Summary
Introduction
There is a need for the development of new biomarkers for diagnosis and prognosis of ovarian cancer, which can ideally serve as targets for new therapeutic modalities and individualization of treatment. The objectives of this study were to determine the prognostic significance of the neutrophil/lymphocyte ratio in the peripheral blood of patients with ovarian cancer and tumor staging, and to associate this marker with the immune expression of a panel of cytokines.
Methods
The study included 24 patients with malignant ovarian neoplasia treated at the Pelvic Mass Outpatient Clinic of the Clinical Hospital of the Federal University of Triângulo Mineiro. The neutrophil/lymphocyte ratio was calculated as the absolute number of neutrophils divided by the absolute number of lymphocytes. Expression of the cytokines was evaluated by the immunohistochemistry method (IL2, IL5, IL6, IL8, IL10 and TNF-R1). Fisher’s statistical test was used for the comparisons of immunohistochemical expression with the neutrophil/lymphocyte ratio, and the unpaired T-Test was used in the analysis of the association of this ratio with tumor staging.
Results
A neutrophil/lymphocyte ratio > 2.6 was significantly higher in the more advanced stages (II-IV) of malignant ovarian neoplasia (p = 0.0098). In addition, this ratio > 2.6 was associated with IL2 stromal immunostaining (1-3) (p = 0.0472).
Conclusion
Stromal IL-2 is associated with higher a neutrophil/lymphocyte ratio, suggesting a worse prognosis in ovarian cancer and its role in tumor immunology; a neutrophil/lymphocyte ratio > 2.6 is associated with more advanced stages of malignant ovarian neoplasia.
Key words: Neutrophil/lymphocyte ratio, Staging, IL-2, Immunohistochemistry, Ovarian cancer
Introduction
Ovarian cancer is one of the types of gynecological tumors that cause the most deaths worldwide, presenting a law overall survival rate. It is an insidious disease and is commonly diagnosed in more advanced stages 1. The American Cancer Society estimates that about 22,440 women will have a new diagnosis of the disease in the United States by 2017, and about 14,080 will die of disease 2,3.
Ovarian cancer has a strong association with inflammation, and it is well known that inflammation is associated with different stages of tumor development, including initiation, promotion, malignant conversion, invasion and metastasis appear to be regulated by cytokines 4,5. Therefore, several systemic inflammatory markers may have potential applications in the prediction of poor prognosis 6-8.
In recent years, laboratory quantification of systemic inflammatory response markers such as C-reactive protein (CRP), absolute leukocyte count, neutrophil/lymphocyte ratio (NLR) and platelet/lymphocyte ratio (RPL) were introduced as prognostic factors in patients with various types of malignant neoplasms, including ovarian cancer 9. Studies involving the neutrophil/lymphocyte (NLR) ratio as an inflammatory marker have shown favorable results, suggesting that it can be used as a significant predictor of malignancy for solid tumors originating from various tissues 10.
There is a need for the development of new biomarkers for diagnosis and prognosis of ovarian cancer, which can ideally serve as targets for new therapeutic modalities and individualization of treatment 11.
The objectives of this study were to determine the prognostic significance of NLR in ovarian cancer patients, and to verify the stromal expression of cytokines (IL2, IL5, IL6, IL8, IL10 and TNF-R1) in malignant ovarian neoplasia.
Materials and methods
We prospectively evaluated 24 patients with malignant ovarian neoplasia treated at the Pelvic Mass Ambulatory of the Discipline of Gynecology and Obstetrics/Oncology Research Institute (IPON), Federal University of Triângulo Mineiro - UFTM, submitted to surgical treatment according to established criteria, from 2009 to December 2013 and with subsequent histological diagnosis of epithelial ovarian cancer confirmed by the pathologist. The criteria for exploratory laparotomy were: anechoic cysts with a maximum diameter of less than 7.0 cm and persistence of alteration for more than 6 months and normal tumor markers; altered tumor markers; anechoic cysts with a maximum diameter greater than or equal to 7.0 cm; ovarian masses with solid contents, presence of intracystic vegetation, thick septa, 2 or more fine septa; Color Doppler with resistance index less than or equal to 0.4 12,13.
The study was analyzed and approved by the Research Ethics Committee of the Federal University of Triângulo Mineiro. Written informed consent was obtained from each patient or their family members.
