Abstract
Background
Kaposiform lymphangiomatosis (KLA), which is a new subtype of generalized lymphatic anomaly, is a rare disease with a poor prognosis. Currently, there is no standard treatment due to the poor understanding of KLA. Sirolimus, which is an inhibitor of mammalian target of rapamycin, has been shown to have promising potential in the treatment of complicated vascular anomalies. The aim of this study was to introduce the use of sirolimus for the treatment of KLA and to highlight the challenges of managing this refractory disease.
Results
We reported seven patients with KLA who received sirolimus therapy in our center. Combined with previously reported cases, 58.3% achieved a partial response, 25.0% had stable disease, and 16.7% experienced disease progression. No severe sirolimus-related adverse events occurred during treatment.
Conclusions
This study suggests that sirolimus is currently an option for the treatment of KLA, and it is hoped that more specific therapies will be developed in the future. Rapid advances in basic science and clinical practice may facilitate the development of important new treatments for KLA.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13023-021-01893-3.
Keywords: Vascular anomaly, Lymphatic malformation, Kaposiform lymphangiomatosis, Sirolimus, Mammalian target of rapamycin
Background
Kaposiform lymphangiomatosis (KLA) is a rare, infiltrative, multifocal or defused lymphatic anomaly that is classified as a new subtype of generalized lymphatic anomaly (GLA) [1]. It usually presents in children with hemorrhagic effusions and respiratory symptoms. Generally, the characteristic dispersed spindled lymphatic endothelial cells are arranged in sheets or clusters with malformed lymphatic vessels that can be differentiated from GLA and kaposiform hemangioendothelioma [2]. However, compared with GLA, KLA has an unfavorable prognosis, and the overall survival rate is approximately 34% [1, 3].
Because the mechanism of KLA remains largely unknown, the management of patients with KLA is clinically challenging. To date, no standard approach has been developed for the treatment of KLA. Surgical therapies, including resection, drainage, pleurodesis and ligation of the thoracic duct, are palliative [1]. Pharmacotherapies for complicated vascular anomalies have been investigated previously, including interferon-alpha, corticosteroids, and vincristine, but the responses to treatment have been varied and unpredictable [1, 4]. Meanwhile, the adverse neurological effects of interferon and vincristine and the effects of long-term corticosteroid use on growth have limited their use in clinical practice [5, 6]. Recent studies have identified sirolimus as a potential treatment for complicated vascular anomalies with good tolerability [7–9]. Some investigators believe that the combination of sirolimus with steroids and/or vincristine is effective at treating KLA [4]. However, most of the data were derived from case reports [9]. Herein, we report a series of patients with KLA in our center who were treated with sirolimus and review reported KLA patients treated with sirolimus from the literature [7–13], with the aim of highlighting the role of sirolimus in KLA and the challenges of managing this refractory disease.
Methods
We performed a retrospective analysis of patients with KLA who presented to our hospital. The institutional review board of West China Hospital of Sichuan University approved this study. All procedures followed the relevant guidelines and regulations. The parents of all patients provided written informed consent. The diagnosis of KLA was established in our hospital on the basis of clinical presentation, imaging data, and histopathology. Some patients were included in our previous study [2]. Patients with KLA who were not treated with sirolimus or lacked sufficient data were excluded. Data collected from medical records included clinical characteristics, treatments and outcomes.
Moreover, considering the rarity of KLA and the small number of patients in our center, we conducted a literature review of the reported KLA cases treated with sirolimus on PubMed and Medline to better understand the role of sirolimus in the treatment of KLA.
Referring to the published literature [7] and taking into account the characteristics of KLA, we defined the treatment response as follows. Progressive disease (PD) was defined as a ≥ 20% increase in target lesions; partial response (PR) was defined as a ≥ 20% and < 100% reduction in the target lesions; complete response (CR) was defined as a 100% reduction in the target lesions; and stable disease (SD) was defined as a < 20% increase and < 20% decrease in the volumes of the target lesions. All adverse events were evaluated using CTCAE V4.0.
Results
Presentation of the cases
Patient 1
A 6-year-old boy was admitted to our hospital because of a right testis mass, abdominal distention and intermittent pain. He had a 4-year history of perineal ecchymosis accompanied by recurrent thrombocytopenia and coagulation disorders. Before admission to our hospital, the patient had been treated with corticosteroids, immunoglobulin and platelet transfusion. Sequential examinations demonstrated persistent consumptive coagulopathy and thrombocytopenia. Five months prior to referral to our hospital, he developed abdominal distention, intermittent pain and jaundice. Laboratory tests revealed that the platelet count was 10,000/μL, and fibrinogen was 0.49 g/L. Magnetic resonance imaging (MRI) revealed extensive heterogeneous enhanced lesions involving the mediastinum, abdominal cavity, pelvic cavity and bilateral inguinal regions and testes (Fig. 1). The diagnosis of KLA was confirmed by biopsy (Additional file 1: Figure S1). A combination treatment with prednisone (2 mg/kg/d), sirolimus (initial dose of 0.8 mg/m2 twice daily) and ademetionine (1000 mg/d) was commenced. However, the patient suffered from progressive tachypnea and abdominal distention even with active treatment. The boy died of multiple system organ failure after 2 months.
