Abstract
Background
Elephantopus scaber (ES) and Sauropus androgynous (SA) have been frequently reported to possess antibacterial activity through in vitro, but in vivo studies about the protective effect of combined ES and SA have acquired less attention.
Objectives
To evaluate protective effect of combined ethanol extract of ES and SA on hormone imbalance and renal and hepatic necrosis formation in Escherichia coli-infected pregnant mice.
Materials and methods
A total of 28 pregnant Balb/c mice were divided into seven groups (n = 4): control, E. coli-infected pregnant mice, infected pregnant mice received 200 mg/kg ES, infected pregnant mice received combined 150 mg/kg ES and 37.5 mg/kg SA (75:25), 100 mg/kg ES and 75 mg/kg SA (50:50), 50 mg/kg ES and 112.5 mg/kg SA (25:75), and only 150 mg/kg SA. Pregnant mice were orally treated with combined ES and SA on day 1–4th of pregnancy. On the 4th day, mice were infected with 107 CFU/mL of E. coli and continuously treated with ES and SA until the 16th day of pregnancy. After treatment, the kidney and liver were prepared for histological examination using H&E staining. The blood serum was collected in each stage of pregnancy and measured by ELISA assays.
Results
Combined ES and SA gave an impact on altering the prolactin level. Combined ES and SA at ratio dose 75:25 was able to restore progesterone to normal levels (P < 0.05). The level of estradiol (E2) was relatively stable in the presence of E. coli and treatment. Treatment with 200 mg/kg ES, combined 50 mg/kg ES and 112.5 mg/kg SA (25:75) and 100 mg/kg ES and 75 mg/kg SA (50:50) demonstrated an immunomodulatory effect on the Gr1+ cell of E. coli treated-pregnant mice. E. coli infection significantly increased renal tubules and hepatic necrosis in pregnant mice compared to control (P < 0.05). Combined SA and ES at ratio dose 75:25 significantly demonstrated remarkable renal and hepatic protection activity in infected pregnant mice.
Conclusion
The present study provided the establishment of combined ES and SA could be used to invent potent hormonal balancing agent and hepato-renal protective agent in infected pregnant mice.
Keywords: E. coli, Elephantopus scaber, Hormonal, Pregnant, Sauropus androgynous
1. Introduction
Escherichia coli commonly lives in the human intestine and plays a crucial role in decomposition [1]. E. coli as a pathogenic bacteria has easily infected human body, especially pregnant women who have a higher risk of developing urinary tract infection [2,3]. The physiological changes of pregnancy are significantly associated with urinary tract infection. Hormonal changes during pregnancy are also believed to affect urinary tract infection. During pregnancy, an increase in progesterone level causes the ureters muscle tone to relax. Decreased bladder capacity significantly results in urinary frequency [4]. In pregnancy, as the uterus starts growing, mechanical compression of the urinary tract begins and has more incidence of infections. Urinary tract infection may cause many complications in pregnant women as well as in neonates [5]. The infection of enterohemorrhagic E. coli exhibited an acute renal failure in the germ-free mouse model. Significant lesions were observed in the kidney and consisted of acute tubular necrosis and glomerular capillary red blood cell sludging accompanied by fibrin thrombi [6]. A possible mechanism may contribute to the progression of E. coli infection in pregnancy is an endotoxin that produced by its bacteria. During infection, estrogen cause an impairment of hepatic function, thus enhance susceptibility to E. coli infection [[7], [8], [9]].
The increasing of progesterone during pregnancy also affect to mature dendritic cell function included inhibition of pro-inflammatory cytokines (TNF-α and IL-1) secretion, downregulation of cell surface protein expression, and decreased T cell proliferation [10]. The combination of these factors makes the kidney and liver in pregnant women more easily infected by E. coli [11,12]. E. coli affect the liver organs through the hepatic port vein from the stomach and intestines [13]. The E. coli cell wall contains two primary proteins, i.e. α-hemolysin (HlyA) and lipopolysaccharide (LPS), which are associated with tissue damage [1]. When E. coli infect the host cell, it causes immune cell activation to eliminate E. coli. Furthermore, there is an increase in cytokine production which results in an increase in interleukin production, such as IL-6 and IL-8 which trigger an inflammatory response that lead to necrosis and impacts on host cell inflammation [14].
Various cases of E. coli infection can be diminished through natural product administration such as Tapak Liman (Elephantopus scaber L./ES) and Katuk (Sauropus androgynus L. Merr./SA). Katuk (S. androgynus) leaves commonly used to increase milk production during lactation in women and consumed as a vegetable in Indonesia [15,16]. This plant also used to treat cardiovascular diseases and hypertension in Malaysia [17]. In China, the leaves of this plant can be used as a natural slimming agent and reduce fever [18]. In Thailand, the root of Katuk could reduce fever, and also used as an antiseptic agent [19]. Furthermore, Indian people traditionally use the leaves of Katuk as an antidiabetic and to treat eye disease, tonsillitis and ulcers [20]. Both plants are believed to minimize the adverse effects of E. coli infection on pregnant women. Flavonoid compounds in S. androgynus leaves consist of two types, i.e. apigenin and luteolin, which have been proven to inhibit the action of LPS from Gram-negative bacteria [21]. E. scaber leaves contain many antimicrobial compounds, i.e. alkaloids, steroids, tannins, and phenols which play a role in inhibiting the growth of E. coli [22]. However, the overconsumption of natural product may have several toxic effects [23]. Liver is the main organ that plays a role in the detoxification process so that it becomes the main target of toxic substances [24]. Meanwhile, the kidneys have a significant role in the secretion process so that toxic compounds will directly affect the kidneys [25].
Currently, the research about the effect ES and SA combination in the infected pregnant mice are still limited. This research aimed to evaluate the protective effects of the combined ethanol leaf extract of ES and SA against renal and hepatic necrosis formation in pregnant mice infected by E. coli. This study also measured the hormonal changes during pregnancy caused by E. coli infection and treatment with combined ES and SA.
2. Materials and methods
2.1. Ethics
All experimental procedures were approved by the Animal Care and Use Committee of Brawijaya University (Approval ref no: 902-KEP-UB). The study was carried out from April 2019 to December 2019.
2.2. Bacterial preparation
E. coli was bought from the Laboratory of Microbiology, Faculty of Medicine, Brawijaya University, Malang, Indonesia. E. coli was grown in NA (Nutrient Agar) media and then inoculated in 50 mL of NB (Nutrient Broth) media. After incubated at room temperature (37 °C) for 2 h, the number of the bacterial cell was counted by hemocytometer. 1 mL of bacteria suspension was centrifuged at 10,000 rpm, 4 °C, for 5 min. The pellet was washed twice and resuspended in 1 mL phosphate buffer saline (PBS). The concentration of bacterial cell suspension was 1 × 107 CFU/mL. Then, 0.1 mL of E. coli were injected intraperitoneally in mice.
