Abstract
Background
Over two billion people around the world suffer from anemia. Majority of populations are using dietary supplements and herbal medicines for the management of the anemic conditions. Many polyherbal formulations such as RaktavardhakKadha (RK), are available in the Indian market as a nutritional supplement and herbal-based medicine for anemia.
Objectives
The present study is aimed at investigating antianemic potential of polyherbal formulation, RK, against phenylhydrazine-induced anemia in rats.
Materials and methods
RK was subjected to preliminary phytochemical analysis and iron estimation. Anemia was induced by phenylhydrazine administration (40 mg/kg, i.p.) for 2 consecutive days. Antianemic activity of RK was investigated at the dose of 1.8 ml/kg, twice daily for 12 days by estimating blood parameters and pathological changes in liver, heart, spleen and bone marrow.
Results
RK was found to contain saponins, steroids, flavonoids, tannins and phenolic compounds. Iron content was found to be 5 mg/100 ml in RK. Anemia induction by phenylhydrazine injections to rats caused significant decrease in red blood cells (RBCs), hemoglobin and hematocrit. These decreased levels of RBCs, hemoglobin and hematocrit in blood was significantly improved by the treatment with RK. Furthermore, RK restored pathological changes in liver, heart, spleen and bone marrow tissues near to normal.
Conclusion
This study suggests antianemic activity of RK, which can be attributed to its iron content and ability to prevent hemolysis.
Keywords: Anemia, Antianemic activity, Phenylhydrazine, Polyherbal formulation, RaktavardhakKadha
1. Introduction
Anemia, a common public health problem, is characterized as decrease in erythrocyte mass or hemoglobin concentration in the blood leading to reduction in it’s oxygen carrying capacity [1]. More than two billion people around the world suffer from anemia [2]. Anemia is more common health problem in the developing countries [3]. Dietary changes and iron supplementation are commonly preferred for the management of anemia. Oral iron therapy has many disadvantages such as insufficient absorption and lack of compliance [4]. Furthermore, consumption of high quantity of these iron supplements can lead to serious health-related complications such as some neurogenic disorders or cancer [5]. All these facts demonstrate the need to have safe and effective alternative for the management of anemia.
Medicinal plants have been a source to control many diseases and anemia is no exception. In traditional systems of medicine including Ayurveda, many plants are claimed to be useful for anemia [6]. Previous studies reported antianemic potentials of several Indian medicinal plants [7]. Few polyherbal formulations are reported to be effective for the treatment of anemia [8]. These herbal-based formulations are preferred by the community as they are cost-effective and have less side effects. Raktavardhak Kadha (RK) is a proprietary Ayurvedic medicine marketed by Shree Swami Samarth Ayurvedic Pharmaceuticals, Nashik, India for the treatment of anemia. It is a polyherbal medicine that contains aqueous extracts of thirteen herbs and Loha Bhasma as summarized in Table 1. With this background, the present study was conducted to investigate the antianemic activity of RK against experimentally-induced anemia in rats.
Table 1.
Contents of Raktavardhak Kadha.
| Ingredients | Botanical Name | Family | Quantity per 200 ml |
|---|---|---|---|
| Bhueavala | Phyllanthus niruri L. | Euphorbiaceae | 3 g |
| Maka | Eclipta alba L. | Asteraceae | 3 g |
| Avala | Emblica officinalis Gaertn. | Euphorbiaceae | 3 g |
| Jire | Cuminum cyminum L. | Apiaceae | 3 g |
| Jeshthamadh | Glycyrrhiza glabra L. | Fabaceae | 3 g |
| Sunth | Zingiber officinale Roscoe | Zingiberaceae | 2 g |
| Ashvagandha | Withania somnifera (L.) Dunal | Solanaceae | 2 g |
| Shatavari | Asparagus racemosus Willd. | Asparagaceae | 2 g |
| Purnanava | Boerhaavia diffusa L. | Nyctaginaceae | 2 g |
| Nagvel | Piper betle L. | Piperaceae | 2 g |
| Vidarikand | Ipomoea digitata L. | Convolvulaceae | 1 g |
| Varahikand | Dioscorea bulbifera L. | Dioscoreaceae | 1 g |
| Manjishtha | Rubia cordifolia L. | Rubiaceae | 1 g |
| Anantmul | Hemidesmus indicus (L.) R. Br. ex Schult. | Asclepiadaceae | 1 g |
| Loha Bhasma | – | – | 500 mg |
2. Materials and methods
2.1. Animals
Wistar albino rats (150–250 g) of either sex supplied by National Institute of Biosciences, Pune, India were used for the study. Animals were acclimatized for ten days under standard housing conditions in an animal house approved by Committee for the Purpose of Control and Supervision of Experiments on Animals. They were housed in polypropylene cages and maintained at 25 ± 2 °C, relative humidity 50 ± 10% under 12 h light/dark cycles. Animals were provided free access to standard pellet food and water ad libitum. They were given standard diet supplied by Nutrivet Life Sciences, Pune, India. The study protocol was approved by the Institutional Animal Ethics Committee (Ref. No- MIP/IAEC/2018-19/M3/02).
