Abstract
Background
Alcohol is a widely abused drug with many health implications, mainly caused by the oxidative and nitrosative stress on different body parts. Ayurvedic herbalism authenticates the multiple therapeutic applications of Terminalia arjuna bark due to its rich phytochemical repertoire.
Objective
To observe the extent of oxidative damage caused to erythrocytes by alcohol and assess the protective ability of T. arjuna bark powder aqueous extract (AETA) against the damage.
Materials and methods
Wister albino rats were categorized into four groups of eight rats per group; first group (control) was fed with glucose, second group was given alcohol at a dose of 20% v/v; 5g alcohol/kg b. wt/day, third group was co-administered with AETA (0.5 g/kg b. wt/day) and alcohol and the fourth group was kept on bark extract alone. Blood samples were collected and evaluated for different biochemical parameters after the completion of the treatment period.
Results
Alcohol significantly increased the erythrocyte membrane protein carbonyl and malondialdehyde (MDA) contents, along with a concomitant decrease in the membrane antioxidant status, when compared to the control group. Chromatographic analysis of the alcohol-treated rat erythrocyte membranes revealed altered membrane individual phospholipid contents and fluidity properties. Alcohol-induced morphological changes in the erythrocytes and its effect on decreasing the resistance of hypotonic shock induced by NaCl are evident from the hemolysis curves. However, AETA administration to alcoholic rats beneficially modulated the membrane properties anvd protected erythrocytes from damage.
Conclusion
Results suggest that AETA protects erythrocytes from alcohol-induced oxidative stress, biophysical, and biochemical changes very effectively.
Keywords: Alcohol, Herbal remedy, Oxidative stress, Erythrocyte membrane, Terminalia arjuna
1. Introduction
Excess alcohol consumption is a known risk factor in more than 200 diseases and thus the misuse of this legalized substance is an urgent concern, globally [1]. Unabated alcohol intake affects adversely all organs and tissues of the body since it has multiple targets. The pathophysiological changes induced by alcohol are initiate through its interactions with biological membranes. Though physiological cell functions are governed by the intracellular environment, changes in cellular metabolism are manifested by alterations in the structure and function of the plasma membrane and other internal membranes [2]. Every small change in the composition of cell membranes can affect the functioning of membrane proteins - ion and water channels which regulate the chemical and physiological balance in the cell. Alcohol is known to initiate the characteristic effects by its perturbing action on the lipid bilayer of biological membranes of RBCs since the majority of alcohol gets systemically circulated through the blood [3]. Although lacking protein synthesizing machinery and comparatively less specialized than many other cells, erythrocyte membranes carry on enough functions in common to that of highly specialized cells and are thus the chosen simple cell model to study complicated biochemical phenomena in the body [4]. Oxidants are known to produce biophysical alterations in the erythrocyte membrane as manifested by a decreased cytoskeletal protein content and production of high molecular weight proteins which can lead to abnormalities in erythrocyte shape and rheological properties [5,6]. Erythrocytes are extremely susceptible to oxidative damage as a result of the high polyunsaturated fatty acids (PUFA) and the high cellular oxygen and hemoglobin (Hb) concentrations in their membranes [7].
Considerable research in understanding the pathogenesis of alcohol-associated organ damage has shifted the attempts of researchers to treatment strategies with prevention and treatment as a goal, intending to reduce the global burden of alcoholic damage and alcohol-associated diseases [8]. Because of the gradual and continuous increase in the number of alcoholics and with the inclusion of new drinkers every year including teenagers, research and development of effective medicines for treating alcoholism/alcoholic damages are warranted. Several attempts have been made to develop clinically useful compounds from chemical synthesis, to ameliorate or cure alcohol-related disorders and it is well documented that these compounds may exhibit severe cytotoxicity, reproductive toxicity, and other side effects like diarrhea, nausea, and headache [9,10]. Therefore developing an alternative therapeutic strategy, especially based on natural products has been the focus of research [11]. Several researchers emphasized a need for extensive studies to forward effective and potential phytomedicines in the field of treatment of alcoholism [12]. Since more than 4000 phenolic compounds with multiple therapeutic applications have been found in vascular plants, they are believed to be the best-known resources of natural medicine [13]. It is evident from the literature that herbal medicines and their active ingredients alleviate ALD and other alcohol-related diseases through multiple mechanisms which involve antioxidant, anti-inflammation, inhibition of lipid synthesis, and increased fatty acid oxidation. The findings suggest that phytomedicines are promising candidates for prevention or treatment of alcohol-related diseases [14,15].
Terminalia arjuna (T.arjuna) is a deciduous tree of the Combretaceae family which is widely distributed throughout India and is well documented in the Indian ayurvedic system as a medicinal tree, with wide therapeutic applications, especially for its bark extract. Thorough screening of ethnobotanical and scientific literature revealed that T. arjuna possess several potent phytoprinciples and has been used to treat a wide range of diseases ranging from simple illnesses to complex degenerative diseases [16] It is also an active ingredient in many Ayurvedic formulations like Arjunarishta, Arjunaghrita, Shankara vati, Arjunadisiddha kshira, kakubhadi kshira, Arjuna Tablets and Liv-52; with many therapeutic applications [[17], [18], [19], [20]]. Bark extract of T. arjuna was reported to contain many specific phytocompounds including Triterpene glycosides - arjunetin, arjunoglucoside - I/II/III, arjunoside I/II/III/IV, arjunolitin and terminolitin; triterpene saponins - arjunic acid, arjunolic acid, arjungenin; flavonoids – arjunone and arjunolone and also it contains non-specific phytocompounds including phytosterols i.e., β-sitosterol, proanthocyanidins and minerals - Ca2+, Mg2+, Zn2+ and Cu2+ [21,22]. These specific and non-specific phytocompounds possess antioxidant [16], anti-haemolytic [23], anti-hyperlipidemic [24], antimicrobial [16], anticarcinogenic [25], anti-inflammatory [26] properties and known to confer the bark extract of T. arjuna with cardioprotective [27], hepato & renal protective [28], gastroprotective [29,30] and wound healing effects [30]. Hepatoprotective and nephroprotective effects of bark powder aqueous extracts of T. arjuna against alcohol-induced system damage in rats is being presented in our earlier works [31] and the current work is an attempt to look at the ameliorative effects of T. arjuna against alcohol-induced biophysical and biochemical damage of erythrocytes membranes.
2. Materials and methods
2.1. Chemicals
Alcohol∼96% (Merck, India), May-Grunwald stain, Giemsa stain (Fluka Research chemicals), Phospholipids standards (Aventi Polar Lipid Co.), and Sodium Chloride 99% was purchased from Sigma (Sigma, India). All the other chemicals were purchased from SDFCL, India, and were of analytical grade.
2.2. Preparation of aqueous extract of T. arjuna (AETA)
Bark of T. arjuna (Roxb.) Wight & Arn. Obtained from local sources and authenticated at National Ayurveda Dietetics Research Institute, Jayanagar, Bangalore, and voucher specimen (Drug Authentication/SMPU/NADRI/BNG/2019–20/1129) was deposited in the herbarium of the institution. Chunks of bark were made into smaller pieces by using mortar and pestle, and further powdered using a blender. For 25 gm of powder taken in a conical flask, approximately 150 ml of distilled water was added to soak the powder completely, followed by the addition of chloroform (1 ml per 100 ml of extract mixture). The extract obtained by incubating the flask overnight on an orbital shaker was filtered using a muslin cloth and the residue was subject to two more rounds of extraction. The Filtrate pooled out of the three extractions was poured into steel trays and concentrated by keeping on a water bath set at 25–30 °C for 24–48 h until the surface appears dried. Thereafter the extract concentrate was scraped into petri plates and further kept in a desiccator for 24–48 h to rid the scrapings of moisture. The dried extract was powdered using a mortar and pestle, and stored in airtight containers. Percentage of yield for the above-mentioned procedure was found to be 35.07%.