2.3. Plant extract preparation
Fresh leaves of E. scaber and S. androgynus were collected from UPT Materia Medica Batu, Malang, East Java, Indonesia. The plants were identified and confirmed by UPT Materia Medica Batu. The specimen number (074/228/102.7/2018; 074/229/102.7/2018) were deposited in the herbarium of the Biology Department for future reference. The leaves were cleaned, dried in the oven at 40 °C and ground into powder using laboratory blender. 100 g of powdered samples from each plant were separately extracted by steeping in 900 mL of 95% ethanol three times overnight and placed in a dark place. At the end of extraction, the samples were filtered by Whatman filter paper No. 1 (Whatman Ltd., England). The filtered extracts were evaporated to dryness using a rotary evaporator at 40 °C and then refrigerated at 4 °C for further analysis. The concentration of crude extract in each plant was prepared at the different concentration for animal treatment.
2.4. Animals
A total of 63 female BALB/c mice (weight 20–25 g, 6 weeks of age) were obtained from LPPT Gadjah Mada University, Yogyakarta, Indonesia. The mice were maintained in cages under the constant condition: free access was allowed to standard diet and water, controlled light cycle (12 h light/12 h dark) and controlled temperature (22 ± 2 °C). All animals were acclimatized for 1 week before the beginning of the study.
2.5. Experimental design
After the acclimatization period, the mice were randomly mated by putting female and male mice into the same cage. After a vaginal plug was found, a vaginal swab was performed to determine the metestrus phase of female mice. The presence of vaginal plug and metestrus phase in female mice was considered as day 1 of gestational period.
A total of 28 pregnant female BALB/c mice were randomly grouped into seven groups (n = 4) and treated as follows: control groups (healthy mice) (N), pregnant mice infected with E. coli (C+), pregnant mice infected with E. coli and treated with 200 mg/kg ES (C1), pregnant mice infected with E. coli and treated with 150 mg/kg ES and 37.5 mg/kg SA (75:25) (C2), pregnant mice infected with E.coli and treated with 100 mg/kg ES and 75 mg/kg SA (50:50) (C3), pregnant mice infected with E. coli and treated with 50 mg/kg ES and 112.5 mg/kg SA (25:75) (C4), and pregnant mice infected with E. coli and treated with 150 mg/kg SA (C5). We also observed the effect of combined ES and SA on histopathological changes at a dose of 100 mg/kg ES and 75 mg/kg SA (50:50) in uninfected pregnant mice.
2.6. E. coli injection and herbal treatment
The pregnant mice received combined ES and SA by oral gavages daily for 16 days of mice pregnancy, starting from 1st to 16th days of pregnancy. The mice were injected by a single-dose intraperitoneal injection of 107 CFU/mL E. coli dissolved in 0.1 mL of phosphate buffer saline (PBS) at 5th days of pregnancy. E. coli infection was confirmed 24 h after injection by collecting tail vein blood. E. coli were detected using the Gram Staining and Catalase Test [26,27]. The results confirmed that E. coli had successfully infected C+ and C1–C5 groups.
2.7. ELISA assay
At the end of the treatment period, the blood serum was collected from the orbit of the mice eye. The progesterone and estrogen kit used in this study is an enzyme-linked immunosorbent assay (ELISA) for the detection of both hormone developed by Elabscience® (USA) with catalogue no: E-EL-0090 for Pg (Progesterone) ELISA kit, catalogue no: E-EL-0065 for E2 (Estradiol) ELISA kit and catalogue no. E-EL-M0083 for PRL (Prolactin).
2.8. Flow cytometry analysis
Bone marrow cells were isolated from femur and tibia of mice by flushing out method. Suspension of the cells was centrifuged at 2500 rpm, 4 °C, for 5 min. Cells were stained with a specific antibody: FITC anti-mouse Ly-6G/Ly-6C (Granulocyte receptor-1/Gr-1) Antibody (Biolegend, San Diego, CA). Each sample was then analyzed using flow cytometer (BD Bioscience FACS Calibur™). Data were analyzed by BD cell quest Pro™ and then performed the statistical analysis.
2.9. Histopathological examination
The mice were sacrificed by cervical dislocation. The kidney and liver were isolated immediately and then washed in PBS. The liver and kidney were fixed in 10% formalin for 24 h. Then, the samples were processed by the standard procedure of paraffin embedding. The sections of about 5 μm were cut and stained with hematoxylin and eosin (H&E) [28,29]. The histological changes of kidney and liver were observed under BX51 light microscope with 10×40 magnification. The necrotic cell was calculated at 10 fields of view. The calculation of the necrotic cell was used as qualitative data. The photomicrograph of liver and kidney histology was also documented [30].
2.10. Statistical analysis
Data were analyzed using Two-Way ANOVA and continued by the Tukey test. P-values <0.05 were considered statistically significant. All statistics were performed using SPSS version 20.0 for windows.
3. Results
3.1. Serum prolactin level
The results showed that the levels of prolactin significantly increased (p < 0.05) at the end of the pregnancy period (day-16) in healthy pregnant mice. However, infection with E. coli demonstrated a high decrease in prolactin levels of pregnant mice at the end of pregnancy (day-16) (Table 1). Administration of 150 mg/kg of SA (C5) exhibited a high level of prolactin at day-12, which indicated that the ethanol extract of SA improving the prolactin level in infected-pregnant mice at mid-pregnancy. Furthermore, at the end of the pregnancy period, combined ES and SA at ratio dose of 25:75 (C4) showed a high level of prolactin. This finding suggests that combined ES and SA also gave an impact on altering the prolactin level.
Table 1.
Effect of combined E. scaber (ES) and S. androgynus (SA) extract on prolactin levels of infected pregnant mice.
| Group | Prolactin levels (ng/mL) |
||
|---|---|---|---|
| Day-8 | Day-12 | Day-16 | |
| N | 15.21 ± 0.47b | 12.76 ± 0.20b | 38.95 ± 0.82d |
| C+ | 11.62 ± 0.51a | 16.74 ± 1.56c | 14.00 ± 0.60a |
| C1 | 19.01 ± 0.85c | 6.66 ± 1.37a | 20.97 ± 1.01b |
| C2 | 20.34 ± 1.53c | 13.67 ± 1.43b | 9.93 ± 0.76a |
| C3 | 28.45 ± 0.48d | 9.82 ± 2.01a | 28.05 ± 1.30c |
| C4 | 23.47 ± 0.56c | 14.99 ± 1.60c | 31.00 ± 1.11c |
| C5 | 13.32 ± 1.13b | 35.44 ± 0.24d | 24.83 ± 0.45c |
Values were expressed as mean ± SD, n = 4. Healthy pregnant mice were not subjected to E. coli infection (N), pregnant mice infected with E. coli (C+), pregnant mice infected with E. coli and treated with 200 mg/kg of ES (C1), pregnant mice infected with E. coli and treated with combined 150 mg/kg ES and 37.5 mg/kg SA (75% ES: 25% SA), 100 mg/kg ES and 75 mg/kg SA (50% ES: 50% SA), 50 mg/kg ES and 112.5 mg/kg SA (25% ES: 75% SA) (C2, C3, C4, respectively), pregnant mice infected with E. coli and treated with 150 mg/kg SA (C5). Different superscript letters indicate statistical significance (p < 0.05).