Fig. 1.
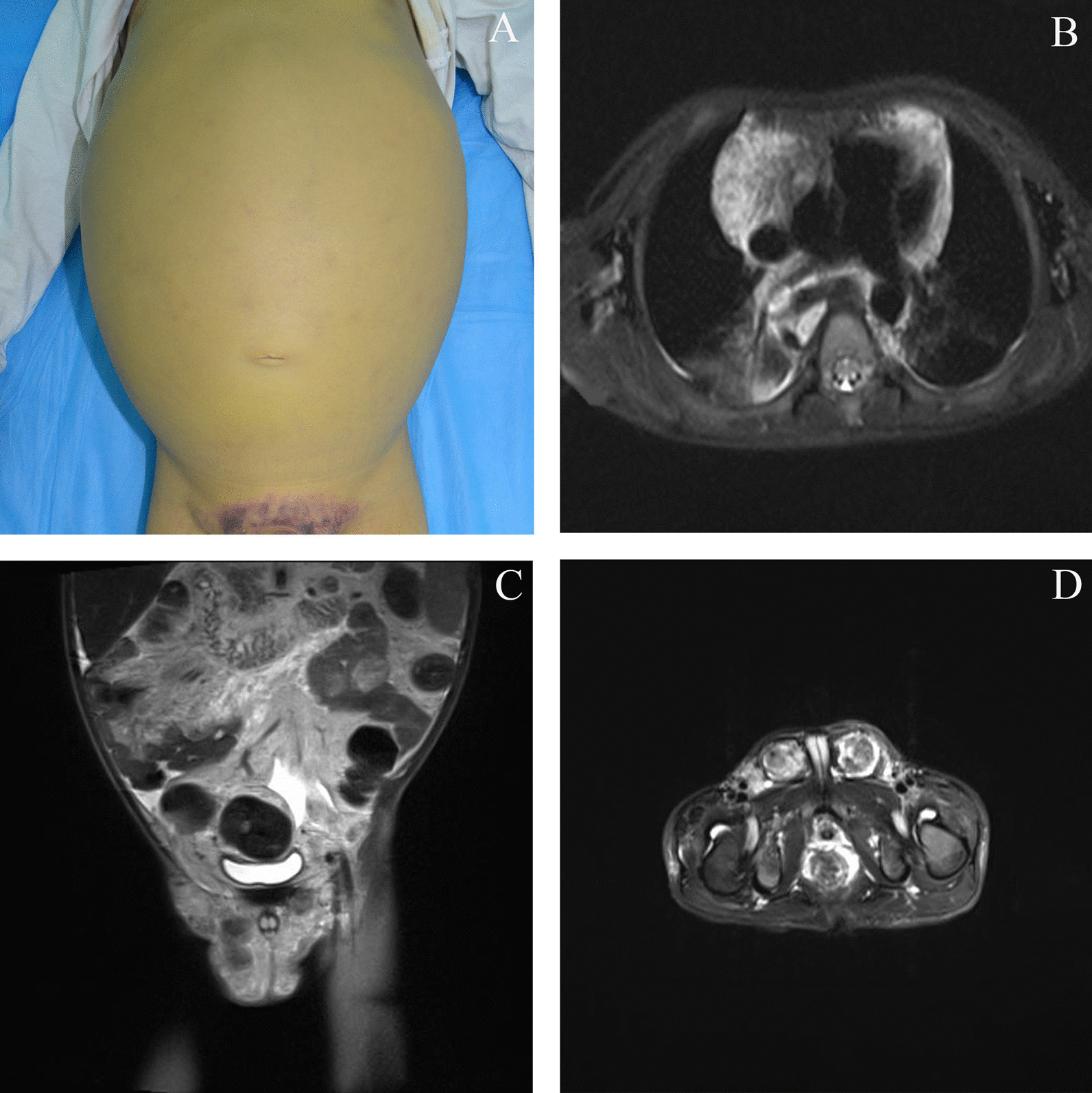
Patient 1 showed a grossly distended abdomen and perineal ecchymosis (A). Axial T2 fat-saturated image showed extensive mediastinal involvement (B). Coronal T2 fat-saturated image showed extensive abdominal cavity and pelvic cavity involvement (C). Axial T2 fat-saturated image showed bilateral inguinal regions, including testis involvement (D)
Patient 2
A 4-month-old girl presented to our hospital because of dyspnea and shortness of breath for 1 month. Image examination indicated severe pleural effusion and heterogeneous enhanced lesions involving the left mediastinum and left lung. The platelet count was 23,000/μL, and fibrinogen was 0.41 g/L. Considering the severe respiratory symptoms of the patient, thoracic drainage was performed. The drained fluid was hemorrhagic, but her pleural effusion was difficult to control. The girl was diagnosed with KLA. Then, sirolimus therapy (initial dose of 0.8 mg/m2 twice daily) combined with prednisone (2 mg/kg/day) was initiated. During the treatment, the plasma concentration of sirolimus was maintained within the range of 5–15 ng/ml. The hematological parameters returned to normal 2 months later, and prednisone was tapered. After 3 months of sirolimus treatment, her respiratory symptoms disappeared. Repeated MRI showed that the thoracic lesions shrunk and pleural effusion decreased significantly after 5 months of sirolimus therapy (Fig. 2). Two self-healing oral mucositis lesions occurred during sirolimus treatment. The patient is currently being treated with sirolimus monotherapy.