2.3. Animals and experimental design
Albino Wistar male rats weighing 180–200 gm were procured from Sri Venkateswara agencies, Bangalore, India. Animals were maintained on a standard pellet diet (M/s. Hindustan Lever Ltd., Mumbai, India) and water ad libitum. After acclimatization for a week, animals were divided into four groups and caged individually. Experimentation and animal maintenance were done with the approval of the institutional ethical committee (Regd. No. 470/01/a/CPCSEA, dt.24th Aug 2019) and as per guidelines. Animals were categorized into four groups of eight rats in each group. Group I (Control rats) received glucose as a caloric equivalent of alcohol; group II (alcoholic rats) received an oral administration of alcohol through a gastric tube (20% alcohol v/v; 5 gm alcohol/kg b. wt/day); group III (Therapeutic group) received a co-administration of AETA (0.5 gm of AETA/kg b. wt/day) along with alcohol (20% alcohol v/v; 5 gm alcohol/kg b. wt/day) and group IV were given AETA (0.5 gm of AETA/kg b. wt/day) alone. All treatments were given for a period of 60 days. Experimental duration, dosage, and mode of administration of alcohol were adopted from earlier studies [32,33] and the dosage of AETA for the present investigation was fixed based on the earlier reports [34,35]. After the completion of the experimental period, all the rats were sacrificed by cervical dislocation. Blood was immediately collected into heparinized tubes by cardiac puncture and plasma was separated for assessing different biochemical parameters.
2.4. Preparation of erythrocyte membrane
Erythrocytes were isolated from the heparinized whole blood as described previously [36], wherein to remove WBC and platelets; blood was diluted with saline and then passed through a cellulose column. The filtrate collected was centrifuged at 1000 rpm for 10 min. The erythrocytes, settled as pellets, were separated by removing the upper supernatant carefully. This washing step was repeated and the finally obtained erythrocytes were used for the preparation of erythrocyte ghosts. Erythrocyte membrane was prepared by using the standard method of Dodge et al., as mentioned earlier [37]. Erythrocyte suspension was washed with phosphate-buffered saline (pH 7.2). Then cells were lysed with 5 mM phosphate buffer (pH.8.0) and were spun at 15,000 g for 30 min. The supernatant was removed carefully and by using the same buffer the latter step was repeated to obtain hemoglobin free ghosts. Membrane protein was estimated by using the standard Lowry’s method.
2.5. Estimation of malondialdehyde (MDA)
Lipid peroxidative extent of the erythrocyte membrane fractions were measured in terms of the formation of MDA, an end product of the lipid peroxidation, by using the standard method of Ohkawa as adapted earlier [38]. Malondialdehyde reacts with TBA to form a chromogenic complex with absorption maxima at 535 nm. The MDA content in the membrane fractions were expressed as μmols MDA formed/mg protein.
2.6. Estimation of protein carbonyl content
The concentration of protein carbonyls in the erythrocyte membrane fractions was determined using 2,4-dinitrophenylhydrazine (DNPH) assay, according to the method of Reznick and Packer, 1994 [39]. Carbonyls react with 2,4-DNPH to form hydrazones, which can be detected spectrophotometrically at 372 nm and the results are expressed as μmols/mg protein.
2.7. Erythrocyte membrane lipid extraction and analysis
Erythrocyte membrane lipids were extracted by adapting the standard method of Folch et al. as mentioned earlier [40]. A known volume of suspension was mixed with 10 ml of chloroform-methanol mixture (2:1v/v) and vigorously shaken, filtered through Whatmann filter paper (No.42) and the filtrate was mixed with 0.2 ml of physiological saline and the mixture was kept overnight undisturbed. The lower phase with lipid was drained off into pre-weighed beakers and the upper phase was re-extracted with more of chloroform-methanol mixture and the extracts were pooled and evaporated to dryness. The pooled lipid fractions were redissolved in 1.0 ml of chloroform-methanol mixture and aliquots were used for the estimation of cholesterol and phospholipids. Erythrocyte membrane individual phospholipids were quantified by HPLC. The chromatographic column was a 300 × 4mm in diameter pre-packed stainless steel micro porasil silica column, which contained silica gel (10 μm particle size). The mobile phase solvent [acetonitrile-methanol-phosphoric acid (85%) (90:3:1, v/v/v)] was thoroughly mixed in advance, filtered through a microporous membrane (0.2 μm) and degassed, and delivered to the column by a computerized solvent delivery system at the flow rate of 0.80 ml per min. The sample volume injected for HPLC analysis was 10 μl. The effluent was detected by a UV detector at 203 nm.
2.8. Analysis of erythrocyte antioxidant status
Hemolysate of erythrocyte was prepared by adapting the method of Beutler et al., as mentioned earlier [41]. Erythrocyte suspension (0.2 ml) in saline was added to 1.8 ml of β-mercaptoethanol-EDTA stabilizing solution (0.05 ml of β-mercaptoethanol and 10 ml of neutralized 10% EDTA to a volume of 1 L with water) to obtain 1:20 hemolysate. The tubes containing hemolysate were stored at 4 °C for assay of enzymes within 1–2 h of preparation unless otherwise stated. Hemoglobin in hemolysate was estimated and activity of the enzyme was expressed as international units/gram haemoglobin (IU/gHb) unless otherwise stated. Activity of catalase enzyme was determined considering extinction coefficient of H2O2 as 0.071 cm−1 mol−1 and expressed as IU x 104/g Hb at 37 °C. Total reduced glutathione content and glutathione peroxidase enzyme activity was measured, wherein the cell lysate was prepared by adding 2.0 ml of water to 0.2 ml of packed erythrocytes. Superoxide dismutase enzyme activity was assayed after removal of hemoglobin from the hemolysate.
2.9. Erythrocyte membrane fluidity studies
Erythrocyte membrane fluidity was quantitatively assessed by fluorescence polarization technique with DPH (1,6-diphenyl-1,3,5-hexatriene) as a fluorescent probe. Freshly prepared membrane filtrate (equivalent to 50 μg protein) was suspended in 50 mmol/L of DPH (solubilized in tetrahydrofuran), and incubated at 37 °C for 30 min. Fluoresence polarization was determined using fluorescence spectrophotometer (Hitachi, Japan). The excitation wavelength was set at 360 nm and emission intensity was read at 435 nm. Polarization (P) and fluorescence anisotropy (γ) were measured as mentioned in our earlier studies [36].
2.10. Erythrocyte osmotic stability studies
Osmotic stability of erythrocytes was assessed by adopting the method of Penha-Silva et al., 2008 [42]. Accordingly, duplicate Eppendorf vials containing 1 ml of NaCl solution taken in different concentrations (0–1 g/dL) were pre-incubated for 10 min at 37 °C. Later 20 μl of whole blood was added to each tube, shaken, sealed, and incubated further for 20 min. Vials were then centrifuged (1300 g, 10 min) at 37 °C. Absorbance (A) at 540 nm for each supernatant was measured and converted into percentage hemolysis using the expression,
| Hemolysis (%) = A/Amax x 100 |
(A = absorbance at 540 nm, Amax = mean maximal absorbance).
The relationship between the percentage of hemolysis and NaCl concentration was statistically adjusted to a sigmoid regression line.
2.11. Erythrocyte morphological studies
Alcohol-induced morphological changes in erythrocytes of rats belonging to different treatment groups were analyzed following the method of Alimi et al., 2012 [43]. Accordingly, 20 μl of the whole blood was spread on slides immediately after blood sampling and the smears were air-dried and stained on a staining rack by flooding with May-Grunwald solution for 3 min. An equal volume of 0.1 M phosphate buffer (pH 7.4) was then added without rinsing the May-Grunwald solution. After a metallic gleam becomes visible, the solution was poured off and thoroughly rinsed with demineralized water and Giemsa solution (in 0.1 M phosphate buffer, pH 7.4) was applied for 20 min. Later the slides were intensively rinsed with demineralized water and blotted. After staining the slides were examined under a microscope (Olympus, lens: 100x with oil immersion). Erythrocytes were morphologically identified and counted to determine the number of deformed erythrocytes in the blood smear sample of a select number of rats from each group. Alcohol-induced morphological changes in erythrocytes were investigated by enumerating different erythrocyte forms and the normal to abnormal ratio of erythrocytes was calculated.
2.12. Statistical analysis
Mean and S.E.M values of all the parameters were determined for each group. ANOVA followed by student “t” test were performed to determine the significant differences among the groups. A p < 0.05 was considered statistically significant.