3.2. Serum estradiol level
At the early pregnancy period (day-8), the level of estradiol in all treated pregnant mice was relatively stable until the end of the pregnancy period (day-16). However, there were not a significant decrease in serum estradiol (E2) levels in pregnant mice after E. coli infection (p > 0.05) until the end of pregnancy (day-16) as compared with the control group (Table 2). Furthermore, there is no significant effect of all treatment group in pregnant mice. The results revealed that 1 × 107 CFU/mL of E. coli did not produce significant changes on the estrogen levels in pregnant mice. The levels of estrogen at all combined ES and SA group was relatively stable.
Table 2.
Effect of combined E. scaber (ES) and S. androgynus (SA) extract on estradiol levels of infected pregnant mice.
| Group | Estradiol levels (pg/mL) |
||
|---|---|---|---|
| Day-8 | Day-12 | Day-16 | |
| N | 3.29 ± 0.008a | 3.42 ± 0.008a | 3.30 ± 0.014a |
| C+ | 3.28 ± 0.010a | 3.26 ± 0.004a | 3.26 ± 0.005a |
| C1 | 3.30 ± 0.025a | 3.33 ± 0.052a | 3.27 ± 0.010a |
| C2 | 3.30 ± 0.020a | 3.30 ± 0.034a | 3.28 ± 0.018a |
| C3 | 3.29 ± 0.047a | 3.37 ± 0.030a | 3.20 ± 0.009a |
| C4 | 3.40 ± 0.116a | 3.33 ± 0.054a | 3.30 ± 0.012a |
| C5 | 3.30 ± 0.018a | 3.42 ± 0.031a | 3.18 ± 0.008a |
Values were expressed as mean ± SD, n = 4. Healthy pregnant mice were not subjected to E. coli infection (N), pregnant mice infected with E. coli (C+), pregnant mice infected with E. coli and treated with 200 mg/kg of ES (C1), pregnant mice infected with E. coli and treated with combined 150 mg/kg ES and 37.5 mg/kg SA (75% ES: 25% SA), 100 mg/kg ES and 75 mg/kg SA (50% ES: 50% SA), 50 mg/kg ES and 112.5 mg/kg SA (25% ES: 75% SA) (C2, C3, C4, respectively), pregnant mice infected with E. coli and treated with 150 mg/kg SA (C5). Different superscript letters indicate statistical significance (p < 0.05).
3.3. Serum progesterone level
We also measured the level of serum progesterone concentration in all treated group. The serum progesterone level was significantly increased (p < 0.05) in healthy pregnant mice at the mid-pregnancy (day-12) and then tend to decrease at the end of pregnancy. This decrease occurred because of the prolactin surge during late pregnancy period (Table 1).
Infection with E. coli at day 5th of pregnancy did not significantly reduce progesterone levels until day 8th of pregnancy. However, the decline in the progesterone levels was observed at the mid-pregnancy (day-12) (Table 3). At this period, the progesterone level was typically increased and then tended to decrease at the end of pregnancy. But, the level of progesterone was significantly reduced (p < 0.05) after E. coli infection by 3.09 pg/mL compared to healthy pregnant mice at the mid-pregnancy. The results proved that 107 CFU/mL of E. coli causes a decrease in the levels of serum progesterone in pregnant mice.
Table 3.
Effect of combined E. scaber (ES) and S. androgynus (SA) extract on progesterone levels of infected pregnant mice.
| Group | Progesterone levels (pg/mL) |
||
|---|---|---|---|
| Day-8 | Day-12 | Day-16 | |
| N | 2.93 ± 0.008a | 3.22 ± 0.008a | 2.92 ± 0.014a |
| C+ | 2.92 ± 0.010a | 3.09 ± 0.004b | 2.93 ± 0.005a |
| C1 | 2.93 ± 0.025a | 3.09 ± 0.052b | 2.94 ± 0.010a |
| C2 | 2.92 ± 0.020a | 3.22 ± 0.034a | 2.91 ± 0.018a |
| C3 | 2.93 ± 0.047a | 3.14 ± 0.030b | 2.92 ± 0.009a |
| C4 | 2.93 ± 0.116a | 3.19 ± 0.054b | 2.92 ± 0.012a |
| C5 | 2.93 ± 0.018a | 3.12 ± 0.031b | 2.90 ± 0.008a |
Values were expressed as mean ± SD, n = 4. Healthy pregnant mice were not subjected to E. coli infection (N), pregnant mice infected with E. coli (C+), pregnant mice infected with E. coli and treated with 200 mg/kg of ES (C1), pregnant mice infected with E. coli and treated with combined 150 mg/kg ES and 37.5 mg/kg SA (75% ES: 25% SA), 100 mg/kg ES and 75 mg/kg SA (50% ES: 50% SA), 50 mg/kg ES and 112.5 mg/kg SA (25% ES: 75% SA) (C2, C3, C4, respectively), pregnant mice infected with E. coli and treated with 150 mg/kg SA (C5). Different superscript letters indicate statistical significance (p < 0.05).
The effect of combined ES and SA in infected pregnant mice was observed at different ratio of ES and SA concentrations which is expected to get the effective dose combination in the modulating hormonal levels. Progesterone levels of C2 group exhibited an increase of 3.22 pg/mL at the middle of pregnancy (day-12) (p < 0.05) (Table 2). These findings revealed that 150 mg/kg ES and 37.5 mg/kg SA (75:25) was able to restore progesterone levels to normal levels. Compared with the other ES and SA combination treatments, the combination of 150 mg/kg ES and 37.5 mg/kg SA (75:25) exhibited a high level of progesterone at the mid-pregnancy.
3.4. Bone marrow Gr1+ cell
During E. coli infection, the bone marrow accelerates the production of granulocytes to support the recruitment of phagocytes cells into the site of inflammation. Mice infected with E. coli showed 40.1% and 41.2% Gr1+ cells in the bone marrow at day-8 and day-16 of pregnancy, respectively, compared with only 30.7% and 31.6% in healthy pregnant mice (Fig. 1A, B). These findings indicated that there was increased recruitment of Gr1+ cells in the response of systemic E. coli infection. The excessive Gr1+ response has consequences on the inflammatory response. Furthermore, the Gr1+ cells recruitment triggered by E. coli was significantly reduced at C1 and C2 group at day-8 (Table 4). At day-16, a significant decrease of Gr1+ was found in C1, C2 and C3 group. It was indicated that ES treatment (C1) and combined ES and SA treatment at ratio dose 75:25 (C2) and 50:50 (C3) demonstrated an immunomodulatory effect on the Gr1+ cell of E. coli treated-mice.