The inclusion criterion was the postoperative diagnosis of primary malignant epithelial ovarian neoplasm by the anatomic-pathological paraffin. Exclusion criteria were torsion of the adnexal pedicle, malignant secondary ovarian neoplasia (metastasis), antineoplastic treatment prior to surgery (Chemotherapy), and relapse.
The anatomopathological study was carried out by the Special Pathology Discipline (UFTM) in paraffin-embedded sections, and the cases were reviewed by an observer of the Special Pathology discipline, in order to choose the best cuts for immunohistochemical study. The anatomopathological evaluation and the tumor staging of the cases were performed according to the International Federation of Gynaecology and Obstetrics (FIGO) criteria 14.
IMMUNOHISTOCHEMISTRY
We performed an immunohistochemical study of the cytokines (IL2, IL5, IL6, IL8, IL10 and TNF-R1) and its reading in the stromal compartment of malignant epithelial ovarian neoplasms, as detailed below.
The specimens obtained by surgical resection were processed in paraffin and reviewed by an experienced pathologist. The selected cases were submitted to new cuts (4 μm) on silanized slides (ATPS - Silano, Sigma® A3648), using the Novolink™ Polymer Detection System. The slides were kept in a greenhouse (56 °C, 24 h) and then dewaxed (3xylol baths, 5 min each) and dehydrated (three baths of absolute alcohol and one 80% alcohol bath, 10 sec each). After, the slides remained in the bath (PBS, pH 7.2, 5 min) for hydration.
Recovery of the antigens was then performed. The slides were placed in cytology tubes containing 10 mM citrate buffer (pH 6.0) or Tris-EDTA, as directed by the manufacturer, and placed into a Pascal pan, which was quenched with distilled water to the limit indicated for 30 minutes at a temperature of 100 °C. Next, the tubes were removed from inside the pan and placed on the bench for its cooling.
To neutralize the endogenous peroxidase the Peroxidase Blocker was used for 5 minutes. Washed in PBS for 5 minutes. Incubated with Protein Blocker for 5 minutes. The slides were washed with PBS for 5 minutes. The sections were incubated overnight at 4°C with a primary antibody.
The primary antibodies used were IL-2 Antibody (H-133; Rabbit polyclonal IgG; sc-7896; Lote #H0811; Santa Cruz Biotechnology, Inc.; Dallas, Texas), IL 5 Antibody (H-85; Rabbit policlonal IgG; sc-7887; Lote D0708; Santa Cruz Biotechnology, Inc.; Dallas, Texas), NLC-L-IL-6 Antibody (Mouse Monoclonal; Lote L155309; Leica Biosystems Nussloch; Nussloch, Germany), IL-8 RB (E-2) Antibody; Mouse Monoclonal IgG; sc-7304; Lote F1510; Santa Cruz Biotechnology, Inc.; Dallas, Texas), IL 10 Antibody (Polyclonal; Lote 11042807; cat nº 250713; ABBIOTEC; San Diego), p-TNF-R1 (ser 274) Antibody (Rabbit policlonal IgG; Lote B0509; sc-130220; Santa Cruz Biotechnology, Inc.; Dallas, Texas).
After overnight incubation at 4° C with specific antibody, the slides were placed at room temperature (15 min), washed (PBS) for 5 minutes and dried. Incubated with Primary Powder for 30 minutes. Washed in PBS for 5 minutes. Incubated with Novolink™ Polymer for 30 minutes. Washed in PBS for 5 minutes.
After washing in PBS, the slides were developed by chromogen solution (Diaminobenzidine-DAB) for 5 min. Following this, the slides were washed (running water) and counterstained in Harris hematoxylin. Finally, the slides were immersed in 3 baths of absolute alcohol (10 seconds each), to remove excess water, 1 xylene bath phenicate and 3 xylol baths (5 minutes each). The coverslips were added on the blades with entellan for further analysis.
Positive and negative controls were used. Two observers evaluated the slides. The intensity of immunostaining in the stroma was subjectively assessed using 0 to 3: 0 (no labeling), 1 (weak labeling), 2 (moderate labeling), 3 (strong labeling) 15.
NEUTROPHIL/LYMPHOCYTE RATIO
The absolute values of neutrophils and lymphocytes were collected from the preoperative hemogram. NLR values were obtained by dividing the absolute number of neutrophils by the absolute number of lymphocytes. It was used as a cutoff value for this relationship 2.6, based on previous studies in patients with ovarian epithelial cancer 6,16,17.