3. Results
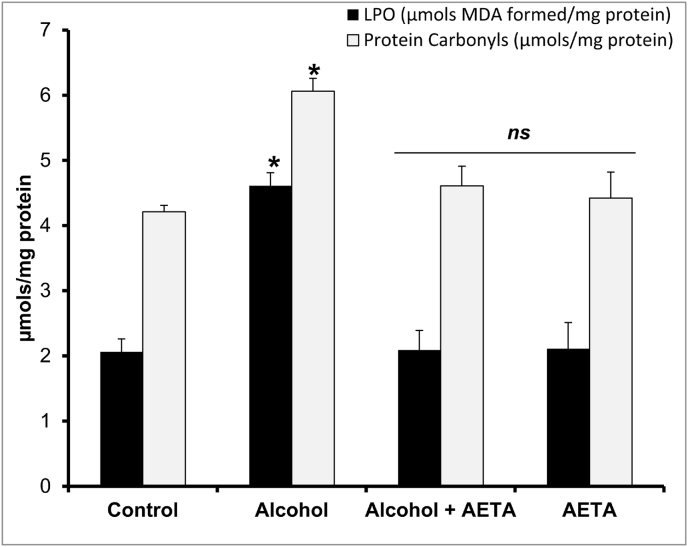
Alcohol-induced biochemical and biophysical alterations in rat erythrocyte membranes, and the protective effects of AETA against those changes, are evident in the present study. A significant increase (p < 0.05) in lipid peroxidation - 124% and protein carbonyl content - 44% (Fig. 1) in red cell membranes from alcoholic rats was observed in comparison with the control group. Administration of AETA to alcohol receiving rats prevented the hike and significantly decreased the values, comparable to the control group of rats.
Fig. 1.
Effect of AETA administration on erythrocyte membrane lipid peroxidation (LPO) and Protein carbonyl contents in control and experimental group of rats. Values are represented as mean ± SEM (n = 8). A p < 0.05 is considered as significantly different between groups. “∗” symbol represents “significant difference” compared to control and “ns” symbol represents “not-significantly different” from control group of rats.
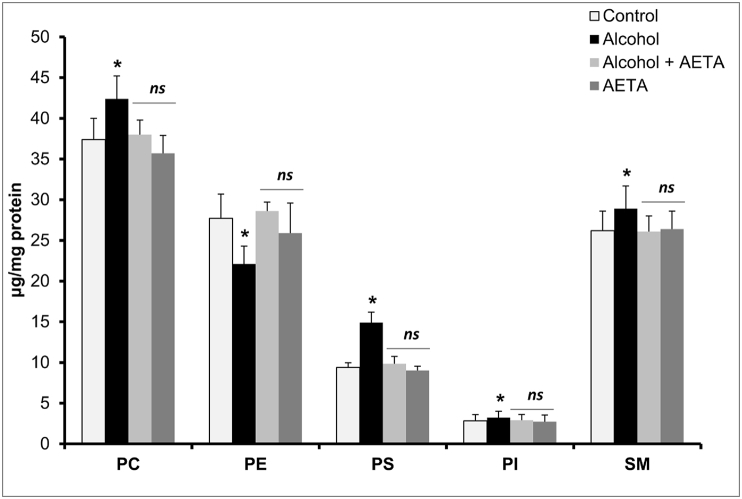
The effect of alcohol on the erythrocyte membrane total cholesterol and total phospholipids is presented in Table 1. Significant elevation in the levels of cholesterol (77%) and phospholipids (17%) were observed in cell membranes of erythrocytes in alcohol-treated rats. Co-administration of AETA along with alcohol resulted in mitigation of the alcohol-induced elevation of the parameters. Moreover increased c/p ratio in membranes from chronic alcoholic rats is also evident from the result obtained. Furthermore, the effect of alcohol on the concentrations of erythrocyte membrane individual phospholipids is studied in the present study. Significant elevations in the levels of PC (13%), PE (29%), PS (59%), PI (14%), and SM (5%) were observed in the alcohol-treated rats, in comparison with the control group (Fig. 2). AETA administration could significantly rectify the changes observed in the alcoholic group by restoring the individual phospholipid levels, comparable to the levels of the phospholipids in control group rats.
Table 1.
Effect of administration of AETA on erythrocyte membrane total cholesterol and total phospholipids in chronic alcoholic rats.
| Parameter | Control | Alcohol | Alcohol + AETA | AETA |
|---|---|---|---|---|
| Total Cholesterol (μg/mg protein) | 89.8 ± 2.9d | 168.0 ± 5.9a | 98.7 ± 3.3c | 83.4 ± 6.6d |
| Total Phospholipids (μg/mg protein) | 103.1 ± 3.7d | 135.0 ± 6.8a | 105.4 ± 4.2c | 99.7 ± 4.2d |
| C/P ratio | 0.87 | 1.24 | 0.93 | 0.83 |
Values are Mean ± SEM of eight rats in each group. Means in a row not sharing a common superscript are significantly different (P < 0.05) among groups.
Fig. 2.
Effect of AETA administration on erythrocyte membrane individual phospholipids; phosphatidyl choline (PC), phosphatidyl ethanolamine (PE), phosphatidyl serine (PS), phosphatidyl inositol (PI) and sphingomyelin (SM) in control and experimental group of rats. Values are represented as mean ± SEM (n = 8). A p < 0.05 is considered as significantly different between groups. “∗” symbol represents “significant difference” compared to control and “ns” symbol represents “not-significantly different” from control group of rats.
Alcohol -induced biochemical alterations in the status of erythrocyte antioxidant machinery - GSH content and the activities of GPx, SOD, and Catalase enzymes are furnished in Table 2. Alcoholic rats showed a significant decrease (p < 0.05) in GSH content (21%) and enzyme activities (26%, 26%, and 28% respectively), in comparison with the control group. Administration of AETA to alcohol receiving rats caused the reversal of these alterations and the enzyme activities were restored to normal level along with the GSH content (see Table 2).
Table 2.
Effect of administration of AETA on erythrocyte membrane antioxidant enzymes in chronic alcoholic rats.
| Parameter | Control | Alcohol | Alcohol + AETA | AETA |
|---|---|---|---|---|
| GSH (μmol/gHb) | 3.69 ± 0.1a | 2.91 ± 0.2c | 3.67 ± 0.1a | 3.86 ± 0.8a |
| GPx (μmol of GSH oxidised/min/mgHb) | 17.0 ± 1.8a | 12.6 ± 1.1c | 17.4 ± 1.4a | 17.3 ± 1.8a |
| SOD (Units/mgHb/min) | 6.14 ± 0.2a | 4.52 ± 0.4c | 5.91 ± 0.7a | 6.27 ± 0.6a |
| Catalase (IUX104/gHb) | 8.44 ± 0.6a | 6.03 ± 0.9c | 8.29 ± 0.9a | 8.61 ± 0.1a |
Values are Mean ± SEM of eight rats in each group. Means in a row not sharing a common superscript are significantly different (P < 0.05) among groups.
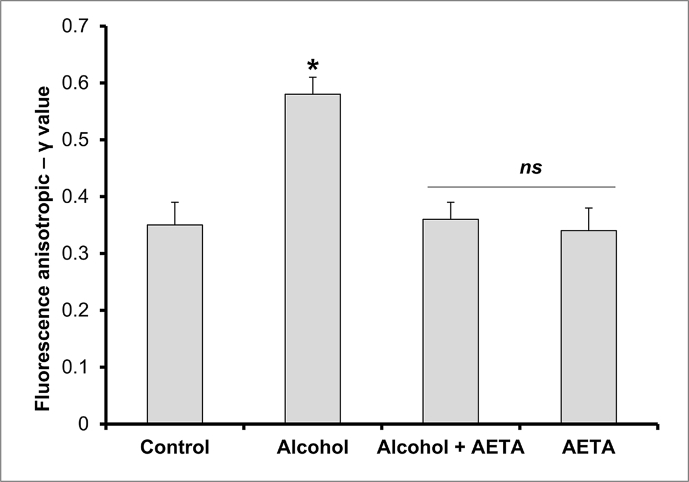
Membrane fluidity property of the erythrocyte membranes was assessed through the anisotropic studies done using DPH as the hydrocarbon probe. Erythrocyte membranes from different treatment groups were assessed for their fluidity properties and the data is depicted in Fig. 3. Compared to control group rats, alcohol-intoxicated rats exhibited a significant rise (p < 0.05) in the anisotropic – γ value (11%); whereas co-administration of AETA along with alcohol resulted in the rectification of the γ – values (8%).
