Abstract
Background and Purpose:
National guidelines recommend prompt identification of candidates for acute ischemic stroke (AIS) treatment, requiring timely neuroimaging with CT and/or MRI. CT is often preferred because of its widespread availability and rapid acquisition. Despite higher diagnostic accuracy of MRI, it commonly involves complex workflows that could potentially cause treatment time-delays. The purpose of this study was to analyze the impact on outcomes of imaging utilization prior to treatment decisions, at comprehensive stroke centers (CSC) for patients presenting with suspected AIS in the anterior circulation with last-known-well-to-arrival time 0–24 hours.
Methods:
Decision-simulation model based on the American Heart Association’s recommendations for AIS-care pathways was developed from a healthcare perspective to compare initial imaging strategies: (a) Stepwise-CT: NCCT at time of presentation; CTA±CTP only in select patients (initial imaging exclude hemorrhage and extensive-ischemia) for mechanical thrombectomy (MT) evaluation, (b) Stepwise-Hybrid: NCCT at time of presentation; MRA±MRP only for MT evaluation, (c) Stepwise-Advanced: NCCT+CTA at presentation; MR-DWI+MRP only for MT evaluation, (d) Comprehensive-CT: NCCT+CTA+CTP at time of presentation, (e) Comprehensive-MR: MR-DWI+MRA+MRP at time of presentation. Model parameters were defined with evidence-based data. Cost-effectiveness and sensitivity analyses were performed.
Results:
The cost-effectiveness analyses revealed that Comprehensive-CT and Comprehensive-MR yield highest lifetime quality-adjusted life-years (QALYs) (4.81 and 4.82, respectively). However, the incremental cost-effectiveness ratio of Comprehensive-MR is $233,000/QALY compared to Comprehensive-CT. Stepwise-CT, Stepwise-Hybrid, and Stepwise-Advanced strategies are dominated yielding lower QALYs and higher costs compared to Comprehensive-CT.
Conclusion:
Performing Comprehensive-CT at presentation is the most cost-effective initial imaging strategy at CSCs.
Summary
Performing Comprehensive-CT (NCCT+CTA+CTP) at initial presentation in patients presenting with suspected acute ischemic stroke in the anterior circulation with last-known-well-to-arrival time 0–24 hours at comprehensive stroke centers is cost-effective compared to Comprehensive-MR and stepwise approaches.
Introduction
Neuroimaging plays a critical role in both diagnosis and treatment decisions in acute ischemic stroke (AIS). Given the recent advances in endovascular treatment of AIS patients presenting with large anterior vessel occlusion, several randomized controlled trials (RCTs) have proven efficacy of mechanical thrombectomy (MT) using imaging findings as inclusion criteria for patient selection[1–6]. After favorable outcomes were also reported in the DAWN[1] and DEFUSE-3[2] trials using advanced imaging for patient selection, the treatment time window was extended up to 24 hours from last-known-well-to-arrival (LKWA). Thus, the number of AIS patients undergoing MT has grown significantly[7], highlighting the need for efficient and timely imaging protocols at comprehensive stroke centers, where a full spectrum of care services to treat complex stroke patients including advanced imaging, neurological, neurosurgical and neuro-intensive care services are available[8].
The American Heart Association (AHA) guidelines[9] provide recommendations for the initial imaging based on the standard level of information needed for AIS treatment decision-making in patients with moderate to severe symptoms (NIH Stroke Scale ≥6) to minimize time-delays because the therapeutic effects from both intravenous thrombolytics (IV-tPA) and MT are time-dependent[10,11]. For patients eligible for IV-tPA, NCCT or MRI is considered an effective initial imaging strategy to exclude intracranial hemorrhage and treatment should not be delayed for additional multimodal neuroimaging (CTA-CTP or MRA-MRP). For patients eligible for MT, noninvasive vessel imaging is recommended during the initial imaging evaluation when feasible. Otherwise, additional imaging should be performed in patients evaluated for MT in a stepwise approach potentially producing workflow inefficiencies[12]. In the LKWA 0–6-hour window, patient selection for MT is recommended based on CT/CTA or MRI/MRA in preference to performance of additional imaging such as perfusion. However, the AHA guidelines do recommend CTP or MRI-DWI with or without perfusion to determine clinical-core mismatch or perfusion-core mismatch for patient selection in LKWA >6–24-hour window. In practice, comprehensive and stepwise initial imaging strategies are implemented at comprehensive stroke centers[13–17]. For example, time efficiency of advanced imaging with angiography and perfusion as a comprehensive initial imaging approach is analyzed in Brunnquell et al.[18]. A stepwise approach with utilization of angiography at presentation and additional MRI-DWI for MT evaluation of select patients is described in Gonzalez et al.[19]. However, there is little evidence demonstrating the clinical value of initial imaging strategies employing advanced imaging in AIS patients in terms of clinical outcomes and costs.
Several questions remain unanswered in stroke imaging utilization regarding modality choice (CT vs MRI), initial imaging utilization approach (NCCT/MRI and/or CTA/MRA and/or CTP/MRP), and potential impact of radiation risks and imaging time-delays on clinical outcomes. Since it is not feasible to adequately evaluate several stroke imaging strategies in a single RCT, we conducted model-based cost-effectiveness analyses to quantify the clinical outcomes and costs for different initial imaging approaches. The purpose of this study was to analyze the impact of the imaging utilization approach prior to treatment decisions at comprehensive stroke centers for patients presenting with moderate-to-severe AIS (NIH Stroke Scale ≥6) in the anterior circulation with LKWA 0–24 hours.
Methods
Target Population, Setting and Study Perspective
We developed a decision-simulation model from a healthcare perspective focused on the initial imaging strategies that could be deployed at comprehensive stroke centers, representing the highest level of stroke care certification where advanced imaging and treatment are available[8]. The modeled cohort represents patients presenting with suspected moderate-to-severe acute ischemic stroke (NIH Stroke Scale ≥6) in the anterior circulation within the treatment windows for intravenous thrombolytics at 0–4.5 hours, mechanical thrombectomy at 0–6 hours (MT), or mechanical thrombectomy at >6–24 hours (Extended-MT) from last-known-well-to-arrival[9]. Patients in the model were assumed to have an average age of 70 years, in accordance with the AHA stroke statistics 2019 update[20]. Treatment and management decisions followed the AHA Class-I recommendations[9]. IRB approval was not required to conduct our study because it did not involve human subjects research.
The initial imaging strategies follow the main principles and recommendations endorsed by the American College of Radiology (ACR), American Society of Neuroradiology (ASNR) and Society of NeuroInterventional Surgery (SNIS)[21], which incorporated stepwise utilization of imaging with advanced imaging only in select patients for MT or Extended-MT evaluation based on initial imaging results (exclusion of hemorrhage and extensive-ischemia, as defined below) or comprehensive utilization of imaging as follows: (a) Stepwise-CT: NCCT at time of presentation in all patients; additional CTA only for MT evaluation; additional CTA+CTP only for Extended-MT evaluation. (b) Stepwise-Hybrid: NCCT at time of presentation in all patients; additional MRA only for MT evaluation; additional MRA+MRP only for Extended-MT evaluation. (c) Stepwise-Advanced: NCCT+CTA at time of presentation in all patients; additional MR-DWI+MRP only for MT and Extended-MT evaluation. (d) Comprehensive-CT: NCCT+CTA+CTP at time of presentation in all patients. (e) Comprehensive-MR: MR-DWI+MRA+MRP at time of presentation in all patients.
Since time-delays to treatment is a main concern in acute ischemic stroke care, we included the following time-delays: (a) re-scan: additional time needed in select patients to perform advanced imaging in Stepwise-CT, Stepwise-Hybrid, and Stepwise-Advanced strategies, (b) MR-workflow: additional time needed for screening and performing MR-based imaging occurring in Stepwise-Hybrid, Stepwise-Advanced, and Comprehensive-MR strategies, (c) perfusion-workflow: additional time needed to perform and post-process perfusion imaging in the 0–6-hour window from last-known-well-to-arrival occurring in Comprehensive-CT, Comprehensive-MR, and Stepwise-Advanced strategies. These time-delays were modeled with 5-minute increments within the range 0–120 minutes.