2.2. Chemicals and equipments
RK was obtained from Shree Swami Samarth Ayurvedic Pharmaceuticals, Nashik, India as a gift sample for research purpose along with no objection certificate of the manufacture. Tablet Livogen XT (Merck Ltd., India) was purchased from Nilesh Medical Stores, Poladpur, India. Phenylhydrazine hydrochloride (Analab Fine Chemicals, India) was purchased from New Neeta Chemicals, Pune, India. Hematoanalyzer (Make: Cell take α, Model: MEK-6550) was used for blood analysis.
2.3. Preliminary phytochemical analysis and iron estimation
Phytochemical analysis of RK was carried out to detect the existence of different classes of phytoconstituents by standard methods [9]. Iron content in RK was estimated by inductively coupled plasma optical emission spectroscopy (ICP-OES).
2.4. Induction of anemia
Anemia was induced by intraperitoneal injections of phenylhydrazine (40 mg/kg) for 2 consecutive days [10]. Rats were considered as anemia-induced when RBC level as well as hemoglobin content of the blood reduced by 30% [11].
2.5. Selection of RK dose
The dose of RK was calculated from human dose using conversion factor based on body surface area [12]. The adult human dose of RK is 20 ml twice daily and therefore dose of 1.8 mg/kg twice daily was selected for the study.
2.6. Experimental design
Rats were divided into four groups containing six rats in each group (n = 6). Group I was normal control and did not receive any treatment. Remaining rats were treated with phenylhydrazine to induce anemia. Group II was anemic control. Group III was a RK-treated group and received RK (1.8 ml/kg, twice daily). Group IV was a standard drug treatment group and received Livogen XT (9 mg of iron/kg, twice daily). Livogen XT and RK were given from day 3–15 after first injection of phenylhydrazine by oral route. Livogen XT tablet contains ferrous ascorbate (iron 100 mg), folic acid (1.5 mg) and zinc sulphate monohydrate (61.8 mg). It is used for the treatment of iron-deficiency anemia and nutritional anemia. Dose of Livogen XT was calculated by extrapolation from the human dose (100 mg of Iron, twice daily) based on surface area ratio method [12].
2.7. Blood estimations
Blood (0.5 ml) was collected from retro-orbital sinus under isoflurane-anesthesia on day 0-before phenylhydrazine administration, and on days 3, 7, 10 and 15 after first injection of phenylhydrazine. Blood was analyzed for red blood cells (RBCs), hemoglobin (Hb) and hematocrit (HCT) levels [10].
2.8. Histopathology
After blood collection on last day, animals were sacrificed to isolate brain, liver, lung, kidney, heart, spleen and femur bone for bone marrow examination. The collected tissues were processed, stained by Hematoxylin and Eosin staining method and examined under light microscope for histological changes.
2.9. Statistical analysis
The statistical analysis was performed using GraphPad Prism 6.0 software. Values of normal control group and anemia control group were compared by Student’s t-test, whereas one-way analysis of variance (ANOVA) followed by Dunnett’s comparison test was used for comparison between drug-treated groups and anemia control group. p < 0.05 was considered significant.
3. Results
Phytochemical analysis of RK revealed the presence of saponins, steroids, flavonoids, tannins and phenolic compounds. Iron content was found to be 5 mg/100 ml of RK.
Induction of anemia by phenylhydrazine administration caused significant (p < 0.001) decrease in RBCs count during experimental period (Table 2). This decrease in RBC count was significantly reversed by treatment with both RK (p < 0.01) as well as Livogen XT (p < 0.001). Livogen XT was found to be more effective than RK in this regard (Table 2).
Table 2.