Fig. 3.
Effect of AETA administration on erythrocyte membrane anisotropic value (γ) in control and experimental group of rats. Values are represented as mean ± SEM (n = 8). A p < 0.05 is considered as significantly different between groups. “∗” symbol represents “significant difference” compared to control and “ns” symbol represents “not-significantly different” from control group of rats.
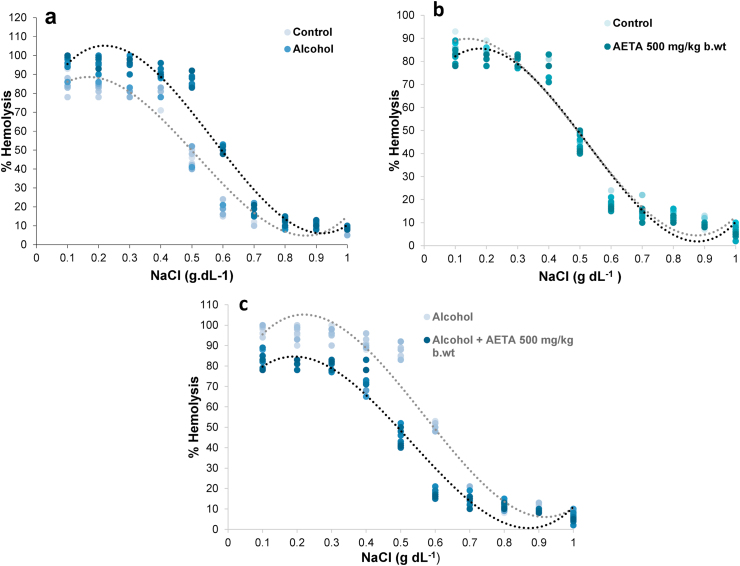
Alcohol-induced changes in the osmotic properties of erythrocyte from rats belonging to different treatment groups and the protective role played by AETA in offering resistance, were investigated in the present study. Osmotic stability of erythrocytes was assessed at different concentrations of NaCl and the changes in percentage hemolysis from the erythrocytes of rats belonging to different treatment groups was presented as the classically represented osmotic stability curves, which were fitted to a sigmoidal plot (Fig. 4). Compared to the control group rats, erythrocytes from the rats treated with alcohol alone showed enhanced susceptibility to hemolysis, as evidenced by a large right-side shift of the alcohol curve from the control curve (Fig. 4a). Erythrocytes from the rats treated with AETA alone showed an enhanced resistance for hemolysis, as evidenced by a slight left shift of the AETA curve in comparison to the control curve (Fig. 4b). Alcoholic rats treated with AETA showed enhanced resistance to the hypotonic shock and hemolysis which was evidenced by a large left shift of alcohol + AETA curve in comparison to the alcohol curve (Fig. 4c).
Fig. 4.
Osmotic stability curves of erythrocytes from rats of different treatment groups, showing the variation of hemolysis percentage at different NaCl concentrations. Alcohol treatment to healthy rats enhanced susceptibility of their erythrocytes to hemolysis, as evident from the right shift of the ethanol curve from the normal curve (4a). Rats treated with AETA alone, showed enhanced resistance to hypotonic shock, depicted by a slight left-shift of AETA curve in comparison with the control curve (4b). Pre-treatment of alcohol-fed rats with AETA, effectively reversed the alcohol induced erythrocyte hemolysis, as evident from the large left-shift of AETA curve, compared to the alcohol curve (4c).
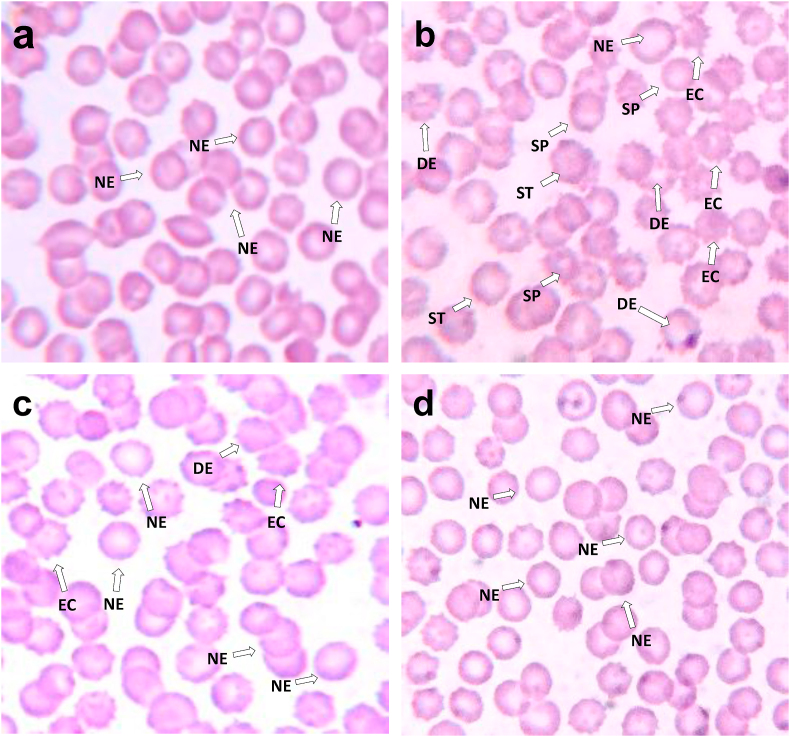
Biophysical effect of alcohol on red blood cell morphology and the therapeutic effect of AETA in the prevention of alcohol-induced biophysical changes is evident from photomicrographs shown in Fig. 5. Alcohol induced erythrocyte deformities, based on changes in erythrocyte morphology (shape) and also the influence of AETA administration on prevention of those deformities and restoration of normal to abnormal erythrocyte cell count ratio is presented in Table 3.
Fig. 5.
Photomicrographs of erythrocytes of control and experimental group of rats, wherein blood smear from control group of rats (5a) showed normal erythrocytes (NE) with the normal biconcave, discoid shape and smooth surface morphology. While smear sample from ethanol-treated rat (5b) showed very few numbers of normal erythrocytes (NE) and a large number of deformed erythrocytes (DE) with a maximum number of them getting transformed into echinocytes (EC), spherocytes (SP) and stomatocytes (ST), the smear sample from alcoholic rats treated with AETA (5c) showed normal morphological features with reduces the number of EC and increased number of NE. Erythrocytes of rats treated with AETA alone (5d) showed normal erythrocyte morphological features like that of control group.
Table 3.
Enumeration of different erythrocyte forms in control and experimental rats.
| Erythrocyte shapes | Control | Alcohol | Alcohol + AETA | AETA |
|---|---|---|---|---|
| Total erythrocyte numbera | 179 | 168 | 173 | 128 |
| Normal erythrocytesb | 90% (161) | 24% (40) | 80% (138) | 95% (122) |
| Stomatocytesb | 0% | 9% (15) | 0% | 0% |
| Echinocytesb | 7.4% (13) | 49% (82) | 21% (36) | 4.9% (6) |
| Spherocytesb | 2.5% (4) | 19% (32) | 0% | 1.9% (2) |
| Deformed erythrocytesb | 8.6% (15) | 76% (128) | 21% (36) | 5.8% (7) |
| Normal: Abnormal ratio | 5:1 | 1:6 | 7:1 | 6:1 |
Values are in number of erythrocytes.
Values are the percentages (number of cells in brackets) of different erythrocyte shapes counted under the microscope.