Stroke-imaging subtypes (Table S1(online)) were defined by the presence or absence of the following stroke-imaging features used to assess acute ischemic stroke treatment eligibility in accordance with the AHA guidelines: 1) Hemorrhage – acute blood products in the brain parenchyma, subarachnoid, subdural, and/or epidural compartments; 2) Extensive-Ischemia – parenchymal signal changes greater than one-third MCA territory; 3) Large anterior vessel occlusion – occlusion of the intracranial ICA and/or the M1 segment of the MCA with or without the occlusion of the A1 segment of the anterior cerebral artery; and 4) Perfusion deficit – mismatch ≥20% between the penumbra and core infarct volumes[4].
Diagnostic accuracy of stroke-imaging subtypes varied in the strategies depending on the individual imaging-test characteristics (sensitivity and specificity). Importantly, treatment triage defined the branching structure of the decision model, which combines clinical metrics recommended in the AHA guidelines with the diagnostic accuracy for the stroke-imaging subtype classification. Therefore, the probability of patient selection for the treatment options differed amongst the imaging strategies. Table 1 describes the initial imaging exams used for treatment triage in the imaging strategies.
Table 1.
Intravenous thrombolytic (IV-tPA) and mechanical thrombectomy (MT) triage rules according to imaging strategy were based on current stroke management guidelines.
| Treatment Triage | Stepwise-CT | Stepwise-Hybrid | Stepwise-Advance | Comprehensive-CT | Comprehensive-MR |
|---|---|---|---|---|---|
|
IV-tPA (LKWA: 0–4.5 hours) |
NCCT*: No Hemorrhage NCCT*: No Extensive-Ischemia |
NCCT*: No Hemorrhage NCCT*: No Extensive-Ischemia |
NCCT*: No Hemorrhage NCCT*: No Extensive-Ischemia |
NCCT*: No Hemorrhage CTP*: No Extensive-Ischemia |
MR-DWI*,†: No Hemorrhage MR-DWI*,‡: No Extensive-Ischemia |
|
MT (LKWA: 0–6 hours) |
CTA†,§: LAVO | MRA†,§,‡: LAVO | CTA: LAVO MR-DWI†,§,‡: No Extensive-Ischemia MRP†,§,‡,ǂ: Perfusion Deficit |
CTA*: LAVO CTP*,ǂ: Perfusion Deficit |
MRA*,‡: LAVO MRP*,‡,ǂ: Perfusion Deficit |
|
Extended-MT (LKWA: >6–24 hours) |
CTA†,§: LAVO CTP†,§: Perfusion Deficit |
MRA†,§,‡: LAVO MRP†,§,‡: Perfusion Deficit |
CTA: LAVO MR-DWI†,§,‡: No Extensive-Ischemia MRP†,§,‡: Perfusion Deficit |
CTA*: LAVO CTP*: Perfusion Deficit |
MRA*,‡: LAVO MRP*,‡: Perfusion Deficit |
Imaging performed at time of presentation in all patients is marked with the symbol *;
additional imaging performed in a subset of patients to assess MT or Extended-MT eligibility is marked with the symbol †.
Re-scan, MR-workflow, and perfusion-workflow time-delays are marked with §, ‡, and ǂ, respectively.
MT = mechanical thrombectomy, LKWA = last-known-well-to-arrival time, LAVO = large anterior vessel occlusion, NCCT = non-contrast CT, CTA/MRA = CT/MR angiography, CTP/MRP = CT/MR perfusion, MR-DWI = MR diffusion-weighted imaging.
The base-case scenario constitutes the initial imaging strategies without time-delays used at comprehensive stroke centers for the assessment of the modeled cohort described above. Stepwise-CT was considered the standard initial imaging strategy.
Health Outcomes
The modified Rankin scale (mRS) (range mRS0 = no disability to mRS5 = severe disability, and mRS6 = stroke-related death)[22] is the most commonly used metric for evaluating stroke clinical outcomes. Furthermore, mRS at 90 days after discharge is used as the end point in randomized clinical trials[1–6,23]. Therefore, the clinical outcome of a stroke intervention (treatment or no treatment) was modeled as the probability of entering a specific health state according to the degree of disability measured with the 90-day mRS scores, conditioned on the stroke-imaging subtype and intervention. Importantly, clinical outcomes were independent of the imaging-test characteristics, and the performance of the imaging strategies relies on the diagnostic accuracy for stroke-imaging subtype classification. Values were based on the highest available evidence in the literature (Table S2(online)). When clinical outcomes for specific stroke-imaging subtypes were not available in the literature, values for similarly behaving stroke-imaging subtypes were used, employing a structured logic based upon the expected natural history of clinical outcomes for ischemic and hemorrhagic stroke (Table S3(online)).
Time Horizon
Markov transition models were incorporated in the simulation-decision model to estimate the lifetime quality-adjusted life-years (QALYs) and lifetime healthcare cost. After stroke intervention, patients entered a specific 90-day mRS state (dictated by the outcome probabilities based on stroke-imaging subtype and intervention) incurring an initial mRS-based utility and combined cost including the imaging utilized in the initial-imaging strategy, stroke intervention (treatment type, or no treatment), and mRS-based acute care costs (Table 2). Although the degree of disability of stroke survivors is expected to improve with rehabilitation[24], the steady state for the mRS score occurs within 3 to 6 months after discharge[25,26]. Consequently, we assume that during each 1-year cycle, patients could either (a) remain in the same mRS state, incurring a 3%-discounted mRS-based utility and mRS-based chronic care cost; (b) experience a recurrent stroke, acquiring a 3%-discounted recurrent-stroke care cost, and transition to a worse mRS state; or (c) death (stroke-related or other causes)[27]. For thorough assessment of imaging risks, the probability of long-term malignant-brain cancer resulting from radiation exposure from NCCT±CTA±CTP was incorporated in the imaging strategies using CT-based techniques with a Markov transition model, similar to the one described above, with possible transition to developing malignant-brain cancer[28]. The age-based risk of mortality was obtained from the United States vital statistics for the general population[29]. The mortality-hazard ratios were used in the model to adjust the mortality risk based on mRS or malignant-brain cancer health states (Table S4(online)). A lifetime time horizon was implemented in the Markov models to properly assess the impact of the diagnostic accuracy of the initial imaging strategies for stroke-imaging subtype classification based on the projected costs and outcomes of the resulting stroke intervention[27].
Table 2.
Imaging test-characteristics (sensitivity and specificity) values for detection of hemorrhage, extensive-ischemia, large anterior vessel occlusion, and perfusion deficit for CT and MR.