STATISTICAL ANALYSIS
The data were analyzed with GraphPad Prism software. Unpaired T-test was used to compare NLR and tumor staging, and Fisher’s exact test was used to compare the cytokines and NLR immunostaining. In the immunohistochemical study, agreement among the three observers was performed through kappa: κ < 0.4: weak agreement; 0.4 ≤ κ < 0.8: moderate agreement; 0, 8 ≤ κ < 1, 0: strong agreement; κ = 1.0: perfect agreement 18. Differences were considered significant at p < 0.05. All discordant cases were reevaluated, and the result was defined by consensus. Fisher’s exact test was used, with a level of significance lower than 0.05.
Results
The mean age of the patients analyzed was 48.3 (± 14.4) years. The histological diagnosis of the 24 cases of malignant ovarian neoplasms was: 9 (37.5%) borderline mucinous tumors, 8 (33.3%) serous cystadenocarcinomas, 2 (8.3%) mucinous cystadenocarcinoma, 1 (4.2%) borderline serous tumor, 2 (8.3%) adenocarcinomas, 1 (4, 2%) clear cell carcinoma and 1 (4.2%) endometrioid adenocarcinoma (Tab. I).
In relation to the staging 14, 12 (50.0%) had staging I, 1 (4.2%) staging II, 9 (37.5%) staging III and 2 (8.3%) staging IV. Regarding tumor grade 14, they were classified in 1, 2 or 3, according to the cell differentiation. Ten (41.7%) patients had histological grade 1, 10 (41.7%) had histological grade 2, and 4 patients had histological grade 3 (16.6%).
NLR > 2.6 was significantly higher in the more advanced stages (II-IV) of malignant ovarian neoplasia (p = 0.0098) compared to stage I (Fig. 1). In addition, NLR > 2.6 had association with stromal expression (staining 1-3) of IL2 compared to immunolabeling 0 (p = 0.0472) (Tab. II).
Fig. 1.
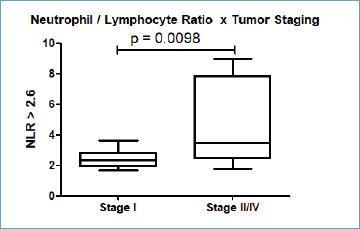
Association of NLR > 2.6 with stages II-IV of ovarian epithelial cancer (p = 0.0098).
There was no statistical significance in relation at other cytokines studied. NLR > 2.6 there was no association with stromal expression (staining 1-3) compared to immunolabeling 0 of IL6 (p = 0.1049), IL5, IL8, IL10 and TNF-R1 (p = 1.0000).
Discussion
Ovarian cancer is associated a low survival rate, and high morbidity and mortality. This is mainly due to late diagnosis and ineffective screening methods. New strategies need to be identified to define new prognostic factors for ovarian neoplasia 19.
Prognostic factors defined by correlation with survival generally reflect the extent of disease (stage), intrinsic tumor biology (type and histologic grade), and the patient’s ability to tolerate treatment for the disease 20.
Inflammatory biomarkers of peripheral blood, such as the neutrophil/lymphocyte ratio, have been used as prognostic markers in solid tumors, including as ovarian tumors 6,9.
Inflammation has been recognized as one of the hallmarks of almost all human cancers. The tumor-related inflammatory microenvironment could facilitate tumor growth and metastasis by sustained proliferation, inhibiting apoptosis, inducing the epithelial-mesenchymal transition, initiating angiogenesis and suppressing host anti-tumor immunity. For epithelial ovarian cancer, epidemiological studies have revealed pelvic inflammatory diseases may increase risk, while inflammation caused by incessant ovulation remains one of the well-accepted hypotheses of its carcinogenesis 6. It is possible to identify an important relation in neutrophil and lymphocyte counts and the risk of patients regarding the complications and poor prognosis in malignant ovarian diseases. This immunological understanding allows new therapies, with prognostic evaluation and improvement in patients’ quality of life 8.
In the study by Li et al. (2017) 6, preoperative NLR was elevated in ovarian epithelial cancer, and was significantly associated with characteristics of high tumor burden and advanced stages of the disease. Also, two independent studies with colorectal cancer added evidence to this hypothesis. Chenet al. (2015) 21 indicated that an NLR > 5 was associated with poor prognosis in metastatic colorectal cancer and was correlated with increased expression of inflammatory cytokines, such as interleukin 6 (IL-6), IL-8, IL-2, HGF, macrophage colony stimulating factor (M-CSF), and epidermal vascular growth factor (VEGF). In our study, when comparing peripheral blood RLN in patients with malignant ovarian neoplasia and its tumor staging, the NLR > 2.6 is associated with more advanced stages of malignant ovarian neoplasia (p = 0.0098).
