Abstract
Background
Dietary inorganic nitrate (NO3−) is a polyatomic ion, which is present in large quantities in green leafy vegetables and beetroot, and has attracted considerable attention in recent years as a potential health-promoting dietary compound. Numerous small, well-controlled laboratory studies have reported beneficial health effects of inorganic NO3− consumption on blood pressure, endothelial function, cerebrovascular blood flow, cognitive function, and exercise performance. Translating the findings from small laboratory studies into ‘real-world’ applications requires careful consideration.
Main body
This article provides a brief overview of the existing empirical evidence basis for the purported health-promoting effects of dietary NO3− consumption. Key areas for future research are then proposed to evaluate whether promising findings observed in small animal and human laboratory studies can effectively translate into clinically relevant improvements in population health. These proposals include: 1) conducting large-scale, longer duration trials with hard clinical endpoints (e.g. cardiovascular disease incidence); 2) exploring the feasibility and acceptability of different strategies to facilitate a prolonged increase in dietary NO3− intake; 3) exploitation of existing cohort studies to explore associations between NO3− intake and health outcomes, a research approach allowing larger samples sizes and longer duration follow up than is feasible in randomised controlled trials; 4) identifying factors which might account for individual differences in the response to inorganic NO3− (e.g. sex, genetics, habitual diet) and could assist with targeted/personalised nutritional interventions; 5) exploring the influence of oral health and medication on the therapeutic potential of NO3− supplementation; and 6) examining potential risk of adverse events with long term high- NO3− diets.
Conclusion
The salutary effects of dietary NO3− are well established in small, well-controlled laboratory studies. Much less is known about the feasibility and efficacy of long-term dietary NO3− enrichment for promoting health, and the factors which might explain the variable responsiveness to dietary NO3− supplementation between individuals. Future research focussing on the translation of laboratory data will provide valuable insight into the potential applications of dietary NO3− supplementation to improve population health.
Keywords: Nitrate, Beetroot juice, Population health, Epidemiology, Randomised controlled trials, Blood pressure, Exercise performance, Translation
Background
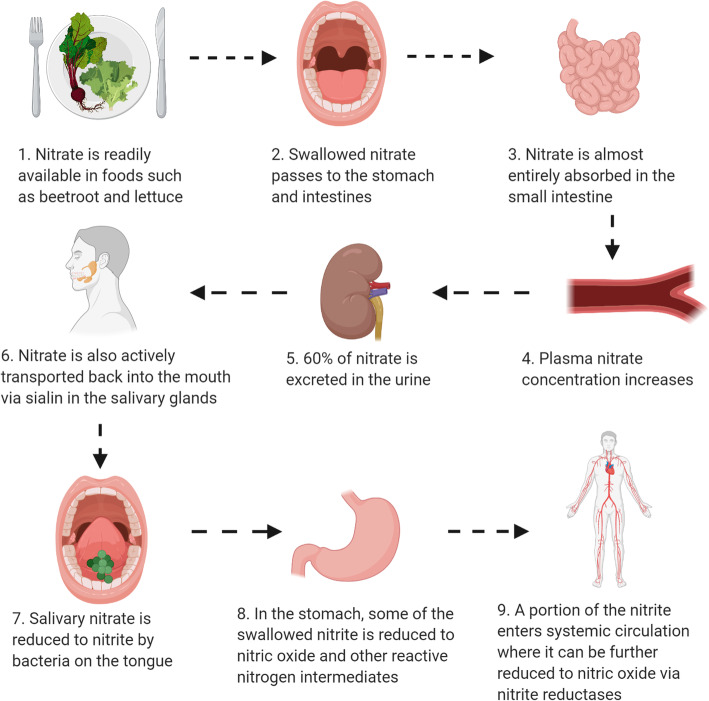
Dietary inorganic nitrate (NO3−) is a polyatomic ion present in large quantities in green leafy vegetables and certain root vegetables such as beetroot [1]. In recent years, inorganic NO3−has attracted substantial attention as a potential health promoting and exercise performance-enhancing dietary compound. These effects have largely been attributed to its ability to serve as a substrate for the ubiquitous gasotransmitter, nitric oxide (NO; Fig. 1) [2]. Following consumption, inorganic NO3−is absorbed in the upper gastrointestinal tract, increasing plasma NO3− concentration [3]. In the blood, exogenous NO3− mixes with endogenous NO3− produced via oxidation of NO. Most (~ 60%) of the ingested NO3− is excreted in the urine [4]. However, ~ 25% is actively taken up by the salivary glands via the transporter protein sialin [5], and secreted into the oral cavity, where it is reduced to nitrite (NO2−) by facultative anaerobic bacteria residing primarily on the dorsal surface of the tongue [6, 7]. Salivary (in the saliva) NO2− is then swallowed and a portion is converted into NO and other nitrogen oxides in the acidic environment of the stomach [2, 8, 9]. A further portion of the swallowed NO2− reaches the systemic circulation, where it can be transported to various tissues and reduced to NO by a range of enzymatic and non-enzymatic catalysis [2, 3]. By increasing the bioavailability of NO and other nitrogen oxides, which play a role in the regulation of multifarious physiological processes, inorganic NO3− has the capacity to elicit far-reaching effects in the human body.
Fig. 1.
A schematic representation of the nitrate-nitrite-nitric oxide pathway. Created with Biorender.com
One of the most well-documented effects following inorganic NO3− consumption is a decrease in blood pressure (BP), an effect which was first demonstrated by Larsen and colleagues from the Karolinska Institute in 2006 [10]. This group reported that 3 days of supplementation with NO3− salts (0.1 mmol/kg/d sodium NO3−) reduced diastolic and mean arterial BP by − 3.7 and − 3.2 mmHg, respectively, in young healthy adults. A number of independent research groups [11–16] has substantiated these promising findings across a range of participant cohorts and using various supplementation strategies, including the provision of whole and juiced vegetables, especially beetroot juice [17]. Over the past 10 years, as this burgeoning research area has expanded, various other potentially beneficial effects of inorganic NO3− consumption have also emerged. Notably, NO3− has been shown to improve a range of cardiovascular risk factors [17], increasing endothelial function [14, 18–21], decreasing arterial stiffness [15, 20, 22, 23], and reducing platelet aggregation [20, 24, 25]. Some [26–28], but not all [18, 29–31] studies have also shown beneficial effects of inorganic NO3− on cognitive function – effects which may be underpinned by alterations in cerebrovascular blood flow [31–33] and could be of value to a range of clinical and healthy populations [34]. Likewise, NO3− has been identified as a potential prebiotic for the oral microbiome [35], with the potential to positively impact oral health [36]. Moreover, NO3− consumption has been demonstrated to improve performance during continuous [12, 13, 29, 37–42], intermittent [43–45] and strength-based [46, 47] exercise, especially in untrained and recreationally active individuals [40, 48–50]. The mechanisms for the ergogenic effects of NO3− have not been fully resolved, but may include: 1) improvements in mitochondrial efficiency (reported by some [51], but not others [52]); 2) enhanced muscle contractile efficiency/ function [53–56]; and 3) augmented tissue blood flow, particularly to areas of low oxygen tensions such as type II muscle fibres (demonstrated in animal models [57, 58], but with less convincing data in humans [59–63]).
Current research has provided valuable insight into optimisation of NO3− supplementation strategies (e.g. pharmacokinetics, dose-response and supplementation duration) [13, 64, 65] and mechanisms of action [51, 53, 57, 66]. Nevertheless, more research is needed to understand whether findings from typically small, well-controlled laboratory studies are likely to translate into clinically relevant improvements in population health. This article highlights key areas for further research that could help in this regard. Such research is warranted to help guide practitioners, influence policy, and form guidelines for the effective and safe consumption of inorganic NO3−.
Main text
Research focus 1: large-scale, longer duration trials
Although NO3− consumption has been linked with a range of positive health outcomes, the majority of trials exploring the salutary effects of inorganic NO3− have involved short-term supplementation regimens, typically a few days in duration. Only a handful of trials have explored the medium- to longer-term effects of NO3− consumption (4 weeks to 6 months), usually focusing on BP or endothelial function as an outcome. Whilst not a universal finding [67, 68], beneficial effects of medium- to longer-term NO3− supplementation protocols have been reported in some trials [69–71]. For example, Siervo et al. [69] found that 2 months supplementation with NO3−-rich beetroot juice (~ 6.5 mmol/d NO3−) decreased 24-h systolic and diastolic BP by − 10.8 and − 5.4 mmHg, respectively, in a Sub-Saharan African setting. Similar effects were also observed when NO3− rich beetroot juice was co-ingested alongside folate. In another study, Mills and colleagues [70] showed that 6 months consumption of NO3−-rich beetroot juice (~ 11 mmol/d NO3−) decreased central systolic pressure by − 2.6 mmHg. Likewise, Kapil et al. [71] reported reductions in 24-h systolic and diastolic BP (7.7 and 5.2 mmHg, respectively) and improved endothelial function and arterial stiffness with 4 weeks NO3−-rich beetroot juice supplementation (6.4 mmol/d NO3−) with no change after placebo. Although focusing on different outcomes to the above trials, a study by Thompson et al. [72] also showed greater adaptations to sprint interval training in individuals consuming NO3− rich beetroot juice (13 mmol/d) over a 4-week period, providing further evidence of a benefit of this supplement when given over prolonged periods.
By contrast, studies by Blekkenhorst [67] and Sundqvist [68] observed no effects of 4- and 5-week NO3− interventions on BP. The lack of effect in these studies could be related to the relatively low NO3− doses administered (2.4 and 4.8 mmol/d NO3−, respectively). Conversely, the source of NO3− (vegetables or NO3− pills rather than NO3−-rich beetroot juice) could be relevant in explaining the lack of effect in these studies, given different foods providing equivalent doses of NO3− appear to have divergent effects on plasma NO2− concentration and BP [16], which could be linked to the (poly) phenol and ascorbate content of these foods [73]. Indeed, in most studies to date NO3− has been administered as beetroot juice, which is also rich in a constellation of different bioactive compounds, particularly (poly) phenols and the betalains [74]. Independent of NO3−, betalains have been shown to possess antioxidant [75], anti-inflammatory [76], and vasodilatory [77] properties, although studies in humans are still scarce. To isolate the effects NO3− from other compounds in beetroot juice, researchers often compare the effects of a NO3− rich beetroot juice to a taste-, smell- and appearance-matched NO3− depleted juice. One limitation of this strategy is that it cannot account for any synergistic interactions between NO3− and the other bioactive compounds that may augment the physiological effects of beetroot juice; in other words, we cannot be certain if the positive effects in these studies are simply due to NO3− or its interactions with the other bioactive compounds present. Thus, studies chiefly aimed at untangling the mechanistic effects of NO3− may prefer to administer NO3− in the form of NO3− salts instead of food-based supplements that contain other compounds. Overall, additional comparisons of NO3− rich beetroot juice and sodium NO2− or NO3− supplements are required. When interpreting the findings of the studies discussed in this review, it is important the reader is aware that studies with NO3− salts and NO3− -rich beetroot juice do not contain the same compounds and therefore different effects are possible. Notwithstanding, as discussed in Section 4 of this review, cross-talk between NO3− and other dietary components or participant-level differences in the response to NO3− could also account for the lack of effect of NO3− in the studies of Blekkenhorst [67] and Sundqvist [68].
Based around the current evidence it is likely that, under the right circumstances (which remain to be fully elucidated), consumption of inorganic NO3− could elicit longer-term health benefits. In order to fully appreciate the potential applications of NO3− on population health, large-scale (e.g. n= > 1000), longer duration (e.g. 2–5 years) trials which focus not only on risk factors (e.g. BP, endothelial function, cognitive function), but also incidence of key non-communicable diseases (e.g. CVD, dementia) are warranted. Specific considerations for the design of such studies are provided in Table 1. Whilst likely to be logistically complex and require substantial financial backing from funders, this research could be justified by the promising evidence from short-term trials and the potential application of findings to ease the unsustainable societal and financial burden of conditions such as CVD (annual global costs ~$863 billion [78]) and dementia (annual global cost ~$1 trillion [79]). Prior to undertaking this research, it is essential to obtain more data on the feasibility and acceptability of different strategies to increase habitual NO3−intake by a sufficient quantity and for a sufficient period to obtain long lasting health benefits. This information is critical for the design of feasible longer-term trials and translation to the general population, and will be explored in more detail in the next section.
Table 1.