Fig. 2.
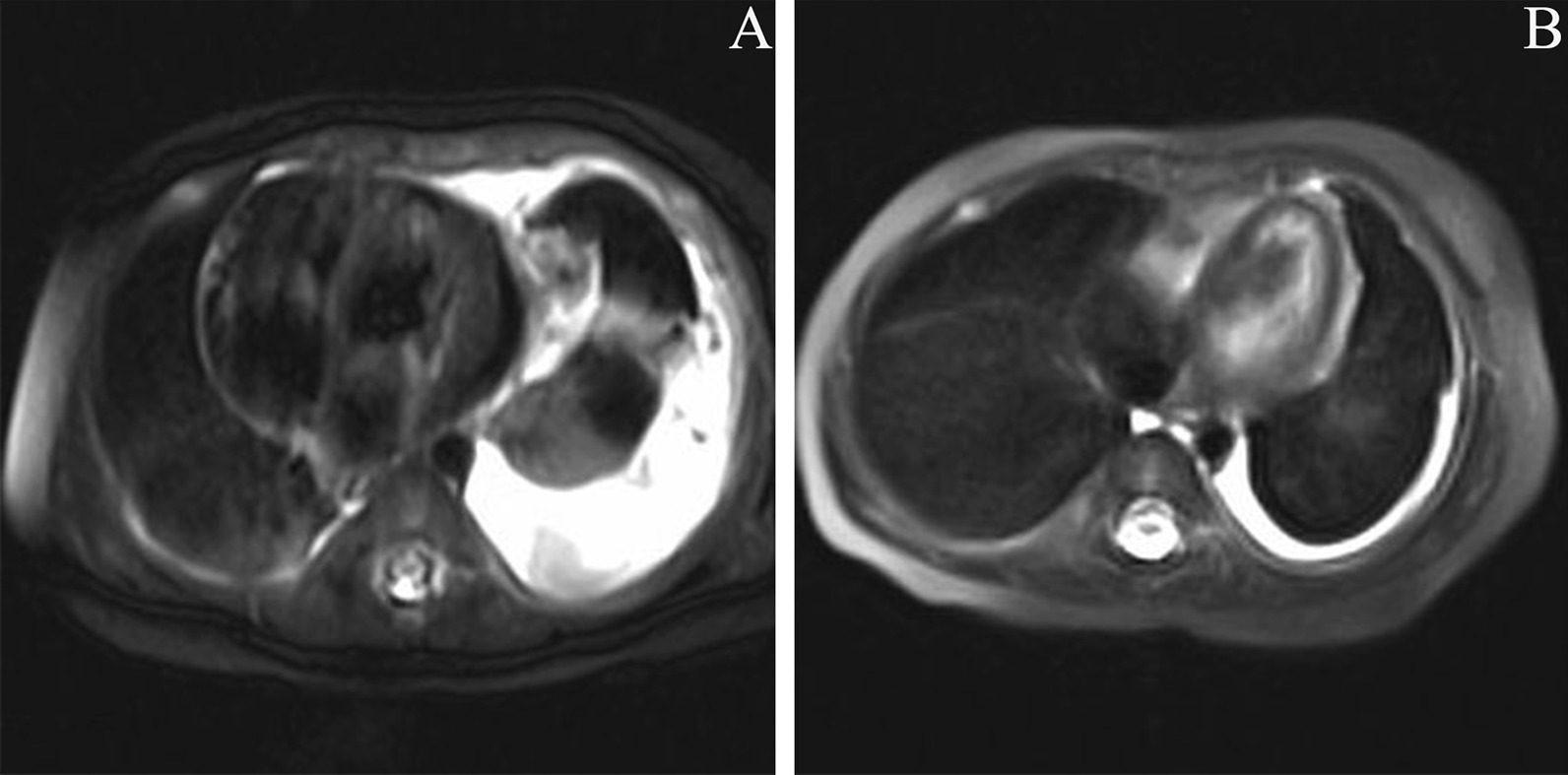
Patient 2 presented to our hospital because of dyspnea, shortness of breath, thrombocytopenia, and coagulopathy. T2-weighted MRI of the chest showed severe pleural effusion and thoracic lesions (A). After 5 months of treatment, thoracic MRI indicated a significant decrease in pleural effusion and a reduction in lesion size (B)
Patient 3
A 5-year-old boy was admitted to our hospital with dyspnea. Image examination suggested massive pleural effusion, and heterogeneous enhanced lesions involving both lungs, mediastinum, and multiple osteolytic lesions were found in vertebral bodies and ribs. The platelet count was 46,000/μL, and fibrinogen was 0.33 g/L. Thoracic drainage and fibrinogen transfusion were conducted to control the symptoms. The diagnosis of KLA was considered clinically and confirmed by biopsy. Then, sirolimus (initial dose of 0.8 mg/m2 twice daily) combined with corticosteroids (2 mg/kg/d) was administered. After 5 months of combined treatment, coagulation function improved, respiratory symptoms disappeared, and corticosteroids tapered. A partial response was achieved with 12 months of sirolimus treatment. He presented with slightly increased liver enzyme levels, which returned to normal after adjustment for the sirolimus dose. The patient is still being treated with sirolimus.