| Parameters | Base-case value | Range | Dist | Source | |||||
|---|---|---|---|---|---|---|---|---|---|
| Hemorrhage detection | |||||||||
| NCCT | SN: 89%; SP: 100% | 70%–97%; 98%–100% | Δ | [33] | |||||
| MRI | SN: 81%; SP: 100% | 61%–93%; 98%–100% | Δ | [33] | |||||
| Extensive-Ischemia detection | |||||||||
| NCCT | SN: 66%; SP: 87% | 2%–87%; 54%–100% | Δ | [49] | |||||
| CTP | SN: 82%; SP: 96% | 75%–88%; 89%–99% | Δ | [50] | |||||
| MR-DWI | SN: 91%; SP: 95% | 88%–100%;75%–100% | Δ | [34] | |||||
| Large anterior vessel occlusion | |||||||||
| CTA | SN: 96.6%; SP: 99.4% | 88.1%–99.6%; 98.1%–99.9% | Δ | [51] | |||||
| MRA | SN: 92%; SP: 91% | 80%–100%; 80%–99% | Δ | [35] | |||||
| Perfusion Deficit | |||||||||
| CTP | SN: 82%; SP: 96% | 75%–88%; 89%–99% | Δ | [50] | |||||
| MRP | SN: 91%; SP: 82% | 87%–96%;81%–91% | Δ | [36] | |||||
| Imaging Cost: | |||||||||
| NCCT | $202 | $194–$242 | γ | [52,53] | |||||
| CTA±CTP | $789 | $757–$947 | γ | [52,53] | |||||
| MR-DWI±MRP | $636 | $611–$763 | γ | [52,53] | |||||
| MRA±MRP | $1,043 | $1,001–$1,252 | γ | [52,53] | |||||
| Treatment Cost: | |||||||||
| IV-tPA | $7,518 | $7,217–$9,022 | γ | [54,53] | |||||
| MT | $15,714 | $15,085–$18,857 | γ | [54,53] | |||||
| Malignant-Brain Cancer | $149,476 | $143,497–$179,371 | γ | [55] | |||||
| Acute-Care Cost: | |||||||||
| mRS0 | $7,614 | $7,309–$9,137 | γ | [54,53] | |||||
| mRS1 | $10,511 | $10,091–$12,613 | γ | [54,53] | |||||
| mRS2 | $16,508 | $15,848–$19,810 | γ | [54,53] | |||||
| mRS3 | $20,416 | $19,599–$24,499 | γ | [54,53] | |||||
| mRS4 | $27,357 | $26,262–$32,828 | γ | [54,53] | |||||
| mRS5 | $32,680 | $31,373–$39,216 | γ | [54,53] | |||||
| mRS6 | $7,681 | $7,374–$9,217 | γ | [54,53] | |||||
| Chronic-Care Cost: | |||||||||
| mRS0 | $10,708 | $10,280–$12,850 | γ | [54,53] | |||||
| mRS1 | $11,026 | $10,585–$13,231 | γ | [54,53] | |||||
| mRS2 | $12,755 | $12,245–$15,306 | γ | [54,53] | |||||
| mRS3 | $21,902 | $21,026–$26,282 | γ | [54,53] | |||||
| mRS4 | $44,330 | $42,557–$53,196 | γ | [54,53] | |||||
| mRS5 | $65,174 | $62,567–$78,209 | γ | [54,53] | |||||
| Malignant-Brain Cancer | $10,140 | $9,734–$12,168 | γ | [55] | |||||
| Recurrent-Care Cost: | |||||||||
| Stroke | $21,903 | $21,027–$26,284 | γ | [54,53] | |||||
| Utility: | |||||||||
| mRS0 | 1.0 | 0.78–1.0 | Ϝ | [56,57] | |||||
| mRS1 | 0.91 | 0.77–0.95 | Ϝ | [56,57] | |||||
| mRS2 | 0.76 | 0.6–0.83 | Ϝ | [56,57] | |||||
| mRS3 | 0.65 | 0.35–0.67 | Ϝ | [56,57] | |||||
| mRS4 | 0.33 | 0.09–0.42 | Ϝ | [56,57] | |||||
| mRS5 | 0.0 | 0.0–0.12 | Ϝ | [56,57] | |||||
| mRS6 | 0.0 | 0.0 | [56,57] | ||||||
Cost and utility parameters used to calculate lifetime healthcare cost and quality-adjusted life-years. NCCT = non-contrast CT, CTA/MRA = CT/MR angiography, CTP/MRP = CT/MR perfusion, MR-DWI = MR diffusion-weighted imaging, IV-tPA = intravenous thrombolytics, MT = Mechanical thrombectomy, mRS = modified Rankin scale, SN = sensitivity, SP = specificity, Dist = probability distribution used in the probabilistic-sensitivity analysis, Δ = Triangular distribution, Ϝ = Fractal distribution.
The model was implemented using TreeAge Pro 2017 R2.0 software[30]; a simplified algorithm of the decision model is provided in Figure 1. Further details are described in Supplemental-Materials(online).
Figure 1.
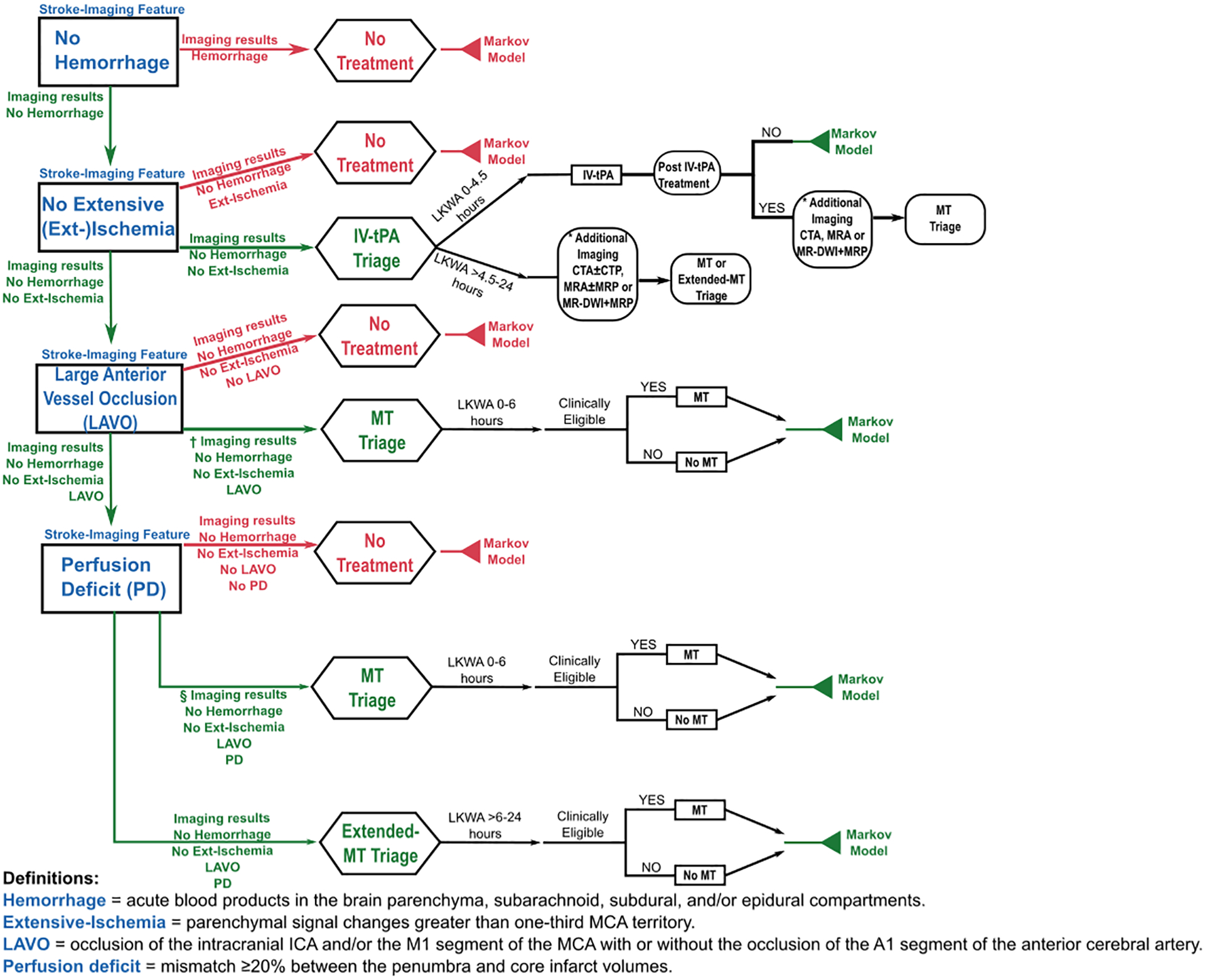
Simplified depiction of the decision model demonstrating the imaging-based algorithmic logic utilized in the model for management and treatment decisions. In this example, the true stroke-imaging subtypes (blue font) evaluated by the imaging strategies include absence of hemorrhage, absence of extensive-ischemia, presence of large anterior vessel occlusion, and presence of perfusion deficit. It can be observed that the proper classification of all the stroke-imaging features (green arrows) leads to the correct treatment triage, as illustrated in the IV-tPA, MT, and Extended-MT branches (green font). However, misclassification of one of the stroke-imaging features (red arrows) could lead to the wrong treatment triage, as shown in the No treatment branches (red font). These clinical-care pathways terminate in a Markov model to evaluate the expected lifetime quality-adjusted life-years and lifetime cost of the imaging strategies. LKWA = last-known-well-to-arrival time, IV-tPA = intravenous thrombolytics, MT = mechanical thrombectomy, CTA/MRA = CT/MR angiography, CTP/MRP = CT/MR perfusion, MR-DWI = MR diffusion-weighted imaging, * = only in Stepwise-CT, Stepwise-Hybrid, and Stepwise-Advanced, † = only in Stepwise-CT and Stepwise-Hybrid, § = only in Stepwise-Hybrid, Comprehensive-CT, and Comprehensive-MR.
Model Assumptions
The model has the following assumptions in the acute ischemic stroke care pathway: (a) Stepwise-CT is the standard initial imaging approach used in practice; (b) MRI is available at comprehensive stroke centers for stroke imaging; (c) Treatment assumptions: 1) Clinical outcomes of patients treated with intravenous thrombolytics were classified as patients with either favorable response to intravenous thrombolytics[31], patients with unfavorable response to intravenous thrombolytics and treated with mechanical thrombectomy, and patients with unfavorable response to intravenous thrombolytics without additional treatment; 2) The conditional probability of patient selection for mechanical thrombectomy is based on the imaging results and other clinical metrics recommended in the guidelines for mechanical thrombectomy selection[9,32]; (d) Imaging assumptions: 1) CT or MRI multi-modal imaging sequences are executed sequentially with NCCT or MRI-DWI followed by angiography (CTA or MRA) followed by perfusion imaging (CTP or MRP); 2) MR sequences included: DWI, GRE, T1WI, T2WI, T2-FLAIR[33,34], 3D TOF[35], contrast-enhanced PWI with 3D T2*WI EPI-gradient[36]; 3) Contraindications to IV contrast for CTA, CTP and MRP were not included as impacting long-term outcomes[37,38].