Fig. 1.
The relative number of Gr1+ cells after combined ethanolic extract of E. scaber (ES) and S. androgynus (SA) administration in pregnant mice with E. coli infection. Flow cytometry analysis of Gr1+ cells in infected pregnant mice at (A). Day-8, and (B). Day- 16 of pregnancy period of mice. Note: N: healthy pregnant mice; C+: pregnant mice infected with E. coli; C1: pregnant mice infected with E. coli and treated with 200 mg/kg of ES; C2–C4: pregnant mice infected with E. coli and treated with combined 150 mg/kg ES and 37.5 mg/kg SA (75:25); 100 mg/kg ES and 75 mg/kg SA (50:50); 50 mg/kg ES and 112.5 mg/kg SA (25:75); and C5: pregnant mice infected with E. coli and treated with 150 mg/kg SA.
Table 4.
Effect of combined E. scaber (ES) and S. androgynus (SA) extract on the relative number of Gr1+ cell of infected pregnant mice.
| Group | Gr1+ cell (%) |
|
|---|---|---|
| Day-8 | Day-16 | |
| N | 30.7 ± 0.008a | 31.6 ± 0.014a |
| C+ | 40.1 ± 0.010b | 41.2 ± 0.005b |
| C1 | 34.4 ± 0.025a | 29.9 ± 0.010a |
| C2 | 34.5 ± 0.020a | 33.1 ± 0.018a |
| C3 | 39.2 ± 0.047b | 31.4 ± 0.009a |
| C4 | 42.4 ± 0.116b | 38.3 ± 0.012b |
| C5 | 39.6 ± 0.018b | 44.0 ± 0.008b |
Values were expressed as mean ± SD, n = 4. Healthy pregnant mice were not subjected to E. coli infection (N), pregnant mice infected with E. coli (C+), pregnant mice infected with E. coli and treated with 200 mg/kg of ES (C1), pregnant mice infected with E. coli and treated with combined 150 mg/kg ES and 37.5 mg/kg SA (75% ES: 25% SA), 100 mg/kg ES and 75 mg/kg SA (50% ES: 50% SA), 50 mg/kg ES and 112.5 mg/kg SA (25% ES: 75% SA) (C2, C3, C4, respectively), pregnant mice infected with E. coli and treated with 150 mg/kg SA (C5). Different superscript letters indicate statistical significance (p < 0.05).
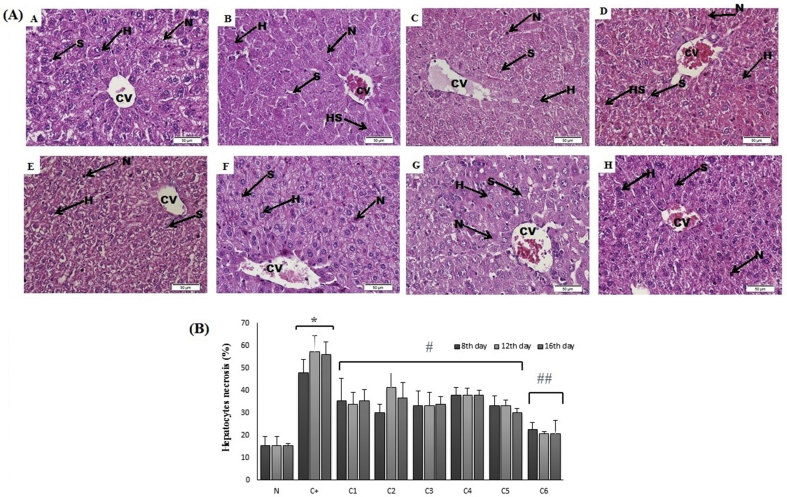
3.5. Hepatic histopathological findings
The liver of healthy pregnant mice showed normal hepatic architecture that indicated by hepatic lobule with a thin wall central vein (CV) and hepatic sinusoid (S) surrounded with normal radiating hepatocytes (Fig. 2A). Normal hepatocytes were arranged in a single cell cord and joined to each other. The shape of normal hepatocytes was polygonal with a small round nucleus and granular cytoplasm. At normal condition, the necrotic cell was also found in normal hepatic tissue, but the level is in a normal range. The level of hepatocytes necrosis in healthy pregnant mice was relatively similar to those found in early, mid and the end of pregnancy period (15.33%) (Fig. 2B).
Fig. 2.
(A) Photomicrograph of liver section stained with H&E (400× magnification). H (hepatocytes), S (sinusoids), CV (central vein), HS (hydropic swelling) and N (necrosis cell); (B) The level of hepatocytes necrosis after combined ethanol extract of E. scaber (ES) and S. androgynus (SA) administration in pregnant mice with E. coli infection. The error bar indicated the standard error of the means. ∗p < 0.05 versus control group, #p < 0.05 versus infected pregnant mice, ##p < 0.05 versus C1–C5. Note: N: healthy pregnant mice; C+: pregnant mice infected with E. coli; C1: pregnant mice infected with E. coli and treated with 200 mg/kg of ES; (C2, C3, C4) pregnant mice infected with E. coli and treated with combined 150 mg/kg ES and 37.5 mg/kg SA (75:25), 100 mg/kg ES and 75 mg/kg SA (50:50), 50 mg/kg ES and 112.5 mg/kg SA (25:75); C5: pregnant mice infected with E. coli and treated with 150 mg/kg SA; and C6: pregnant mice treated with 100 mg/kg ES and 75 mg/kg SA (50:50).
However, the liver tissue of infected pregnant mice showed a liver architecture destruction along with dis-arrangement of hepatocytes. Hepatocytes around central vein showed a high level of necrosis. Furthermore, a high level of the injured cell was found in the infected liver of pregnant mice which indicated by hydropic cell swelling (Fig. 2B). The hydropic cell swelling was characterized by swollen hepatocytes with loss of brush border between the cell and increasing of nuclei away from the normal position. The level of hepatocytes necrosis was significantly increased (p < 0.05) in infected pregnant mice in a time-dependent manner of pregnancy period. The level of hepatocytes necrosis at early, mid and the end of pregnancy period was 48%, 57.33% and 56%, respectively (Fig. 2B). Furthermore, the infection of E. coli causes severe fetal absorption indicated the loss of fetal at the end of the pregnancy period (16th days of mice pregnancy, data not shown).
