that the rs10165869 variant affects the upregulation of ACKR3 in the lung, resulting in more severe dyspnoea symptoms than seen in asthma patients with the wild type, in whom exertional dyspnoea is purely a hypoxic condition due to chronic asthma. Previous GWAS also suggested an important role for rs7607316 and rs144060362 near the ACKR3 region as genetic risk factors for airflow obstruction related to COPD (p=3×10−6, 2×10−6 respectively) [29, 30]. Our finding was not replicated in COPDGene.
Our study has several limitations. First, answers to questions about respiratory symptoms are subjective and thus influenced by both the perception of symptoms and recall bias. However, the misclassification seeming non-differential may lead to null or weaker associations. Moreover, these concerns are ameliorated by replication of our results for exertional dyspnoea in an independent multi-ethnic cohort of children living in a different geographic location. Second, this is a cross-sectional analysis. Although genotypes do not vary over time, we were unable to assess the temporal stability or variable severity of the reported symptoms in children with asthma. Third, we lack data on the use of controller medications for asthma in GACRS, which would affect respiratory symptoms. Despite these challenges, our analysis highlights the significance of symptom-based GWAS to identify the genetic determinants of respiratory symptoms.
In conclusion, our study identified that SNP rs10165869 is associated with exertional dyspnoea among children with asthma, enabling a better understanding of exertional dyspnoea. We hope that our finding motivates the association analysis of a wider range of phenotypes that characterise respiratory symptoms in other airway diseases/studies.
Supplementary Material
Regions of chromatin contact for rs10165869 in lung tissue. Blue lines reflect -log(p-value) for the one-tailed test of whether observed Hi-C counts (black lines) were greater than the expected number of Hi-C counts (solid red lines). ACKR3 is significantly associated with rs10165869 after tissue-specific False Discovery Rate (FDR, dashed red) and Bonferroni (dashed purple) corrections.
Regional plot for an exertional dyspnea-associated locus in 400 kb upstream and downstream regions. Purple diamond represents the top-ranked SNP, rs10165869 and other SNPs are colored according to their r2 value in relation to that SNP. The recombination rate correlations and LD map were estimated on the basis of the 1000 Genomes Project EUR datasets.
CXCR4 (A) and ACKR3 (B) expression in pulmonary and cardiac microvascular endothelial cell (human) response to hypoxia in vitro: time course up to 48 hours
Acknowledgements
Molecular data for the Trans-Omics in Precision Medicine (TOPMed) programme was supported by the National Heart, Lung, and Blood Institute (NHLBI). Genome Sequencing for “NHLBI TOPMed: Genetic Epidemiology of Asthma in Costa Rica” (phs000988.v4.p1) was performed at the Northwest Genomics Center (HHSN268201600032I, 3R37HL066289-13S1). Genome Sequencing for “NHLBI TOPMed: Childhood Asthma Management Program (CAMP)” (phs001726.v1.p1) was performed at the Northwest Genomics Center (HHSN268201600032I). Genome sequencing for “NHLBI TOPMed: Genetic Epidemiology of COPD Study” (phs000951.v4.p4) was performed at the Broad Institute Genomics Platform (HHSN268201500014C) and the Northwest Genomics Center (3R01HL089856-08S1). Core support including centralised genomic read mapping and genotype calling, along with variant quality metrics and filtering, were provided by the TOPMed Informatics Research Center (3R01HL-117626-02S1; contract HHSN268201800002I). Core support including phenotype harmonisation, data management, sample-identity QC and general programme coordination were provided by the TOPMed Data Coordinating Center (R01HL-120393; U01HL-120393; contract HHSN268201800001I). We gratefully acknowledge the studies and participants who provided biological samples and data for TOPMed. The COPDGene project described was supported by Award Number U01 HL089897 and Award Number U01 HL089856 from the NHLBI. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI or the National Institutes of Health. The COPDGene project is also supported by the COPD Foundation through contributions made to an Industry Advisory Board comprising AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens and Sunovion. A full listing of COPDGene investigators can be found in the supplementary material. The TOPMed Banner Authorship List is provided in the supplementary material.