Model Verification
We performed analyses to validate that the model results were independent of the decision-model structure[39]. We compared model outputs by assuming perfect imaging (100% sensitivity, 100% specificity) or imperfect imaging (0% sensitivity, 0% specificity), forcing all strategies to follow fixed patient triage rules, and the simulated patient cohort was stratified by stroke-imaging subtypes. Our analyses showed that under these extreme conditions all the strategies had the same behavior and model outcomes showed minimal deviations from values reported in the literature per stroke-imaging subtype.
Time-Dependent Outcome Probabilities
We introduced time-dependent outcome probabilities at stroke-imaging subtype nodes for the treatment branches to account for potential imaging time-delays caused by re-scan, MR-workflow, and/or perfusion time-delays.
Time-dependent outcomes for successful intravenous thrombolytics treatment[40] decreased at the rate based on the human-data study from Kim et al.[41]. Time-dependent outcome probabilities for successful mechanical thrombectomy treatment were defined with the decrease rate of independent outcome (mRS0–2) and increase rate of stroke-related mortality obtained with data from the MR CLEAN registry[42], which reported similar rate values as in the HERMES study[11], with the advantage of appropriate statistical power for the sensitivity analyses (Table S5(online)). Finally, we assumed the time-dependent outcome behavior of mechanical thrombectomy in the >6–24-hour window from last-known-well-to-arrival could be described with data from the 0–6-hour window given the lack of published literature. Further details are described in Supplemental-Materials(online).
Cost-effectiveness and Sensitivity Analyses
We used conventional cost-effectiveness rules to conduct incremental cost-effectiveness analyses with expected lifetime QALYs and costs projected for each imaging strategy from a healthcare perspective[43]. We conducted one-way sensitivity and probabilistic-sensitivity (PSA) analyses to evaluate the cost-effectiveness results under variations that could potentially occur in the model parameters. Willingness-to-pay (WTP) thresholds of $100,000/QALY and $50,000/QALY were used in the analyses[44]. However, acceptability curves were generated with WTP thresholds ranging from $50,000/QALY to $250,000/QALY for robustness of the analysis[45,46].
Results
Model Inputs
The input parameters of the model were based on the highest level of evidence in the literature with a preference to meta-analyses and RCTs data. Costs in the model included imaging, treatment, acute and chronic care costs. Cost values were based on Medicare reimbursement rates reported in 2013–2017 U.S. dollars and were converted to 2019 U.S dollars using the consumer price index[47]. A 3% annual-discount rate was applied to the costs and utilities used in the model to estimate lifetime QALYs and lifetime healthcare costs[48]. The key supporting data and reference sources are available in Tables 2, S2–S6 (online).
Base-Case Scenario
The cost-effectiveness analysis of the model revealed that Comprehensive-CT and Comprehensive-MR yield the highest expected lifetime QALYs per patient (4.81 and 4.82, respectively) at an expected lifetime healthcare cost per patient of $223,003 and $224,280, respectively. The Stepwise-CT, Stepwise-Hybrid and Stepwise-Advanced strategies yield lower QALYs at slightly higher average cost per patient than Comprehensive-CT; thus, these strategies are considered dominated (Table 3). The incremental cost-effectiveness ratio analysis show that Comprehensive-CT is the preferred strategy at both WTP=$100,000/QALY and $50,000/QALY.
Table 3.
Cost-effectiveness analyses of initial imaging strategies for the base-case scenario.
| Stepwise-CT | Stepwise-Hybrid | Stepwise-Advance | Comprehensive-CT | Comprehensive-MR | |
|---|---|---|---|---|---|
| Dominated | Yes | Yes | Yes | No | No |
|
Cost QALY |
$223,691 4.78 |
$223,670 4.78 |
$223,323 4.79 |
$223,003 4.81 |
$224,280 4.82 |
| ICER | -- | -- | -- | Reference | $233,000/QALY |
Projected lifetime cost and quality-adjusted life-years (QALYs) of the strategies correspond to the expected lifetime healthcare cost and QALYs per patient gained after stroke intervention (treatment or no treatment). Dominated strategies are removed from the incremental cost-effectiveness ratio (ICER) analyses and the first-ranked strategy is designated as the reference in the ICER analyses.
These model results are intended for interpretation from a population-based healthcare perspective. For example, the incidence of ischemic stroke in the United States in 2019 was approximately 660,000[20]. Implementing Stepwise-CT, Comprehensive-CT and Comprehensive-MR would project a total cost in billions of $147.6, $147.1, and $148, respectively. Therefore, Comprehensive-CT would generate a cost savings of $454 million compared to Stepwise-CT which translates into saving $32,300 per QALY gained per patient. However, Comprehensive-MR would require an investment of $875 million compared to Comprehensive-CT which translates into an investment of $233,000 per QALY gained per patient.
Sensitivity Analyses
Cost and Utility Parameters
One-way sensitivity analyses on the cost and utility parameters identified that Comprehensive-CT and Comprehensive-MR dominate Stepwise-CT, Stepwise-Hybrid, and Stepwise-Advanced at WTP=$100,000/QALY and $50,000/QALY. The dominant performance of the comprehensive-imaging strategies is mainly driven by their QALYs compared to the stepwise-imaging strategies yielding lower QALYs. However, the sensitivity analyses show that Comprehensive-CT is the preferred strategy at WTP=$100,000/QALY and $50,000/QALY due to the small differences in both costs and QALYs between Comprehensive-CT and Comprehensive-MR.
PSA analyses on costs and utilities with 100,000 iterations showed that the cost-effectiveness range of Comprehensive-CT varied from 100% of the iterations at WTP threshold of $50,000/QALY to 53% at WTP threshold of $250,000/QALY given its diagnostic accuracy in defining stroke-imaging subtypes (Figure 2).
Figure 2.

Acceptability curves of cost-effectiveness calculated with probabilistic-sensitivity analyses on cost and utility parameters with 100,000 iterations. The analyses show the robustness of the cost-effectiveness performance of Comprehensive-CT at willingness-to-pay threshold values ranging from $50,0000/QALY to $250,000/QALY. QALY = quality-adjusted life-year.
Imaging-Test Characteristics
One-way sensitivity analyses on the imaging-test characteristics determined that Comprehensive-CT and Comprehensive-MR yield higher QALYs than Stepwise-CT, Stepwise-Hybrid, and Stepwise-Advanced at WTP=$100,000/QALY and $50,000/QALY. Comprehensive-CT is cost-effective at WTP=$50,000/QALY; however, the analyses show that there is a preference switch from Comprehensive-CT to Comprehensive-MR only at WTP=$100,000/QALY under deterministic variation of both CT and MR specificity of extensive-ischemia, sensitivity of large anterior vessel occlusion, and sensitivity of perfusion deficit (Figure 3).
Figure 3.