Treatment with combined ES and SA at all dose performed protective effect in the liver of infected pregnant mice indicated by the decreasing of necrotic cells. Combined ES and SA at dose 100 mg/kg ES and 75 mg/kg SA (C3) significantly (p < 0.05) decreased the level of necrosis cell in the liver of infected pregnant mice compared to infected pregnant mice. The decreasing of necrotic cells level were found at early (8th days of pregnancy), mid (12th days of pregnancy) and the end of pregnancy period (16th days of gestation) with the average of the necrotic cell of 33.3%, 33.3% and 34%, respectively. The number of necrotic cells at the C3 group was lower than the other group with combined ES and SA at a different ratio.
Administration of ES (C1) or SA (C5) alone also gave the protective effect to the liver of infected pregnant mice indicated by the decreasing of hepatocytes necrosis at early, mid and the end of pregnancy period (ES: 35.33%, 34%, and 35.33%, respectively; SA: 33.33%,33.33%, and 30%, respectively). In this study, we also measured the level of necrosis in the pregnant mice treated with combined ES and SA at ratio 50:50 (100 mg/kg ES and 75 mg/kg SA) to know the toxicity of both plant extract. The results showed that combined ES and SA at appropriate combination did not perform toxicity which indicated by the lowest necrotic cell in the liver and reached the normal level of the necrotic cell.
3.6. Renal histopathological findings
This study found that normal pregnant mice showed a normal structure of renal cortex and glomerular tufts (Fig. 3A). Healthy pregnant mice also showed the normal structure of renal corpuscle with normal glomeruli and tubules. Most of the cells were normal, and both tubules (proximal and distal) were distinguished. After infection with E. coli, the kidney of pregnant mice showed several changes in the structure of most renal corpuscles, including diminished and distorted glomeruli, dilated tubules, renal tubule necrosis and entrapped red blood cells. The percentage of renal tubules necrosis and glomerular capillary red blood cell entangled in infected pregnant mice was higher than healthy pregnant mice (Fig. 3B).
Fig. 3.
(A) Light micrograph of the kidney section from different treatment groups. G (glomerulus), P (proximal tubule), D (distal tubule), and N (necrosis cell). Original magnification ×400. (B) The level of hepatocytes necrosis. The error bar indicated the standard error of the means. ∗p < 0.05 versus control group, #p < 0.05 versus infected pregnant mice, ##p < 0.05 versus C1–C4 and C6. Note: N: healthy pregnant mice; C+: pregnant mice infected with E. coli; C1: pregnant mice infected with E. coli and treated with 200 mg/kg of ES; (C2, C3, C4) pregnant mice infected with E. coli and treated with combined 150 mg/kg ES and 37.5 mg/kg SA (75:25), 100 mg/kg ES and 75 mg/kg SA (50:50), 50 mg/kg ES and 112.5 mg/kg SA (25:75); C5: pregnant mice infected with E. coli and treated with 150 mg/kg SA; and C6: pregnant mice treated with 100 mg/kg ES and 75 mg/kg SA (50:50).
Histopathological studies of the kidney sections of infected pregnant mice treated with all dose of combined ES and SA showed restoration of normal renal architecture with the disappearance of tubules necrosis and glomerular capillary red blood cell entrapped at 8th, 12th and 16th of pregnancy period (Fig. 3A). A kidney of combined ES and SA at C1, C2, C3, and C4 showed a lower number of tubule necrosis compared to infected pregnant mice (p < 0.05) (Fig. 3B). The administration of combined ES and SA in pregnant mice without E. coli infection increased the percentage of renal tubules necrosis but lower than infected pregnant mice.
4. Discussion
An effect of E. coli infection in pregnant mice has been observed in this study and have an impact on the outcome of pregnancy such as fetal reabsorption (data not shown), hormonal changes and histopathological changes of kidney and liver. The low levels of estradiol increase the susceptibility to E. coli infection in pregnancy [31]. Lack of estrogen levels in infected pregnant mice causes miscarriage (data not shown). However, the levels of estrogen did not significantly decrease in this study.
We also observed the progesterone serum levels in pregnant mice and its secretion depends on LH stimulation which increases during the first few days of pregnancy. On the 10th day of pregnancy, the decrease in progesterone concentration in blood circulation is related to the prolactin surge during pregnancy. Progesterone secretion commonly increases at a maximum level before late pregnancy. On the 18th day, the concentration of progesterone decreased significantly and continued until birth [31,32]. In this study, hormonal patterns in pregnant mice exhibited a similar pattern. According to Barkley et al. [32], the serum progesterone tends to increase at the mid-pregnancy and continued to decrease at the end of the pregnancy period.
Aisemberg et al. [33] stated that progesterone plays a vital role in the reproductive system and maintaining pregnancy. The significance of this hormone for the successful pregnancy is indicated by inhibiting the hormonal binding sites which cause abortion in humans and some animal species. There was a close relationship between the levels of progesterone in the blood circulation with the mice pregnancy. LPS injection on the 7th day of Balb/c mice pregnancy showed embryo reabsorption after 24 h LPS infection. The level of progesterone at 12 h and 24 h after LPS injection significantly decreased 60%. Furthermore, when injected with synthetic progesterone, embryo reabsorption levels were increased [33]. Aisemberg’s study [33] supported our research that the presence of E. coli has an impact on the level of progesterone in Balb/c mice during pregnancy. This results indicated that progesterone is significantly needed in the pregnancy, especially in the presence of E. coli infection in pregnant condition.
An increase in progesterone levels is vital for maintaining pregnancy and embryonic development, which resulted in the preventing of miscarriage. Only C2 group produced the synergistic effect to increase progesterone levels in infected pregnant mice at mid-gestation. At the end-gestation, the progesterone levels tend to decrease because of the presence of progesterone at this stage, usually at a low level. It is indicated by decreasing progesterone concentration in all treatment group at the end of pregnancy period (16th day) (Table 3).
The necrotic cells found in the infected pregnant mice were caused by E. coli infection resulted in the damaging of liver tissue. E. coli is a type of bacteria which easily infect the digestive system of the host. E. coli infection in the gastrointestinal tract could spread to the liver through the hepatic port vein from the stomach and intestines [13]. During pregnancy, the mice were more easily infected with E. coli because of an increase in progesterone which suppresses TNF-α secretion, inhibits cytokine production and suppress innate immune responses such as macrophages and NK (Natural killer) cells [11]. Therefore, there were found more necrotic liver cells in the infected pregnant mice compared to healthy mice and extract combination treatment.
The administration of combined ES and SA extract could play a role in reducing hepatocytes necrosis due to E. coli infection. Tapak Liman (E. scaber/ES) and Katuk (S. androgynus/SA) contains a high anti-inflammatory and antibacterial compounds which significantly prevent the negative effect of E. coli infection [34]. Katuk (SA) leaves were exhibited to have the highest content of flavonoids i.e quercetin, myricetin, luteolin, apigenin, and kaempferol which detected as a strong antibacterial agent [35]. The study by Usman et al. [36] showed that luteolin exhibited antibacterial activity against methicillin-resistant S. aureus and its antibacterial activity could be enhanced by quercetin addition.