Effect of RK on blood levels of RBCs, hemoglobin and HCT in anemia-induced rats.
| Days | Group-I (Normal Control) |
Group-II (Anemic Control) |
Group-III (RK Treated) |
Group-IV (Livogen-XT Treated) |
|---|---|---|---|---|
| RBCs count (106/μl) | ||||
| 0 | 8.18 ± 0.26 | 8.86 ± 0.17 | 8.21 ± 0.19 | 8.93 ± 0.38 |
| 3 | 8.35 ± 0.50 | 2.80 ± 0.17a∗∗∗ | 3.87 ± 0.16 | 3.15 ± 0.16 |
| 7 | 7.30 ± 0.32 | 3.48 ± 0.29a∗∗∗ | 5.23 ± 0.27b∗∗ | 5.54 ± 0.32b∗∗∗ |
| 10 | 7.33 ± 0.43 | 4.10 ± 0.38a∗∗∗ | 5.85 ± 0.23b∗∗ | 6.35 ± 0.34b∗∗∗ |
| 15 | 7.65 ± 0.24 | 5.85 ± 0.18a∗∗∗ | 7.03 ± 0.27b∗∗ | 7.26 ± 0.19b∗∗∗ |
| Hemoglobin content (g/dl) | ||||
| 0 | 13.13 ± 0.31 | 14.50 ± 0.22 | 14.70 ± 0.29 | 15.05 ± 0.36 |
| 3 | 13.50 ± 0.95 | 9.01 ± 0.48a∗∗ | 10.25 ± 0.48 | 9.92 ± 0.37 |
| 7 | 12.59 ± 0.26 | 9.22 ± 0.33a∗∗∗ | 11.15 ± 0.44b∗∗ | 11.56 ± 0.37b∗∗ |
| 10 | 12.63 ± 0.23 | 10.35 ± 0.27a∗∗∗ | 11.60 ± 0.17b∗∗ | 12.25 ± 0.20b∗∗∗ |
| 15 | 13.03 ± 0.26 | 11.95 ± 0.21a∗∗ | 13.67 ± 0.40b∗∗ | 14.25 ± 0.28b∗∗∗ |
| HCT count (%) | ||||
| 0 | 42.28 ± 2.43 | 45.33 ± 0.55 | 47.97 ± 1.17 | 46.70 ± 1.14 |
| 3 | 42.28 ± 2.39 | 25.15 ± 1.10a∗∗∗ | 22.47 ± 1.78 | 24.72 ± 1.00 |
| 7 | 41.16 ± 1.01 | 27.64 ± 1.33a∗∗∗ | 40.22 ± 1.52b∗∗∗ | 40.73 ± 1.33b∗∗∗ |
| 10 | 39.47 ± 2.00 | 30.17 ± 1.05a∗∗ | 37.47 ± 1.09b∗∗∗ | 41.75 ± 0.81b∗∗∗ |
| 15 | 40.98 ± 1.18 | 35.35 ± 1.15a∗∗ | 43.28 ± 1.16b∗∗∗ | 46.12 ± 0.79b∗∗∗ |
Values are expressed as mean ± SEM, n = 6, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. avs. normal control, bvs. anemic control. SEM=Standard error of mean. RBCs-Reb blood cells, HCT-Hematocrit.
Phenylhydrazine treatment caused significant decrease (p < 0.001) in Hb content during experimental period (Table 2). This decrease in Hb content was significantly reversed by treatment with both RK (p < 0.01) as well as Livogen XT (p < 0.001). Livogen XT was found to be more effective as compared to RK in increasing Hb content of anemic rats (Table 2).
The HCT count was significantly (p < 0.001) decreased in anemia-induced rats as compared to normal control rats (Table 2). This decrease in HCT count was significantly (p < 0.001) reversed by treatment with RK and Livogen XT. Livogen XT as well as RK found to be equipotent in this regard (Table 2).