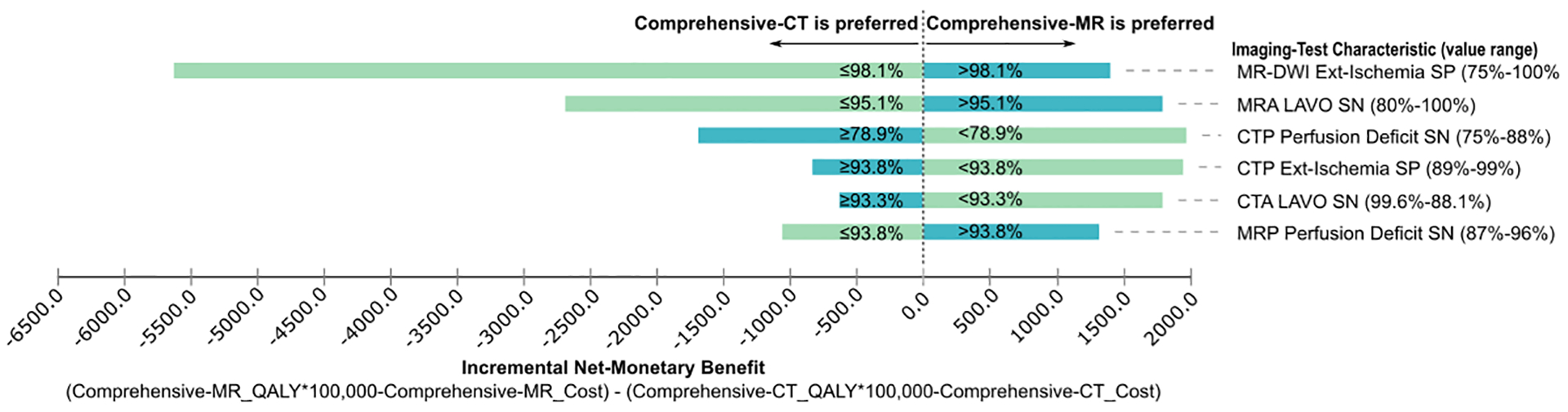
Net-monetary benefit diagram of the sensitivity analyses evaluating plausible variations in the imaging-test characteristics. The labeled values indicate the CT/MRI sensitivity and specificity thresholds where strategy preference changes between Comprehensive-CT and Comprehensive-MR at willingness-to-pay threshold of $100,000/QALY. MR-DWI = MR diffusion-weighted imaging, CTA/MRA = CT/MR angiography, CTP/MRP = CT/MR perfusion, LAVO = large vessel occlusion, Ext-Ischemia = extensive ischemia, QALY = quality-adjusted life-year, SN = sensitivity, SP = specificity.
PSA analyses on the imaging-test characteristics using 100,000 iterations showed that Comprehensive-CT has the highest probability of being cost-effective at WTP thresholds values between $50,000/QALY-$200,000/QALY. However, Comprehensive-MR could be cost-effective at higher WTP values (>$206,000/QALY-$250,000/QALY). For example, the probability of the imaging strategies being cost-effective at WTP=$50,000/QALY (WTP=$100,000/QALY) is Comprehensive-CT 82.9% (61.5%), Comprehensive-MR 12.3% (33.2%), Stepwise-Advanced 3.2% (3.9%), Stepwise-Hybrid 0.9% (0.6%), and Stepwise-CT 0.8% (0.9%) (Figure 4A). The analyses based on cost and utility parameters indicted a lower degree of sensitivity of the model on these parameters. Additional, PSA analyses including imaging-test characteristics, costs and utilities confirmed that the cost-effectiveness of the imaging strategies were mostly driven by the imaging-test characteristics (Figure 4B). Furthermore, Comprehensive-CT has the highest probability of being cost-effective at WTP thresholds values between $50,000/QALY-$250,000/QALY.
Figure 4.
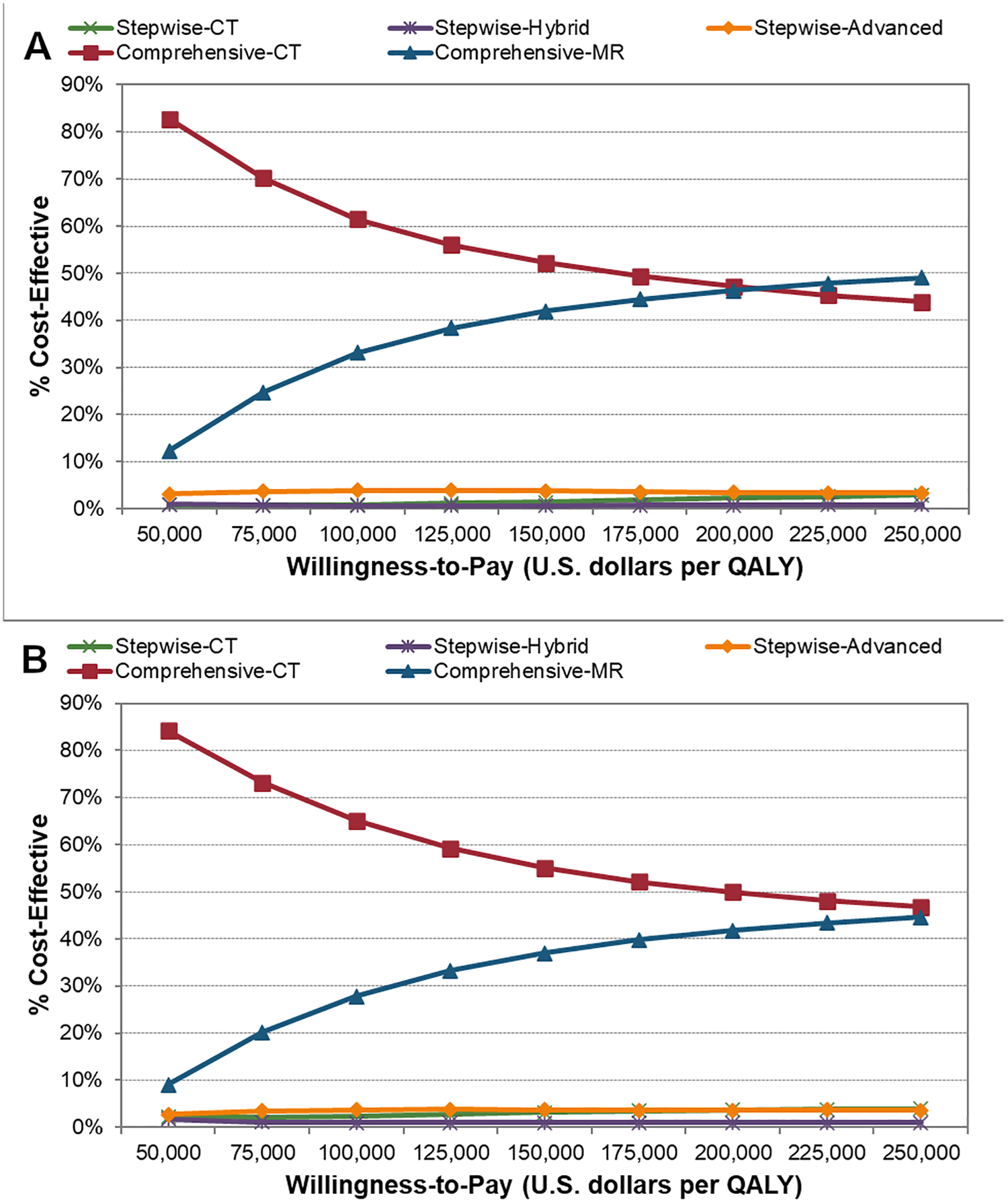
Acceptability curves of cost-effectiveness calculated with probabilistic-sensitivity analyses on: (A) imaging-test characteristics with 100,000 iterations (B) imaging-test characteristics, cost, and utility with 100,000 iterations. The analyses emphasize the added value of information from comprehensive utilization of imaging dominating strategies with a stepwise utilization approach (A,B). However, Comprehensive-CT and Comprehensive-MR are more likely to be cost-effective at WTP threshold values ranging from $50,000/QALY-$200,000/QALY and >$206,000/QALY-$250,000/QALY, respectively (A). Comprehensive-CT is more likely to be cost-effective at WTP threshold values of $50,000/QALY-$200,000/QALY when cost and utilities parameters are included in the PSA analysis (B) Furthermore, the cost-effective performance of the model behaves similarly without variations on cost and utility parameters (A) or with them (B). WTP = willingness-to-pay, QALY = quality-adjusted life-year.
The cost-effective performance of the imaging strategies observed in the PSA is dependent on the possible combinations of the imaging-test characteristics that could compromise or improve the diagnostic accuracy of stroke-imaging subtypes. For example, Comprehensive-CT yields higher QALYs at lower cost (dominates) compared to Stepwise-CT in 59.7% (61.6% including variations on costs and utilities) of the possible iterations (Figure 5A). Furthermore, Comprehensive-CT dominates Comprehensive-MR in 36.5% (38.6% including variations on costs and utilities) of the possible iterations (Figure 5B).
Figure 5.