Key considerations for future randomised controlled trials exploring the health effects of NO3− ingestion
| Consideration | Recommendation |
|---|---|
| Dose | Consumption of a NO3− dose ≥8 mmol |
| NO3− form | Provision of NO3− salts or vegetables, with NO3− content independently verified |
| Study duration | Longer duration (e.g., months-to-years) warranted |
| Participant cohort | ‘At risk’ cohort studied (e.g., individuals with hypertension for studies exploring effects of NO3− on cardiovascular disease risk) |
| Genetics/ microbiome | Consider recruitment of T allele carriers with G894T polymorphism in the eNOS gene |
| Microbiome | Consider recruitment of individuals with greater abundance of NO3− reducing oral bacteria |
| Mouthwash | Avoidance of mouthwash prior to and during the study |
| Dietary controls | Avoidance of thiocyanate and sulphate rich foods in conjunction with NO3− |
| Other lifestyle factors | Avoidance of smoking |
| Outcomes | Inclusion of hard clinical endpoints (e.g., CVD or dementia incidence) to build upon promising findings on risk factors for these conditions |
Research focus 2: feasibility and acceptability of different strategies to facilitate prolonged, increased consumption of nitrate
To date, a limited number of studies have reported data on the feasibility and acceptability of beetroot juice as a vehicle for increasing habitual NO3− intake. Mixed findings have been reported. For example, Ormesher and colleagues [80] gave 40 pregnant women 70 mL/d concentrated beetroot juice (~ 400 mg of NO3−) and, after 8 days of ingestion, 97% of participants indicated they would consume the supplement again, if they were experiencing benefits. However, only 62% of participants reported finding it easy to consume the beetroot juice and just over half of the participants rated the drink as palatable (54%). These findings suggest that longer-term consumption of beetroot juice may be difficult in this cohort, which could impede longer-term adherence. More recently, Kandhari et al. [81] evaluated the feasibility of a 60-day concentrated beetroot juice and folate intervention to treat hypertension in Sub-Saharan Africa. No serious adverse events were reported, and compliance was > 90%, suggesting beetroot juice was well accepted in this population. In addition, all participants rated the taste as “good” or “very good” and most participants (~ 87%) indicated a preference for beetroot juice over BP medication. The studies by Ormesher et al. [80] and Kandhari et al. [81] both administered the same brand of concentrated beetroot juice, such that the different findings cannot be attributed to a different type of supplement administered. Alternatively, it is possible that the different findings of Ormesher et al. [80], which was conducted in the UK, and Kandhari et al. [81], which was conducted in Tanzania, reflect cultural/ regional differences in food preference. However, it is noteworthy that participants in the Ormesher et al. [80] study were also pregnant, which may have further contributed towards the difference in palatability given pregnancy is known to influence taste [82].
In another study, Babateen et al. [83] examined the feasibility of different doses of concentrated beetroot juice in overweight and obese older adults over a 13-week period. Compliance was high, no adverse events were reported, and the attrition rate was 19%, which is similar or lower than the dropout rates reported in other human intervention trials [84, 85]. Collectively, these studies suggest that beetroot juice may represent an acceptable strategy to facilitate increased consumption of NO3−, at least in certain cohorts. However, future studies need to evaluate the feasibility and acceptability of beetroot juice consumption over longer periods (e.g., > 6 months) and in other populations.
Concentrated, commercially available beetroot juice shots have the advantage of being readily available (they are now sold in many major supermarket chains) and contain a standardised dose of NO3− sufficient to influence myriad health outcomes. This form of beetroot juice has also been shown to be more effective at reducing BP (and presumably eliciting other physiological changes) than non-concentrated beetroot juice when the same dose is administered [86]. In addition, as mentioned in the previous section, beetroot juice also contains other bioactive compounds that may contribute to overall health. Nevertheless, as participants do not always enjoy the taste of beetroot juice and the relatively high cost of commercially available beetroot ‘shots’ (~£1–2 or $2–3 each) may be prohibitive to some users, it is essential for researchers to explore the feasibility and acceptability of other strategies to increase NO3− consumption. This could include other NO3−-rich foods (e.g. lettuce, rhubarb, spinach, radish), gels, powders, crystals, capsules and non-beetroot drinks. To this end, both Blekkenhorst et al. [67] (> 98% compliance) and Sundqvist et al. [68] (> 97% compliance) demonstrated excellent compliance to 4 and 5 week interventions, respectively, with NO3−-rich vegetables, which were well tolerated with minimal side effects. Importantly, Sundqvist et al. [68] reported similar compliance between NO3−-rich vegetables and NO3−-containing pills (> 97% vs. > 98%). Nevertheless, neither Blekkenhorst et al. [67] nor Sundqvist et al. [68] reported beneficial physiological effects of their interventions, which could be related to the relatively modest NO3− doses provided (~ 2.4 and 4.8 mmol/d respectively) or other methodological factors which were discussed in Section 1 of this review. A comprehensive investigation of patient preferences and the real and perceived barriers of adopting a high-NO3− diet or consuming NO3−-rich supplements warrants further investigation. In addition, studies need to determine the amount of NO3−-rich vegetables required to elicit beneficial physiological effects, whether this is achievable for different populations, and whether effects are superior to non-vegetable NO3− sources. Finally, it is worth exploring whether there are regional and population preferences, as this knowledge could be used to develop more targeted NO3− products.
Research focus 3: nitrate intake and health outcomes in epidemiological studies
The role of dietary NO3− for human health has gradually shifted over the last five decades. Indeed, this compound was initially considered as a risk factor for cancer, endocrine disorders and infant methaemoglobinaemia. However, the stigma attached to dietary NO3− has gradually dwindled, and NO3− is now viewed by many a potential health-promoting compound (see Section 6 for further details). The initial results suggesting a harmful role of dietary NO3− intake (from food) were mostly derived from animal models and weakly designed epidemiological studies which have had a prominent, almost demonizing, influence on defining the role of dietary NO3− for human health [87]. These initial studies informed the still contentious WHO nutritional recommendations for dietary NO3− intake in humans which was set at 3.7 mg/ kg body weight [88]. The perception of dietary NO3− as a risk factor started to change with the discovery of the role of NO3− as key substrate for the NO3−-NO2−-NO pathway and the evidence of a beneficial effect of NO3− on health parameters such as BP.
After the study by Larsen et al. [10] in 2006, which first demonstrated a BP lowering effect of sodium NO3−, there was a rapid surge in research testing the effects of dietary NO3− on health outcomes [89]. However, the research strategy in the last decade has almost taken an inverse approach to that typically adopted in nutritional science as the conduction of clinical trials have surpassed epidemiological investigations, which are generally considered as a first step to validate research hypotheses [90–92]. One of the primary reasons for the inverse trend is the lack of reliable and representative food databases of NO3− content to support an accurate dietary assessment [93]. An additional limitation is the severe lack of validation studies testing the accuracy of dietary assessment methods against valid biomarkers of NO3− intake (e.g. 24-h urinary NO3− concentrations) [94]. This is compounded by the fact that the NO3− content of vegetables will vary by farming method (whether NO3− fertiliser is used or not), growing conditions, time of year the crop is harvested, and storage conditions [1], such that there is likely to be a degree of error in estimated NO3− intake values [95]. Several research groups have developed independent databases by collecting data on NO3− food content from published sources in an attempt to obtain valid estimates of NO3− intake and evaluate associations with health outcomes [96, 97]. Although this is a step in the right direction, it remains difficult to accurately estimate long-term habitual dietary NO3− intake for the reasons mentioned above. In addition, NO3− concentrations measured in biological fluids have been used in some analysis as indirect markers of NO3− intake [98]. Whether these objective markers of NO3− intake show stronger links with health outcomes compared with subjective, self-reported NO3− intake values, is the subject of ongoing research. A summary of the key non-cancer related epidemiological studies testing the association of inorganic NO3− with health outcomes is provided in Table 2.
Table 2.
Key epidemiological studies exploring associations between inorganic nitrate consumption and non-cancer related health outcomes
| Author, year | Population Size | Study Design | Duration of Follow up (y) | Nitrate Assessment | Health Outcome | Key Findings |
|---|---|---|---|---|---|---|
| Bahadoran et al., [99] | 4920 | Prospective (Tehran Lipid and Glucose Study) | 5.8 | FFQ | Type 2 Diabetes (T2D) | No significant association between NO3− intake and the risk of T2D in fully adjusted model |
| Kang et al. [100] |
Nurses’ Health Study (63,893 women) Health Professionals Follow-up Study (41,094 men) |
Prospective | ~ 30 years for both | FFQ | Primary open-angle glaucoma (POAG) | Higher dietary NO3− and green leafy vegetable intake was associated with a lower POAG risk, particularly POAG with early paracentral VF loss at diagnosis. |
| Mirmiran et al. [101] | 1546 | Prospective (Tehran Lipid and Glucose Study) | 3 | FFQ | Chronic Kidney Disease (CKD) | At baseline, higher intake of high-vegetable NO3− intake was associated with a 48% higher chance of having CKD (OR 1.48, 95% CI 1.05–2.13). After 3 years of follow-up, there was no significant association with the occurrence of CKD |
| Blekkenhorst et al. [102] | 1227 | Prospective (Perth Longitudinal Study of Aging in Women) | 15 | FFQ | Atherosclerotic vascular disease (ASVD) mortality | A high vegetable NO3− intake was associated with a lower risk of ASVD (HR: 0.79 95% CI: 0.68, 0.93, P = 0.004) and all-cause mortality (HR: 0.87 95% CI: 0.78, 0.97, P = 0.011) |
| Bondonno et al. [103] | 1226 | Prospective (Perth Longitudinal Study of Aging in Women) | 14.5 | FFQ | CCA-IMT, plaque severity and risk of an ischemic cerebrovascular disease event | Higher intake of vegetable NO3− was associated with 17% lower risk of cerebrovascular disease events (P = 0.02) and lower CCA-IMT (P = 0.002). |
| Gumanova et al. [104] | 1087 | Cross-sectional (Stress Aging and Health Study) | – | Plasma NOx | Diabetes type II, hyperthyroidism, coronary heart disease, gout and thrombosis/stroke, osteoporosis, cancer | NOx over 44.7 μM were associated with increased prevalence of diabetes type II, hyperthyroidism, coronary heart disease, gout and thrombosis/stroke |
| Kuhnle et al. [105] | 7598 | Cross-sectional (EPIC Norfolk) | – | Drinking water NO3− concentrations | Blood pressure (BP) | At low sulfate concentrations, NO3− was inversely associated with BP (− 4 mmHg in top quintile) whereas this was reversed at higher concentrations (+ 3 mmHg in top quintile) |
| Maas et al. [106] | 2855 | Prospective (Framingham Offspring Study) | 17.3 | Plasma NO3− | All-cause mortality and incident CVD | Plasma NO3− was weakly associated with an increased risk of death (HR, 1.16; 95%CI, 1.00–1.35 P = 0.057) but not with incident CVD |
| Smallwood et al. [107] | 919 | Cross-Sectional (InChianti) | – | 24-h urinary NO3− | Blood pressure | Systolic blood pressure in the ≥2 mmol urinary NO3− excretion group was 3.9 (CI: − 7.1 to − 0.7) mm Hg lower than in the comparison < 1 mmol excretion group. |
| Liu et al. [108] | 2900 |
Prospective (Blue Mountains Eye Study) |
15 | FFQ | CVD mortality | In multivariable-adjusted analysis, participants in quartile 4 [> 137.8 mg/d; HR 0.63 (95% CI 0.41, 0.95)] of vegetable NO3− intake had lower hazards for CVD mortality compared to participants in quartile 1 (< 69.5 mg/d) |
| Mendy et al. [109] | 17,618 | Prospective (NHANES) | 4.3 | Urinary NO3− in spot urine samples | Hypertension and CVD prevalence and all-cause mortality | 1-unit increase in log-transformed urinary NO3− was associated with a > 30% decrease in the odds of hypertension (odds ratio, 0.67; 95% confidence interval [CI], 0.55–0.81), stroke (OR, 0.61, 95% CI, 0.43–0.87) and cardiovascular mortality (HR, 0.44; 95% CI, 0.26–0.73) |
| Jackson et al. [110] | 5324 | Prospective (Australian Longitudinal Study on Women’s Health) | 15 | FFQ | Incidence of self-reported CVD-related complications | Women reporting higher total dietary NO3− intakes (Q4 > 78.2 mg/d) and vegetable NO3− intakes (Q4 > 64.4 mg/d) were 25 and 27% reduced risk of developing CVD-related complications, respectively. |
| Jackson et al. [111] | Nurses’ Health Study and Health (62,535 women) | Prospective | 26 | FFQ | Coronary heart disease | Dietary NO3− intake was not related to risk of CHD after adjustment for other lifestyle and non-vegetable dietary factors |
| Sim et al. [112] | 1420 | Cross-sectional (Perth Longitudinal Study of Aging in Women) | – | FFQ | Hand-grip strength and time up and go (TUG) | Higher NO3− intake (31.2 mg/d) was associated with lower odds for weak grip strength (OR 0.84, 95% CI 0.74–0.95, P = 0.005) and slow TUG (OR 0.86, 95% CI 0.76–0.98, P = 0.021) |
| Riddell et al. [113] | 2656 | Prospective | 1.5 |
Urinary NO3− to creatinine ratio (uNCR) |
Prediction of renal transplant rejection | Overall uNCR was highly variable with no diagnostic threshold for kidney transplant rejection |
| Wu et al. [114] 2020 | 14,894 | Cross-sectional (NHANES) | – | Urinary NO3− in spot urine samples | Congestive heart failure, coronary heart disease, angina pectoris, myocardial infarction | Significant association between urinary NO3− and congestive heart failure (OR = 0.651, 95% CI 0.507–0.838, P < 0.001) |
| Pereira et al. [98] | 1015 | Cross-sectional (NHANES) | – | Urinary NO3− in spot urine samples | Cognitive function | Urinary NO3− concentrations were not associated with cognitive performance on any of the cognitive tests. |
EPIC European Prospective Investigation of Cancer, FFQ Food Frequency Questionnaire, CCA-IMT Common Carotid Intimal Medial Thickness, NO3− Nitrate, NO2− nitrite, NOx Nitrate + Nitrite Concentration, CVD Cardiovascular Disease, OR Odds Ratio, HR Hazard Ratio, NHANES National Health and Nutrition Examination Survey, uNCR Urinary nitrate to creatinine ratio
The first studies to evaluate the association between dietary NO3− intake and health outcomes were conducted in 2016 in Iran (two studies) [99, 101] and in the United States (one study) [100]. The former evaluated the association of vegetable NO3− intake with risk of chronic kidney disease in the Tehran Lipid and Glucose Study and found a higher prevalence of chronic kidney disease (CKD) at baseline (cross-sectional analysis) in the high- NO3− intake group whereas no significant association with CKD risk was observed after a 3-year follow up [101]. Using the same dataset, Bahadoran et al. [99] found that dietary NO3− intake, overall and from animal sources, was not associated with prospective risk of diabetes. The US study was conducted in a very large sample (> 100,000 participants) and assessed dietary NO3− intake in the Nurses’ Health Study and the Health Professionals Follow-up Study [100]. The results showed a significantly lower risk of primary open-angle glaucoma in participants with higher NO3− intake [100]. However, a subsequent analysis conducted in the Nurses’ Health Study found a non-significant association between dietary NO3− and prospective risk of coronary heart disease [111]. More recently, several cross-sectional and longitudinal studies have observed significant associations between high NO3− intake or urinary NO3− concentrations (as a proxy for NO3− intake) with cardiovascular outcomes including lower BP [107], risk of hypertension [109], common carotid intimal medial thickness [103], congestive heart failure [114] and CVD mortality [109]. Conversely, higher plasma NO3− concentrations in the Framingham Offspring Study [106] were associated with an increased risk of all-cause mortality, which may be explained by the rise in plasma NO3− concentrations in participants with impaired kidney function included in the analysis and highlights the potential risk of reverse causality in these investigations. The improvements in physical performance and cognition observed in some of the NO3− supplementation trials were also explored in two cross sectional studies [98, 112]. Improved hand-grip strength and timed up and go tests (a test of functional mobility) were observed in middle-aged and older Australian participants with a higher NO3− intake [112] whereas NO3− concentrations measured in spot urine samples were not associated with improved cognition in 1015 older Americans participants enrolled in the National Health and Nutrition Examination Survey [98]. The NIH workshop on dietary NO3− held in 2016 [115] advocated for more epidemiological research to be conducted to better define the predictive role of dietary NO3− consumption for the prevention as well as treatment of chronic diseases. The consensus statement also encouraged the development of detailed and country-specific NO3− food composition tables for a more accurate assessment of the exposure to dietary NO3− [115]. The current epidemiological evidence points towards a protective role of dietary NO3− intake for cardiovascular events and mortality whereas the predictive role for cancer risk is still undefined as latest meta-analyses on the topic indicate a lack of association between dietary NO3− consumption and cancer risk [116, 117]. There is still scarce or no data from prospective studies on the association of dietary NO3− intake with other chronic conditions with established links with NO3−/NO2− and NO pathways such as diabetes, hypertension, physical disability or dementia. Further epidemiological studies in this area are therefore warranted. Such research will complement the findings from RCTs, by providing information on the effectiveness of a NO3− for disease reduction in real-world circumstances with greater sample sizes and longer follow up than is logistically feasible in most RCTs [90, 91].