The role of SA also exhibited the protective effect on the liver, kidney and spleen histopathology during AF exposure by decreasing of the severe liver necrosis and degeneration, and also prevent the necrotic cell and inflammation of tubules epithelial kidney [36]. Methanolic extract of S. androgynus exhibited antibacterial activity against Salmonella typhimurium and Klebsiella pneumonia [37]. Apigenin and luteolin contained in SA inhibit the effect of lipopolysaccharide proteins from Gram-negative bacteria [38]. Katuk (S. androgynus/SA) plays an essential role in the hemato–protective activity, which can repair tissue damage in the liver. Flavonoid compounds in SA act as antioxidants by stabilizing the free radicals which are accumulated in the liver due to exposure to hazardous compounds [39]. Flavonoids eliminated free radicals by releasing hydrogen atoms from their hydroxyl groups. The phenolic hydroxyl (OH) group from flavonoids functions as reducing compound which can accommodate hydroxyl and superoxide radicals; thus, it protects membrane lipids from free radical reactions which can damage tissue [40,41].
Tapak liman (E. scaber/ES) contains many bioactive compounds responsible for antibacterial effect [42]. The terpenoid derivates of E. scaber has been reported its efficacy in urinary tract infection by inhibiting the growth of Extended Spectrum β Lactamase (ESBL)-producing Methicillin Resistant Staphylococcus Aureus (MRSA) bacteria [43]. In silico study also found that the novel terpenoid isolated from ES exhibited antibacterial activity by inhibiting the activity of autolysin and forming a strong atomic interaction with the active site residues for treating S. aureus. E. scaber could prevent autolysin from remodelling the cell wall by binding to peptidoglycan, therefore inhibit bacterial growth [44]. Tannins are known as a general antimicrobial agent and antioxidant [45]. Tannin in ES extract could act as an antimicrobial agent by degrading the bacterial cell membranes. E. scaber root ethanol extract exhibits the highest activity against E. coli, S. aureus, E. faecalis, P. aeruginosa and P. mirabilis followed by E. scaber leaves ethanol extract [46]. The previous study revealed that herbal supplement formula of E. scaber and S. androgynus promotes IL-2 cytokine produced by CD4+ T cells in pregnant mice with S. typhi infection. The combination of both plant extract lead to synergistic effects in stimulating the immune system by modulating IL-2 secretion [47,48].
The previous study showed that E. scaber 75% and S. androgynus 25% was able to modulate the activation of macrophage and B lymphocytes in the bone marrow of pregnant mice during bacterial infection [49]. In this study, we proved that at the proper dose of combined ES and SA did not cause toxicity in the liver and kidney. All doses of treated pregnant mice with combined ES and SA showed a low renal tubules necrosis compared to the positive control. Treatment with 150 mg/kg ES and 37.5 mg/kg SA (75% ES: 25% SA) on infected pregnant mice have the lowest level of renal tubule necrosis compared to other treated pregnant mice. Combined ES and SA at ratio dose of 75% ES: 25% SA also decreased the level of necrosis cell in the liver of infected pregnant mice.
Flow cytometry analysis revealed that there was an increase in GR1+ cell level after E. coli challenge in pregnant mice compared to untreated pregnant mice (P < 0.05). During bacterial infection, the bone marrow accelerates the production of granulocytes to support the rapid recruitment of these cells into the blood circulation [50,51]. E. coli infection stimulated proliferation of granulocytes precursor, markedly by a high production of immature Gr1lo cells in the bone marrow [52,53]. This study showed that flow cytometric gates for each sample defined a fixed range of fluorescence intensities for Gr1+ cells in the bone marrow. An interesting observation in this study was that ES alone, combined ES and SA at ratio 75:25 and 50:50 triggered the release of mature granulocytes from the bone marrow into the systemic circulation in the presence of bacterial infection.
For determine which phytocompounds responsible for the antibacterial effect, we refers to related literature [54], which conducted the screening methods that ethanol extract of ES possessed an antibacterial activity against E. coli, S. aureus, P. aeruginosa and Vibrio sp. Then Nonci et al. [54] performed the Thin Layer Chromatography (TLC) and found that ethanol extract of ES contains alkaloid, flavonoid and steroid compound which responsible for antibacterial effect. This antibacterial activity is also due to the presence of multivitamins, peptides, glycosides, alkaloids, saponins, terpenoids, and flavonoids in ethanol extract of SA [50] which able to inhibit the growth of E. coli in the pregnant mice. If the growth of bacteria was reduced, it would be reduce the cell damage in the kidney and hepar caused by E. coli. Furthermore, the hormonal condition was also improved. Our study also performed that the phenolic content of the S. androgynus and E. scaber ethanol extracts were considerabily high, which strongly correlated with the high antioxidant activity of both plant (data not shown). Therefore, the combination of both demonstrated therapeutic potential for reducing hepatic and renal damage and also improving the hormonal changes caused by E. coli infection in pregnant mice.
5. Conclusion
The present study provided the establishment of combined E. scaber (ES) and S. androgynus (SA) that could be used to develop new and potent hormonal balancing agent and hepato-renal protective agent in infected pregnant mice. This research showed that treatment at a ratio dose of 150 mg/kg ES and 37.5 mg/kg SA (75:25) could delay and protect the liver and kidney damage due to bacterial infection during pregnancy. Furthermore, the combination of 150 mg/kg ES and 37.5 mg/kg SA (75:25) was able to restore progesterone levels to normal levels. We also observed that the highest level of estrogen was found in the treatment of SA alone. Combined ES and SA also demonstrated an immunomodulatory effect on the Gr1+ cell of E. coli treated-pregnant mice.
Source(s) of funding
This work supported by the Indonesian Ministry of Research, Technology, and Higher Education, Jakarta, Indonesia [grant no. 167/SP2H/LT/DRPM/2019].
Conflict of interest
None.