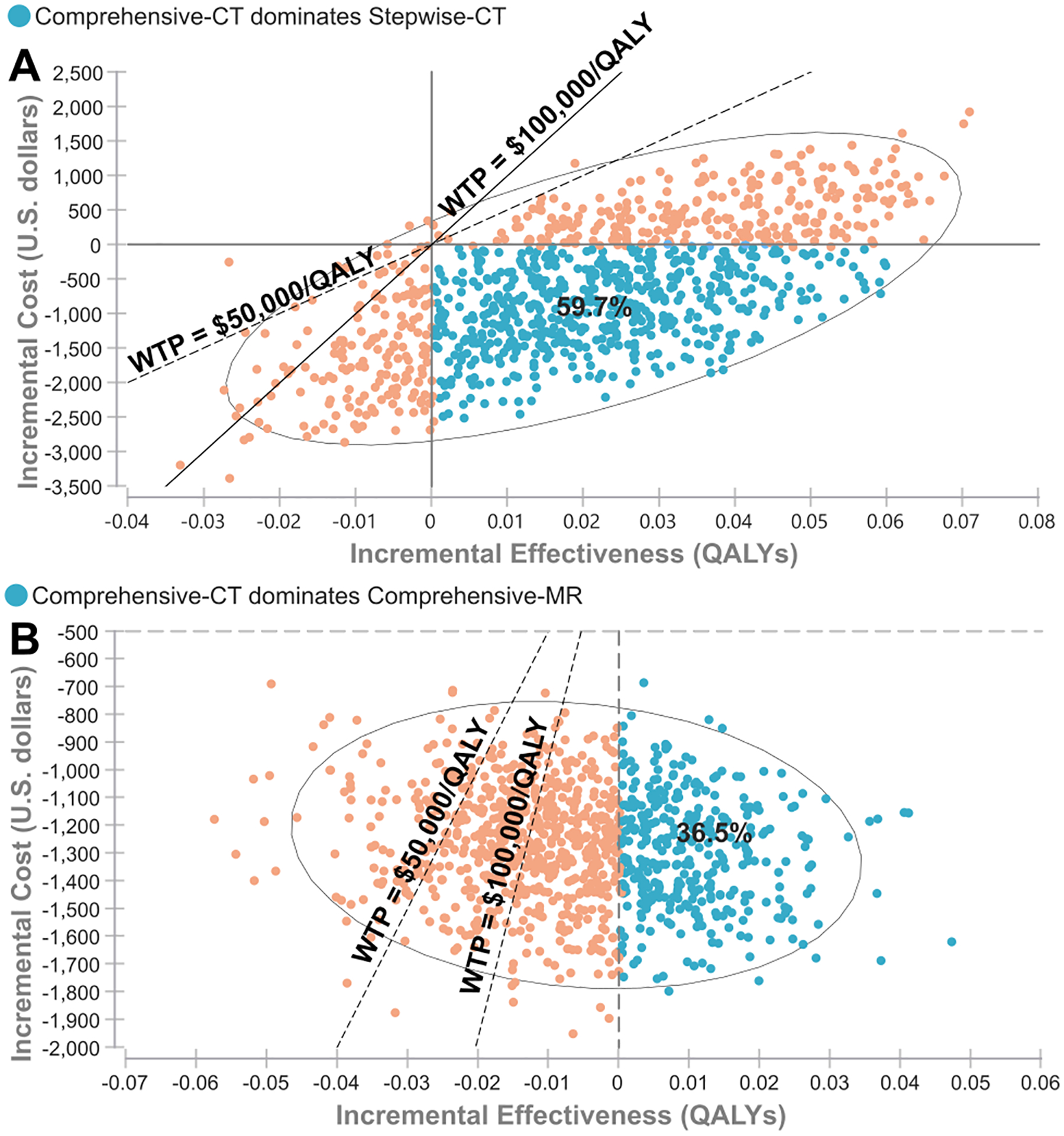
Probabilistic-sensitivity analyses (PSA) on imaging-test characteristics. The PSA results are shown in scatter plots of incremental cost-effectiveness ratio (ICER). The blue dots show where Comprehensive-CT dominates the comparator resulting in negative ICER values due to the comparator being more costly and less effective. The orange dots show where the ICER values for Comprehensive-CT vary as positive or negative due to the comparator being less costly or more effective. ICER plots: (A) Comprehensive-CT compared to Stepwise-CT and (B) Comprehensive-CT compared to Comprehensive-MR. When accounting for the plausible variations of all imaging-test parameters, Comprehensive-CT dominates Stepwise-CT and Comprehensive-MR, yielding higher QALYs and lower cost, in 59.7% (A) and 36.5% (B) of the PSA iterations (blue dots), respectively. Furthermore, Comprehensive-CT is cost-effective at willingness-to-pay thresholds of $50,000/QALY ($100,000/QALY) compared to Stepwise-CT and Comprehensive-MR in 87.8% (84.8%) (A) and 86.3% (64.9%) (B) of the PSA iterations, respectively. QALY = quality-adjusted life-year, WTP = willingness-to-pay threshold.
Treatment Time-Delay
We conducted sensitivity analyses on time-dependent outcome probabilities to analyze the impact of time-delays on the performance of: 1) Comprehensive-CT and Comprehensive-MR subject to perfusion-workflow time-delays compared to Stepwise-CT (base-case scenario); 2) Comprehensive-MR subject to MR-workflow and perfusion-workflow time-delays compared to Comprehensive-CT subject to perfusion-workflow time-delays.
The analyses determined that Comprehensive-CT and Comprehensive-MR yield higher QALYs compared to Stepwise-CT if perfusion-workflow time-delays are minimized below 50 and 63 minutes, respectively. However, Comprehensive-CT has lower cost compared to both Stepwise-CT and Comprehensive-MR for perfusion-workflow time-delays between 0–120 minutes. Furthermore, Comprehensive-MR yields slightly higher QALYs and higher cost compared to Comprehensive-CT if time-delays caused by MR-workflow are minimized below 10 minutes for perfusion-workflow time-delays between 0–50 minutes. Otherwise, Comprehensive-MR yields lower QALYs and higher cost than Comprehensive-CT. Consequently, Comprehensive-CT is the preferred strategy at both WTP=$100,000/QALY and $50,000/QALY.
Comprehensive-CT Radiation Risk
Sensitivity analyses on the radiation-induced-cancer risk after exposure to NCCT+CTA+CTP (base-case 27.4×10−5, range: 2×10−5-80.2×10−5)[28] showed that CT-imaging radiation-risk does not impact the model results at WTP=$100,000/QALY and $50,000/QALY. Therefore, the benefits of implementing Comprehensive-CT outweighs the radiation risks.
Discussion
We conducted cost-effectiveness analyses from the healthcare perspective accounting for different approaches to imaging utilization prior to treatment decisions which can be implemented for patients with acute ischemic stroke in the anterior circulation presenting with moderate-to-severe symptoms in the 0–24-hour window from last-known-well-to-arrival at comprehensive stroke centers. The stroke-care pathways and the structure of imaging strategies were based on the AHA guidelines[9] and recommendations endorsed by the ACR, ASNR, and SNIS[21], respectively.
The analyses reveal that a comprehensive approach to imaging utilization at presentation with CT or MR (Comprehensive-CT, Comprehensive-MR) yields better clinical outcomes than a stepwise approach (Stepwise-CT, Stepwise-Hybrid, Stepwise-Advanced). Furthermore, the sensitivity analyses emphasize that Comprehensive-CT dominates the stepwise approach as well as Comprehensive-MR, yielding higher QALYs and lower cost across plausible variations in imaging-test characteristics, costs, utilities, and time-delays. Thus, Comprehensive-CT is the preferred strategy at both WTP=$100,000/QALY and $50,000/QALY.
Interestingly, the probabilistic-sensitivity analyses indicated a higher sensitivity of the model for the imaging-test characteristics. This highlights the importance of the diagnostic accuracy for stroke-imaging subtype classification, which is one of the main criteria used for intravenous thrombolytics and/or mechanical thrombectomy treatment selection. Furthermore, Comprehensive-CT was the strategy with the highest probability of being cost-effective at WTP thresholds values between $50,000/QALY-$250,000/QALY.
To our knowledge, there are no prior studies in the literature on model-based cost-effectiveness analyses evaluating the diagnostic accuracy of stroke-imaging subtype classification. A main strength of our study is the novel in-depth modeling of the imaging-test characteristics which enables the cost-effectiveness evaluation of initial imaging strategies on patient selection for intravenous thrombolytics and/or mechanical thrombectomy in the 0–24-hour window from last-known-well-to-arrival. Specifically, our study highlights the value of perfusion imaging for mechanical thrombectomy selection in the 0–6-hour window from last-known-well-to-arrival. The main driver of the results is the value of added information from angiography and perfusion regarding large anterior vessel occlusion and perfusion deficit status at presentation, allowing more accurate and rapid patient selection for mechanical thrombectomy; thus, enabling more patients to receive potential benefits from treatment while avoiding unnecessary treatment time-delays.