Research focus 4: inter-individual differences in the response to nitrate
At the individual participant level, several groups have suggested the existence of possible ‘responders’ and ‘non-responders’ to NO3−, irrespective of the vehicle used to provide this inorganic anion [64, 118, 119]. It is important to note that random within-subject variation could explain much of the variability in response to NO3− supplementation between individuals [120, 121]. Similarly, issues may also arise when attempting to establish whether an individual is a dependable ‘responder’ or ‘non-responder’ on different occasions [122, 123]. Nevertheless, several factors have been identified which could explain genuine differences in the response to NO3− between individuals. These include individual characteristics such as age [124, 125], health [126] and exercise training status [40, 49], sex [14], genetic factors [127], and differences in the oral microbiome (explored further in Section 5 of this review). In addition, between-participant differences in potentially plastic lifestyle factors such as smoking status [128], use of mouthwash [129], and habitual diet [49, 130] might also impact an individual’s response to NO3−. We briefly review the impact of these variables on the effects of NO3− below.
Individuals with lower aerobic fitness levels may respond more favourably to NO3− supplementation [40, 131]. This theory stemmed from several studies reporting that while NO3− supplementation from any source enhanced exercise performance in recreational level athletes (V̇O2peak 40–60 ml/kg/min), such effects were less pronounced or non-existent in well-trained and elite endurance athletes (typically manifesting a V̇O2max > 60 ml/kg/min) [132–135]. Porcelli et al. [40] provide the most convincing evidence to support this notion and demonstrated that, when all other methodological factors such as the exercise test and NO3− dose are held content, individuals with a higher aerobic fitness status are less responsive to the ergogenic effects of NO3−. Indeed, those authors reported beneficial effects of sodium NO3− on 3 km running performance in individuals with low (V̇O2peak: 28.2–44.1 ml/kg/min), and moderate (V̇O2peak: 45.5–57.1 ml/kg/min), but not high (V̇O2peak: 63.9–81.1 ml/kg/min) aerobic fitness levels. Several possible explanations have been put forth to try and explain why high fitness levels might render NO3− supplementation less effective, and these are discussed in detail elsewhere [40, 131, 136]. One prominent explanation is that elite endurance athletes might produce more NO via the canonical NOS pathways and are therefore less reliant on NO3− as a substrate for NO generation [132]. Furthermore, recent evidence indicates that NO3− might elicit preferential effects on type II compared with type I muscle fibres [54, 57, 58]. Well-trained endurance athletes might therefore benefit less from NO3− supplementation given a lower proportion of type II, and a higher proportion of type I, muscle fibres compared with recreationally active individuals [137, 138]. In contrast, some studies have shown a beneficial effect of NO3− in well-trained athletes [42, 139–141]. Jonvik et al., (2015) suggested that methodological limitations of some studies could at least partly explain the null findings in some studies with elite athletes. Notably, there are far less studies assessing the effects of NO3− supplementation, irrespective of vehicle, in well-trained athletes in comparison to healthy, physically active, individuals. This is likely because well-trained athletes are only a small fraction of the population, and are logistically harder to test and recruit to studies due to their desire to avoid potential training interruptions. Thus, more research is still required to ascertain the influence of aerobic fitness levels on the responsiveness to NO3− supplementation.
Women are underrepresented in research into the health effects of dietary NO3− [142]. Nevertheless, preliminary evidence suggests potentially differential effects of NO3− (at least in regard to the effects of NO3− on BP) between the sexes, which warrants further investigation. Women have been demonstrated to have greater oral NO3− reducing capacity than men due to an oral microflora composition that is more conducive for NO3− reduction to NO2− [143]. Nevertheless, Kapil et al. [14] and Coles and Clifton [144] both demonstrated BP-lowering effects of NO3− (potassium NO3− and beetroot juice, respectively) in men with higher baseline BP and lower plasma NO2− concentrations but not in women. Likewise, in a meta-analysis by Jackson et al. [17], BP reductions with NO3− were greater in studies with more male participants. Those authors speculated that this could be related to a greater vascular production of NO in pre-menopausal women due to oestrogen-related release and activity of NO [145], diminishing the response to supplemental NO3− in women compared with men.
Although studies remain scarce, there is some evidence that the heterogeneous responses to NO3− supplementation are partly explained by polymorphisms in the eNOS gene. This was first explored by Hobbs et al., [127], who examined the effects of NO3− supplementation on BP in patients with and without a specific polymorphism in the eNOS gene (G894T), which has been suggested to inhibit NO production from eNOS [127]. Although findings are equivocal [146], the G894T polymorphism, alongside being a T allele carrier, has been associated with cardiovascular disease [147–149], of which a key risk factor is diminished NO bioavailability [150, 151]. Intriguingly, despite the small sample size (n = 14), Hobbs et al., [127] found that NO3− supplementation (beetroot bread) only reduced BP in patients who were both T allele carriers and had the G894T polymorphism in the eNOS gene. A more recent study examined the influence of the G894T polymorphism and NO3− therapy on mortality in chronic heart failure patients [146]. Somewhat at odds with the findings of Hobbs et al., [127], Azzam et al. [146] found that NO3− therapy (source not specified) increased the risk of mortality in patients with the G894T polymorphism, and to a greater extent in G allele carriers, suggesting that NO3− therapy might increase mortality in advanced heart failure. However, as this study was observational, cause-effect relationships cannot be established. Moreover, the findings are at contrast to the beneficial effects of NO3− shown in most [152–155], but not all [156, 157], short term intervention trials which show that NO3− improves cardiac function and/or exercise capacity in heart failure patients. Clearly, more studies with larger cohorts are required to determine the extent to which genetic variation influences the responsiveness to NO3− supplementation, but the findings from these two studies raise the possibility that that genetic factors could contribute towards the inter-individual variability reported by many studies.
Smoking has been shown to increase plasma and salivary concentrations of thiocyanate [158], a compound which competitively inhibits uptake of NO3− into the salivary glands [159], potentially reducing the amount of ‘substrate’ available to the oral bacteria for reduction into NO2−. Consequently, it is possible that smokers will experience compromised NO3− metabolism and thus a diminished physiological response to NO3− supplementation versus non-smokers. Indeed, Bailey et al. [128] demonstrated a smaller increase in salivary NO3−, plasma NO3− and NO2− concentration, and an attenuated BP response, following a NO3− bolus (beetroot juice) in smokers compared to non-smoking controls.
It is possible that supplemental NO3− is ineffective at eliciting meaningful physiological changes in individuals habitually consuming a high NO3− diet. Nevertheless, as population intake of NO3− is typically low — Babateen et al. [93] reported a median intake of 108 mg/d in healthy individuals. With very few individuals regularly consuming NO3− levels to match those provided through supplementation [160], high habitual NO3− intake is unlikely to explain a lack of response to NO3− supplementation in most ‘non-responders’. Alternatively, there is compelling evidence to suggest that consumption of other dietary compounds alongside NO3− may have the capacity to influence response to this compound, such that an individual’s background diet could determine (at least transiently) their status as a NO3− ‘responder’ or ‘non-responder’. For example, consumption of glucosinolate-rich vegetables, such as those from the Brassica family like broccoli, cauliflower, and cabbage, proximal to consumption of NO3−-rich vegetables was shown to blunt the BP lowering response of the latter [130]. Interestingly, this appears to be related to a similar mechanism to which smoking attenuates the effect of NO3−. Specifically, during processes that result in plant cell membrane damage such as mastication, glucosinolates are exposed to the enzyme myrosinase, which catalyses the hydrolysis of glucosinolates into thiocyanate [161]. Although consumption of thiocyanate-rich vegetables leads to lower salivary and plasma thiocyanate concentrations compared with smoking, Dewhurst-Trigg et al. [130] showed that the BP-lowering effect of a NO3−-rich smoothie was attenuated by the presence of thiocyanate rich vegetables. In that study, thiocyanate did not seem to interfere with NO3− transport into the mouth (as evident by similar salivary NO3− concentrations when NO3− was consumed alongside vegetables that were both high and low in thiocyanate), suggesting that thiocyanate may influence other aspects of NO3− metabolism. Specifically, co-ingestion of thiocyanate synthesising vegetables and NO3−-rich vegetables lowered salivary NO2− concentration compared to ingestion of NO3−-rich vegetables alone. This suggests that some Brassica vegetables might transiently alter the oral microbiome, consistent with the antimicrobial effects of thiocyanate derivatives in the oral cavity [162].
A study by Hughan et al. [163] found that the co-ingestion of sodium NO3− alongside conjugated linoleic acid, an unsaturated fatty acid particularly abundant in dairy and meat products, attenuated the rise in plasma NO3− and NO2− concentrations and supressed the BP-lowering and platelet-inhibiting effects that were apparent when supplements were administered in isolation. Mechanistically, co-consumption of conjugated linoleic acid altered the metabolic fate of ingested NO3− leading to the formation of conjugated linoleic acid nitration products, which do not appear to have the same vasodilatory and platelet inhibiting properties as NO2− and NO. Likewise, Bailey et al. [164] found that the ingestion of iodide, which is fortified in many foods [165] and known to compete for salivary NO3− uptake [159], lowered salivary NO3− concentration when co ingested with NO3− rich beetroot juice. However, the increase in salivary and plasma NO2− concentration, alongside the lowering of BP, were similar compared with NO3− alone. Finally, a possible interaction between dietary NO3− and sulphate was identified by Kuhnle et al. [105] who indicated that when estimated sulphate intake was low, higher dietary NO3− intake was associated with lower BP. Conversely, when sulphate intake was high, this association was reversed, such that greater NO3− intake was actually associated with higher BP. The mechanistic basis through which sulphate could modulate the BP lowering effects of dietary NO3− is presently unknown.
Collectively, the evidence presented above indicates that the response to NO3− is unlikely to be uniform between individuals, and could also potentially differ within individuals based around malleable lifestyle factors such as habitual diet. Better understanding the factors that influence responsiveness to NO3− is crucial to maximise the efficacy of NO3−-based interventions and will facilitate the development of targeted interventions for individuals most likely to benefit from consumption of this compound. Given many of the factors which appear to moderate the effectiveness of NO3− impact the oral conversion of this compound into NO2−, future research could also explore the potential physiological effects of direct NO2− administration (for a recent example, see [166]), which does not require processing in the mouth and could theoretically elicit more consistent responses between individuals. Nevertheless, caution should be taken to ensure such a strategy does not increase formation of potentially carcenogenic nitrosamines [167].
Research focus 5: oral microbiota and oral health
Once in the oral cavity, NO3− is reduced to NO2− during the anaerobic respiration of facultative and obligate bacteria which are particularly abundant on the dorsal surface of the tongue [168]. The oral microbiome collectively comprises over 700 individual species or phylotypes of bacteria that are organised in a series of complex interdependent communities [169]. To date, 14 species of bacteria have been identified as NO3− reducers, the majority of which are from the genera Veillonella, Prevotella, Neisseria, and Haemophilus [170]. A greater relative abundance of these bacteria on the tongue has been shown to augment the rate and magnitude of salivary NO2− production following the ingestion of NO3− rich beetroot juice [171]. Conversely, disruption of the oral microbiome by antibacterial mouthwash causes a transient loss of viable NO3−-reducing bacteria [172] and severely blunts the generation of NO2− in the saliva [173]. Strong antibacterial mouthwash has also been shown to increase BP, likely due to suppression of NO production from the NO3−-NO2−-NO pathway [174–176]. These data confirm the essential role of the oral bacteria in NO homeostasis and support the hypothesis that oral and systemic health are inextricably linked [177].