Support statement: This work was supported by the Industrial Core Technology Development Program (20000134) funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea); the Bio-Synergy Research Project (NRF-2017M3A9C4065964) of the Ministry of Science, ICT and Future Planning through the National Research Foundation; Cure Alzheimer’s Fund; the National Human Genome Research Institute (R01HG008976, 2U01HG008685); and the National Heart, Lung, and Blood Institute (P01HL132825, U01HL089856, U01HL089897, P01HL120839). Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: S. Lee has nothing to disclose. J.A. Lasky-Su has nothing to disclose. C. Lange has nothing to disclose. W. Kim has nothing to disclose. P.L. Kumar has nothing to disclose. M-L.N. McDonald has nothing to disclose. C.A. Vaz Fragoso has nothing to disclose. C. Laurie has nothing to disclose. B.A. Raby has nothing to disclose. J.C. Celedon has received research materials from GSK and Merck (inhaled steroids) and Pharmavite (vitamin D and placebo capsules), in order to provide medications free of cost to participants in NIH-funded studies, unrelated to the current work. M.H. Cho has nothing to disclose. S. Won has nothing to disclose. S.T. Weiss reports that he is an author for UpToDate, PI of several NIH grants and an unpaid advisor to Novartis Pharmaceuticals. J. Hecker has nothing to disclose.
Footnotes
This article has an online supplement.
Conflict of Interest Statements
References
- 1.Martinez FD, Vercelli D. Asthma. Lancet 2013: 382(9901): 1360–1372. [DOI] [PubMed] [Google Scholar]
- 2.Papadopoulos NG, Arakawa H, Carlsen KH, Custovic A, Gern J, Lemanske R, Le Souef P, Makela M, Roberts G, Wong G, Zar H, Akdis CA, Bacharier LB, Baraldi E, van Bever HP, de Blic J, Boner A, Burks W, Casale TB, Castro-Rodriguez JA, Chen YZ, El-Gamal YM, Everard ML, Frischer T, Geller M, Gereda J, Goh DY, Guilbert TW, Hedlin G, Heymann PW, Hong SJ, Hossny EM, Huang JL, Jackson DJ, de Jongste JC, Kalayci O, Ait-Khaled N, Kling S, Kuna P, Lau S, Ledford DK, Lee SI, Liu AH, Lockey RF, Lodrup-Carlsen K, Lotvall J, Morikawa A, Nieto A, Paramesh H, Pawankar R, Pohunek P, Pongracic J, Price D, Robertson C, Rosario N, Rossenwasser LJ, Sly PD, Stein R, Stick S, Szefler S, Taussig LM, Valovirta E, Vichyanond P, Wallace D, Weinberg E, Wennergren G, Wildhaber J, Zeiger RS. International consensus on (ICON) pediatric asthma. Allergy 2012: 67(8): 976–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fritz GK, McQuaid EL, Kopel SJ, Seifer R, Klein RB, Mitchell DK, Esteban CA, Rodriguez-Santana J, Colon A, Alvarez M, Canino G. Ethnic differences in perception of lung function: a factor in pediatric asthma disparities? Am J Respir Crit Care Med 2010: 182(1): 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcus BS, McAvay G, Gill TM, Vaz Fragoso CA. Respiratory symptoms, spirometric respiratory impairment, and respiratory disease in middle-aged and older persons. J Am Geriatr Soc 2015: 63(2): 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim KW, Ober C. Lessons Learned From GWAS of Asthma. Allergy Asthma Immunol Res 2019: 11(2): 170–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vicente CT, Revez JA, Ferreira MAR. Lessons from ten years of genome-wide association studies of asthma. Clin Transl Immunology 2017: 6(12): e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng X, Vonk JM, de Jong K, Xu X, Huo X, Boezen HM. No convincing association between genetic markers and respiratory symptoms: results of a GWA study. Respir Res 2017: 18(1): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijkstra AE, Boezen HM, van den Berge M, Vonk JM, Hiemstra PS, Barr RG, Burkart KM, Manichaikul