Abstract
Radiotherapy (RT) is one of the mainstay treatments for prostate cancer (PCa), a highly prevalent neoplasm among males worldwide. About 30% of newly diagnosed PCa patients receive RT with a curative intent. However, biochemical relapse occurs in 20–40% of advanced PCa treated with RT either alone or in combination with adjuvant-hormonal therapy. Epigenetic alterations, frequently associated with molecular variations in PCa, contribute to the acquisition of a radioresistant phenotype. Increased DNA damage repair and cell cycle deregulation decreases radio-response in PCa patients. Moreover, the interplay between epigenome and cell growth pathways is extensively described in published literature. Importantly, as the clinical pattern of PCa ranges from an indolent tumor to an aggressive disease, discovering specific targetable epigenetic molecules able to overcome and predict PCa radioresistance is urgently needed. Currently, histone-deacetylase and DNA-methyltransferase inhibitors are the most studied classes of chromatin-modifying drugs (so-called ‘epidrugs’) within cancer radiosensitization context. Nonetheless, the lack of reliable validation trials is a foremost drawback. This review summarizes the major epigenetically induced changes in radioresistant-like PCa cells and describes recently reported targeted epigenetic therapies in pre-clinical and clinical settings.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13148-021-01111-8.
Keywords: Epigenetics, Epidrugs, Radioresistance, DNA repair, Prostate cancer
Introduction
Prostate cancer (PCa) remains highly prevalent among males worldwide. Despite relatively low mortality rates, this malignancy is the second most common cancer in men, mostly due to the current widespread intensive prostate-specific antigen (PSA) screening [1, 2]. According to Globocan estimates, around 1.4 million new cases of PCa were diagnosed in 2020 [2].
Radiotherapy (RT) using external beam radiation therapy (EBRT) is considered a first-line standard treatment with curative intent for PCa patients, and is often performed with moderate hypofractionation therapeutic schemes using fraction sizes larger than 2 Gy delivered daily [3]. Relapse is a major clinical problem in locally advanced PCa and for both intermediate-risk and high-risk PCa (HRPC) patient’s, prognosis is significantly worse. Between 20 and 40% of patients treated with RT experience long-term recurrence within a 5-year follow-up [4]. Despite considerable efforts to develop effective therapeutic strategies, improvements in precision medicine techniques are still needed and represent the next step toward enhancing clinical management of resistance to first-line therapies.
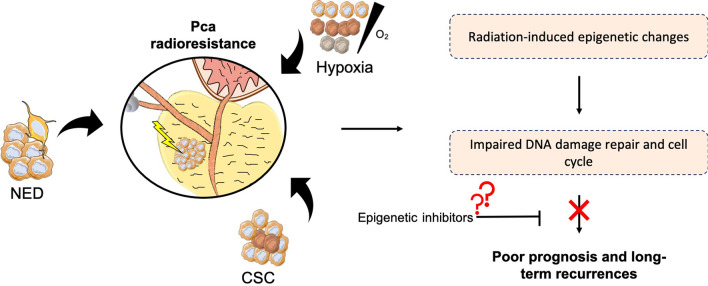
Dynamics of ionizing radiation (IR) response is mainly associated with DNA damage pathways [5]. Hypoxic foci, PCa stem cell (PCSC) population, and neuroendocrine differentiation (NED) involved in DNA repair/apoptosis and cell cycle deregulation play a major role in radioresistance [6]. Epigenetic reprograming in PCa may also contribute to the regulation of these functional pathways [7]. Understanding how this phenotypic switch occurs and enhances radioresistance in a PCa subpopulation has been a critical research focus during the past years. Since epigenetic dysregulation is a key mechanism underlying cancer cell death escape after RT, the identification of novel epigenetic targets and new epidrugs might increment personalized clinical management [7].
In this review, we discuss the latest pre-clinical and clinical insights into the role of epigenetics in Pca radioresistance. Specifically, we focus on recent advancements in PCa’s understanding, from the molecular machineries driving radiation response based on tumor biology to epigenetic alterations involved in cell death and DNA damage pathways, all of which might impact Pca patient outcome. We also described the benefits of strategies using so-called epidrugs concurrently administered with standard RT.
The importance of radiotherapy in prostate cancer care
Unlike other cancers, slowly proliferating tumors, such as PCa, could be highly responsive to larger radiation fraction sizes and in many cases RT constitutes the standard of care for PCa [8]. Although a meta-analysis of 25 studies including > 14.000 patients concluded that hypofractionated RT could be more effective than conventional fractions of 1.8/2 Gy [9] the major phase III trials of moderate hypofractionation did not demonstrated superiority in terms of both outcomes and toxicity (HYPRO, phase III randomized trial, ISRCTN85138529). At the same time, the hypofractionated approach, due to the added advantages of being more convenient for patients with a lower cost, has become the standard practice in the clinical management of PCa patients. Similar to normal healthy tissues with low proliferative rates, such as, kidney, lung, rectum, bladder, and brain, PCa has a long cell cycle, which confers higher damage repair efficiency and capability [8]. In fact, PCa exhibits a longer doubling time than other tumors, with a much lower fraction size (α/β ratio) of 1–2 Gy [9]. Thus, taking together radiobiological assumptions for tumor control and dose limitations of surrounding normal tissue, hypofractionation schedules in RT treatment planning might improve PCa therapeutic efficacy [8].