Patient 4
A 6-month-old boy was admitted to the hospital for abdominal distention and vomiting. MRI showed the extent of his lesions, including the lungs, mediastinum, spleen, liver, pancreas and mesentery. Laboratory tests revealed abnormal coagulation. A biopsy was performed and KLA confirmed. Aspirin (5 mg/kg/d) and vincristine (0.03 mg/kg once a week) plus corticosteroids (2 mg/kg/d) were administered successively, but the disease continued to progress. Considering the promising potential in treating complicated vascular anomalies, sirolimus (initial dose of 0.8 mg/m2 twice daily) was administered, and the lesions were stable, but the coagulation disorders did not improve until the use of corticosteroids (2 mg/kg/d). Six month later, corticosteroids tapered. No sirolimus-related adverse events occurred during treatment. To date, he has been treated with sirolimus for 41 months and has stable disease.
Patient 5
A 14-month-old boy was admitted to the hospital with complaints of cough and unexplained severe coagulopathy. Further examination found pleural and pericardial effusion and extensive lesions of the lungs and spleen. Pericardiocentesis and platelet transfusion were performed to control symptoms. Coagulation function improved, but respiratory function gradually deteriorated after splenectomy. At the same time, the diagnosis of KLA was established. Sirolimus (initial dose of 0.8 mg/m2 twice daily) monotherapy was administered, and the lesions of the lungs shrunk with symptoms ameliorated after 6 months of treatment, suggesting that sirolimus was effective in this case. He experienced no sirolimus-related adverse events during the treatment period. He is still being treated with sirolimus.
Patient 6
This 4-year-old boy presented with cough and was found to have left lung and mediastinal lesions with coagulopathy. After multidisciplinary discussion, the diagnosis of KLA was confirmed. He was treated with vincristine, but he had no response. He was subsequently treated with sirolimus (initial dose of 0.8 mg/m2 twice daily) plus short-term (4 months) prednisone (2 mg/kg/d) and had disease stabilization during 4 years of follow-up. He experienced upper respiratory tract infection and self-healing oral mucositis. Currently, he is still undergoing sirolimus monotherapy.
Patient 7
A 2-year-old boy presented with dyspnea and cough without fever, and laboratory examinations demonstrated unexplained coagulation disorders and thrombocytopenia. Initial imaging examination revealed massive pericardial effusion. Further MRI found extensive heterogeneous enhanced lesions involving the mediastinum, lungs, neck and spleen (Fig. 3). The diagnosis was changed from GLA to KLA by pathological examination. Treatment with sirolimus (initial dose of 0.8 mg/m2 twice daily), propranolol (2 mg/kg/d) and prednisone (2 mg/kg/d) was initiated and followed up regularly. No significant changes in the disease were seen during the first 6 months of treatment. At the seventh month of follow-up, coagulation function improved gradually, and platelets returned to normal. Then, propranolol and prednisone tapered. Currently, he is still being treated with sirolimus monotherapy, and his coagulation function is normal, while his foci lesions are stable.
Fig. 3.
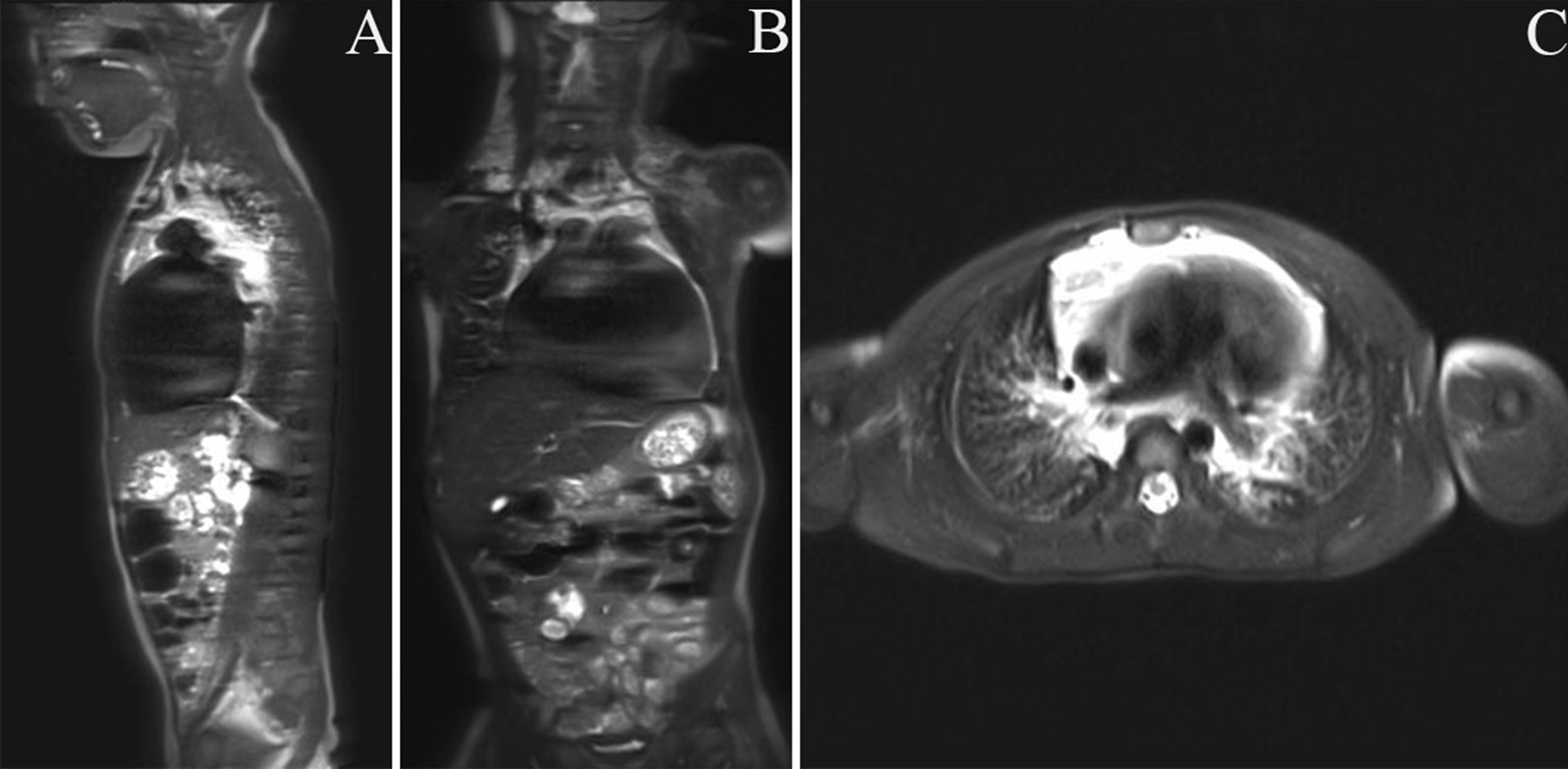
Patient 7 presented with dyspnea and cough. Imaging tests showed extensive lesions involving the mediastinum, lungs, neck and spleen
The summary of all KLA patients is shown in Table 1.
Table 1.
Summary of KLA patients
| N | Age at start /sex | Location of target lesions | Major signs, symptoms, and/or complications | Previous treatments | Duration of sirolimus treatment (months) | Range (mean) of trough concentrations (ng/mL) | Response to sirolimus |