The mouth is continually exposed to the external environment and is regularly subjected to brushing, flossing, and nutrient intake, all of which may influence the physiological conditions inside the oral cavity and alter the composition of the bacterial milieu [178]. Ageing is known to cause a reduction in salivary flow rate [179] and has been reported to alter the composition of the oral microbiome in some [180, 181] but not all [182] studies. Other factors may also be expected to influence the abundance and activity of oral bacteria, including exercise, diet, oral and systemic diseases, haemodialysis [183] and peritoneal dialysis [184] and medication (particularly antibiotics). In particular, the ingestion of NO3−-rich beetroot juice has been shown to increase salivary pH and cause meaningful alterations to the oral microbiome in favour of oral health [182, 185]. Given the multitude of potential modifiers, it is perhaps unsurprising that there is profound between-individual variation in the abundance of NO3−-reducing bacteria [121]. Of note, these authors also reported significant within-individual week-to-week variability in the abundance of these bacteria and the magnitude by which plasma NO2− increased following the ingestion of NO3−-rich beetroot juice. This was despite participants standardising their diet, physical activity, use of mouthwash, teeth brushing, and tongue cleaning between visits. The unpredictability in how different individuals respond to NO3− supplementation and how the same individual responds across repeated visits poses a particular challenge for researchers who wish to explore the therapeutic effects of this dietary intervention.
While recent advancements in genomic sequencing techniques have greatly enhanced our understanding of human bacterial interactions in the context of NO homeostasis, several important questions remain unanswered. To date, the majority of the research exploring links between the oral microbiome and health outcomes has only reported the relative abundance of phyla, genera, or species. Although this quantifies the proportional makeup of the community structure it does not reveal the metabolic activities of individual bacterial species [186] which may vary depending on substrate availability, metabolite expression from neighbouring microbes and host cells, and the impact of environmental conditions [187]. Future research should deploy meta-transcriptomic analysis to determine how factors such as diet, medication, physical activity, ageing, and disease influence NO2− and NO3− reductase gene expression of the oral bacteria. Furthermore, data from epidemiological studies and short-term intervention trials seem to support the notion that increasing habitual dietary intake of NO3− can improve markers of oral health and reduce the incidence of caries [185, 188, 189]. It remains to be established whether dietary NO3− supplementation may also be an effective treatment method for those already suffering from oral diseases such as chronic periodontitis.
Research focus 6: risks versus rewards
NO3− is increasingly recognised as a beneficial ion that protects against chronic disease, yet, as noted in Section 3 of this review, historically, it was considered a food contaminant with adverse health effects, particularly increased risk of certain cancers and methaemoglobinaemia [1, 88]. While the aforementioned WHO ADI for NO3− of 3.7 mg/kg of body mass remain in place today, the discovery of multiple positive health effects of NO3− have prompted a re-examination of these claims.
In 2004 the WHO reaffirmed their restrictions on NO3− intake yet, in 2008, a panel of experts from the European Food Safety Authority, concluded that the epidemiological evidence did not support an association between NO3− and cancer risk [190]. Similarly, in 2010, the International Agency for Research on Cancer confirmed that there was inadequate evidence to suggest NO3− from food or water was carcinogenic in humans [191]. Evidence that NO3− might cause infant methemoglobinemia, which was first mooted in the 1940s [192], has also been questioned. Indeed, an investigation conducted on behalf of the WHO in 2004 found no exposure-response relationship between dietary NO3− and methemoglobinemia in infants [193]. It is also worth noting that although some studies report mild adverse symptoms with high NO3− intake such as nausea and sickness, to the authors knowledge, no serious adverse events have ever been reported in clinical trials administering NO3− [1, 93].
Notwithstanding, the available evidence does not rule out the possibility that prolonged consumption of NO3− above the ADI could harm health. Currently, at least with short to medium term intakes, research suggests that doses exceeding the ADI are needed to optimise vascular health or exercise performance [17, 48]. Because most human trials have only examined the acute health effects (< 4 weeks) of increased NO3− intake, the long-term safety of consuming NO3− in amounts that exceed the ADI is not well understood. At present, epidemiological studies provide the strongest evidence that prolonged, high intakes of NO3− are safe. Indeed, these indicate that rather than being harmful, dietary NO3− intake is inversely associated with cardiovascular disease risk [102, 194] and certain cancers [117]. Furthermore, diets and dietary patterns high in fruits and vegetables are linked to greater longevity [195, 196], protection against type 2 diabetes [197] and chronic obstructive pulmonary disease [198], and improved cardiovascular [92, 199, 200] and cognitive health [201, 202]. This suggests that higher intake of dietary NO3−, at least through plants, is more likely to be associated with health benefits than adverse effects.
Some animal studies have explored the longer-term effects of high dietary NO3− intake on health. In a study in rats, 10 weeks of a low sodium NO3− dose (0.1 mmol/kg/d), which the authors suggest is equivalent to amounts achievable in the human diet, reduced BP, whereas a much higher dose (1 mmol/kg/d), elevated BP [203]. Interestingly, this study found that the high NO3− dose down-regulated eNOS activity, not only suggesting a crosstalk between the canonical and NO3−- NO2−-NO pathway, but also that any vascular benefits afforded by NO3− supplementation could wane over time. Nonetheless, these findings were not supported by a more recent animal study from the same group. Hezel and colleagues [204] fed mice the human equivalent of 350 mg/d or 26 mg/d of sodium NO3−. After 17 months, mice consuming the high NO3− diet did not have elevated BP or any other adverse health effects, despite the fact the dose exceeded the WHO recommended ADI for an adult under ~ 95 kg. On the contrary, the high NO3− diet decreased plasma insulin and modulated inflammation, findings consistent with the metabolic benefits observed in acute human studies [205]. These effects need to be verified in humans but support the notion that prolonged increases in NO3− intake are not harmful to health.
It is important to note that any carcinogenic risk attributed to NO3− intake could be mitigated by the intake of antioxidants such as vitamin C or (poly) phenols, which are present in most fruits and vegetables. Studies have shown that vitamin C and E are effective inhibitors of nitrosamine formation [167]. In addition, (poly) phenols, which are abundant in commonly consumed NO3− sources such as spinach and beetroot [206], can also abrogate nitrosamine formation [73]. Thus, increasing NO3− intake through a greater vegetable intake may significantly lessen the risk of any NO3− induced nitrosamine formation. This could partly explain why diets high in vegetables are associated with a reduced and not heightened risk of cancer.
Health concerns have also been raised over the high oxalate content of NO3−-rich vegetables [207, 208]. Oxalates are present in several foods, but particularly high in spinach, beetroot, and rhubarb [208, 209]. Intake of these foods increases urinary oxalate excretion, a risk factor for renal stone formation [209–211], thus, it is currently recommended that foods rich in dietary oxalates are consumed in moderation [208, 210]. However, the link between dietary oxalates and kidney stone formation remains equivocal. Although consuming oxalate rich foods increases oxalate excretion, a large prospective study (> 190,000 participants) found only modest non-significant associations between dietary oxalate intake and kidney stone risk, concluding that dietary oxalate intake is not a major risk factor for the formation of kidney stones in younger or older adults [212]. Furthermore, the Dietary Approaches to Stop Hypertension (DASH) diet, which is high in oxalates and NO3−-rich vegetables [1], was recently shown to increase urinary oxalate excretion but reduce the risk of kidney stone formation in ~ 260 patients [213]. The authors attributed these findings to the high calcium and magnesium content of the diet limiting oxalate absorption. This is supported by previous research showing that oxalates from beetroot have low bioavailability (< 1%), owing to their high calcium content [209]. While more prospective human trials are needed, evidence that oxalate rich vegetables increase the risk of kidney stone formation is limited.
To summarise, claims that dietary NO3− promotes cancer or methemoglobinemia, or that dietary oxalates cause kidney stones are weak and unsubstantiated. Rather, there is compelling evidence that dietary NO3− has salutary health effects and warrants consideration as a long-term therapeutic treatment strategy to manage vascular and metabolic health. Notwithstanding, longer-term studies in humans are lacking and thus it cannot be ruled out that a prolonged increase in NO3− intake, above the WHO recommended ADI, may have adverse effects for some individuals. Thus, it is incumbent that researchers examine the long-term safety of increasing dietary NO3− consumption in a range of contexts and populations. This research will be vital for convincing the public and regulators that NO3− consumption is safe and that current recommendations to limit dietary NO3− intake should be re-considered.
Conclusions
This article has briefly outlined the current state of knowledge around the potential health effects of dietary inorganic NO3−. Six key areas worthy of future research were identified to enhance understanding of the potential role of NO3− in improving population health. As such, it is hoped that this article will help direct researchers to further explore the role of NO3− as a potential health-promoting dietary component.
Acknowledgements
Not applicable.
Abbreviations
- ADI
Acceptable daily intake
- BP
Blood pressure
- CVD
Cardiovascular disease
- CKD
Chronic kidney disease
- DASH
Dietary approach to stop hypertension
- eNOS
Endothelial nitric oxide synthase
- NIH
National Institutes of Health
- NO3−
Nitrate
- NO
Nitric oxide
- NO2−
Nitrite
- V̇O2peak
Peak oxygen uptake
- WHO
World Health Organisation
Authors’ contributions
This review was conceived by OMS and TC, and designed by OMS, CE, AIS, MS, SJB and TC. OMS, CE, AIS, MS, SJB and TC drafted and critically revised the manuscript. OMS created the schematic. All authors approved the final version of the manuscript prior to submission.