Acknowledgement
The authors thank Brawijaya University for facilitating this research.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Forson A.O., Tsidi W.B., Nana-Adjei D., Quarchie M.N., Obeng-Nkrumah N. Escherichia coli bacteriuria in pregnant women in Ghana: antibiotic resistance patterns and virulence factors. BMC Res Notes. 2018;11(901):1–8. doi: 10.1186/s13104-018-3989-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganzle M., Yang L. Mechanisms of pressure mediated cell death and injury in Escherichia coli: from fundamentals to food applications. Front Microbiol. 2015;15(6):1–10. doi: 10.3389/fmicb.2015.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matuszkiewicz-Rowińska J., Małyszko J., Wieliczko M. Urinary tract infections in pregnancy: old and new unresolved diagnostic and therapeutic problems. Arch Med Sci. 2015;11(1):67–77. doi: 10.5114/aoms.2013.39202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Habak P.J., Griggs R.P. StatPearls Publishing LLC; USA: 2020. Urinary tract infection in pregnancy. [PubMed] [Google Scholar]
- 5.Konapa L.A., Vesalapu V., Kolakota R.K., Mugada V. Pregnancy and hormonal effects on urinary tract infections in women: a scoping review. Int J Res Rev. 2018;5(10):407–420. [Google Scholar]
- 6.Eaton K.A., Friedman D.I., Francis G.J., Tyler J.S., Young V.B., Haeger J. Pathogenesis of renal disease due to enterohemorrhagic Escherichia coli in germ-free mice. Infect Immun. 2008;76(7):3054–3063. doi: 10.1128/IAI.01626-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nolan J.P. The role of endotoxin in liver injury. Gastroenterol. 1975;69:1346–1356. [PubMed] [Google Scholar]
- 8.Lee M., Bozzo P., Einarson A., Koren G. Urinary tract infections in pregnancy. Can Fam Physician. 2008;54(2):853–854. [PMC free article] [PubMed] [Google Scholar]
- 9.Loh K., Sivalingam N. Urinary tract infections in pregnancy. Malays Fam Physician. 2007;2(2):54–57. [PMC free article] [PubMed] [Google Scholar]
- 10.Das M., Sabio G., Jiang F., Rincón M., Flavell R.A., Davis R.J. Induction of hepatitis by JNK-mediated expression of TNF-alpha. Cell. 2009;136(2):249–260. doi: 10.1016/j.cell.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson D.P., Kelin S.L. Pregnancy and pregnancy associated hormones alter immune responses and disease pathogenesis. Horm Behav. 2012;62(3):263–271. doi: 10.1016/j.yhbeh.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bien J., Sokolova O., Bozko P. Role of uropathogenic Escherichia coli virulence factors in development of urinary tract infection and kidney damage. Internet J Nephrol. 2012;12(10):1–15. doi: 10.1155/2012/681473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruns T., Zimmermann H.W., Stallmach A. Risk factors and outcome of bacterial infections in cirrhosis. World J Gastroenterol. 2014;20(10):2542–2554. doi: 10.3748/wjg.v20.i10.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jahnukainen T., Chen M., Celsi G. Mechanisms of renal damage owing to infection. Pediatr Nephrol. 2005;20(5):1043–1053. doi: 10.1007/s00467-005-1898-5. [DOI] [PubMed] [Google Scholar]
- 15.Soka S., Alam H., Boenjamin N., Agustina T.W., Suhartono M.T. Effect of Sauropus androgynus leaf extracts on the expression of prolactin and oxytocin genes in lactating BALB/C Mice. J Nutrigenetics Nutrigenomics. 2010;3(1):31–36. doi: 10.1159/000319710. [DOI] [PubMed] [Google Scholar]
- 16.Andarwulan N., Kurniasih D.L.S., Apriady R.A., Rahmat H., Roto A.V., Bolling B.W. Polyphenols, carotenoids, and ascorbic acid in underutilized medicinal vegetables. J Funct Foods. 2012;4(1):339–347. doi: 10.1016/j.jff.2012.01.003. [DOI] [Google Scholar]
- 17.Ong H.C. Utusan Publications and Distributors; Kuala Lumpur, Malaysia: 2003. Sayuran: Khasiat Makanan & Ubatan. [Google Scholar]
- 18.Li B., Qiu H., Ma J., Zhu H., Gilbert M.G., Esser H. Euphorbiaceae. In: Wu Z.Y., Raven P.H., Hong D.Y., editors. Flora of China Vol. 11 (Oxalidaceae through Aceraceae) Missouri Botanical Garden Press; St. Louis, Mo, USA: 2008. pp. 163–314. [Google Scholar]
- 19.Benjapak N., Swatsitang P., Tanpanich S. Determination of antioxidant capacity and nutritive values of Pak-Wanban (Sauropus androgynus L. Merr.) Khon-Kean Univ Sci J. 2008;36:279–289. [Google Scholar]
- 20.Sai K.S., Srividya N. Blood glucose lowering effect of the leaves of Tinospora cordifolia and Sauropus androgynus in diabetic subjects. J Nat Remedies. 2002;2(1):28–32. [Google Scholar]
- 21.Bunawan H., Amin N.M., Bunawan S.N., Baharum S.N., Moor N.M. Ficus deltoidea Jack: a review on its phytochemical and pharmacological importance. J Evid-Based Compl Altern Med. 2014;9(2):1–8. doi: 10.1155/2014/902734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Setyari W., Sudjarwo S.A. Potensi analgesik dan antiinflamasi dari ekstrak tapak liman (Elephantophus scaber) J Penelit Med Eksakta. 2008;7(1):16–22. [Google Scholar]
- 23.Erawati A.M. Bogor: Faculty of Veterinary Medicine, Institute of Agriculture; 2011. Gambaran Histopatologi Hepar dan Ginjal Tikus Laktasi Setelah Mengkonsumsi Ekstrak dan Fraksi Sauropus androgynus (L.) Merr Sejak Bunting Sampai 10 Hari Postpartus. Essay. [Google Scholar]
- 24.Guan Y.-S., He Q. Plants consumption and liver health. Evid Based Compl Alternat Med. 2015;2015:824185. doi: 10.1155/2015/824185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.George B., You D., Joy M.S., Aleksunes L.M. Xenobiotic transporters and kidney injury. Adv Drug Deliv Rev. 2017;116:73–91. doi: 10.1016/j.addr.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen G.N. Springer Science & Business Media; New York: 2011. Microbial biochemistry. [Google Scholar]
- 27.Mohan S.K. AuthorHouse; India: 2009. Gram stain: looking Beyond Bacteria to find fungi in Gram stained smear. [Google Scholar]
- 28.Djati M.S., Rahma Y.A., Dwijayanti D.R., Rifa’i M., Rahayu S. Synergistic effect of Elephantopus scaber L and Sauropus androgynus L merr extracts in modulating prolactin hormone and erythropoiesis in pregnant typhoid mice. Trop J Pharmaceut Res. 2017;16(8):1789–1795. doi: 10.4314/tjpr.v16i8.6. [DOI] [Google Scholar]
- 29.Shi S., Key M.E., Kalra K.L. Antigen retreviel in formalin-fixed, paraffin-embedded tissues: an enhancement method for histological staining. J Histochem Cytochem. 1991;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- 30.Sudatri N., Setyawati I., Suartini N., Yulihastuti D. Penurunan fungsi hepar tikus betina (Rattus norvegivus L.) yang diinjeksi white vitamin c dosis tinggi dalam jangka waktu lama ditinjau Dari kadar SPGT, SGOT serta gambaran histologi hati. J Biol Sci. 2016;3(1):44–51. doi: 10.24843/metamorfosa.2016.v03.i01.p07. [DOI] [Google Scholar]