Importantly, the sensitivity analyses on time-dependent outcome probabilities show the importance of implementing efficient imaging workflows at comprehensive stroke centers with prompt access to MRI for acute ischemic stroke imaging. Furthermore, our analyses show that radiation risk from CT does not impact clinical outcomes for acute ischemic stroke patients whose average age is 70 years, highlighting the safety profile of Comprehensive-CT in stroke imaging.
Our findings are consistent with prior studies that performed similar investigations focused in select subsets of our modeled cohort. Young et al.[58] cost-effectiveness analysis demonstrated the benefit of multimodal CT for intravenous thrombolytics or mechanical thrombectomy selection for patients presenting in the 0–3-hour window from last-known-well-to-arrival. John et al.[59] reported that mechanical thrombectomy selection for acute ischemic stroke patients in the 0–8-hour window from last-known-well-to-arrival based on MR-DWI±MRP translated into reduced healthcare cost due to better patient outcomes and reduced utilization of healthcare resources compared to patients selected based on NCCT+CTA. Furthermore, the prospective study by Kim et al.[60] did not observe significant differences in clinical outcomes of patients selected for mechanical thrombectomy in the 0–6-hour window from last-known-well-to-arrival time based on MR-DWI±MRP or NCCT+CTA, despite the observed median time-delay of 22 minutes in the MRI group. For the modeled cohort in our analysis, the results indicated an MRI-workflow time-delay tolerance of 10 minutes. However, our time-delay analysis showed a 20 minute tolerance when constraining the modeled cohort as in Kim et al.[60].
The relationship between the diagnostic accuracy of the imaging strategies for stroke-imaging-subtype classification and clinical outcomes was achieved through detailed modeling of the outcome probabilities. Consequently, limitations of our study include that we extrapolated data to model clinical outcomes of some stroke-imaging subtypes as well as the association between achieving independent outcome (mRS02) and time to successful mechanical thrombectomy in the >6–24-hour window from last-known-well-to-arrival time because adequate data did not exist in the literature. Importantly, our model reflects stroke-care pathways recommended by the AHA guidelines[9] with workflow from RCTs which reports arrival-to-treatment times between 74–148 minutes[11]. Thus, other variations in stroke-care pathways and workflow that could lead to potential time-saving as in the optimized MR-workflow analyzed in Sakamoto et al.[61] or direct-to-angiography stroke-care pathways as proposed in Mendez et al.[62] are not explicitly accounted for in the model. In addition, our model assumed that MR is readily available at comprehensive stroke centers, which may not always be the case. Furthermore, the results of our analyses could be conservative since the Markov models used to estimate lifetime QALYs assumed that a recurrent stroke increases the degree of disability[63–65]. Therefore, further studies may include analyzing MR-workflow feasibility, patient-specific factors that could modify imaging preferences, and other patient contexts and treatments.
Supplementary Material
Take-Home Points.
Comprehensive utilization of imaging with angiography and perfusion at presentation dominated stepwise approaches. Furthermore, Comprehensive-CT dominates Comprehensive-MR across possible variations on model parameters.
Radiation risks from CT imaging has minimal impact on clinical outcomes for stroke patients whose average age is 70 years.
The cost-effectiveness analyses highlighted the added value of perfusion imaging prior to treatment decision in the first six hours from symptom onset time.
Sensitivity analyses emphasized the importance of implementing efficient imaging workflows at comprehensive stroke centers for acute stroke imaging to achieve better clinical outcomes.
Sources of Support
Research partnership from Siemens Healthineers and The Feinstein Institutes for Medical Research, Northwell Health, National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R56NS114275.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Data Access and Integrity
The authors declare that they had full access to all the data in this study and the authors take complete responsibility for the integrity of the data and the accuracy of the analysis.
REFERENCES
- 1.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018;378(1):11–21. [DOI] [PubMed] [Google Scholar]
- 2.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med 2018;378:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–30. [DOI] [PubMed] [Google Scholar]
- 4.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular Therapy for Ischemic Stroke with Perfusion-Imaging Selection. New England Journal of Medicine. 2015;372:1009–18. [DOI] [PubMed] [Google Scholar]
- 5.Jovin T, Chamorro A, Cobo E, et al. Thrombectomy within 8 Hours after Symptom Onset in Ischemic Stroke. N Engl J Med. 2015;372(11):2296–1306. [DOI] [PubMed] [Google Scholar]
- 6.Saver JL, Goyal M, Bonafe A, et al. Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. N Engl J Med 2015;372:2285–2295. [DOI] [PubMed] [Google Scholar]
- 7.Saber H, Navi BB, Grotta JC, et al. Real-World Treatment Trends in Endovascular Stroke Therapy. Stroke. 2019;50(3):683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Heart Association. Comprehensive Stroke Center Certification. https://www.heart.org/HEARTORG/Professional/HospitalAccreditationCertification/ComprehensiveStrokeCenterCertification/Comprehensive-Stroke-Center-ertification_UCM_455446_SubHomePage.jsp
- 9.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019;50:e344–e418. [DOI] [PubMed] [Google Scholar]
- 10.Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309(23):2480–8. [DOI] [PubMed] [Google Scholar]
- 11.Saver JL, Goyal M, van der Lugt A, et al. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. Jama. 2016;316(12):1279–88. [DOI] [PubMed] [Google Scholar]
- 12.Lee JS, Demchuk AM. Choosing a Hyperacute Stroke Imaging Protocol for Proper Patient Selection and Time Efficient Endovascular Treatment: Lessons from Recent Trials. J Stroke. 2015;17(3):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell BC, Parsons MW. Imaging selection for acute stroke intervention. Int J Stroke. 2018; 13(6):554–567. [DOI] [PubMed] [Google Scholar]
- 14.Yu W, Jiang WJ. A Simple Imaging Guide for Endovascular Thrombectomy in Acute Ischemic Stroke: From Time Window to Perfusion Mismatch and Beyond. Front Neurol. 2019; 10:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turk AS, Magarick JA, Frei D, et al. CT perfusion-guided patient selection for endovascular recanalization in acute ischemic stroke: a multicenter study. J Neurointerv Surg. 2013; 5(6):523–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen S, Lansberg MG. CT perfusion in acute stroke: Practical guidance for implementation in clinical practice. J Cereb Blood Flow Metab. 2019; 39(9):1664–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munich Stephan A., Shakir Hakeem J., et al. Role of CT perfusion in acute stroke management. Cor et Vasa. 2016; 58(2):e215–e224. [Google Scholar]
- 18.Brunnquell CL, Avey GD, Szczykutowicz TP. Objective Evaluation of CT Time Efficiency in Acute Stroke Response. J Am Coll Radiol. 2018;15(6):876–880. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez RG, Copen WA, Schaefer PW, et al. The Massachusetts General Hospital acute stroke imaging algorithm: an experience and evidence based approach. J Neurointerv Surg. 2013; 5 Suppl 1(Suppl 1):i7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9):e139–e596. [DOI] [PubMed] [Google Scholar]
- 21.Wintermark M, Sanelli PC, Albers GW, et al. Imaging recommendations for acute stroke and transient ischemic attack patients: A joint statement by the American Society of Neuroradiology, the American College of Radiology, and the Society of NeuroInterventional Surgery. AJNR Am J Neuroradiol. 2013;34(11):E117–E127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broderick JP, Adeoye O, Elm J. Evolution of the Modified Rankin Scale and Its Use in Future Stroke Trials. Stroke. 2017; 48(7):2007–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007. March;38(3):1091–6. [DOI] [PubMed] [Google Scholar]