|---|---|---|---|---|---|---|---|
| 1a | 2 years/M | Mediastinum, abdominal cavity, pelvic cavity, bilateral inguinal regions and testes | Abdominal distention, pain and coagulation disorder | Corticosteroid, immune globulin and platelet transfusion | 6 | 7.8–12.7 (10.4) | PD |
| 2a | 4 months/F | Left mediastinum and left lung | Dyspnea pleural effusion and coagulation disorder | Thoracic drainage | 18 | 6.1–15.7 (10.2) | PR |
| 3a | 5 years/M | Lungs, vertebrae, ribs and mediastinum | Pleural effusion and coagulation disorder | Thoracic drainage, fibrinogen transfusion, exploratory thoracotomy | 42 | 10.7–14.9 (13.3) | PR |
| 4a | 6 months/M | Lungs, mediastinum, spleen, liver, pancreas and mesentery | Abdominal distention, vomiting and coagulation disorder | Vincristine plus corticosteroid, aspirin | 41 | 5.1–13.7 (11.6) | SD |
| 5a | 14 months/M | Lungs and spleen | Cough, respiratory distress, pleural effusion, pericardial effusion and coagulation disorder | Pericardiocentesis, corticosteroid, splenectomy and platelet transfusion | 14 | 5.9–14.3 (12.1) | PR |
| 6a | 4 years/M | Left lung and mediastinum | Cough and coagulation disorder | Sirolimus, sirolimus plus steroids, sirolimus and vincristine | 21 | 9.4–15.8 (10.6) | SD |
| 7a | 2 years/M | Mediastinum, lungs, neck and spleen | Dyspnea, cough and coagulation disorder | Propranolol and prednisone | 21 | 10.0–14.7 (13.2) | SD |
| 8b | 8 years/M | Bone, thoracic and mediastinal | Chylothorax and coagulation disorder | Interferon and propranolol | 30 (cessation) | 4.4–9.0 (7.5) | PR |
| 9b | 8 years/M | Bone, thoracic and mediastinal | Scoliosis, chylothorax and coagulation disorder | Steroids and propranolol | 24 | 8.1–12.4 (11.2) | SD |
| 10b | 20 years/M | Bone, thoracic and right chest wall | Gastrointestinal hemorrhage and coagulation disorder | Steroids | 12 | 4.7–6.0 (5.5) | PR |
| 11b | –/M | Thorax | NG | NG | NG | NG | PD |
| 12b | 7 years/M | Left scapular, left arm and thoracic vertebral bodies | Dyspnea, shortness of breath, pleural effusion and coagulation disorder | Chinese traditional medicine, pleurocentesis and albumin | 8 | 9.3–13.4 (12.3) | PR |
| 13b | NG | NG | NG | NG | 12 | 10.0–15.0 (NG) | PR |
| 14b | NG | NG | NG | NG | 12 | 10.0–15.0 (NG) | PR |
| 15b | NG | NG | NG | NG | 12 | 10.0–15.0 (NG) | PR |
| 16b | NG | NG | NG | NG | 12 | 10.0–15.0 (NG) | PR |
| 17b | NG | NG | NG | NG | 12 | 10.0–15.0 (NG) | PR |
| 18b | NG | NG | NG | NG | 12 | 10.0–15.0 (NG) | PR |
| 19b | NG | NG | NG | NG | 12 | 10.0–15.0 (NG) | PD |
| 20b | 7 years/M | Abdominal cavity, lower neck, mediastinum, bone and left upper arm | Cephalohematoma and coagulation disorder | Steroids, vincristine and zoledronate | 12 | 9.4–14.8 (NG) | PR |
| 21b | 13 years/F | Bone, mediastinum, spleen | Cough, fever, fatigue, intermittent wheezing, pleural effusion and coagulation disorder | Thoracostomy | 3 | NG | PR |
| 22b | 20 months/M | Mediastinum and spleen | Cough, pleural effusion, pericardial effusion, epidural hematoma and coagulation disorder | Diaphragmatic fenestrations | 7 | NG | PD |
| 23b | 11 years/F | Mediastinum, bone and spleen | Cough, chest pain, fatigue, listlessness, weight loss, coagulation disorder and pericardial effusion, coagulation disorder | Pericardiocentesis | 31 | NG | SD |
| 24b | 4 years/M | Bone, spleen and mediastinum | Fatigue, decreased appetite, weight loss, cough, spontaneous bruising, back pain, coagulation disorder and pleural effusion | Cryoprecipitate, platelet infusions and prednisolone | NG | NG | SD |
M male, F female, NG not given, PD progressive disease, PR partial response, SD stable disease