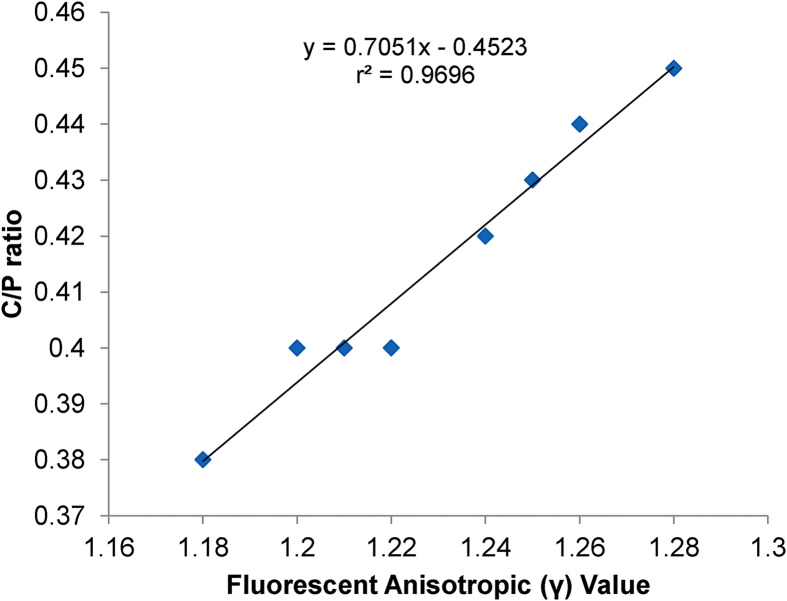
Fig. 6 shows the correlation between C/P ratio and fluorescent anisotropic values in alcohol-treated groups. The results show a strong positive correlation between the parameters with r2 value of 0.9696 and p < 0.001.
Fig. 6.
Correlation analysis (r values) between the fluorescent anisotropic values (γ) and C/P ratios of the alcoholic group of rats (n = 8); determined by Pearson correlation analysis method.
4. Discussion
Alcohol is a xenobiotic substance that can disturb cellular metabolism, leading to considerable changes in cell membrane structure and the associated biological functions. Alcohol’s interactions with lipid bilayers are wholly, if not largely, responsible for its characteristic effects such as euphoria, tolerance, dependence, adaptions, toxicity, disease, damage, inflammation, and pathological conditions [44,45]. Alcohol is a widely used and abused psychoactive drug which can impact almost all the organ systems. Though alcohol has a very simple chemical composition and structure, its pleiotropic effects on multiple body parts are primarily because of its pharmacological role in disordering the membrane lipids and proteins in any biomembrane [46,47]. Red blood cell membranes, in particular, are readily exposed to alcohol for prolonged durations and are the known targets of alcohol actions [36]. Acetaldehyde, the major product of alcohol metabolism is reported to be the compound responsible for the typical alcohol-induced erythrocyte damage [36].
Safer medications and treatments are in demand to prevent alcoholic disorders and modern treatment protocols emphasize the use of natural medicines. Contrary to the synthetic medicines many traditional medicines and natural products have been shown to have preventive and therapeutic effects for alcoholism/alcohol-related diseases. Available scientific literature strongly attests with the guarantee of consistency and repeatability of experimental results (using modern analytical and biochemical approaches) that phytoextracts are more potential and suitable to fight against alcohol-related diseases and disorders and/or to prevent/ameliorate or decrease the burden of alcoholic damage [14]. Many herbal remedies have been researched to look at their protective role against oxidative damage of erythrocytes caused by various oxidant stressor agents [[48], [49], [50], [51], [52]]. Attempts were also made to probe the protective role of various phytocompounds especially against ethanol-induced erythrocyte damage [36,53,54]. Current work is an attempt to specifically look at the protective effect of a herbal remedy against alcohol-induced changes in erythrocyte membrane fluidity and osmotic stability properties. We looked at the efficacy of T. arjuna - bark powder aqueous extract, in treating the different physicochemical changes in erythrocytes induced by alcohol. Our earlier in-vitro studies clearly proved the presence of a rich antioxidant repertoire in the bark of T. arjuna, which confer protection against free radical-induced damage of human erythrocytes [23], and this work is an extension to investigate its therapeutic potential in-vivo.
Erythrocytes have elevated oxygen tension and iron content which is the reason for its susceptibility to oxidative damage initiated by reactive oxygen species [36]. Radical induced peroxidation of membrane polyunsaturated fatty acids is reported to be the reason for erythrocyte membranes damage. Results of the present study clearly demonstrate that alcohol ingestion causes erythrocyte membrane damage mainly by releasing free radicals and many other damaging metabolites like acetaldehyde, which sets-off with a cascade of biochemical alterations like the elevation of erythrocyte membrane MDA content (a classical indicator for assessing the extent of lipid peroxidation) and also the elevation of membrane protein carbonyl content, respectively. AETA when administered to alcohol-fed rats, a significant reduction occurs in the levels of MDA and protein carbonyl contents, which clearly indicates the membrane protective ability of T. arjuna.
Membrane cholesterol: phospholipid (C/P) ratio and saturated: unsaturated fatty acid ratio are known to predominantly determine the membrane fluidity property [[55], [56], [57]]. In the present study, there is a significant increase of C/P ratio in erythrocyte membranes of alcohol-fed rats which indicates a decrease in membrane fluidity as per the earlier observations done on both human and animal models [66]. We observed a positive correlation (Fig. 6) between C/P ratio and fluorescent anisotropic (γ) value and the results are in agreement with earlier reports [33]. Membrane fluidity is observed to be restored in the alcoholic group of rats fed with AETA, since T. arjuna bark extracts is a proven therapeutic intervention for treating hyperlipidemia. Earlier reports show that the hypolipidemic potential of T. arjuna is specifically because of its inhibitory or down regulatory action on HMG-CoA reductase enzyme [58].
Phospholipids are the most essential membrane entities which provide proper polar and special arrangement for optimal activity of membrane-bound enzymes [59]. Literature reveals that lipid distribution in membranes is heterogeneous which differs across organelles and the fate of each lipid is closely linked with a variety of physiological phenomena [57]. Alcohol treatment to rats in the present study has resulted in a considerable alteration of phospholipid setting of the erythrocyte membrane which is known to affect the lipid and protein packing, orientation, and subsequently functioning. It is clear from this study that the elevated levels of PC, PS, PI, SM and decreased levels of PE observed in erythrocyte membranes of alcohol-treated rats might have contributed for the changes in the membrane fluidity and probably would also have facilitated the mediation of transphosphatidylation reaction, leading to the formation of phosphatidylethanol, which is mentioned to be an unusual phospholipid that plays a significant role in rearranging the phospholipid subclasses during prolonged alcohol exposure [56]. AETA could restore the phospholipid microenvironment in alcoholic rats probably because of its rich phytoprinciples.
Possible impact of alcohol on erythrocytes cannot be verified by just assaying standard parameters viz., antioxidant status, osmotic resistance, and MDA production alone, since studies [60] also reveal that oxidative damage inflicted to erythrocytes can also correspond to the dysfunctioning of the membrane transport system. Therefore, erythrocyte membrane fluidity alterations are being assessed and quantified in the present study by using fluorescent polarization technique. A significant increase in the anisotropic (γ) values for the alcohol-treated rats is being observed, which in turn indicates a reduction in the membrane fluidity as evidenced by the reduction in DPH polarization. The observations are in agreement with the earlier studies [36]. Results of this studies suggested an increase in microviscosity of the membrane domains of alcoholic rats indicating a decrease in fluidity or increased rigidification which was rectified/restored to that of control in alcoholic rats receiving AETA, which is probably because of the potential ion quenching activity of phytoconstituents present in AETA, which rendered nonavailability of the broken down adduct products of alcohol to cause any alterations to the erythrocyte membrane. The functions of proteins embedded in erythrocyte membranes are modulated by the composition and properties of the bilayer lipid environment, where lipids play a pivotal role in conferring fluidity property to the membrane. Our previous studies also revealed that the fluidity of the lipid environment influences physiologically important membrane-bound enzymes such as Na+/K+ATPase activity [59]. In the present study, altered levels of membrane cholesterol and phospholipids were strongly corresponding to the decreased membrane fluidity. To find out the relationship between C/P ratio and anisotropic value, person correlation analysis was performed and we noticed that there was a strong positive correlationship between the parameters.