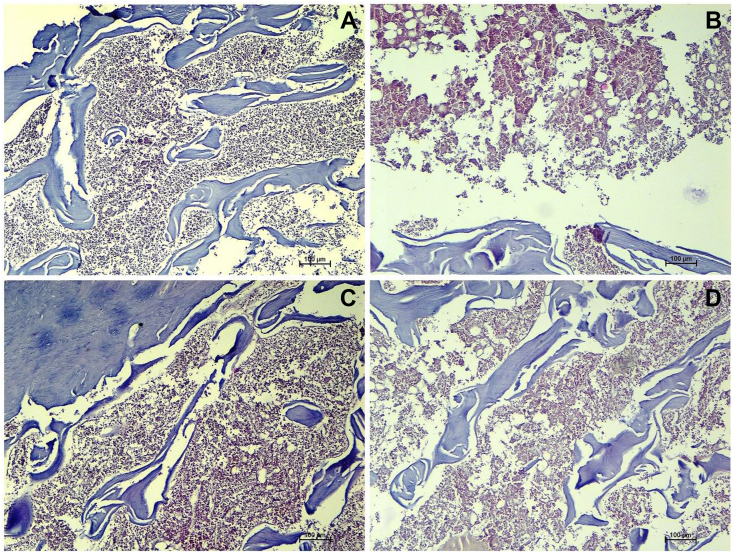
Liver tissue of anemia-induced rats showed minimal and focal degenerative changes of hepatocytes around central vein (Fig. 1-B). Focal areas of cellular swelling of hepatocytes were noted with enlarged nucleus and granular cytoplasmic changes. Focal vacuolar changes were also observed in few of the hepatocytes around central vein. Treatment with RK and Livogen XT prevented these histological changes and restored normal histomorphological features of liver (Fig. 1-C and D).
Fig. 1.
Representative images of liver sections from animals of (A) normal control showing normal architecture of liver tissue; (B) anemic control indicating minimal and focal degenerative changes of hepatocytes around central vein. Focal areas of cellular swelling of hepatocytes were noted with enlarged nucleus and granular cytoplasmic changes; (C) RK treated and (D) Livogen XT treated group (H and E × 100).
Induction of anemia by phenylhydrazine administration caused focal degenerative changes of mild nature of cardiac muscle with dilation of cardiac fibers (Fig. 2-B). Treatment with RK and Livogen XT prevented these histological changes and restored normal histomorphological features of cardiac muscles (Fig. 2-C and D).
Fig. 2.
Representative images of heart sections from animals of (A) normal control showing normal architecture of heart tissue with cardiac muscle fibers in the myocardium; (B) anemic control showing focal degenerative changes of moderate to severe nature of cardiac muscle with dilation of cardiac fibers; (C) RK treated and (D) Livogen XT treated groups. (H and E × 100).
Normal architecture of spleen with presence of normal features of red pulp, white pulp and adequate lymphoid cellular population was observed in normal control animals (Fig. 3- A). Induction of anemia by phenylhydrazine administration caused focal depletion of cellular population in splenic parenchyma (Fig. 3-B). This histological change was prevented by treatment with RK and Livogen XT (Fig. 3-C and D).
Fig. 3.
Representative images of spleen sections from animals of (A) normal control showing presence of normal features of red pulp, white pulp and adequate lymphoid cellular population; (B) anemic control indicating focal depletion of cellular population in splenic parenchyma; (C) RK treated and (D) Livogen XT treated groups. (H and E × 100).
There was no difference in the histology of kidney, lungs and brain tissues of normal control, anemic control, RK treated and Livogen XT treated rats. All samples showed normal histomorphology of tissues.
Bone marrow tissue of anemia-induced rats showed presence of certain foci of minimal grade containing adipose tissue (Fig. 4-B). This is suggestive of depletion of bone marrow cellular population. Treatment with RK and Livogen XT prevented this histological change (Fig. 4-C and D).
Fig. 4.
Representative images of bone marrow sections from animals of (A) normal control showing presence of adequate number of bone marrow cells; (B) anemic control indicating presence of certain foci of minimal grade in bone marrow containing adipose tissue; (C) RK treated and (D) Livogen XT treated groups. (H and E × 100).
4. Discussion
Several animal models of anemia are available for screening of antianemic activity of the test preparations. Out of all, phenylhydrazine rat model is rapid, reliable and therefore widely used method [13]. Wistar albino rat is one of the most commonly used animals in biomedical study. Rats have been used in many experimental studies from a long time which have added information about diseases, genetics, effects of drugs, and other related topics in health and medicine. Therefore, we have chosen phenylhydrazine-induced anemia model in rats for screening of antianemic activity of RK.
Phenylhydrazine is a non-immunogenic chemical that induces hemolytic type of anemia by selectively destroying matured RBCs through oxidative stress, denaturation of red cell Hb, membrane phospholipids and enzymes involved in energy metabolism [14]. Phenylhydrazine-induced anemia is characterized by erythropenia, lowered Hb and reduced HCT levels in the blood [15].