Funding
No specific funding was received to support the writing of this review.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
T.C is an Associate Editor for BMC Sport Science, Medicine & Rehabilitation. All other authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr. 2009;90:1–10. doi: 10.3945/ajcn.2008.27131. [DOI] [PubMed] [Google Scholar]
- 2.Kapil V, Khambata RS, Jones DA, Rathod K, Primus C, Massimo G, et al. The noncanonical pathway for in vivo nitric oxide generation: the nitrate-nitrite-nitric oxide pathway. Pharmacol Rev. 2020;72:692–766. doi: 10.1124/pr.120.019240. [DOI] [PubMed] [Google Scholar]
- 3.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 4.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 5.Qin L, Liu X, Sun Q, Fan Z, Xia D, Ding G, et al. Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc Natl Acad Sci U S A. 2012;109:13434–13439. doi: 10.1073/pnas.1116633109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, et al. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1995;1:546–551. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 7.Hezel M, Weitzberg E. The oral microbiome and nitric oxide homoeostasis. Oral Dis. 2015;21:7–16. doi: 10.1111/odi.12157. [DOI] [PubMed] [Google Scholar]
- 8.Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, Golden M, et al. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 10.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 11.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, et al. Acute blood pressure lowering, vasoprotective and anti-platelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, DiMenna FJ, Wilkerson DP, et al. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol. 2009;107:1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 13.Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, et al. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1121–R1131. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- 14.Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, et al. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56:274–281. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 15.Ashor AW, Shannon OM, Werner A-D, Scialo F, Gilliard CN, Cassel KS, et al. Effects of inorganic nitrate and vitamin C co-supplementation on blood pressure and vascular function in younger and older healthy adults: a randomised double-blind crossover trial. Clin Nutr. 2020;39:708–717. doi: 10.1016/j.clnu.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Jonvik KL, Nyakayiru J, Pinckaers PJ, Senden JM, van Loon LJ, Verdijk LB. Nitrate-rich vegetables increase plasma nitrate and nitrite concentrations and lower blood pressure in healthy adults. J Nutr. 2016;146:986–993. doi: 10.3945/jn.116.229807. [DOI] [PubMed] [Google Scholar]
- 17.Jackson JK, Patterson AJ, MacDonald-Wicks LK, Oldmeadow C, McEvoy MA. The role of inorganic nitrate and nitrite in cardiovascular disease risk factors: a systematic review and meta-analysis of human evidence. Nutr Rev. 2018;76:348–371. doi: 10.1093/nutrit/nuy005. [DOI] [PubMed] [Google Scholar]
- 18.Bondonno CP, Downey LA, Croft KD, Scholey A, Stough C, Yang X, et al. The acute effect of flavonoid-rich apples and nitrate-rich spinach on cognitive performance and mood in healthy men and women. Food Funct. 2014;5:849. doi: 10.1039/C3FO60590F. [DOI] [PubMed] [Google Scholar]
- 19.Heiss C, Meyer C, Totzeck M, Hendgen-Cotta UB, Heinen Y, Luedike P, et al. Dietary inorganic nitrate mobilizes circulating angiogenic cells. Free Radic Biol Med. 2012;52:1767–1772. doi: 10.1016/j.freeradbiomed.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 20.Velmurugan S, Gan JM, Rathod KS, Khambata RS, Ghosh SM, Hartley A, et al. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. Am J Clin Nutr. 2016;103:25–38. doi: 10.3945/ajcn.115.116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shepherd AI, Costello JT, Bailey SJ, Bishop N, Wadley AJ, Young-Min S, et al. “Beet” the cold: beetroot juice supplementation improves peripheral blood flow, endothelial function, and anti-inflammatory status in individuals with Raynaud’s phenomenon. J Appl Physiol. 2019;127:1478–1490. doi: 10.1152/japplphysiol.00292.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bahra M, Kapil V, Pearl V, Ghosh S, Ahluwalia A. Inorganic nitrate ingestion improves vascular compliance but does not alter flow-mediated dilatation in healthy volunteers. Nitric Oxide. 2012;26:197–202. doi: 10.1016/j.niox.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J-K, Moore DJ, Maurer DG, Kim-Shapiro DB, Basu S, Flanagan MP, et al. Acute dietary nitrate supplementation does not augment submaximal forearm exercise hyperemia in healthy young men. Appl Physiol Nutr Metab. 2015;40:122–128. doi: 10.1139/apnm-2014-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velmurugan S, Kapil V, Ghosh SM, Davies S, McKnight A, Aboud Z, et al. Antiplatelet effects of dietary nitrate in healthy volunteers: involvement of cGMP and influence of sex. Free Radic Biol Med. 2013;65:1521–1532. doi: 10.1016/j.freeradbiomed.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson G, Hicks SL, O’Byrne S, Frost MT, Moore K, Benjamin N, et al. The ingestion of inorganic nitrate increases gastric S-nitrosothiol levels and inhibits platelet function in humans. Nitric Oxide. 2002;7:24–29. doi: 10.1016/S1089-8603(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 26.Gilchrist M, Winyard PG, Fulford J, Anning C, Shore AC, Benjamin N. Dietary nitrate supplementation improves reaction time in type 2 diabetes: development and application of a novel nitrate-depleted beetroot juice placebo. Nitric Oxide. 2014;40:67–74. doi: 10.1016/j.niox.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Wightman EL, Haskell-Ramsay CF, Thompson KG, Blackwell JR, Winyard PG, Forster J, et al. Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Physiol Behav. 2015;149:149–158. doi: 10.1016/j.physbeh.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 28.Thompson C, Wylie LJ, Fulford J, Kelly J, Black MI, McDonagh STJ, et al. Dietary nitrate improves sprint performance and cognitive function during prolonged intermittent exercise. Eur J Appl Physiol. 2015. 10.1007/s00421-015-3166-0. [DOI] [PubMed]
- 29.Shannon OM, Duckworth L, Barlow MJ, Deighton K, Matu J, Williams EL, et al. Effects of dietary nitrate supplementation on physiological responses, cognitive function, and exercise performance at moderate and very-high simulated altitude. Front Physiol. 2017;8:401. doi: 10.3389/fphys.2017.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly J, Fulford J, Vanhatalo A, Blackwell JR, French O, Bailey SJ, et al. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. Am J Physiol Regul Integr Comp Physiol. 2013;304:R73–R83. doi: 10.1152/ajpregu.00406.2012. [DOI] [PubMed] [Google Scholar]
- 31.Thompson KG, Turner L, Prichard J, Dodd F, Kennedy DO, Haskell C, et al. Influence of dietary nitrate supplementation on physiological and cognitive responses to incremental cycle exercise. Respir Physiol Neurobiol. 2014;193:11–20. doi: 10.1016/j.resp.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Aamand R, Dalsgaard T, Ho Y-CL, Møller A, Roepstorff A, Lund TE. A NO way to BOLD?: dietary nitrate alters the hemodynamic response to visual stimulation. NeuroImage. 2013;83:397–407. doi: 10.1016/j.neuroimage.2013.06.069. [DOI] [PubMed] [Google Scholar]
- 33.Presley TD, Morgan AR, Bechtold E, Clodfelter W, Dove RW, Jennings JM, et al. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide. 2011;24:34–42. doi: 10.1016/j.niox.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clifford T, Babateen A, Shannon OM, Capper T, Ashor A, Stephan B, et al. Effects of inorganic nitrate and nitrite consumption on cognitive function and cerebral blood flow: a systematic review and meta-analysis of randomised clinical trials. Crit Rev Food Sci Nutr. 2019;59:2400–2410. doi: 10.1080/10408398.2018.1453779. [DOI] [PubMed] [Google Scholar]
- 35.Rosier BT, Buetas E, Moya-Gonzalvez EM, Artacho A, Mira A. Nitrate as a potential prebiotic for the oral microbiome. Sci Rep. 2020;10:12895. doi: 10.1038/s41598-020-69931-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jockel-Schneider Y, Goßner SK, Petersen N, Stölzel P, Hägele F, Schweiggert RM, et al. Stimulation of the nitrate-nitrite-NO-metabolism by repeated lettuce juice consumption decreases gingival inflammation in periodontal recall patients: a randomized, double-blinded, placebo-controlled clinical trial. J Clin Periodontol. 2016;43:603–608. doi: 10.1111/jcpe.12542. [DOI] [PubMed] [Google Scholar]
- 37.Lansley KE, Winyard PG, Bailey SJ, Vanhatalo A, Wilkerson DP, Blackwell JR, et al. Acute dietary nitrate supplementation improves cycling time trial performance. Med Sci Sports Exerc. 2011;43:1125–1131. doi: 10.1249/MSS.0b013e31821597b4. [DOI] [PubMed] [Google Scholar]
- 38.Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, et al. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. J Appl Physiol. 2011;110:591–600. doi: 10.1152/japplphysiol.01070.2010. [DOI] [PubMed] [Google Scholar]
- 39.Cermak NM, Gibala MJ, van Loon LJ, et al. Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int J Sport Nutr Exerc Metab. 2012;22:64. doi: 10.1123/ijsnem.22.1.64. [DOI] [PubMed] [Google Scholar]
- 40.Porcelli S, Ramaglia M, Bellistri G, Pavei G, Pugliese L, Montorsi M, et al. Aerobic fitness affects the exercise performance responses to nitrate supplementation. Med Sci Sports Exerc. 2015;47(8):1643–51. 10.1249/MSS.0000000000000577. [DOI] [PubMed]
- 41.Shannon OM, Barlow MJ, Duckworth L, Williams E, Wort G, Woods D, et al. Dietary nitrate supplementation enhances short but not longer duration running time-trial performance. Eur J Appl Physiol. 2017;117:775–785. doi: 10.1007/s00421-017-3580-6. [DOI] [PubMed] [Google Scholar]
- 42.Shannon OM, Duckworth L, Barlow MJ, Woods D, Lara J, Siervo M, et al. Dietary nitrate supplementation enhances high-intensity running performance in moderate normobaric hypoxia, independent of aerobic fitness. Nitric Oxide. 2016;59:63–70. doi: 10.1016/j.niox.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Wylie LJ, Bailey SJ, Kelly J, Blackwell JR, Vanhatalo A, Jones AM. Influence of beetroot juice supplementation on intermittent exercise performance. Eur J Appl Physiol. 2016;116:415–425. doi: 10.1007/s00421-015-3296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson C, Vanhatalo A, Jell H, Fulford J, Carter J, Nyman L, et al. Dietary nitrate supplementation improves sprint and high-intensity intermittent running performance. Nitric Oxide. 2016. 10.1016/j.niox.2016.10.006. [DOI] [PubMed]
- 45.Nyakayiru J, Jonvik KL, Trommelen J, Pinckaers PJM, Senden JM, van Loon LJC, et al. Beetroot juice supplementation improves high-intensity intermittent type exercise performance in trained soccer players. Nutrients. 2017;9:314. doi: 10.3390/nu9030314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mosher SL, Sparks SA, Williams EL, Bentley DJ, Mc Naughton LR. Ingestion of a nitric oxide enhancing supplement improves resistance exercise performance. J Strength Cond Res. 2016;30:3520–3524. doi: 10.1519/JSC.0000000000001437. [DOI] [PubMed] [Google Scholar]
- 47.de Oliveira GV, Morgado M, Conte-Junior CA, Alvares TS. Acute effect of dietary nitrate on forearm muscle oxygenation, blood volume and strength in older adults: a randomized clinical trial. PLoS One. 2017;12:e0188893. doi: 10.1371/journal.pone.0188893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Senefeld JW, Wiggins CC, Regimbal RJ, Dominelli PB, Baker SE, Joyner MJ. Ergogenic effect of nitrate supplementation: a systematic review and meta-analysis. Med Sci Sports Exerc. 2020;52:2250–2261. doi: 10.1249/MSS.0000000000002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones AM. Influence of dietary nitrate on the physiological determinants of exercise performance: a critical review. Appl Physiol Nutr Metab. 2014;39:1019–1028. doi: 10.1139/apnm-2014-0036. [DOI] [PubMed] [Google Scholar]
- 50.Shannon O, McGawley K, Nybäck L, Duckworth L, Barlow MJ, Woods D, et al. ‘Beet-ing’ the mountain: a review of the physiological and performance effects of dietary nitrate supplementation at simulated and terrestrial altitude. Sports Med. 2017;47:2155–2169. doi: 10.1007/s40279-017-0744-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, et al. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 52.Whitfield J, Ludzki A, Heigenhauser GJF, Senden JMG, Verdijk LB, van Loon LJC, et al. Beetroot juice supplementation reduces whole body oxygen consumption but does not improve indices of mitochondrial efficiency in human skeletal muscle. J Physiol. 2016;594:421–435. doi: 10.1113/JP270844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, et al. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J Appl Physiol. 2010;109:135–148. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 54.Hernandez A, Schiffer TA, Ivarsson N, Cheng AJ, Bruton JD, Lundberg JO, et al. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle. J Physiol. 2012;590:3575–3583. doi: 10.1113/jphysiol.2012.232777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haider G, Folland JP. Nitrate supplementation enhances the contractile properties of human skeletal muscle. Med Sci Sports Exerc. 2014;46:2234–2243. doi: 10.1249/MSS.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 56.Bailey SJ, Gandra PG, Jones AM, Hogan MC, Nogueira L. Incubation with sodium nitrite attenuates fatigue development in intact single mouse fibres at physiological PO2. J Physiol. 2019;597:5429–5443. doi: 10.1113/JP278494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, et al. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J Physiol. 2013;591:547–557. doi: 10.1113/jphysiol.2012.243121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferguson SK, Holdsworth CT, Wright JL, Fees AJ, Allen JD, Jones AM, et al. Microvascular oxygen pressures in muscles comprised of different fiber types: impact of dietary nitrate supplementation. Nitric Oxide. 2015;48:38–43. doi: 10.1016/j.niox.2014.09.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bentley RF, Walsh JJ, Drouin PJ, Velickovic A, Kitner SJ, Fenuta AM, et al. Dietary nitrate restores compensatory vasodilation and exercise capacity in response to a compromise in oxygen delivery in the noncompensator phenotype. J Appl Physiol. 2017;123:594–605. doi: 10.1152/japplphysiol.00953.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Craig JC, Broxterman RM, Smith JR, Allen JD, Barstow TJ. Effect of dietary nitrate supplementation on conduit artery blood flow, muscle oxygenation, and metabolic rate during handgrip exercise. J Appl Physiol (1985) 2018;125:254–262. doi: 10.1152/japplphysiol.00772.2017. [DOI] [PubMed] [Google Scholar]