Antioxidant enzymes are understood to be the first line of defense against free radical-induced oxidative stress [59]. A significant decrease of GSH levels and also the activities of antioxidant enzymes GPx, SOD, and CAT in alcohol-fed rats in the present group indicate the excess oxidative stress conditions induced by alcohol and these findings agree with the studies of Senthilkumar et al., 2002 [61]. Literature also attests to the fact that alcohol significantly alters the erythrocyte glutathione enzymatic machinery [62] and GSH being a major non-protein thiol playing an important role in coordinating the antioxidant defense system, the observed results are justifiable. AETA administration has efficiently conferred protection to the antioxidant machinery of erythrocyte membrane in alcohol-fed rats, clearly because of its rich phytochemical composition, which is proved to possess antioxidant and radical scavenging abilities.
Chronic ethanol consumption is known to significantly decrease the erythrocyte resistance to hypnotic shock which is evidenced in the present study as a large right shift in the osmotic stability curve of erythrocytes from rats receiving alcohol alone in comparison with the control group. The results are in agreement with earlier reports [42,50]. A possible reason for the observed susceptibility of erythrocytes during chronic ethanol consumption might be the formation of membrane pores leading to morphological changes in the shapes of erythrocytes. AETA administration is understood to be safe for consumption as the erythrocytes from rats receiving plant extract alone showed enhanced resistance to the hemolysis and further AETA could effectively avoid the osmotic susceptibility of erythrocyte, as observed in alcoholic rats treated with AETA.
These observations are further supported by the morphological studies using whole blood smears which revealed changes in the shape of RBCs in alcoholic rats. Red cells from AETA receiving alcoholic rats did not show any significant changes in morphology and shape. It is evident from the reports of the present study that alcohol-induced deformities in red blood cells were not found in AETA receiving rats. While alcoholic rat blood smear showed normal to abnormal ratio of 1:6, AETA treatment brought the ratio to almost normal by decreasing the count of abnormal cells and the reports are in agreement with earlier reports [42]. These observations indicate the efficacy of AETA in decreasing the alcohol-induced oxidative stress on the erythrocyte membrane, reflecting not only the free radical and antioxidant principles of bark extract but also its cytoprotective properties.
5. Conclusion
The present study confirms the protective effect of AETA in conferring protection against alcohol-induced oxidative stress and damage to the erythrocyte membranes. However, there is further scope for looking at the synergistic pattern in which these phytocompounds act to confer protection against alcohol-induced membrane damage. Investigations are needed at the molecular level to understand the effect of AETA in ameliorating the alcohol-induced alterations of erythrocyte cytoskeletal protein network: spectrin, ankyrin, band 3, band 4.1 & 4.2, actin, G3PDH, and stomatin. Moreover, human clinical trials are warranted to establish the correspondence between the experimental dose and a possible dose that could be used in humans.
Source(s) of funding
None declared.
Conflict of interest
None.
Acknowledgments
Authors are thankful to Dr. Vipin Nair, Department of Chemistry, REVA University, Biosciences, Bengaluru; for his valuable inputs.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Rehm J., Room R., Monteiro M., Gmel G., Graham K., Rehn N. Alcohol as a risk factor for global burden of disease. Eur Addiction Res. 2003 Oct;9(4):157–164. doi: 10.1159/000072222. PMID: 12970584. [DOI] [PubMed] [Google Scholar]
- 2.Adachi J., Asano M., Ueno Y., Niemelä O., Ohlendieck K., Peters T.J. Alcoholic muscle disease and biomembrane perturbations (review) J Nutr Biochem. 2003 Nov;14(11):616–625. doi: 10.1016/s0955-2863(03)00114-1. PMID: 14629892. [DOI] [PubMed] [Google Scholar]
- 3.Dickey A.N., Faller R. How alcohol chain-length and concentration modulate hydrogen bond formation in a lipid bilayer. Biophys J. 2007 Apr 1;92(7):2366–2376. doi: 10.1529/biophysj.106.097022. Epub 2007 Jan 11. PMID: 17218462; PMCID: PMC1864837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asaro R.J., Zhu Q. Vital erythrocyte phenomena: what can theory, modeling, and simulation offer? Biomech Model Mechanobiol. 2020 Feb 10;19:1361–1388. doi: 10.1007/s10237-020-01302-x. [DOI] [PubMed] [Google Scholar]
- 5.Hatherill J.R., Till G.O., Ward P.A. Mechanisms of oxidant-induced changes in erythrocytes. Agents Actions. 1991 Mar;32(3–4):351–358. doi: 10.1007/BF01980898. PMID: 1862753. [DOI] [PubMed] [Google Scholar]
- 6.Hebbel R.P., Leung A., Mohandas N. Oxidation-induced changes in microrheologic properties of the red blood cell membrane. Blood. 1990 Sep 1;76(5):1015–1020. PMID: 2393710. [PubMed] [Google Scholar]
- 7.Cruz Silva M.M., Madeira V.M., Almeida L.M., Custódio J.B. Hemolysis of human erythrocytes induced by tamoxifen is related to disruption of membrane structure. Biochim Biophys Acta. 2000 Mar 15;1464(1):49–61. doi: 10.1016/s0005-2736(99)00237-0. PMID: 10704919. [DOI] [PubMed] [Google Scholar]
- 8.Seitz H.K., Bataller R., Cortez-Pinto H., Gao B., Gual A., Lackner C. Alcoholic liver disease. Nat Rev Dis Prim. 2018 Aug 16;4(1):16. doi: 10.1038/s41572-018-0014-7. Erratum in: Nat Rev Dis Primers. 2018 Aug 28;4(1):18. PMID: 30115921. [DOI] [PubMed] [Google Scholar]
- 9.Kiefer F., Mann K. New achievements and pharmacotherapeutic approaches in the treatment of alcohol dependence. Eur J Pharmacol. 2005 Dec 5;526(1–3):163–171. doi: 10.1016/j.ejphar.2005.09.028. Epub 2005 Nov 2. PMID: 16266700. [DOI] [PubMed] [Google Scholar]
- 10.Goh E.T., Morgan M.Y. Review article: pharmacotherapy for alcohol dependence - the why, the what and the wherefore. Aliment Pharmacol Ther. 2017 Apr;45(7):865–882. doi: 10.1111/apt.13965. Epub 2017 Feb 20. PMID: 28220511. [DOI] [PubMed] [Google Scholar]
- 11.Tomczyk M., Zovko-Koncić M., Chrostek L. Phytotherapy of alcoholism. Nat Prod Commun. 2012 Feb;7(2):273–280. PMID: 22474979. [PubMed] [Google Scholar]
- 12.Abenavoli L., Capasso F., Addolorato G. Phytotherapeutic approach to alcohol dependence: new old way? Phytomedicine. 2009 Jun;16(6–7):638–644. doi: 10.1016/j.phymed.2008.12.013. Epub 2009 Feb 11. PMID: 19216063. [DOI] [PubMed] [Google Scholar]
- 13.Xu B.J., Zheng Y.N., Sung C.K. Natural medicines for alcoholism treatment: a review. Drug Alcohol Rev. 2005 Nov;24(6):525–536. doi: 10.1080/09595230500293795. PMID: 16361209. [DOI] [PubMed] [Google Scholar]
- 14.Ding R.B., Tian K., Huang L.L., He C.W., Jiang Y., Wang Y.T. Herbal medicines for the prevention of alcoholic liver disease: a review. J Ethnopharmacol. 2012 Dec 18;144(3):457–465. doi: 10.1016/j.jep.2012.09.044. Epub 2012 Oct 9. PMID: 23058988. [DOI] [PubMed] [Google Scholar]