Previous reports suggested that intraperitoneal injections of phenylhydrazine to rats at the dose of 40 mg/kg for 2 consecutive days induce the anemic condition [13]. Therefore, we selected 40 mg/kg dose of phenylhydrazine by intraperitoneal route for 2 consecutive days for induction of anemic condition in rats [10].
The phenylhydrazine-induced erythropenia lowered Hb and reduced HCT levels which persisted for 8–12 days [13]. Furthermore, phenylhydrazine also induces pathological changes in tissues such as heart, kidney, liver and spleen [16]. Therefore to screen the antianemic potential of RK, we evaluated levels of RBCs, Hb and HCT during experimental period as well as histopathological studies of major organs at the end of experimental period.
RBCs are the cellular component of blood. The main function of RBCs is the transportation of oxygen into the body tissues. In some pathological conditions such as anemia, the function of RBC gets modified which may be detrimental for the normal functioning of the body. In the present study, phenylhydrazine administration caused decline in RBCs count of blood during experimental period which is consistent with earlier reports [13,17,18]. This decrease in RBCs count was improved by treatment with RK. Phenylhydrazine causes selective destruction of matured RBCs through oxidative stress [14]. Therefore, the beneficial effect of RK to improve RBCs count may be due to its ability to prevent phenylhydrazine-induced hemolysis.
Hb is a major parameter for screening of antianemic drugs as low level of Hb causes decline in oxygen-carrying capacity of blood. In the present study, phenylhydrazine administration caused decrease in Hb content of blood during experimental period which is consistent with earlier reports [13,17,18]. This decline in blood Hb content was improved by treatment with RK. Phenylhydrazine is reported to induce anemia by oxidative denaturation of Hb initiated by free radicals. Therefore, antioxidant potential of RK may be responsible for the observed effect of RK to improve blood Hb content.
HCT, also known as the packed cell volume, is the ratio of volume of packed RBCs to the total blood volume. A low HCT is an indicator of anemic condition. In the present study, there was significant decrease of HCT in anemic control rats due to phenylhydrazine-induced hemolysis which is consistent with earlier reports [13,19]. This decrease in HCT was reversed by treatment with RK. This effect may be due to protective effect of RK against phenylhydrazine-induced hemolysis.
Histopathological evaluation indicated that anemia-induction by phenylhydrazine administration caused pathological changes of moderate grade in liver, heart, spleen and bone marrow tissues. These pathological changes were reversed by RK treatment and restored histomorphological features of these tissues near to normal. The observed effect may be due to potent antianemic potential of RK.
It has been reported that phenylhydrazine increases formation of reactive oxygen species and thereby causes oxidative damage to RBCs. However, flavonoids have potent antioxidant property and have capacity to prevent or repair this damage to red cells [20]. Phytochemical evaluation of RK showed the presence of saponins, steroids, flavonoids, tannins and phenolic compounds. Thus, it appears that the presence of flavonoids or other active principles may be responsible for the observed antianemic activity of RK.
Quantitative iron estimation showed the presence of iron in the RK. Therefore, RK is also useful for the treatment of iron-deficiency type of anemia. Iron content of RK and its ability to prevent phenylhydrazine-induced hemolytic anemia provides that the RK has beneficial effect against both iron deficiency as well as hemolytic types of anemia.
5. Conclusion
It is concluded that the phenylhydrazine injections by intraperitoneal route at the dose of 40 mg/kg for 2 consecutive days induces anemic condition in rats. RK improved phenylhydrazine-induced decrease in RBCs, Hb and HCT levels. Furthermore, it also reversed pathological changes in tissues of liver, heart, spleen and bone marrow. Hence, RK has significant antianemic activity against phenylhydrazine induced anemia in rats. Iron estimation showed the presence of iron in RK which is useful against iron-deficiency anemia. Thus, RK has beneficial effect against both iron deficiency as well as hemolytic types of anemia. Further studies on quantitative estimation of phytoconstituents along with in vivo evaluations are necessary for elucidating the exact mechanism of action of the observed antianemic effect.
Source(s) of funding
None declared.