aData from our center
bData from literature
Discussion
Recently, KLA has been proposed as a new entity separate from GLA. However, although KLA has characteristics of malformations in histologic appearance, it has a more invasive nature [1, 4, 14]. The distinguishing features of KLA are intrathoracic lesions and hemorrhagic effusions [1]. KLA is multifocal or diffuse and usually involves the thoracic cavity, bone, retroperitoneum, abdominal viscera and soft tissues, which overlap with GLA [1, 15]. Because of its rarity, knowledge of KLA is extremely limited. In addition, the pathogenesis of KLA is not completely understood. Most recently, several studies detected the NRAS p. Q61R mutations in most patients with KLA [16, 17]. There is evidence suggesting that NRAS mutations are associated with cell proliferation through the MAPK and PI3K/AKT signaling pathways [18]. Similar to other vascular anomalies caused by mutations, NRAS somatic mutations may contribute to the occurrence of KLA.
Until now, no standard treatment regimen has been established due to the lack of firm evidence. Patients with KLA have poor outcomes despite aggressive management; the 5-year survival rate is 51%, and the overall survival rate is 34% [1]. Surgery is often impracticable due to its diffuse infiltrative nature, location and severe coagulation disorder [1]. Temporary improvement can be obtained via symptomatic treatments, but the lesions may rebound quickly [1]. Therefore, for most patients with KLA, pharmacologic interventions are an alternative treatment. Interferon-ɑ, corticosteroids, vincristine and sirolimus have been used to treat KLA in the past several years [1, 4]. Although there are various therapeutic approaches to KLA, the response is variable and suboptimal.
Sirolimus, which is an inhibitor of mammalian target of rapamycin (mTOR), plays a significant role in cell proliferation and migration through PI3K/AKT pathways. Recent studies have proven that sirolimus has promising potential in the treatment of complicated vascular anomalies [1, 7–9]. An in vitro experiment suggested that the PI3K/AKT and MAPK pathways are candidate therapeutic targets for KLA and that sirolimus could effectively inhibit KLA-derived cell proliferation [18]. Some experts recommend sirolimus combined with steroids and/or vincristine to treat KLA, but the relevant data are scarce.
In the present study, 3 of 7 patients partially responded to sirolimus. In these 4 patients, 3 patients obtained stable disease, and only 1 patient had progressive disease. Of the 24 reported patients with KLA treated with sirolimus [7–13], including 7 patients in our center, 14 (58.3%) achieved partial response, 6 (25.0%) showed stable disease, and only 4 (16.7%) experienced disease progression. Given the rarity of some diseases, such as KLA, repurposing existing drugs intended for the treatment of other conditions can be an option. Consistent with other studies, sirolimus was often administered after conventional treatments were ineffective. Notably, most KLA patients accepted combined therapy initially to control disease rather than sirolimus monotherapy. At the same time, the adverse events related to sirolimus during treatment in children suggest that sirolimus is well tolerated. For KLA patients, multidisciplinary collaborative supportive treatments are essential. Severe coagulopathy, pleural effusion and pericardial effusion require aggressive maneuvers to resolve them. Similar to kaposiform hemangioendothelioma, short-term combined therapy contributes to controlling the disease [19]. Later sirolimus monotherapy is sufficient. Considering the highly refractory nature of KLA, sirolimus is an optional medication at present.