- 15.Dhiman R.K., Chawla Y.K. Herbal medicines for liver diseases. Dig Dis Sci. 2005 Oct;50(10):1807–1812. doi: 10.1007/s10620-005-2942-9. PMID: 16187178. [DOI] [PubMed] [Google Scholar]
- 16.Mandal S., Patra A., Samanta A., Roy S., Mandal A., Mahapatra T.D. Analysis of phytochemical profile of Terminalia arjuna bark extract with antioxidative and antimicrobial properties. Asian Pac J Trop Biomed. 2013 Dec;3(12):960–966. doi: 10.1016/S2221-1691(13)60186-0. PMID: 24093787; PMCID: PMC3805097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shengule S.A., Mishra S., Joshi K., Apte K., Patil D., Kale P. Anti-hyperglycemic and anti-hyperlipidaemic effect of Arjunarishta in high-fat fed animals. J Ayurveda Integr Med. 2018 Jan-Mar;9(1):45–52. doi: 10.1016/j.jaim.2017.07.004. . Epub 2017 Dec 15. PMID: 29249636; PMCID: PMC5884182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dube N., Nimgulkar C., Bharatraj D.K. Validation of therapeutic anti-inflammatory potential of Arjuna Ksheera Paka - a traditional Ayurvedic formulation of Terminalia arjuna. J Tradit Complement Med. 2016 Dec 29;7(4):414–420. doi: 10.1016/j.jtcme.2016.11.006. PMID: 29034188; PMCID: PMC5634724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cota D., Mishra S., Shengule S. Arjunarishta alleviates experimental colitis via suppressing proinflammatory cytokine expression, modulating gut microbiota and enhancing antioxidant effect. Mol Biol Rep. 2020 Sep 3;47:7049–7059. doi: 10.1007/s11033-020-05766-z. [DOI] [PubMed] [Google Scholar]
- 20.Cota Damita L., Mishra Sanjay, Shengule Sushant A., Patil Dhanashree. Assessment of in vitro biological activities of Terminalia arjuna Roxb. bark extract and Arjunarishta in inflammatory bowel disease and colorectal cancer. Indian J Exp Biol. May 2020;58:306–313. [Google Scholar]
- 21.Ghosh J., Sil P.C. Arjunolic acid: a new multifunctional therapeutic promise of alternative medicine. Biochimie. 2013 Jun;95(6):1098–1109. doi: 10.1016/j.biochi.2013.01.016. Epub 2013 Feb 10. PMID: 23402784. [DOI] [PubMed] [Google Scholar]
- 22.Dwivedi S. Terminalia arjuna Wight & Arn.--a useful drug for cardiovascular disorders. J Ethnopharmacol. 2007 Nov 1;114(2):114–129. doi: 10.1016/j.jep.2007.08.003. Epub 2007 Aug 10. PMID: 17875376. [DOI] [PubMed] [Google Scholar]
- 23.Hebbani A.V., Reddy V.D., Nallanchakravarthula V. In vitro anti-hemolytic activity of Terminalia arjuna (Roxb.) Wt. and Arn. bark powder aqueous extract. Indian J Adv Chem Sci. 2014;3:102–108. [Google Scholar]
- 24.Subramaniam S., Ramachandran S., Uthrapathi S., Gnamanickam V.R., Dubey G.P. Anti-hyperlipidemic and antioxidant potential of different fractions of Terminalia arjuna Roxb. bark against PX- 407 induced hyperlipidemia. Indian J Exp Biol. 2011 Apr;49(4):282–288. PMID: 21614892. [PubMed] [Google Scholar]
- 25.Sivalokanathan S., Ilayaraja M., Balasubramanian M.P. Antioxidant activity of Terminalia arjuna bark extract on N-nitrosodiethylamine induced hepatocellular carcinoma in rats. Mol Cell Biochem. 2006 Jan;281(1–2):87–93. doi: 10.1007/s11010-006-0433-8. PMID: 16328960. [DOI] [PubMed] [Google Scholar]
- 26.Halder S., Bharal N., Mediratta P.K., Kaur I., Sharma K.K. Anti-inflammatory, immunomodulatory and antinociceptive activity of Terminalia arjuna Roxb bark powder in mice and rats. Indian J Exp Biol. 2009 Jul;47(7):577–583. PMID: 19761042. [PubMed] [Google Scholar]
- 27.Bishop S., Liu S.J. Cardioprotective action of the aqueous extract of Terminalia arjuna bark against toxicity induced by doxorubicin. Phytomedicine. 2017 Dec 1;36:210–216. doi: 10.1016/j.phymed.2017.10.007. Epub 2017 Oct 20. PMID: 29157817. [DOI] [PubMed] [Google Scholar]
- 28.Sinha Mahua, Manna Prasenjit, Sil Parames C. Aqueous extract of the bark of Terminalia arjuna plays a protective role against sodium-fluoride-induced hepatic and renal oxidative stress. J Nat Med. 2007;61(3):251–260. [Google Scholar]
- 29.Devi R.S., Narayan S., Vani G., Shyamala Devi C.S. Gastroprotective effect of Terminalia arjuna bark on diclofenac sodium induced gastric ulcer. Chem Biol Interact. 2007 Apr 5;167(1):71–83. doi: 10.1016/j.cbi.2007.01.011. Epub 2007 Feb 2. PMID: 17327128. [DOI] [PubMed] [Google Scholar]
- 30.Cota D., Mishra S., Shengule S. Beneficial role of Terminalia arjuna hydro-alcoholic extract in colitis and its possible mechanism. J Ethnopharmacol. 2019 Feb 10;230:117–125. doi: 10.1016/j.jep.2018.10.020. Epub 2018 Oct 24. PMID: 30367989. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhari M., Mengi S. Evaluation of phytoconstituents of Terminalia arjuna for wound healing activity in rats. Phytother Res. 2006 Sep;20(9):799–805. doi: 10.1002/ptr.1857. PMID: 16835874. [DOI] [PubMed] [Google Scholar]
- 32.Hebbani Ananda Vardhan, Bulle Saradamma, Reddy Kanu Venkateswarlu, Malini Asha Balachandrababu, Reddy Vaddi Damodara, Nallan Chakravarthula Varadacharyulu. Nephro-protective activity of wheatgrass juice against alcohol-induced oxidative damage in rats. Toxicol Mech Methods. 2020 Sep;30(9):679–686. doi: 10.1080/15376516.2020.1810837. [DOI] [PubMed] [Google Scholar]
- 33.Bulle S., Reddy V.D., Hebbani A.V., Padmavathi P., Challa C., Puvvada P.K. Nephro-protective action of P. santalinus against alcohol-induced biochemical alterations and oxidative damage in rats. Biomed Pharmacother. 2016 Dec;84:740–746. doi: 10.1016/j.biopha.2016.09.103. Epub 2016 Oct 3. PMID: 27710898. [DOI] [PubMed] [Google Scholar]
- 34.Raghavan B., Kumari S.K. Effect of Terminalia arjuna stem bark on antioxidant status in liver and kidney of alloxan diabetic rats. Indian J Physiol Pharmacol. 2006 Apr-Jun;50(2):133–142. PMID: 17051732. [PubMed] [Google Scholar]
- 35.Hebbani A.V., Reddy V.D., Varadacharyulu N.C. Protective effect of aqueous bark extract of Terminalia arjuna against alcohol-induced hepato and nephrotoxicity in rats. Int J Phytomed 20157. 2015;2:142–153. [Google Scholar]
- 36.Reddy V.D., Padmavathi P., Paramahamsa M., Varadacharyulu N. Modulatory role of Emblica officinalis against alcohol induced biochemical and biophysical changes in rat erythrocyte membranes. Food Chem Toxicol. 2009 Aug;47(8):1958–1963. doi: 10.1016/j.fct.2009.05.014. Epub 2009 May 18. PMID: 19454300. [DOI] [PubMed] [Google Scholar]
- 37.Begum S.M., Padmavathi P., Saradamma B., Maturu P., Vardhan A.H., Varadacharyulu N.C. Effect of green tea consumption on RBC morphology, membrane properties and antioxidant status in chronic cigarette smokers. Indian J Biochem Biophys. 2018 Aug;55:256–263. [Google Scholar]
- 38.Maturu P., Reddy V.D., Padmavathi P., Varadacharyulu N. Ethanol induced adaptive changes in blood for the pathological and toxicological effects of chronic ethanol consumption in humans. Exp Toxicol Pathol. 2012 Nov;64(7–8):697–703. doi: 10.1016/j.etp.2011.01.002. Epub 2011 Feb 1. PMID: 21282047. [DOI] [PubMed] [Google Scholar]