The presence of high levels of IL-2 receptors has been found in the serum of patients with various types of solid tumors 22,23. In malignant ovarian neoplasia, an increase in IL-2 receptor concentrations can be found in more advanced stages and may play a role in predicting the risk of recurrence 24. Serum IL-2 receptor levels have been found to be significantly elevated in ovarian cancer patients compared to benign gynecological tumors and healthy patients, and may reflect the status of the immune system and the severity of the disease, being a possible marker of prognosis 25. Jammal et al. (2016) 26 demonstrated stronger IL-2 immunostaining in malignant neoplasms compared to benign ovarian neoplasms, and there was a strong immunostaining relationship with histological grade 3 in malignant ovarian neoplasms. Our findings are consistent with these studies; stromal immunoexpression of IL-2 (1-3) was associated with NLR > 2.6.
There was no statistical significance in relation at other cytokines studied. It is important to note that there is a heterogeneous response of the cytokine expression profile, in these pathways may reflect underlying differences in stroma biology and inflammatory response intrinsic to the various sites 27.
The main limitation of the study is the small sample of patients. Other studies with a larger number of patients are needed to clarify the role of IL-2 and other cytokines in the prognosis and progression of ovarian cancer.
Conclusion
Stromal IL-2 is associated with a high neutrophil/lymphocyte ratio, suggesting poorer prognosis in ovarian cancer and its role in tumor immunology; a neutrophil/lymphocyte ratio > 2.6 is associated with more advanced stages of malignant ovarian neoplasia.
Figures and tables
Tab. I.
Histological diagnosis and histological grade (FIGO) of the malignant and borderline ovarian neoplasms (Raspolini et al., 2007) 4.
| Malignant and borderline ovarian neoplasms (n = 24) | ||
|---|---|---|
| n | % | |
| Borderline mucinous tumors Grade 1 = 9 (100%) |
9 | 37.5 |
| Serous cystadenocarcinoma Grade 2 = 7 (87.5%) Grade 3 = 1 (12.5%) |
8 | 33.3 |
| Mucinous cystadenocarcinoma Grade 2 = 2 (100%) |
2 | 8.3 |
| Adenocarcinoma Grade 2 = 1 (50%) Grade 3 = 1 (50%) |
2 | 8.3 |
| Endometrioid adenocarcinoma Grade 3 = 1 (100%) |
1 | 4.2 |
| Clearcell carcinoma Grade 3 = 1 (100%) |
1 | 4.2 |
| Borderline serous tumor Grade 1 = 1 (100%) |
1 | 4.2 |
Tab. II.
Association of stromal IL-2 immunostaining with NLR.
| IL-2 staining 0 | IL-2 staining 1, 2, 3 | |
|---|---|---|
| NLR > 2, 6 (n = 11) | 2 (18.2%) | 9 (81.8%)* |
| NLR ≤ 2, 6 (n = 13) | 8 (61.5%) | 5 (38.5%) |
Fisher’s exact test, *p = 0.0472.
ACKNOWLEDGEMENTS
The authors wish to acknowledge the funding received from the CNPq, FUNEPU and the FAPEMIG.
Footnotes
CONFLICT OF INTEREST STATEMENT
None declared.
References
- 1.Corvigno S, Wisman GB, Mezheyeuski A, et al. Markers of fibroblast-rich tumor stroma and perivascular cells in serous ovarian cancer: Inter- and intra-patient heterogeneity and impact on survival. Oncotarget 2016;7:18573-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Cancer Society [homepage on the internet]. Cancer facts & figures 2013. Atlanta, GA, 2017. [Cited 2017 May 04]. Available from: http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/ACSPC-036845.