Conflict of interest
None.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Hoffbrand A.V., Moss P., Pettit J.E. Blackwell Publishing; UK: 2006. Erythropoiesis and general aspects of anaemia. Essential Haematology. [Google Scholar]
- 2.Hashim N., Farooqi M., Naqvi S., Jaffery H.F. Anemia: moderate to severe during pregnancy. Prof Med J. 2014;32:247–252. [Google Scholar]
- 3.Tolentino K., Friedman J.F. An update on anemia in less developed countries. Am J Trop Med Hyg. 2007;77:44–51. [PubMed] [Google Scholar]
- 4.Maladkar M., Sankar S., Yadav A. A novel approach for iron deficiency anaemia with liposomal iron: concept to clinic. J Biosci Med (Irvine) 2020;8:27–41. [Google Scholar]
- 5.Saha U., Dharwadkar P.S., Sur S., Vishaharini V., Malleshappa M. Plant extracts as an astounding remedy to anemia - a review. Ann Plant Sci. 2018;7:2166–2171. [Google Scholar]
- 6.Aduwamai U.H., Abimbola M.M. Effect of Solanum nigrum methanol leaf extract on phenylhydrazine induced anemia in Rats. Jordan J Biol Sci. 2018;11:65–71. [Google Scholar]
- 7.Patil R.R., Navghare A.A. Medicinal plants for treatment of anemia: a brief review. World J Pharmaceut Res. 2019;8:701–717. [Google Scholar]
- 8.Aslam M.S., Ahmad M.S., Mamat A.S., Ahmad M.Z., Salam F. An update review on polyherbal Formulation: a global perspective. Sys Rev Pharm. 2016;7:35–41. [Google Scholar]
- 9.Khandelwal K.R. 9th ed. Nirali Prakashan; New Delhi: 2002. Practical pharmacognosy. [Google Scholar]
- 10.Diallo A., Gbeassor M., Vovor A., Eklu-Gadegbeku K., Aklikokou K., Agbonon A. Effect of Tectona grandis on phenylhydrazine-induced anaemia in rats. Fitoterapia. 2008;79:332–336. doi: 10.1016/j.fitote.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Koffuor G., Amoateng P., Andey T. Immunomodulatory and erythropoietic effects of aqueous extract of the fruits of Solanum torvum Swartz (Solanaceae) Pharmacogn Res. 2011;3:130–134. doi: 10.4103/0974-8490.81961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh M.N. 2nd ed. Scientific Book Agency; Calcutta: 1984. Fundamentals of experimental pharmacology. [Google Scholar]
- 13.Gheith I., El-Mahmoudy A. Laboratory evidence for the hematopoietic potential of Beta vulgaris leaf and stalk extract in a phenylhydrazine model of anemia. Braz J Med Biol Res. 2018;51:1–3. doi: 10.1590/1414-431X20187722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adebayo M.A., Enitan S.S., Owonikoko W.M., Igogo E., Ko A. Haematinic properties of methanolic stem bark and fruit extracts of Ficus sur in rats pre-exposed to phenylhydrazine-induced hemolytic anaemia. Afr J Biomed Res. 2017;20:85–92. [Google Scholar]
- 15.Itano H.A., Hirota K., Hosokawa K. Mechanism of induction of haemolytic anaemia by phenyl- hydrazine. Nature. 1975;256:665–667. doi: 10.1038/256665a0. [DOI] [PubMed] [Google Scholar]
- 16.Elaby S., Ali J. The anti-anemic effect of dried beet green in phenylhydrazine treated rats. Archiv Pharmaceut Sci Ain Shams Univ. 2018;2:54–69. [Google Scholar]
- 17.Sridhar Prasad Y.P., Hari Padmaja, Shajina M., Mirshad P.V., Fasalu Rahiman O.M. Hematinic and antioxidant potential of aqueous extract of Sesamum indicum seeds against phenylhydrazine-induced hemolytic anemia in albino rats. Natl J Physiol Pharm Pharmacol. 2018;8:1092–1096. [Google Scholar]
- 18.Gupta D., Kushwah C., Joshi A., Malvia S., Kharia A. Anti-anemic activity of hydro-alcoholic extract of Lycium barbarum in phenyl hydrazine induced anemic rats. Int J Res Pharm Sci. 2018;8:1–3. [Google Scholar]
- 19.Lee H.W., Kim H., Ryuk J.A., Kil K.J., Ko B.S. Hemopoietic effect of extracts from constituent herbal medicines of Samul-tang on phenylhydrazine-induced hemolytic anemia in rats. Int J Clin Exp Pathol. 2014;7:6179–6185. [PMC free article] [PubMed] [Google Scholar]
- 20.Ogbe R.J., Adoga G.I., Abu A.H. Antianaemic potentials of some plant extracts on phenyl hydrazine- induced anaemia in rabbits. J Med Plants Res. 2010;4:680–684. [Google Scholar]