- 39.Reznick A.Z., Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357–363. doi: 10.1016/s0076-6879(94)33041-7. PMID: 8015470. [DOI] [PubMed] [Google Scholar]
- 40.Padmavathi P., Reddy V.D., Kavitha G., Paramahamsa M., Varadacharyulu N. Chronic cigarette smoking alters erythrocyte membrane lipid composition and properties in male human volunteers. Nitric Oxide. 2010 Nov 1;23(3):181–186. doi: 10.1016/j.niox.2010.05.287. [DOI] [PubMed] [Google Scholar]
- 41.Maturu P., Reddy V.D., Padmavathi P., Varadacharyulu N. Ethanol induced adaptive changes in blood for the pathological and toxicological effects of chronic ethanol consumption in humans. Exp Toxicol Pathol. 2012 Nov 1;64(7–8):697–703. doi: 10.1016/j.etp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Penha-Silva N., Arvelos L.R., Cunha C.C., Aversi-Ferreira T.A., Gouvêa-e-Silva L.F., Garrote-Filho M.S. Effects of glycerol and sorbitol on the thermal dependence of the lysis of human erythrocytes by ethanol. Bioelectrochemistry. 2008 Jun;73(1):23–29. doi: 10.1016/j.bioelechem.2008.04.002. Epub 2008 Apr 13. PMID: 18495554. [DOI] [PubMed] [Google Scholar]
- 43.Alimi H., Hfaeidh N., Bouoni Z., Sakly M., Ben Rhouma K. Protective effect of Opuntia ficus indica f. inermis prickly pear juice upon ethanol-induced damages in rat erythrocytes. Alcohol. 2012 May;46(3):235–243. doi: 10.1016/j.alcohol.2011.09.024. Epub 2012 Mar 24. PMID: 22445806. [DOI] [PubMed] [Google Scholar]
- 44.Patra M., Salonen E., Terama E., Vattulainen I., Faller R., Lee B.W. Under the influence of alcohol: the effect of ethanol and methanol on lipid bilayers. Biophys J. 2006 Feb 15;90(4):1121–1135. doi: 10.1529/biophysj.105.062364. Epub 2005 Dec 2. PMID: 16326895; PMCID: PMC1367264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gabbianelli R., Cifani C., Massi M., Polidori C., Falcioni G. Oxidative damage in rat erythrocyte membranes following ethanol intake: effect of ethyl pyruvate. Chem Biol Interact. 2007 Aug 30;169(2):122–131. doi: 10.1016/j.cbi.2007.06.001. Epub 2007 Jun 12. PMID: 17644081. [DOI] [PubMed] [Google Scholar]
- 46.Ingólfsson H.I., Andersen O.S. Alcohol’s effects on lipid bilayer properties. Biophys J. 2011 Aug 17;101(4):847–855. doi: 10.1016/j.bpj.2011.07.013. PMID: 21843475; PMCID: PMC3175087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palmieri V.O., Cicco G., Minerva F., Portincasa P., Grattagliano I., Memeo V. Red blood cells (RBC) deformability and aggregability: alterations in alcoholism. Adv Exp Med Biol. 2006;578:125–131. doi: 10.1007/0-387-29540-2_20. PMID: 16927681. [DOI] [PubMed] [Google Scholar]
- 48.Dobrzyńska I., Szachowicz-Petelska B., Ostrowska J., Skrzydlewska E., Figaszewski Z. Protective effect of green tea on erythrocyte membrane of different age rats intoxicated with ethanol. Chem Biol Interact. 2005 Sep 10;156(1):41–53. doi: 10.1016/j.cbi.2005.07.002. PMID: 16098958. [DOI] [PubMed] [Google Scholar]
- 49.Brahmi N., Saoudi M., Kadri Y., Kallel C., Euch A.E., Ayadi F.M. Protective effect of Chaetomorpha gracilis aqueous extract against erythrocytes oxidative damage induced by high fat diet in treated mice. Arch Physiol Biochem. 2019 Jul;125(3):220–227. doi: 10.1080/13813455.2018.1448997. Epub 2018 Mar 15. PMID: 29544357. [DOI] [PubMed] [Google Scholar]
- 50.Salami H.A., John A.I., Ekanem A.U. The effect of aqueous preparation of Allium cepa (onion) and Allium sativa (garlic) on erythrocyte osmotic fragility in Wistar rats: in vivo and in vitro studies. Niger J Physiol Sci. 2012 Jun 7;27(1):29–34. PMID: 23235305. [PubMed] [Google Scholar]
- 51.López-Revuelta A., Sánchez-Gallego J.I., Hernández-Hernández A., Sánchez-Yagüe J., Llanillo M. Membrane cholesterol contents influence the protective effects of quercetin and rutin in erythrocytes damaged by oxidative stress. Chem Biol Interact. 2006 May 15;161(1):79–91. doi: 10.1016/j.cbi.2006.03.004. Epub 2006 Mar 12. PMID: 16620793. [DOI] [PubMed] [Google Scholar]
- 52.Yang H.L., Chen S.C., Chang N.W., Chang J.M., Lee M.L., Tsai P.C. Protection from oxidative damage using Bidens pilosa extracts in normal human erythrocytes. Food Chem Toxicol. 2006 Sep;44(9):1513–1521. doi: 10.1016/j.fct.2006.04.006. Epub 2006 Apr 25. PMID: 16765500. [DOI] [PubMed] [Google Scholar]
- 53.Lins P.G., Marina Piccoli Pugine S., Scatolini A.M., de Melo M.P. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon. 2018 Sep 20;4(9) doi: 10.1016/j.heliyon.2018.e00805. PMID: 30255162; PMCID: PMC6148714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gonçalves A.C., Bento C., Silva B.M., Silva L.R. Sweet cherries from Fundão possess antidiabetic potential and protect human erythrocytes against oxidative damage. Food Res Int. 2017 May;95:91–100. doi: 10.1016/j.foodres.2017.02.023. Epub 2017 Feb 28. PMID: 28395830. [DOI] [PubMed] [Google Scholar]
- 55.Daleke D.L. Regulation of phospholipid asymmetry in the erythrocyte membrane. Curr Opin Hematol. 2008 May;15(3):191–195. doi: 10.1097/MOH.0b013e3282f97af7. PMID: 18391783. [DOI] [PubMed] [Google Scholar]
- 56.Paxman J., Hunt B., Hallan D., Zarbock S.R., Woodbury D.J. Drunken membranes: short-chain alcohols alter fusion of liposomes to planar lipid bilayers. Biophys J. 2017 Jan 10;112(1):121–132. doi: 10.1016/j.bpj.2016.11.3205. PMID: 28076803; PMCID: PMC5232861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamaji-Hasegawa A., Tsujimoto M. Asymmetric distribution of phospholipids in biomembranes. Biol Pharm Bull. 2006 Aug;29(8):1547–1553. doi: 10.1248/bpb.29.1547. PMID: 16880602. [DOI] [PubMed] [Google Scholar]
- 58.Patil R.H., Prakash K., Maheshwari V.L. Hypolipidemic effect of Terminalia arjuna (L.) in experimentally induced hypercholesteremic rats. Acta Biol Szeged. 2011 Jan 1;55(2):289–293. [Google Scholar]
- 59.Maturu P., Vaddi D.R., Pannuru P., Nallanchakravarthula V. Alterations in erythrocyte membrane fluidity and Na+/K+ - ATPase activity in chronic alcoholics. Mol Cell Biochem. 2010 Jun;339(1–2):35–42. doi: 10.1007/s11010-009-0367-z. Epub 2010 Jan 3. PMID: 20047071. [DOI] [PubMed] [Google Scholar]
- 60.Morabito R., Romano O., La Spada G., Marino A. H2O2-induced oxidative stress affects SO4= transport in human erythrocytes. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0146485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Senthilkumar R., Sengottuvelan M., Nalini N. Protective effect of glycine supplementation on the levels of lipid peroxidation and antioxidant enzymes in the erythrocyte of rats with alcohol-induced liver injury. Cell Biochem Funct. 2004 Mar-Apr;22(2):123–128. doi: 10.1002/cbf.1062. PMID: 15027101. [DOI] [PubMed] [Google Scholar]
- 62.Loguercio C., Del Vecchio Blanco C., Coltorti M., Nardi G. Alteration of erythrocyte glutathione, cysteine and glutathione synthetase in alcoholic and non-alcoholic cirrhosis. Scand J Clin Lab Invest. 1992 May;52(3):207–213. doi: 10.3109/00365519209088787. PMID: 1411253. [DOI] [PubMed] [Google Scholar]