A, Pottinger TD, Silverman EK, Cho MH, Crapo JD, Beaty TH, Bakke P, Gulsvik A, Lomas DA, Bosse Y, Nickle DC, Pare PD, de Koning HJ, Lammers JW, Zanen P, Smolonska J, Wijmenga C, Brandsma CA, Groen HJ, Postma DS, LifeLines Cohort Study g. Dissecting the genetics of chronic mucus hypersecretion in smokers with and without COPD. Eur Respir J 2015: 45(1): 60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayden LP, Cho MH, Raby BA, Beaty TH, Silverman EK, Hersh CP, Investigators CO. Childhood asthma is associated with COPD and known asthma variants in COPDGene: a genome-wide association study. Respir Res 2018: 19(1): 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hunninghake GM, Lasky-Su J, Soto-Quiros ME, Avila L, Liang C, Lake SL, Hudson TJ, Spesny M, Fournier E, Sylvia JS, Freimer NB, Klanderman BJ, Raby BA, Celedon JC. Sex-stratified linkage analysis identifies a female-specific locus for IgE to cockroach in Costa Ricans. Am J Respir Crit Care Med 2008: 177(8): 830–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunninghake GM, Soto-Quiros ME, Avila L, Ly NP, Liang C, Sylvia JS, Klanderman BJ, Silverman EK, Celedon JC. Sensitization to Ascaris lumbricoides and severity of childhood asthma in Costa Rica. J Allergy Clin Immunol 2007: 119(3): 654–661. [DOI] [PubMed] [Google Scholar]
- 12.Weiss ST. NHLBI TOPMed: The Genetic Epidemiology of Asthma in Costa Rica. [Google Scholar]
- 13.Covar RA, Fuhlbrigge AL, Williams P, Kelly HW, the Childhood Asthma Management Program Research G. The Childhood Asthma Management Program (CAMP): Contributions to the Understanding of Therapy and the Natural History of Childhood Asthma. Curr Respir Care Rep 2012: 1(4): 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. COPD 2010: 7(1): 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taliun D, Harris DN, Kessler MD, et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. bioRxiv 2019; preprint [ 10.1101/563866] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen WM. Robust relationship inference in genome-wide association studies. Bioinformatics 2010: 26(22): 2867–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laird NM, Horvath S, Xu X. Implementing a unified approach to family-based tests of association. Genet Epidemiol 2000: 19 Suppl 1: S36–42. [DOI] [PubMed] [Google Scholar]
- 18.Vaz Fragoso CA, McAvay G, Van Ness PH, Casaburi R, Jensen RL, MacIntyre N, Yaggi HK, Gill TM, Concato J. Phenotype of Spirometric Impairment in an Aging Population. Am J Respir Crit Care Med 2016: 193(7): 727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horvath S, Xu X, Lake SL, Silverman EK, Weiss ST, Laird NM. Family-based tests for associating haplotypes with general phenotype data: application to asthma genetics. Genet Epidemiol 2004: 26(1): 61–69. [DOI] [PubMed] [Google Scholar]
- 20.Fu L, Freishtat RJ, Gordish-Dressman H, Teach SJ, Resca L, Hoffman EP, Wang Z. Natural progression of childhood asthma symptoms and strong influence of sex and puberty. Ann Am Thorac Soc 2014: 11(6): 939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffmann TJ, Lange C, Vansteelandt S. Gene-environment interaction tests for dichotomous traits in trios and sibships. Genet Epidemiol 2009; 33: 691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssens R, Struyf S, Proost P. Pathological roles of the homeostatic chemokine CXCL12. Cytokine Growth Factor Rev 2018: 44: 51–68. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Martin L, Sanchez-Mateos P, Cabanas C. CXCR7 impact on CXCL12 biology and disease. Trends Mol Med 2013: 19(1): 12–22. [DOI] [PubMed] [Google Scholar]