However, no complete response was obtained in these patients, and only half of KLA patients achieved a partial response. Therefore, more attention needs to be given to this refractory disease. There is evidence suggesting that MEK inhibitors may be effective in patients with KLA after NRAS mutations are detected, but there are currently no clinical trials. Furthermore, trametinib has been reported to be effective in the treatment of KLA patients without NRAS mutations [20].
Early diagnosis is difficult in patients with KLA due to unspecific symptoms and lack of specific diagnostic indicators. Potential biomarkers such as angiogenic cytokines may be helpful in early diagnosis [21]. Therefore, patients with KLA who do not receive timely treatment may suffer poor outcomes. Furthermore, we believe that molecular diversity may contribute to the different effects of sirolimus and other targeted medicines, which was a limitation of this study. More studies should be conducted in the future to determine the progress of the abnormal development of lymphatic vessels and resolve current problems.
Conclusions
In conclusion, initial short-term combined therapy and later long-term sirolimus monotherapy are currently optional strategies for the treatment of KLA, although their role is challenging in some patients. In the future, more studies are needed to better understand the pathophysiology of KLA, with the aim of supporting the development of more specific therapeutic approaches. It is likely that rapid advances in basic science and clinical practice will facilitate the development of important new approaches for the treatment of KLA.
Supplementary Information
Additional file 1. Figure S1. The specimen of patient 1 was resected as a right testicular, which shows spindle lymphatic endothelial cells in clusters associated with malformed lymphatic channels (H&E staining, magnification × 200) (A). Immunostaining of anti-CD31 showed that the spindle cells were positive for CD31 (magnification × 100) (B). Immunostaining of anti-CD34 showed that the spindle cells were positive for CD34 (magnification × 100) (C). Immunostaining of D2-40 revealed proliferating lymphatic vessels (magnification × 100) (D).
Acknowledgements
We thank the patients and their parents for their cooperation and support and for providing consent for publication. We also thank the study and hospital nurses for their assistance with the management of the patients.
Abbreviations
- KLA
Kaposiform lymphangiomatosis
- mTOR
Mammalian target of rapamycin
- GLA
Generalized lymphatic anomaly
- MRI
Magnetic resonance imaging
- PD
Progressive disease
- PR
Partial response
- CR
Complete response
- SD
Stable disease
Authors' contributions
JYZ, KYY, SYC and YJ were involved in the clinical management of these patients and collected clinical details of this study. JYZ and KYY reviewed the literature and drafted the manuscript. YJ and SYC reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Numbers 81400862 and 81401606), the Key Project in the Science and Technology Program of Sichuan Province (Grant Number 2019YFS0322), the Science Foundation for The Excellent Youth Scholars of Sichuan University (Grant Number 2015SCU04A15), and the 1·3·5 Project for Disciplines of Excellence-Clinical Research Incubation Project, West China Hospital of Sichuan University (Grant Numbers 2019HXFH056 and 2020HXFH048).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethical approval and consent to participate
The study was approved by the Ethics Committee of the West China Hospital of Sichuan University. Written informed consent was obtained from the patients’ parents, according to the provisions of the Declaration of Helsinki.
Consent for publication
Written informed consent for publication of this study was obtained from the patients’ parents. Copies of the signed informed consent forms are available for review by the Series Editor.
Competing interests
The authors declare that they have no competing interests, either financial or nonfinancial, that could be perceived as prejudicing the impartiality of the research reported.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Siyuan Chen, Email: siy_chen@163.com.
Yi Ji, Email: jijiyuanyuan@163.com.