- 61.Richards JC, Racine ML, Hearon CM, Kunkel M, Luckasen GJ, Larson DG, et al. Acute ingestion of dietary nitrate increases muscle blood flow via local vasodilation during handgrip exercise in young adults. Physiol Rep. 2018;6. 10.14814/phy2.13572. [DOI] [PMC free article] [PubMed]
- 62.de Vries CJ, DeLorey DS. Effect of acute dietary nitrate supplementation on sympathetic vasoconstriction at rest and during exercise. J Appl Physiol. 2019;127:81–88. doi: 10.1152/japplphysiol.01053.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hughes WE, Kruse NT, Ueda K, Feider AJ, Hanada S, Bock JM, et al. Dietary nitrate does not acutely enhance skeletal muscle blood flow and vasodilation in the lower limbs of older adults during single-limb exercise. Eur J Appl Physiol. 2020;120:1357–1369. doi: 10.1007/s00421-020-04368-8. [DOI] [PubMed] [Google Scholar]
- 64.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, et al. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol. 2013;115:325–336. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]
- 65.Flueck JL, Bogdanova A, Mettler S, Perret C. Is beetroot juice more effective than sodium nitrate?-the effects of equimolar nitrate dosages of nitrate-rich beetroot juice and sodium nitrate on oxygen consumption during exercise. Appl Physiol Nutr Metab. 2016;4:421–429. doi: 10.1139/apnm-2015-0458. [DOI] [PubMed] [Google Scholar]
- 66.Carlström M, Lundberg JO, Weitzberg E. Mechanisms underlying blood pressure reduction by dietary inorganic nitrate. Acta Physiol. 2018;224:e13080. doi: 10.1111/apha.13080. [DOI] [PubMed] [Google Scholar]
- 67.Blekkenhorst LC, Lewis JR, Prince RL, Devine A, Bondonno NP, Bondonno CP, et al. Nitrate-rich vegetables do not lower blood pressure in individuals with mildly elevated blood pressure: a 4-wk randomized controlled crossover trial. Am J Clin Nutr. 2018;107:894–908. doi: 10.1093/ajcn/nqy061. [DOI] [PubMed] [Google Scholar]
- 68.Sundqvist ML, Larsen FJ, Carlström M, Bottai M, Pernow J, Hellénius M-L, et al. A randomized clinical trial of the effects of leafy green vegetables and inorganic nitrate on blood pressure. Am J Clin Nutr. 2020;111:749–756. doi: 10.1093/ajcn/nqaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siervo M, Shannon O, Kandhari N, Prabhakar M, Fostier W, Köchl C, et al. Nitrate-rich beetroot juice reduces blood pressure in Tanzanian adults with elevated blood pressure: a double-blind randomized controlled feasibility trial. J Nutr. 2020;150:2460–2468. doi: 10.1093/jn/nxaa170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mills CE, Govoni V, Faconti L, Casagrande M-L, Morant SV, Crickmore H, et al. A randomised, factorial trial to reduce arterial stiffness independently of blood pressure: proof of concept? The VaSera trial testing dietary nitrate and spironolactone. Br J Clin Pharmacol. 2020;86:891–902. doi: 10.1111/bcp.14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320–327. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson C, Wylie LJ, Blackwell JR, Fulford J, Black MI, Kelly J, et al. Influence of dietary nitrate supplementation on physiological and muscle metabolic adaptations to sprint interval training. J Appl Physiol. 2017;122(3):642–52. [DOI] [PMC free article] [PubMed]
- 73.Rocha BS, Nunes C, Pereira C, Barbosa RM, Laranjinha J. A shortcut to wide-ranging biological actions of dietary polyphenols: modulation of the nitrate-nitrite-nitric oxide pathway in the gut. Food Funct. 2014;5:1646–1652. doi: 10.1039/C4FO00124A. [DOI] [PubMed] [Google Scholar]
- 74.Clifford T, Howatson G, West DJ, Stevenson EJ. The potential benefits of red beetroot supplementation in health and disease. Nutrients. 2015;7:2801–2822. doi: 10.3390/nu7042801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tesoriere L, Allegra M, Butera D, Livrea MA. Absorption, excretion, and distribution of dietary antioxidant betalains in LDLs: potential health effects of betalains in humans. Am J Clin Nutr. 2004;80:941–945. doi: 10.1093/ajcn/80.4.941. [DOI] [PubMed] [Google Scholar]
- 76.Martinez RM, Longhi-Balbinot DT, Zarpelon AC, Staurengo-Ferrari L, Baracat MM, Georgetti SR, et al. Anti-inflammatory activity of betalain-rich dye of Beta vulgaris: effect on edema, leukocyte recruitment, superoxide anion and cytokine production. Arch Pharm Res. 2015;38:494–504. doi: 10.1007/s12272-014-0473-7. [DOI] [PubMed] [Google Scholar]
- 77.Nemzer BV, Pietrzkowski Z, Hunter JM, Robinson JL, Fink B. A Betalain-rich dietary supplement, but not PETN, increases vasodilation and nitric oxide: a comparative, single-dose, randomized, placebo-controlled, blinded, crossover pilot study. J Food Res. 2020;10:p26. doi: 10.5539/jfr.v10n1p26. [DOI] [Google Scholar]
- 78.World Economic Forum . The global economic burden of non-communicable diseases. Harvard School of Public Health. 2011. [Google Scholar]
- 79.Prince M, Wimo A, Ali G, Wu Y, Prina M. World Alzheimer report 2015: the global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International; 2015. [Google Scholar]
- 80.Ormesher L, Myers JE, Chmiel C, Wareing M, Greenwood SL, Tropea T, et al. Effects of dietary nitrate supplementation, from beetroot juice, on blood pressure in hypertensive pregnant women: a randomised, double-blind, placebo-controlled feasibility trial. Nitric Oxide. 2018;80:37–44. doi: 10.1016/j.niox.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 81.Kandhari N, Prabhakar M, Shannon O, Fostier W, Koehl C, Rogathi J, et al. Feasibility and acceptability of a nutritional intervention testing the effects of nitrate-rich beetroot juice and folic acid on blood pressure in Tanzanian adults with elevated blood pressure. Int J Food Sci Nutr. 2020;72:195–207. doi: 10.1080/09637486.2020.1776226. [DOI] [PubMed] [Google Scholar]
- 82.Choo E, Dando R. The impact of pregnancy on taste function. Chem Senses. 2017;42:279–286. doi: 10.1093/chemse/bjx005. [DOI] [PubMed] [Google Scholar]
- 83.Babateen AM, Rubele S, Shannon O, Okello E, Smith E, McMahon N, et al. Protocol and recruitment results from a 13-week randomized controlled trial comparing the effects of different doses of nitrate-rich beetroot juice on cognition, cerebral blood flow and peripheral vascular function in overweight and obese older people. Contemp Clin Trials Commun. 2020;18:100571. doi: 10.1016/j.conctc.2020.100571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van der Wurff ISM, Meyer BJ, de Groot RHM. A review of recruitment, adherence and drop-out rates in omega-3 polyunsaturated fatty acid supplementation trials in children and adolescents. Nutrients. 2017;9(5):474. doi: 10.3390/nu9050474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kemmler G, Hummer M, Widschwendter C, Fleischhacker WW. Dropout rates in placebo-controlled and active-control clinical trials of antipsychotic drugs: a meta-analysis. Arch Gen Psychiatry. 2005;62(12):1305–1312. doi: 10.1001/archpsyc.62.12.1305. [DOI] [PubMed] [Google Scholar]
- 86.McDonagh STJ, Wylie LJ, Webster JMA, Vanhatalo A, Jones AM. Influence of dietary nitrate food forms on nitrate metabolism and blood pressure in healthy normotensive adults. Nitric Oxide. 2018;72:66–74. doi: 10.1016/j.niox.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 87.Katan MB. Nitrate in foods: harmful or healthy? Am J Clin Nutr. 2009;90:11–12. doi: 10.3945/ajcn.2009.28014. [DOI] [PubMed] [Google Scholar]
- 88.Shannon OM, Grisotto G, Babateen A, McGrattan A, Brandt K, Mathers JC, et al. Knowledge and beliefs about dietary inorganic nitrate among UK-based nutrition professionals: development and application of the KINDS online questionnaire. BMJ Open. 2019;9:e030719. doi: 10.1136/bmjopen-2019-030719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Siervo M, Lara J, Ogbonmwan I, Mathers JC. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: a systematic review and meta-analysis. J Nutr. 2013;143:818–826. doi: 10.3945/jn.112.170233. [DOI] [PubMed] [Google Scholar]
- 90.Faraoni D, Schaefer ST. Randomized controlled trials vs. observational studies: why not just live together? BMC Anesthesiol. 2016;16. 10.1186/s12871-016-0265-3. [DOI] [PMC free article] [PubMed]
- 91.Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996;312:1215–1218. doi: 10.1136/bmj.312.7040.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cowell OR, Mistry N, Deighton K, Matu J, Griffiths A, Minihane AM, et al. Effects of a Mediterranean diet on blood pressure: a systematic review and meta-analysis of randomized controlled trials and observational studies. J Hypertens. 2020;39:729–739. doi: 10.1097/HJH.0000000000002667. [DOI] [PubMed] [Google Scholar]
- 93.Babateen AM, Fornelli G, Donini LM, Mathers JC, Siervo M. Assessment of dietary nitrate intake in humans: a systematic review. Am J Clin Nutr. 2018;108:878–888. doi: 10.1093/ajcn/nqy108. [DOI] [PubMed] [Google Scholar]
- 94.McMahon N, Pavey T, Desbrow B, Leveritt M. Developing a nitrate, nitrite, and nitrosamine food and beverage composition database for use with a nitrate food frequency questionnaire: a systematic review. J Sci Med Sport. 2017;20:22. doi: 10.1016/j.jsams.2017.09.231. [DOI] [Google Scholar]
- 95.de González MTN, Osburn WN, Hardin MD, Longnecker M, Garg HK, Bryan NS, et al. A survey of nitrate and nitrite concentrations in conventional and organic-labeled raw vegetables at retail. J Food Sci. 2015;80:C942–C949. doi: 10.1111/1750-3841.12858. [DOI] [PubMed] [Google Scholar]
- 96.Blekkenhorst LC, Prince RL, Ward NC, Croft KD, Lewis JR, Devine A, et al. Development of a reference database for assessing dietary nitrate in vegetables. Mol Nutr Food Res. 2017;61. https://onlinelibrary.wiley.com/doi/abs/10.1002/mnfr.201600982. [DOI] [PubMed]
- 97.Bahadoran Z, Ghasemi A, Mirmiran P, Mehrabi Y, Azizi F, Hadaegh F. Estimation and validation of dietary nitrate and nitrite intake in iranian population. Iran J Public Health. 2019;48:162–170. [PMC free article] [PubMed] [Google Scholar]
- 98.Pereira LCR, Shannon OM, Mazidi M, Babateen AM, Ashor AW, Stephan BCM, et al. Relationship between urinary nitrate concentrations and cognitive function in older adults: findings from the NHANES survey. Int J Food Sci Nutr. 2021;4:1–11. doi: 10.1080/09637486.2020.1868411. [DOI] [PubMed] [Google Scholar]
- 99.Bahadoran Z, Mirmiran P, Ghasemi A, Carlström M, Azizi F, Hadaegh F. Vitamin C intake modify the impact of dietary nitrite on the incidence of type 2 diabetes: a 6-year follow-up in Tehran lipid and glucose study. Nitric Oxide. 2017;62:24–31. doi: 10.1016/j.niox.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 100.Kang JH, Willett WC, Rosner BA, Buys E, Wiggs JL, Pasquale LR. Association of dietary nitrate intake with primary open-angle glaucoma: a prospective analysis from the nurses’ health study and health professionals follow-up study. JAMA Ophthalmol. 2016;134:294–303. doi: 10.1001/jamaophthalmol.2015.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mirmiran P, Bahadoran Z, Golzarand M, Asghari G, Azizi F. Consumption of nitrate containing vegetables and the risk of chronic kidney disease: Tehran lipid and glucose study. Ren Fail. 2016;38:937–944. doi: 10.3109/0886022X.2016.1165118. [DOI] [PubMed] [Google Scholar]
- 102.Blekkenhorst LC, Bondonno CP, Lewis JR, Devine A, Woodman RJ, Croft KD, et al. Association of dietary nitrate with atherosclerotic vascular disease mortality: a prospective cohort study of older adult women. Am J Clin Nutr. 2017;106(1):207–216. doi: 10.3945/ajcn.116.146761. [DOI] [PubMed] [Google Scholar]
- 103.Bondonno CP, Blekkenhorst LC, Prince RL, Ivey KL, Lewis JR, Amanda D, et al. Association of vegetable nitrate intake with carotid atherosclerosis and ischemic cerebrovascular disease in older women. Stroke. 2017;48:1724–1729. doi: 10.1161/STROKEAHA.117.016844. [DOI] [PubMed] [Google Scholar]
- 104.Gumanova NG, Deev AD, Klimushina MV, Kots AY, Shalnova SA. Serum nitrate and nitrite are associated with the prevalence of various chronic diseases except cancer. Int Angiol. 2017;36:160–166. doi: 10.23736/S0392-9590.16.03674-9. [DOI] [PubMed] [Google Scholar]
- 105.Kuhnle GG, Luben R, Khaw K-T, Feelisch M. Sulfate, nitrate and blood pressure – an EPIC interaction between sulfur and nitrogen. Pharmacol Res. 2017;122:127–129. doi: 10.1016/j.phrs.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maas R, Xanthakis V, Göen T, Müller J, Schwedhelm E, Böger RH, et al. Plasma nitrate and incidence of cardiovascular disease and all-cause mortality in the community: the Framingham offspring study. J Am Heart Assoc. 2017;6:e006224. doi: 10.1161/JAHA.117.006224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smallwood MJ, Ble A, Melzer D, Winyard PG, Benjamin N, Shore AC, et al. Relationship between urinary nitrate excretion and blood pressure in the InChianti cohort. Am J Hypertens. 2017;30:707–712. doi: 10.1093/ajh/hpx035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu AH, Bondonno CP, Russell J, Flood VM, Lewis JR, Croft KD, et al. Relationship of dietary nitrate intake from vegetables with cardiovascular disease mortality: a prospective study in a cohort of older Australians. Eur J Nutr. 2019;58(7):2741–53. [DOI] [PubMed]