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2017;67:7-30. [DOI] [PubMed] [Google Scholar]
- 4.Raspollini MR, Taddei GL. Tumor markers in ovarian carcinoma. Int J Gynecol Obstet 2007;97:175-81. [DOI] [PubMed] [Google Scholar]
- 5.Plewka D, Kowalczyk AE, Jakubiec-Bartnik B, et al. Immunohistochemical visualization of pro-inflammatory cytokines and enzymes in ovarian tumors. Folia Histochem Cytobiol 2014;52:124-37. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Hong N, Robertson M, et al. Preoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancer. Sci Rep 2017;7:43001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [DOI] [PubMed] [Google Scholar]
- 8.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;17;357:539-45. [DOI] [PubMed] [Google Scholar]
- 9.Bishara S, Griffin M, Cargill A, et al. Pre-treatment white blood cell subtypes as prognostic indicators in ovarian cancer. Eur J Obstet Gynecol Reprod Biol 2008;138:71-5. [DOI] [PubMed] [Google Scholar]
- 10.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- 11.Athanassiadou P, Grapsa D, Athanassiades P, et al. The prognostic significance of COX-2 and survivin expression in ovarian cancer. Pathol Res Pract 2008;204:241-9. [DOI] [PubMed] [Google Scholar]
- 12.Murta EF, da Silva CS, Gomes RA, et al. Ultrasonographic criteria and tumor marker assay are good procedures for the diagnosis of ovarian neoplasia in preselected outpatients. Eur J Gynaecol Oncol 2004;25:707-12. [PubMed] [Google Scholar]
- 13.Murta EFC, Nomelini RS. Early diagnosis and predictors of malignancy in the evaluation of adnexal mass. Curr Opin Obstet Gynecol 2006;18:14-9. [DOI] [PubMed] [Google Scholar]
- 14.Zeppernick F, Meinhold-Heerlein I. The new FIGO staging system for ovarian, fallopian tube, and primary peritoneal cancer. Arch Gynecol Obstet 2014;290:839-42. [DOI] [PubMed] [Google Scholar]
- 15.Ozel E, Peştereli HE, Simşek T, et al. Expression of cyclooxygenase-2 and inducible nitric oxide synthase in ovarian surface epithelial carcinomas: is there any correlation with angiogenesis or clinicopathologic parameters? Int J Gynecol Cancer 2006;16:549-55. [DOI] [PubMed] [Google Scholar]
- 16.Asher V, Lee J, Innamaa A, et al. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol 2011;13:499-503. [DOI] [PubMed] [Google Scholar]
- 17.Thavaramara T, Phaloprakarn C, Tangjitgamol S, et al. Role of neutrophil to lymphocyte ratio as a prognostic indicator for epithelial ovarian cancer. J Med Assoc Thai 2011;94:871-7. [PubMed] [Google Scholar]
- 18.Arango EG. Bioestatística teórica e computacional. In: Arango EG. Bioestatística teórica e computacional. Rio de Janeiro: Ed. Guanabara Koogan; 2001, pp. 93-113. [Google Scholar]
- 19.Rezaeifard S, Razmkhah M, Robati M, et al. Cytokines, chemokines, and chemokine receptors quantitative expressions in patients with ovarian cancer. Iran J Med Sci 2015;40:225-32. [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal R, Kaye SB. Prognostic factors in ovarian cancer: how close are we to a complete picture? Ann Oncol 2005;16:4-6. [DOI] [PubMed] [Google Scholar]
- 21.Chen ZY, Raghav K, Lieu CH, et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br J Cancer 2015;112:1088-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lissoni P, Barni S, Rovelli F, et al. The biological significance of soluble interleukin-2 receptors in solid tumors. Eur J Cancer 1990;26:33-6. [DOI] [PubMed] [Google Scholar]
- 23.Brunetti G, Bossi A, Baiardi P, et al. Soluble interleukin 2 receptor (sIL-2R) in monitoring advanced lung cancer during chemotherapy. Lung Cancer 1999;23:1-9. [DOI] [PubMed] [Google Scholar]
- 24.Gebauer G, Rieger M, Jäger W, et al. Prognostic relevance of soluble interleukin-2 receptors in patients with ovarian tumors. Anticancer Res 1999;19:2509-11. [PubMed] [Google Scholar]
- 25.Gadducci A, Ferdeghini M, Malagnino G, et al. Elevated serum levels of neopterin and soluble interleukin-2 receptor in patients with ovarian cancer. Gynecol Oncol 1994;52:386-91. [DOI] [PubMed] [Google Scholar]
- 26.Jammal MP, Martins-Filho A, Silveira TP, et al. Cytokines and prognostic factors in epithelial ovarian cancer. Clin Med Insights Oncol 2016;10:71-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagemann AR, Hagemann IS, Cadungog M, et al. Tissue-based immune monitoring II: Multiple tumor sites reveal immunologic homogeneity in serous ovarian carcinoma. Cancer Biol Ther 2011;15;12:367-77. [DOI] [PMC free article] [PubMed] [Google Scholar]