References
- 1.Croteau SE, Kozakewich HPW, Perez-Atayde AR, et al. Kaposiform lymphangiomatosis: a distinct aggressive lymphatic anomaly. J Pediatr. 2014;164:383–388. doi: 10.1016/j.jpeds.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji Y, Chen S, Peng S, Xia C, Li L. Kaposiform lymphangiomatosis and kaposiform hemangioendothelioma: similarities and differences. Orphanet J Rare Dis. 2019;14:165–265. doi: 10.1186/s13023-019-1147-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozeki M, Fujino A, Matsuoka K, Nosaka S, Kuroda T, Fukao T. Clinical features and prognosis of generalized lymphatic anomaly, kaposiform lymphangiomatosis, and Gorham–Stout disease. Pediatr Blood Cancer. 2016;63:832–838. doi: 10.1002/pbc.25914. [DOI] [PubMed] [Google Scholar]
- 4.Adams DM, Ricci KW. Vascular anomalies: diagnosis of complicated anomalies and new medical treatment options. Hematol Oncol Clin N Am. 2019;33:455–470. doi: 10.1016/j.hoc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Pineda I, Lopez-Gutierrez JC, Chocarro G, Bernabeu-Wittel J, Ramirez-Villar GL. Long-term outcome of vincristine–aspirin–ticlopidine (VAT) therapy for vascular tumors associated with Kasabach–Merritt phenomenon. Pediatr Blood Cancer. 2013;60:1478–1481. doi: 10.1002/pbc.24543. [DOI] [PubMed] [Google Scholar]
- 6.Michaud AP, Bauman NM, Burke DK, Manaligod JM, Smith RJ. Spastic diplegia and other motor disturbances in infants receiving interferon-alpha. Laryngoscope. 2004;114:1231–1236. doi: 10.1097/00005537-200407000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Adams DM, Trenor CC, III, Hammill AM, et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics. 2016;137:e20153257. doi: 10.1542/peds.2015-3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triana P, Dore M, Cerezo VN, et al. Sirolimus in the treatment of vascular anomalies. Eur J Pediatr Surg. 2017;27:86–90. doi: 10.1055/s-0036-1597655. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Li K, Yao W, Dong K, Xiao X, Zheng S. Successful treatment of kaposiform lymphangiomatosis with sirolimus. Pediatr Blood Cancer. 2015;62:1291–1293. doi: 10.1002/pbc.25422. [DOI] [PubMed] [Google Scholar]
- 10.Crane J, Manfredo J, Boscolo E, et al. Kaposiform lymphangiomatosis treated with multimodal therapy improves coagulopathy and reduces blood angiopoietin-2 levels. Pediatr Blood Cancer. 2020;67:e28529. doi: 10.1002/pbc.28529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozeki M, Nozawa A, Yasue S, et al. The impact of sirolimus therapy on lesion size, clinical symptoms, and quality of life of patients with lymphatic anomalies. Orphanet J Rare Dis. 2019;14:141. doi: 10.1186/s13023-019-1118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes VM, Fargo JH, Saini S, et al. Kaposiform lymphangiomatosis: unifying features of a heterogeneous disorder. Pediatr Blood Cancer. 2015;62:901–904. doi: 10.1002/pbc.25278. [DOI] [PubMed] [Google Scholar]
- 13.Raam MS, Festekjian A, Elkhunovich MA. Point-of-care thoracic ultrasonography in the diagnosis and management of kaposiform lymphangiomatosis. Pediatr Emerg Care. 2016;32:888–891. doi: 10.1097/PEC.0000000000000968. [DOI] [PubMed] [Google Scholar]
- 14.Adams DM, Brandão LR, Peterman CM, et al. Vascular anomaly cases for the pediatric hematologist oncologists-an interdisciplinary review. Pediatr Blood Cancer. 2018;65:e26716. doi: 10.1002/pbc.26716. [DOI] [PubMed] [Google Scholar]
- 15.Goyal P, Alomari AI, Kozakewich HP, et al. Imaging features of kaposiform lymphangiomatosis. Pediatr Radiol. 2016;46:1282–1290. doi: 10.1007/s00247-016-3611-1. [DOI] [PubMed] [Google Scholar]
- 16.Barclay SF, Inman KW, Luks VL, et al. A somatic activating NRAS variant associated with kaposiform lymphangiomatosis. Genet Med. 2019;21:1517–1524. doi: 10.1038/s41436-018-0390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozeki M, Aoki Y, Nozawa A, et al. Detection of NRAS mutation in cell-free DNA biological fluids from patients with kaposiform lymphangiomatosis. Orphanet J Rare Dis. 2019;14:215–315. doi: 10.1186/s13023-019-1191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boscolo E, Pastura P, Glaser K, et al. Signaling pathways and inhibitors of cells from patients with kaposiform lymphangiomatosis. Pediatr Blood Cancer. 2019;66:e27790. doi: 10.1002/pbc.27790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji Y, Chen S, Xiang B, et al. Sirolimus for the treatment of progressive kaposiform hemangioendothelioma: a multicenter retrospective study. Int J Cancer. 2017;141:848–855. doi: 10.1002/ijc.30775. [DOI] [PubMed] [Google Scholar]
- 20.Foster JB, Li D, March ME, et al. Kaposiform lymphangiomatosis effectively treated with MEK inhibition. EMBO Mol Med. 2020;12:e12324. doi: 10.15252/emmm.202012324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozeki M, Nozawa A, Kawamoto N, Fujino A, Hirakawa S, Fukao T. Potential biomarkers of kaposiform lymphangiomatosis. Pediatr Blood Cancer. 2019;66:e27878–e27978. doi: 10.1002/pbc.27878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Figure S1. The specimen of patient 1 was resected as a right testicular, which shows spindle lymphatic endothelial cells in clusters associated with malformed lymphatic channels (H&E staining, magnification × 200) (A). Immunostaining of anti-CD31 showed that the spindle cells were positive for CD31 (magnification × 100) (B). Immunostaining of anti-CD34 showed that the spindle cells were positive for CD34 (magnification × 100) (C). Immunostaining of D2-40 revealed proliferating lymphatic vessels (magnification × 100) (D).
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