- 24.Chang EY, Chang EH, Cragg S, Cramer SC. Predictors of Gains During Inpatient Rehabilitation in Patients with Stroke- A Review. Crit Rev Phys Rehabil Med. 2013;25(3–4):203–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samsa GP, Reutter RA, Parmigiani G, Ancukiewicz, et al. Performing cost-effectiveness analysis by integrating randomized trial data with a comprehensive decision model: application to treatment of acute ischemic stroke. J Clin Epidemiol. 1999;52(3):259–71. [DOI] [PubMed] [Google Scholar]
- 26.Earnshaw SR, Jackson D, Farkouh R, Schwamm L. Cost-effectiveness of patient selection using penumbral-based MRI for intravenous thrombolysis. Stroke. 2009;40(5):1710–20. [DOI] [PubMed] [Google Scholar]
- 27.Earnshaw SR, Wilson M, Mauskopf J, Joshi AV. Model-based cost-effectiveness analyses for the treatment of acute stroke events: a review and summary of challenges. Value Health. 2009;12(4):507–20. [DOI] [PubMed] [Google Scholar]
- 28.Ivanidze J, Charalel RA, Shuryak I, et al. Effects of Radiation Exposure on the Cost-Effectiveness of CT Angiography and Perfusion Imaging in Aneurysmal Subarachnoid Hemorrhage. AJNR Am J Neuroradiol. 2017;38:462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arias E, Xu J. United States Life Tables, 2017. NVSR Volume 68, Number 7. 66pp. [PubMed] [Google Scholar]
- 30.TreeAge Pro 2018, R2. TreeAge Software, Williamstown. TreeAge
- 31.Bhaskar S, Stanwell P, Cordato D, Attia J, Levi C. Reperfusion therapy in acute ischemic stroke: dawn of a new era?. BMC Neurol. 2018; 18(1):8. doi: 10.1186/s12883-017-1007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mokin M, Pendurthi A, Ljubimov V, Burgin WS, Siddiqui AH, Levy E, et al. ASPECTS, Large Vessel Occlusion, and Time of Symptom Onset: Estimation of Eligibility for Endovascular Therapy. Neurosurgery. 2018; 83(1):122–127. [DOI] [PubMed] [Google Scholar]
- 33.Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. The Lancet. 2007; 369(9558):293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiebach JB, Schellinger PD, Jansen O, et al. CT and diffusion-weighted MR imaging in randomized order: diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke. 2002; 33(9):2206–10. [DOI] [PubMed] [Google Scholar]
- 35.Hirai T, Korogi Y, Ono K, et al. Prospective evaluation of suspected stenoocclusive disease of the intracranial artery: combined MR angiography and CT angiography compared with digital subtraction angiography. American Journal of Neuroradiology. 2002; 23(1):93–101. [PMC free article] [PubMed] [Google Scholar]
- 36.Zaro-Weber O, Moeller-Hartmann W, Heiss WD, et al. MRI perfusion maps in acute stroke validated with 15O-water positron emission tomography. Stroke. 2010; 41(3):443–9. [DOI] [PubMed] [Google Scholar]
- 37.Brinjikji W, Demchuk AM, Murad MH, Rabinstein AA, et al. Neurons Over Nephrons: Systematic Review and Meta-Analysis of Contrast-Induced Nephropathy in Patients With Acute Stroke. Stroke. 2017. July;48(7):1862–1868. [DOI] [PubMed] [Google Scholar]
- 38.Dittrich R, Akdeniz S, Kloska SP, Fischer T, et al. Low rate of contrast-induced Nephropathy after CT perfusion and CT angiography in acute stroke patients. J Neurol. 2007;254(11):1491–7 [DOI] [PubMed] [Google Scholar]
- 39.Eddy DM, Hollingworth W, Caro JJ, et al. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making. 2012;32(5):733–743. [DOI] [PubMed] [Google Scholar]
- 40.Apoil M, Turc G, Tisserand M, et al. Clinical and magnetic resonance imaging predictors of very early neurological response to intravenous thrombolysis in patients with middle cerebral artery occlusion. J Am Heart Assoc. 2013; 2(6):e000511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YD, Nam HS, Kim SH, et al. Time-Dependent Thrombus Resolution After Tissue-Type Plasminogen Activator in Patients With Stroke and Mice. Stroke. 2015;46(7):1877–82. [DOI] [PubMed] [Google Scholar]
- 42.Mulder MJHL, Jansen IGH, Goldhoorn RB, et al. Time to Endovascular Treatment and Outcome in Acute Ischemic Stroke: MR CLEAN Registry Results. Circulation. 2018;138(3):232–240. [DOI] [PubMed] [Google Scholar]
- 43.Hunink MM, Weinstein MC, Wittenberg E, Drummond MF, Pliskin JS, Wong JB, et al. Decision Making in Health and Medicine: Integrating Evidence and Values, 2nd Ed. Cambridge, UK: Cambridge University Press; 2014. [Google Scholar]
- 44.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(21):2304–2322. [DOI] [PubMed] [Google Scholar]
- 45.Neumann PJ, Cohen JT, Weinstein M. Updating Cost-Effectiveness — The Curious Resilience of the $50,000-per-QALY Threshold. N Engl J Med. 2014;371:796–797. [DOI] [PubMed] [Google Scholar]
- 46.Vanness DJ, Lomas J, Ahn H. A Health Opportunity Cost Threshold for Cost-Effectiveness Analysis in the United States. Ann Intern Med. 2020. [DOI] [PubMed] [Google Scholar]
- 47.United States Department of Labor—Bureau of Labor Statistics Consumer Price Index (CPI).
- 48.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316(10):1093–1103. [DOI] [PubMed] [Google Scholar]
- 49.Wardlaw JM, Mielke O. Early signs of brain infarction at CT: observer reliability and outcome after thrombolytic treatment—systematic review. Radiology. 2005; 235(2):444–53. [DOI] [PubMed] [Google Scholar]
- 50.Shen J, Li X, Li Y, et al. Comparative accuracy of CT perfusion in diagnosing acute ischemic stroke: A systematic review of 27 trials. PloS one. 2017; 12(5):e0176622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duffis EJ, Jethwa P, Gupta G, et al. Accuracy of computed tomographic angiography compared to digital subtraction angiography in the diagnosis of intracranial stenosis and its impact on clinical decision-making. J Stroke Cerebrovasc Dis. 2013;22(7):1013–7. [DOI] [PubMed] [Google Scholar]
- 52.Burke JF. Cost and Utility in the Diagnostic Evaluation of Stroke. Continuum. 2014; 20(2 Cerebrovascular Disease): 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.NYS Health and Health Care Cost Institute. 2019. 2017 Health Care Spending, Prices, and Utilization for Employer-Sponsored Insurance in New York Report. https://nyshealthfoundation.org/resources/.
- 54.Kunz WG, Hunink MG, Dimitriadis K, et al. Cost-effectiveness of Endovascular Therapy for Acute Ischemic Stroke: A Systematic Review of the Impact of Patient Age. Radiology. 2018;288(2):518–526. [DOI] [PubMed] [Google Scholar]
- 55.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(8):699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaisinanunkul N, Adeoye O, Lewis RJ, Grotta JC, Broderick J, Jovin TG, et al. Adopting a Patient-Centered Approach to Primary Outcome Analysis of Acute Stroke Trials Using a Utility-Weighted Modified Rankin Scale. Stroke. 2015;46(8):2238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rethnam V, Bernhardt J, Dewey H, et al. Utility-weighted modified Rankin Scale: Still too crude to be a truly patient-centric primary outcome measure?. Int J Stroke. 2020;15(3):268–27.7 [DOI] [PubMed] [Google Scholar]
- 58.Young KC, Benesch CG, Jahromi BS. Cost-effectiveness of multimodal CT for evaluating acute stroke. Neurology. 2010;75:1678–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.John S, Thompson NR, Lesko T, et al. Cost Analysis of the Addition of Hyperacute Magnetic Resonance Imaging for Selection of Patients for Endovascular Stroke Therapy. Intervent Neurol 2017;6:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim JT, Cho BH, Choi KH, et al. Magnetic Resonance Imaging Versus Computed Tomography Angiography Based Selection for Endovascular Therapy in Patients With Acute Ischemic Stroke. Stroke. 2019; 50(2):365–372. [DOI] [PubMed] [Google Scholar]
- 61.Sakamoto Y, Suzuki K, Abe A, et al. Reducing door-to-reperfusion time in acute stroke endovascular therapy using magnetic resonance imaging as a screening modality. J Neurointerv Surg. 2020:neurintsurg-2019–015625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mendez B, Requena M, Aires A, Martins N, et al. Direct Transfer to Angio-Suite to Reduce Workflow Times and Increase Favorable Clinical Outcome. Stroke. 2018. November;49(11):2723–2727. [DOI] [PubMed] [Google Scholar]
- 63.Stahmeyer JT, Stubenrauch S, Geyer S, Weissenborn K, et al. The Frequency and Timing of Recurrent Stroke: An Analysis of Routine Health Insurance Data. Dtsch Arztebl Int. 2019;116(42):711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ganesh A, Luengo-Fernandez R, Wharton RM, Gutnikov S, et al. Time Course of Evolution of Disability and Cause-Specific Mortality After Ischemic Stroke: Implications for Trial Design. J Am Heart Assoc. 2017;6(6):e005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Y, Shi YZ, Zhang N, Wang S, et al. The Disability Rate of 5-Year Post-Stroke and Its Correlation Factors: A National Survey in China. PLoS One. 2016;11(11):e0165341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