- 24.Chang HC, Huang PH, Syu FS, Hsieh CH, Chang SL, Lu J, Chen HC. Critical involvement of atypical chemokine receptor CXCR7 in allergic airway inflammation. Immunology 2018: 154(2): 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ngamsri KC, Muller A, Bosmuller H, Gamper-Tsigaras J, Reutershan J, Konrad FM. The Pivotal Role of CXCR7 in Stabilization of the Pulmonary Epithelial Barrier in Acute Pulmonary Inflammation. J Immunol 2017: 198(6): 2403–2413. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, Qiu L, Zhang Y, Xu D, Zheng JC, Jiang L. CXCL12 enhances angiogenesis through CXCR7 activation in human umbilical vein endothelial cells. Sci Rep 2017: 7(1): 8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costello CM, Howell K, Cahill E, McBryan J, Konigshoff M, Eickelberg O, Gaine S, Martin F, McLoughlin P. Lung-selective gene responses to alveolar hypoxia: potential role for the bone morphogenetic antagonist gremlin in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2008: 295(2): L272–284. [DOI] [PubMed] [Google Scholar]
- 28.Costello CM, McCullagh B, Howell K, Sands M, Belperio JA, Keane MP, Gaine S, McLoughlin P. A role for the CXCL12 receptor, CXCR7, in the pathogenesis of human pulmonary vascular disease. Eur Respir J 2012: 39(6): 1415–1424. [DOI] [PubMed] [Google Scholar]
- 29.Lutz SM, Cho MH, Young K, Hersh CP, Castaldi PJ, McDonald ML, Regan E, Mattheisen M, DeMeo DL, Parker M, Foreman M, Make BJ, Jensen RL, Casaburi R, Lomas DA, Bhatt SP, Bakke P, Gulsvik A, Crapo JD, Beaty TH, Laird NM, Lange C, Hokanson JE, Silverman EK, Investigators E, Investigators CO. A genome-wide association study identifies risk loci for spirometric measures among smokers of European and African ancestry. BMC Genet 2015: 16: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilk JB, Shrine NR, Loehr LR, Zhao JH, Manichaikul A, Lopez LM, Smith AV, Heckbert SR, Smolonska J, Tang W, Loth DW, Curjuric I, Hui J, Cho MH, Latourelle JC, Henry AP, Aldrich M, Bakke P, Beaty TH, Bentley AR, Borecki IB, Brusselle GG, Burkart KM, Chen TH, Couper D, Crapo JD, Davies G, Dupuis J, Franceschini N, Gulsvik A, Hancock DB, Harris TB, Hofman A, Imboden M, James AL, Khaw KT, Lahousse L, Launer LJ, Litonjua A, Liu Y, Lohman KK, Lomas DA, Lumley T, Marciante KD, McArdle WL, Meibohm B, Morrison AC, Musk AW, Myers RH, North KE, Postma DS, Psaty BM, Rich SS, Rivadeneira F, Rochat T, Rotter JI, Soler Artigas M, Starr JM, Uitterlinden AG, Wareham NJ, Wijmenga C, Zanen P, Province MA, Silverman EK, Deary IJ, Palmer LJ, Cassano PA, Gudnason V, Barr RG, Loos RJ, Strachan DP, London SJ, Boezen HM, Probst-Hensch N, Gharib SA, Hall IP, O’Connor GT, Tobin MD, Stricker BH. Genome-wide association studies identify CHRNA5/3 and HTR4 in the development of airflow obstruction. Am J Respir Crit Care Med 2012: 186(7): 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Regions of chromatin contact for rs10165869 in lung tissue. Blue lines reflect -log(p-value) for the one-tailed test of whether observed Hi-C counts (black lines) were greater than the expected number of Hi-C counts (solid red lines). ACKR3 is significantly associated with rs10165869 after tissue-specific False Discovery Rate (FDR, dashed red) and Bonferroni (dashed purple) corrections.
Regional plot for an exertional dyspnea-associated locus in 400 kb upstream and downstream regions. Purple diamond represents the top-ranked SNP, rs10165869 and other SNPs are colored according to their r2 value in relation to that SNP. The recombination rate correlations and LD map were estimated on the basis of the 1000 Genomes Project EUR datasets.
CXCR4 (A) and ACKR3 (B) expression in pulmonary and cardiac microvascular endothelial cell (human) response to hypoxia in vitro: time course up to 48 hours