- 109.Mendy A. Association of urinary nitrate with lower prevalence of hypertension and stroke and with reduced risk of cardiovascular mortality. Circulation. 2018;137:2295–2297. doi: 10.1161/CIRCULATIONAHA.118.034168. [DOI] [PubMed] [Google Scholar]
- 110.Jackson JK, Patterson AJ, MacDonald-Wicks LK, Forder PM, Blekkenhorst LC, Bondonno CP, et al. Vegetable nitrate intakes are associated with reduced self-reported cardiovascular-related complications within a representative sample of middle-aged Australian women, prospectively followed up for 15 years. Nutrients. 2019;11. https://www.mdpi.com/2072-6643/11/2/240. [DOI] [PMC free article] [PubMed]
- 111.Jackson JK, Zong G, MacDonald-Wicks LK, Patterson AJ, Willett WC, Rimm EB, et al. Dietary nitrate consumption and risk of CHD in women from the nurses’ health study. Br J Nutr. 2019;121:831–838. doi: 10.1017/S0007114519000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sim M, Lewis JR, Blekkenhorst LC, Bondonno CP, Devine A, Zhu K, et al. Dietary nitrate intake is associated with muscle function in older women. J Cachexia Sarcopenia Muscle. 2019;10:601–610. doi: 10.1002/jcsm.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Riddell A, Kirkwood J, Smallwood M, Winyard P, Knight B, Romanczuk L, et al. Urinary nitrate concentration as a marker for kidney transplant rejection. BMC Nephrol. 2020;21:441. doi: 10.1186/s12882-020-02096-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wu Z, Tian T, Ma W, Gao W, Song N. Higher urinary nitrate was associated with lower prevalence of congestive heart failure: results from NHANES. BMC Cardiovasc Disord. 2020;20:498. doi: 10.1186/s12872-020-01790-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Amrita A, Mark G, Coleman Gary D, Norman H, George H, Kim-Shapiro Daniel B, et al. Dietary nitrate and the epidemiology of cardiovascular disease: Report from a National Heart, Lung, and Blood Institute Workshop. J Am Heart Assoc. 2016;5:e003402. doi: 10.1161/JAHA.116.003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Song P, Wu L, Guan W. Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: a meta-analysis. Nutrients. 2015;7:9872–9895. doi: 10.3390/nu7125505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xie L, Mo M, Jia H-X, Liang F, Yuan J, Zhu J. Association between dietary nitrate and nitrite intake and sitespecific cancer risk: evidence from observational studies. Oncotarget. 2016;7:56915–56932. doi: 10.18632/oncotarget.10917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bourdillon N, Fan J-L, Uva B, Müller H, Meyer P, Kayser B. Effect of oral nitrate supplementation on pulmonary hemodynamics during exercise and time trial performance in normoxia and hypoxia: a randomized controlled trial. Front Physiol. 2015;6. 10.3389/fphys.2015.00288. [DOI] [PMC free article] [PubMed]
- 119.James PE, Willis GR, Allen JD, Winyard PG, Jones AM. Nitrate pharmacokinetics: taking note of the difference. Nitric Oxide. 2015. 10.1016/j.niox.2015.04.006. [DOI] [PubMed]
- 120.Atkinson G, Batterham AM. True and false interindividual differences in the physiological response to an intervention. Exp Physiol. 2015;100:577–588. doi: 10.1113/EP085070. [DOI] [PubMed] [Google Scholar]
- 121.Liddle L, Burleigh MC, Monaghan C, Muggeridge DJ, Sculthorpe N, Pedlar CR, et al. Variability in nitrate-reducing oral bacteria and nitric oxide metabolites in biological fluids following dietary nitrate administration: an assessment of the critical difference. Nitric Oxide. 2019;83:1–10. doi: 10.1016/j.niox.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 122.Betts JA, Gonzalez JT. Personalised nutrition: what makes you so special? Nutr Bull. 2016;41:353–359. doi: 10.1111/nbu.12238. [DOI] [Google Scholar]
- 123.Deighton K, King JA, Stensel DJ, Jones B. Expanding the investigation of meaningful effects in physiology research. Future Sci OA. 2017;3(3):FSO218. doi: 10.4155/fsoa-2017-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ahmed KA, Kim K, Ricart K, Van Der Pol W, Qi X, Bamman MM, et al. Potential role for age as a modulator of oral nitrate reductase activity. Nitric Oxide. 2021;108:1–7. doi: 10.1016/j.niox.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stanaway L, Rutherfurd-Markwick K, Page R, Wong M, Jirangrat W, Teh KH, et al. Acute supplementation with nitrate-rich beetroot juice causes a greater increase in plasma nitrite and reduction in blood pressure of older compared to younger adults. Nutrients. 2019;11:1683. doi: 10.3390/nu11071683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ashworth A, Mitchell K, Blackwell JR, Vanhatalo A, Jones AM. High-nitrate vegetable diet increases plasma nitrate and nitrite concentrations and reduces blood pressure in healthy women. Public Health Nutr. 2015;18:2669–2678. doi: 10.1017/S1368980015000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hobbs DA, George TW, Lovegrove JA. Differential effect of beetroot bread on postprandial DBP according to Glu298Asp polymorphism in the eNOS gene: a pilot study. J Hum Hypertens. 2014;28:726–730. doi: 10.1038/jhh.2014.16. [DOI] [PubMed] [Google Scholar]
- 128.Bailey SJ, Blackwell JR, Wylie LJ, Holland T, Winyard PG, Jones AM, et al. Nitric Oxide. 2016;61:29–37. doi: 10.1016/j.niox.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 129.Alzahrani HS, Jackson KG, Hobbs DA, Lovegrove JA. The role of dietary nitrate and the oral microbiome on blood pressure and vascular tone. Nutr Res Rev. 2020;7:1–18. [DOI] [PubMed]
- 130.Dewhurst-Trigg R, Yeates T, Blackwell JR, Thompson C, Linoby A, Morgan PT, et al. Lowering of blood pressure after nitrate-rich vegetable consumption is abolished with the co-ingestion of thiocyanate-rich vegetables in healthy normotensive males. Nitric Oxide. 2018;74:39–46. doi: 10.1016/j.niox.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 131.Jones AM. Dietary nitrate supplementation and exercise performance. Sports Med. 2014;44:35–45. doi: 10.1007/s40279-014-0149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wilkerson DP, Hayward GM, Bailey SJ, Vanhatalo A, Blackwell JR, Jones AM. Influence of acute dietary nitrate supplementation on 50 mile time trial performance in well-trained cyclists. Eur J Appl Physiol. 2012;112:4127–4134. doi: 10.1007/s00421-012-2397-6. [DOI] [PubMed] [Google Scholar]
- 133.Peacock O, TjøNna AE, James P, WisløFf U, Welde B, BöHlke N, et al. Dietary nitrate does not enhance running performance in elite cross-country skiers. Med Sci Sports Exerc. 2012;44:2213–2219. doi: 10.1249/MSS.0b013e3182640f48. [DOI] [PubMed] [Google Scholar]
- 134.Nybäck L, Glännerud C, Larsson G, Weitzberg E, Shannon OM, McGawley K. Physiological and performance effects of nitrate supplementation during roller-skiing in normoxia and normobaric hypoxia. Nitric Oxide. 2017;70:1–8. doi: 10.1016/j.niox.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 135.Boorsma RK, Whitfield J, Spriet LL. Beetroot juice supplementation does not improve performance in elite 1500-m runners. Med Sci Sports Exerc. 2014;46:2326–2334. doi: 10.1249/MSS.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 136.Jonvik KL, Nyakayiru J, van Loon LJC, Verdijk LB. Can elite athletes benefit from dietary nitrate supplementation? J Appl Physiol. 2015;119:759–761. doi: 10.1152/japplphysiol.00232.2015. [DOI] [PubMed] [Google Scholar]
- 137.Gollnick PD, Matoba H. The muscle fiber composition of skeletal muscle as a predictor of athletic success. An overview. Am J Sports Med. 1984;12:212–217. doi: 10.1177/036354658401200309. [DOI] [PubMed] [Google Scholar]
- 138.Tesch PA, Karlsson J. Muscle fiber types and size in trained and untrained muscles of elite athletes. J Appl Physiol. 1985;59:1716–1720. doi: 10.1152/jappl.1985.59.6.1716. [DOI] [PubMed] [Google Scholar]
- 139.Hoon MW, Jones AM, Johnson NA, Blackwell JR, Broad EM, Lundy B, et al. The effect of variable doses of inorganic nitrate-rich beetroot juice on simulated 2,000 m rowing performance in trained athletes. Int j sports physiol perform. 2014;9:615–620. doi: 10.1123/ijspp.2013-0207. [DOI] [PubMed] [Google Scholar]
- 140.Peeling P, Cox GR, Bullock N, Burke LM. Beetroot juice improves on-water 500 m time-trial performance, and laboratory-based paddling economy in national and international-level kayak athletes. Int J Sport Nutr Exerc Metab. 2014;25:278–284. doi: 10.1123/ijsnem.2014-0110. [DOI] [PubMed] [Google Scholar]
- 141.Rokkedal-Lausch T, Franch J, Poulsen MK, Thomsen LP, Weitzberg E, Kamavuako EN, et al. Chronic high-dose beetroot juice supplementation improves time trial performance of well-trained cyclists in normoxia and hypoxia. Nitric Oxide. 2019;85:44–52. doi: 10.1016/j.niox.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 142.Wickham KA, Spriet LL. No longer beeting around the bush: a review of potential sex differences with dietary nitrate supplementation 1. Appl Physiol Nutr Metab. 2019;44:915–924. doi: 10.1139/apnm-2019-0063. [DOI] [PubMed] [Google Scholar]
- 143.Kapil V, Rathod KS, Khambata RS, Bahra M, Velmurugan S, Purba A, et al. Sex differences in the nitrate-nitrite-NO• pathway: role of oral nitrate-reducing bacteria. Free Radic Biol Med. 2018;126:113–121. doi: 10.1016/j.freeradbiomed.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 144.Coles LT, Clifton PM. Effect of beetroot juice on lowering blood pressure in free-living, disease-free adults: a randomized, placebo-controlled trial. Nutr J. 2012;11:106. doi: 10.1186/1475-2891-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Forte P, Kneale BJ, Milne E, Chowienczyk PJ, Johnston A, Benjamin N, et al. Evidence for a difference in nitric oxide biosynthesis between healthy women and men. Hypertension. 1998;32:730–734. doi: 10.1161/01.HYP.32.4.730. [DOI] [PubMed] [Google Scholar]
- 146.Azzam N, Zafrir B, Fares F, Smith Y, Salman N, Nevzorov R, et al. Endothelial nitric oxide synthase polymorphism and prognosis in systolic heart failure patients. Nitric Oxide. 2015;47:91–96. doi: 10.1016/j.niox.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 147.Lembo G, De Luca N, Battagli C, Iovino G, Aretini A, Musicco M, et al. A common variant of endothelial nitric oxide synthase (Glu298Asp) is an independent risk factor for carotid atherosclerosis. Stroke. 2001;32:735–740. doi: 10.1161/01.STR.32.3.735. [DOI] [PubMed] [Google Scholar]
- 148.Tian G-X, Zeng X-T, Wang X-B, Zhang L, Zhang W, Wei W-L. Association between the endothelial nitric oxide synthase gene Glu298Asp polymorphism and coronary heart disease: a meta-analysis of 39 case-control studies. Mol Med Rep. 2013;7:1310–1318. doi: 10.3892/mmr.2013.1301. [DOI] [PubMed] [Google Scholar]
- 149.Jiménez-Morales AI, Ruano J, Delgado-Lista J, Fernandez JM, Camargo A, López-Segura F, et al. NOS3 Glu298Asp polymorphism interacts with virgin olive oil phenols to determine the postprandial endothelial function in patients with the metabolic syndrome. J Clin Endocrinol Metab. 2011;96:E1694–E1702. doi: 10.1210/jc.2011-1056. [DOI] [PubMed] [Google Scholar]
- 150.Naseem KM. The role of nitric oxide in cardiovascular diseases. Mol Asp Med. 2005;26:33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 151.Siervo M, Scialò F, Shannon OM, Stephan BCM, Ashor AW. Does dietary nitrate say NO to cardiovascular ageing? Current evidence and implications for research. Proc Nutr Soc. 2018;77:112–123. doi: 10.1017/S0029665118000058. [DOI] [PubMed] [Google Scholar]
- 152.Woessner MN, Levinger I, Allen JD, McIlvenna LC, Neil C. The effect of dietary inorganic nitrate supplementation on cardiac function during submaximal exercise in men with heart failure with reduced ejection fraction (HFrEF): a pilot study. Nutrients. 2020;12:2132. doi: 10.3390/nu12072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zamani P, Rawat D, Shiva-Kumar P, Geraci S, Bhuva R, Konda P, et al. The effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation. 2015;131:371–380. doi: 10.1161/CIRCULATIONAHA.114.012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zamani P, Tan V, Haideliza S-C, Melissa B, Brandimarto Jeffrey A, Lien T, et al. Pharmacokinetics and pharmacodynamics of inorganic nitrate in heart failure with preserved ejection fraction. Cir Res. 2017;120:1151–1161. doi: 10.1161/CIRCRESAHA.116.309832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chirinos JA, Londono-Hoyos F, Zamani P, Beraun M, Haines P, Vasim I, et al. Effects of organic and inorganic nitrate on aortic and carotid haemodynamics in heart failure with preserved ejection fraction. Eur J Heart Fail. 2017;19:1507–1515. doi: 10.1002/ejhf.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hirai DM, Zelt JT, Jones JH, Castanhas LG, Bentley RF, Earle W, et al. Dietary nitrate supplementation and exercise tolerance in patients with heart failure with reduced ejection fraction. Am J Physiol Regul Integr Comp Physiol. 2016;312:R13–R22. doi: 10.1152/ajpregu.00263.2016. [DOI] [PubMed] [Google Scholar]
- 157.Shaltout HA, Eggebeen J, Marsh AP, Brubaker PH, Laurienti PJ, Burdette JH, et al. Effects of supervised exercise and dietary nitrate in older adults with controlled hypertension and/or heart failure with preserved ejection fraction. Nitric Oxide. 2017. 10.1016/j.niox.2017.05.005. [DOI] [PMC free article] [PubMed]