- 31.Kumar P., Magon N. Hormones in pregnancy. Niger Med J. 2012;53(4):179–183. doi: 10.4103/0300-1652.107549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barkley M.S., Geschwind, Bradford G.E. The gestational pattern of estradiol, testosterone and progesterone secretion in selected strains of mice. Biol Reprod. 1979;20(4):733–738. doi: 10.1095/biolreprod20.4.733. [DOI] [PubMed] [Google Scholar]
- 33.Aisemberg J., Vercelli C.A., Bariani M.V., Billi S.S.C., Wolfson M.L., Franchi A.M. Progesterone is essential for protecting against LPS-induced pregnancy loss. LIF as a potential mediator of the anti-inflammatory effect of progesterone. PloS One. 2013;8(2) doi: 10.1371/journal.pone.0056161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suprayogi A. Studies of the biological effect of Sauropus androgynus (L).Merr: effect of milk production and the possibilities of induced pulmonary disorder in lactating sheep. J Pharmacol. 2000;40(8):931–941. [Google Scholar]
- 35.Bunawan H., Bunawan S.N., Baharum S.N., Noor N.M. Sauropus androgynus (L.) merr. Induced bronchiolitis obliterans: from botanical studies to toxicology. Evid-Based Compl Altern Med. 2015;2015(714158):1–7. doi: 10.1155/2015/714158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Usman A.M., Khurram M., Khan T.A., Faidah H.S., Ullah Shah Z., Ur Rahman S. Effects of luteolin and quercetin in combination with some conventional antibiotics against methicillin-resistant Staphylococcus aureus. Int J Mol Sci. 2016;17(11):1947. doi: 10.3390/ijms17111947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prakoso Y.A., Puspitasari, Rini C.S., Aliviameita A., Salasia S.I.O., Kurniasih The role of Sauropus androgynus (L.) merr. Leaf powder in the broiler chickens fed a diet naturally contaminated with aflatoxin. J Toxicol. 2018;2018:2069073. doi: 10.1155/2018/2069073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ariharan V.N., Devi V.N.M., Prasad P.N. Antibacterial activity of sauropus and rogynous leaf extracts against some pathogenic bacteria. Rasayan J Chem. 2013;6(2):134–137. [Google Scholar]
- 39.Rezai-Zadeh K., Ehrhart J., Bai Y., Sanberg P.R., Bickford P., Tan J. Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J Neuroinflammation. 2008;5:41. doi: 10.1186/1742-2094-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei L.S., Wendy W., Julius Y.F.S., Desy F.S. Characterization of antimicrobial, antioxidant, anticancer properties and chemical composition of Sauropus androgynus stem extract. Acta Med Litu. 2011;12(1):12–16. doi: 10.6001/actamedica.v18i1.1808. [DOI] [Google Scholar]
- 41.Rubin R., David S.R., Emanuel R. Lippincott Williams & Wilkins; New York: 2011. Rubin’s pathology: clinicopathologic foundations of medicine. [Google Scholar]
- 42.Jasmine R., Daisy P., Selvakumar B.N. Evaluating the antibacterial activity of Elephantopus scaber extracts on clinical isolates of β-lactamase producing methicillin resistant Staphylococcus aureus from UTI patients. Int J Pharmacol. 2007;3:165–169. doi: 10.3923/ijp.2007.165.169. [DOI] [Google Scholar]
- 43.Avani K., Neeta S. A study of the antimicrobial activity of Elephantopus scaber. Indian J Pharmacol. 2005;37:126–127. doi: 10.4103/0253-7613.15115. [DOI] [Google Scholar]
- 44.Arora D.S., Kaur G.J. Antibacterial activity of some Indian medicinal plants. J Nat Med. 2007;61:313–317. doi: 10.1007/s11418-007-0137-8. [DOI] [Google Scholar]
- 45.Daisy P., Mathew S., Suveena S., Rayan N.A. A novel terpenoid from Elephantopus scaber – antibacterial activity on Staphylococcus aureus: a substantiate computational approach. Int J Biomed Sci. 2008;4(3):196–203. [PMC free article] [PubMed] [Google Scholar]
- 46.Rievere C., Hong V.N., Pieters L., Dejaegher B., Heyden Y.V., Van M.C. Polyphenols isolated from antiradical extracts of Mallotus metcalfianus. Phytochemistry. 2009;70(1):86–94. doi: 10.1016/j.phytochem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 47.Anitha V.T., Antonisamy J.M., Jeeva S. Anti-bacterial studies on Hemigraphis colorata (blume) H.G. Hallier and Elephantopus scaber L. Asian Pac J Trop Med. 2012;5(1):52–57. doi: 10.1016/S1995-7645(11)60245-9. [DOI] [PubMed] [Google Scholar]
- 48.Djati M.S. Alternative formulations of E. scaber and S. androgynous extracts as immunomodulatory agents for suppression of bacterial infection in pregnant mice: a case of traditional herbal formulas in reproduction. AIP Conf Proc. 2019;2019(1) doi: 10.1063/1.5061908. [DOI] [Google Scholar]
- 49.Djati M.S., Dwijayanti D.R., Rifai M. Herbal supplement formula of Elephantopus scaber and Sauropus androgynus promotes IL-2 cytokine production of CD4+ T cells in pregnant mice with typhoid fever. Open Life Sci. 2016;22:211–219. doi: 10.1515/biol-2016-0029. [DOI] [Google Scholar]
- 50.Djati M.S., Dwijayanti D.R., Nurmamulyosari L.D., Fuadah Y., Basyarudin M., Jannah N. Elephantopus scaber and Sauropus androgynus regulate macrophages and B lymphocyte cells during Salmonella typhi infection. UNEJ e-Proc. 2017;Sl:42–44. [Google Scholar]
- 51.Terashima T., Wiggs B., English D., Hogg J.C., van Eeden S.F. Polymorphonuclear leukocyte transit times in bone marrow during streptococcal pneumonia. Am J Physiol. 1996;271:L587–L592. doi: 10.1152/ajplung.1996.271.4.L587. [DOI] [PubMed] [Google Scholar]
- 52.Hartmann D.W., Entringer M.A., Robinson W.A., Vasil M.L., Drebing C.J., Morton N.J. Regulation of granulopoiesis and distribution of granulocytes in early phase of bacterial infection. J Cell Physiol. 1981;109(1):17–24. doi: 10.1002/jcp.1041090103. [DOI] [PubMed] [Google Scholar]
- 53.Shi X., Lin Y.P., Gao B., Zhang P. Impairment of hematopoietic precursor cell activation during the granulopoietic response to bacteremia in mice with chronic-plus-binge alcohol administration. Infect Immun. 2017;85(11) doi: 10.1128/IAI.00369-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nonci F.Y., Rusli R., Atqiyah A. Antimikroba ekstrak ethanol Daun tapak liman (Elephantopus scaber L.) Dengan menggunakan metode KLT bioautografi. JF FIK UINAM. 2014;2(4):144–148. doi: 10.24252/.v2i4.2160. [DOI] [Google Scholar]