- 158.Tsuge K, Kataoka M, Seto Y. Cyanide and thiocyanate levels in blood and saliva of healthy adult volunteers. J Health Sci. 2000;46:343–350. doi: 10.1248/jhs.46.343. [DOI] [Google Scholar]
- 159.Edwards DA, Fletcher K, Rowlands EN. Antagonism between perchlorate, iodide, thiocyanate and nitrate for secretion in human saliva. Analogy with the iodide trap of the thyroid. Lancet. 1954;263:498–499. doi: 10.1016/S0140-6736(54)91196-4. [DOI] [PubMed] [Google Scholar]
- 160.Jonvik KL, Nyakayiru J, Van Dijk J-W, Wardenaar FC, Van Loon LJC, Verdijk LB. Habitual dietary nitrate intake in highly trained athletes. Int J Sport Nutr Exerc Metab. 2016;27:148–157. doi: 10.1123/ijsnem.2016-0239. [DOI] [PubMed] [Google Scholar]
- 161.Angelino D, Dosz EB, Sun J, Hoeflinger JL, Van Tassell ML, Chen P, et al. Myrosinase-dependent and –independent formation and control of isothiocyanate products of glucosinolate hydrolysis. Front Plant Sci. 2015;6:831. doi: 10.3389/fpls.2015.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ashby MT. Inorganic chemistry of defensive peroxidases in the human oral cavity. J Dent Res. 2008;87:900–914. doi: 10.1177/154405910808701003. [DOI] [PubMed] [Google Scholar]
- 163.Hughan KS, Wendell SG, Delmastro-Greenwood M, Helbling N, Corey C, Bellavia L, et al. Conjugated linoleic acid modulates clinical responses to oral nitrite and nitrate. Hypertension. 2017;7:634–644. doi: 10.1161/HYPERTENSIONAHA.117.09016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bailey SJ, Blackwell JR, Wylie LJ, Emery A, Taylor E, Winyard PG, et al. Influence of iodide ingestion on nitrate metabolism and blood pressure following short-term dietary nitrate supplementation in healthy normotensive adults. Nitric Oxide. 2017;63:13–20. doi: 10.1016/j.niox.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 165.Fiore E, Tonacchera M, Vitti P. Influence of iodization programmes on the epidemiology of nodular goitre. Best Pract Res Clin Endocrinol Metab. 2014;28:577–588. doi: 10.1016/j.beem.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 166.Rossman MJ, Gioscia-Ryan RA, Santos-Parker JR, Ziemba BP, Lubieniecki KL, Johnson LC, et al. Inorganic nitrite supplementation improves endothelial function with aging. Hypertension. 2021;77:1212–1222. doi: 10.1161/HYPERTENSIONAHA.120.16175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mirvish SS. Blocking the formation of N-nitroso compounds with ascorbic acid in vitro and in vivo. Ann N Y Acad Sci. 1975;258:175–180. doi: 10.1111/j.1749-6632.1975.tb29277.x. [DOI] [PubMed] [Google Scholar]
- 168.Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci. 2005;113:14–19. doi: 10.1111/j.1600-0722.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 169.Kilian M, Chapple ILC, Hannig M, Marsh PD, Meuric V, Pedersen AML, et al. The oral microbiome – an update for oral healthcare professionals. Br Dent J. 2016;221:657–666. doi: 10.1038/sj.bdj.2016.865. [DOI] [PubMed] [Google Scholar]
- 170.Hyde ER, Andrade F, Vaksman Z, Parthasarathy K, Jiang H, Parthasarathy DK, et al. Metagenomic analysis of nitrate-reducing bacteria in the oral cavity: implications for nitric oxide homeostasis. PLoS One. 2014;9:e88645. doi: 10.1371/journal.pone.0088645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Burleigh MC, Liddle L, Monaghan C, Muggeridge DJ, Sculthorpe N, Butcher JP, et al. Salivary nitrite production is elevated in individuals with a higher abundance of oral nitrate-reducing bacteria. Free Radic Biol Med. 2018;120:80–88. doi: 10.1016/j.freeradbiomed.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 172.Bescos R, Ashworth A, Cutler C, Brookes ZL, Belfield L, Rodiles A, et al. Effects of chlorhexidine mouthwash on the oral microbiome. Sci Rep. 2020;10:5254. doi: 10.1038/s41598-020-61912-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 174.Bondonno CP, Liu AH, Croft KD, Considine MJ, Puddey IB, Woodman RJ, et al. Antibacterial mouthwash blunts oral nitrate reduction and increases blood pressure in treated hypertensive men and women. Am J Hypertens. 2015;28:572–575. doi: 10.1093/ajh/hpu192. [DOI] [PubMed] [Google Scholar]
- 175.Woessner M, Smoliga JM, Tarzia B, Stabler T, Van Bruggen M, Allen JD. A stepwise reduction in plasma and salivary nitrite with increasing strengths of mouthwash following a dietary nitrate load. Nitric Oxide. 2016;54:1–7. doi: 10.1016/j.niox.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 176.McDonagh STJ, Wylie LJ, Winyard PG, Vanhatalo A, Jones AM. The effects of chronic nitrate supplementation and the use of strong and weak antibacterial agents on plasma nitrite concentration and exercise elood pressure. Int J Sports Med. 2015;36:1177–1185. doi: 10.1055/s-0035-1554700. [DOI] [PubMed] [Google Scholar]
- 177.Jansson L, Lavstedt S, Frithiof L. Relationship between oral health and mortality rate. J Clin Periodontol. 2002;29:1029–1034. doi: 10.1034/j.1600-051X.2002.291108.x. [DOI] [PubMed] [Google Scholar]
- 178.Hall MW, Singh N, Ng KF, Lam DK, Goldberg MB, Tenenbaum HC, et al. Inter-personal diversity and temporal dynamics of dental, tongue, and salivary microbiota in the healthy oral cavity. NPJ BiofilmsMicrobiomes. 2017;3:1–7. doi: 10.1038/s41522-016-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Affoo RH, Foley N, Garrick R, Siqueira WL, Martin RE. Meta-analysis of salivary flow rates in young and older adults. J Am Geriatr Soc. 2015;63:2142–2151. doi: 10.1111/jgs.13652. [DOI] [PubMed] [Google Scholar]
- 180.Percival RS, Challacombe SJ, Marsh PD. Age-related microbiological changes in the salivary and plaque microflora of healthy adults. J Med Microbiol. 1991;35:5–11. doi: 10.1099/00222615-35-1-5. [DOI] [PubMed] [Google Scholar]
- 181.Xu X, He J, Xue J, Wang Y, Li K, Zhang K, et al. Oral cavity contains distinct niches with dynamic microbial communities. Environ Microbiol. 2015;17:699–710. doi: 10.1111/1462-2920.12502. [DOI] [PubMed] [Google Scholar]
- 182.Vanhatalo A, Blackwell JR, L’Heureux JE, Williams DW, Smith A, van der Giezen M, et al. Nitrate-responsive oral microbiome modulates nitric oxide homeostasis and blood pressure in humans. Free Radic Biol Med. 2018;124:21–30. doi: 10.1016/j.freeradbiomed.2018.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kho H-S, Lee S-W, Chung S-C, Kim Y-K. Oral manifestations and salivary flow rate, pH, and buffer capacity in patients with end-stage renal disease undergoing hemodialysis. Oral Surg Oral Med Oral Path Oral Radiol Endod. 1999;88:316–319. doi: 10.1016/S1079-2104(99)70035-1. [DOI] [PubMed] [Google Scholar]
- 184.Schmidt RJ, Yokota S, Tracy TS, Sorkin MI, Baylis C. Nitric oxide production is low in end-stage renal disease patients on peritoneal dialysis. Am J Phys. 1999;276:F794–F797. doi: 10.1152/ajprenal.1999.276.5.F794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Burleigh M, Liddle L, Muggeridge DJ, Monaghan C, Sculthorpe N, Butcher J, et al. Dietary nitrate supplementation alters the oral microbiome but does not improve the vascular responses to an acute nitrate dose. Nitric Oxide. 2019;89:54–63. doi: 10.1016/j.niox.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 186.Belstrøm D, Constancias F, Liu Y, Yang L, Drautz-Moses DI, Schuster SC, et al. Metagenomic and metatranscriptomic analysis of saliva reveals disease-associated microbiota in patients with periodontitis and dental caries. NPJ Biofilms Microbiomes. 2017;3:23. doi: 10.1038/s41522-017-0031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jeong H, Arif B, Caetano-Anollés G, Kim KM, Nasir A. Horizontal gene transfer in human-associated microorganisms inferred by phylogenetic reconstruction and reconciliation. Sci Rep. 2019;9:5953. doi: 10.1038/s41598-019-42227-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Doel JJ, Hector MP, Amirtham CV, Al-Anzan LA, Benjamin N, Allaker RP. Protective effect of salivary nitrate and microbial nitrate reductase activity against caries. Eur J Oral Sci. 2004;112:424–428. doi: 10.1111/j.1600-0722.2004.00153.x. [DOI] [PubMed] [Google Scholar]
- 189.Burleigh MC, Sculthorpe N, Henriquez FL, Easton C. Nitrate-rich beetroot juice offsets salivary acidity following carbohydrate ingestion before and after endurance exercise in healthy male runners. PLoS One. 2020;15:e0243755. doi: 10.1371/journal.pone.0243755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Statement on possible public health risks for infants and young children from the presence of nitrates in leafy vegetables. EFSA Journal. 2010.
- 191.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2010. [PMC free article] [PubMed] [Google Scholar]
- 192.Comly HH. Cyanosis in infants caused by nitrates in well water. JAMA. 1945;129:112–116. doi: 10.1001/jama.1945.02860360014004. [DOI] [PubMed] [Google Scholar]
- 193.Fewtrell L. Drinking-water nitrate, methemoglobinemia, and global burden of disease: a discussion. Environ Health Perspect. 2004;112(14):1371–1374. doi: 10.1289/ehp.7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Liu AH, Bondonno CP, Russell J, Flood VM, Lewis JR, Croft KD, et al. Relationship of dietary nitrate intake from vegetables with cardiovascular disease mortality: a prospective study in a cohort of older Australians. Eur J Nutr. 2019;58:2741–2753. doi: 10.1007/s00394-018-1823-x. [DOI] [PubMed] [Google Scholar]
- 195.Shannon OM, Ashor AW, Scialo F, Saretzki G, Martin-Ruiz C, Lara J, et al. Mediterranean diet and the hallmarks of ageing. Eur J Clin Nutr. 2021. 10.1038/s41430-020-00841-x. [DOI] [PubMed]
- 196.Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014;17:2769–2782. doi: 10.1017/S1368980013003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ. 2010;341:c4229. doi: 10.1136/bmj.c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hirayama F, Lee AH, Binns CW, Zhao Y, Hiramatsu T, Tanikawa Y, et al. Do vegetables and fruits reduce the risk of chronic obstructive pulmonary disease? A case–control study in Japan. Prev Med. 2009;49:184–189. doi: 10.1016/j.ypmed.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 199.Shannon OM, Stephan BCM, Minihane A-M, Mathers JC, Siervo M. Nitric oxide boosting effects of the Mediterranean diet: a potential mechanism of action. J Gerontol A Biol Sci Med Sci. 2018;73:902–904. doi: 10.1093/gerona/gly087. [DOI] [PubMed] [Google Scholar]
- 200.Shannon OM, Mendes I, Köchl C, Mazidi M, Ashor AW, Rubele S, et al. Mediterranean diet increases endothelial function in adults: a systematic review and meta-analysis of randomized controlled trials. J Nutr. 2020;150(5):1151–1159. doi: 10.1093/jn/nxaa002. [DOI] [PubMed] [Google Scholar]
- 201.Shannon OM, Stephan BCM, Granic A, Lentjes M, Hayat S, Mulligan A, et al. Mediterranean diet adherence and cognitive function in older UK adults: the European prospective investigation into Cancer and nutrition-Norfolk (EPIC-Norfolk) study. Am J Clin Nutr. 2019;110:938–948. doi: 10.1093/ajcn/nqz114. [DOI] [PubMed] [Google Scholar]
- 202.Stevenson EJ, Shannon OM, Minihane AM, Adamson A, Burns A, Hill T, et al. NuBrain: UK consortium for optimal nutrition for healthy brain ageing. Nutr Bull. 2020;45:223–229. doi: 10.1111/nbu.12429. [DOI] [Google Scholar]
- 203.Carlström M, Liu M, Yang T, Zollbrecht C, Huang L, Peleli M, et al. Cross-talk between nitrate-nitrite-NO and NO synthase pathways in control of vascular NO homeostasis. Antioxid Redox Signal. 2015;23:295–306. doi: 10.1089/ars.2013.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hezel MP, Liu M, Schiffer TA, Larsen FJ, Checa A, Wheelock CE, et al. Effects of long-term dietary nitrate supplementation in mice. Redox Biol. 2015;5:234–242. doi: 10.1016/j.redox.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lundberg JO, Carlström M, Weitzberg E. Metabolic effects of dietary nitrate in health and disease. Cell Metab. 2018;28:9–22. doi: 10.1016/j.cmet.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 206.Wootton-Beard PC, Ryan L. A beetroot juice shot is a significant and convenient source of bioaccessible antioxidants. J Funct Foods. 2011;3:329–334. doi: 10.1016/j.jff.2011.05.007. [DOI] [Google Scholar]
- 207.EFSA (European Food Safety Authority) Annual report of the emerging risks exchange network European Food Safety Authority. EFSA Supporting publication. 2016. [Google Scholar]
- 208.Mitchell T, Kumar P, Reddy T, Wood KD, Knight J, Assimos DG, et al. Dietary oxalate and kidney stone formation. Am J Physiol Renal Physiol. 2019;316:F409–F413. doi: 10.1152/ajprenal.00373.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hanson CF, Frankos VH, Thompson WO. Bioavailability of oxalic acid from spinach, sugar beet fibre and a solution of sodium oxalate consumed by female volunteers. Food Chem Toxicol. 1989;27:181–184. doi: 10.1016/0278-6915(89)90067-7. [DOI] [PubMed] [Google Scholar]
- 210.Noonan SC. Oxalate content of foods and its effect on humans. Asia Pac J Clin Nutr. 1999;8:64–74. doi: 10.1046/j.1440-6047.1999.00038.x. [DOI] [PubMed] [Google Scholar]
- 211.Siener R, Seidler A, Voss S, Hesse A. The oxalate content of fruit and vegetable juices, nectars and drinks. J Food Comp Anal. 2016;45:108–112. doi: 10.1016/j.jfca.2015.10.004. [DOI] [Google Scholar]
- 212.Taylor EN, Curhan GC. Oxalate intake and the risk for nephrolithiasis. J Am Soc Nephrol. 2007;18:2198–2204. doi: 10.1681/ASN.2007020219. [DOI] [PubMed] [Google Scholar]
- 213.Maddahi N, Aghamir SMK, Moddaresi SS, Mirzaei K, Alizadeh S, Yekaninejad MS. The association of dietary approaches to stop hypertension-style diet with urinary risk factors of kidney stones formation in men with nephrolithiasis. Clin Nutr ESPEN. 2020;39:173–179. doi: 10.1016/j.clnesp.2020.06.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.