Recent innovative three-dimensional RT techniques, such as intensity-modulated radiation therapy (IMRT) and volumetric-modulated arc therapy (VMAT) using image-guided RT (IGRT), are overcoming previous limitations of higher dose administration schemes that resulted in late complications and tumor-related morbidities, considering the proximity of prostate gland to bladder and bowel wall [10–12]. Although conventional EBRT doses are delivered in 37–40 fractions with total delivery doses of 76–80 Gy [13], moderate to extreme hypofractionation schemes using larger daily fractions with ablative doses are reported to be more convenient in terms of costs and convenience for the patients, being non inferior in terms of both outcomes and acute/late toxicity [10].
Another approach of increasing the therapeutic ratio of RT for PCa consists of dose-escalation. Zelensky et al. in a retrospective analysis of 2551 patients with different risk categories demonstrated that biochemical disease free survival (bDFS) was significantly improved by dose escalation (above 81 Gy for the intermediate and HRPC) [14]. The FLAME trial is currently evaluating the efficacy of an integrated boost delivery of 95 Gy in multiparametric (mp) MRI-defined tumors and has shown no significant toxicities increment. Another approach of dose-escalation consists of the delivery of a high-dose rate boost of brachytherapy after, with good outcomes in terms of control of the disease [8].
Several findings support the advantages of androgen-deprivation therapies (ADT; gonadotropin-releasing hormone agonists/antagonists, abiraterone, and anti-androgens/androgen receptor [AR] antagonists) in concomitance with RT to improve overall survival rates and reduce the risk of long-term biochemical recurrences [15]. The combination of ADT with RT has definitively proven its superiority compared with RT alone followed by salvage ADT in different phase III RCTs (RTOG8610 and TROG96.01, randomized clinical trials), so that for intermediate risk PCa patients a short duration of around 6 months is advised, whereas a longer one (2–3 years) is needed for HRPC patients.
Another strategy that could improve the therapeutic efficacy in HRPC patients is the addition of chemotherapy (docetaxel) to standard RT plus ADT for a defined subset of HRPC (STAMPEDE, randomized controlled trial), although subsequent meta-analysis did not confirm the advantage in overall survival [16].
Despite the aforementioned different strategies, a significant percentage of patients progress to a castration-resistant phenotype after 2–3 years of ADT initiation, worsening the patient’s prognosis [16]. Notwithstanding advances in therapeutic management, PCa patients with high-grade tumor burden display high progression rates and consequently a greater risk of treatment failure [16]. Hence, the discovery of critical targets of unravelled molecular patterns is urgently required to predict and overcome radioresistance in this malignancy.
Intrinsic molecular pathways involved in PCa during therapeutic radiation exposure
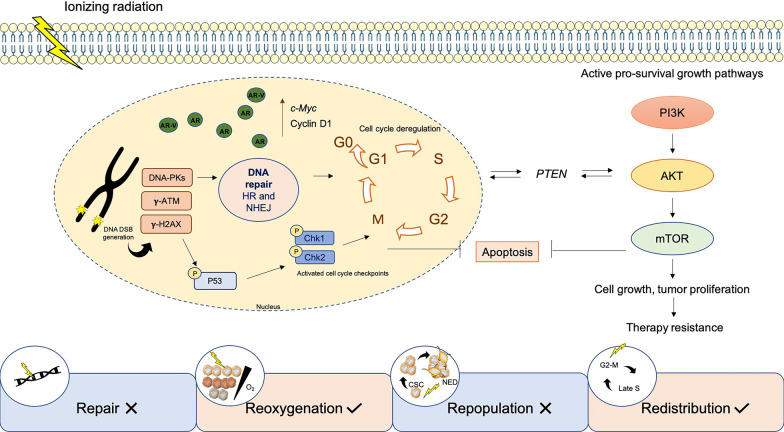
Considering the high degree of PCa’s histopathologic heterogeneity, RT resistance poses a major clinical challenge due to the likely development of an aggressive disease. The dynamics of successful tumor irradiation are defined by the classic R’s of radiobiology: Repair of sublethal damage, Redistribution of sensitive cell cycle phases, Repopulation, and Reoxygenation of hypoxic tumor cells (Fig. 1) [17]. As discussed above, slow proliferating tumors as PCa have a greater capacity for damage repair due to intrinsic longer cell division time [8]. All aforementioned R’s can be affected by the establishment of innovative therapeutic schemes [18]. Herein, hypofractionated approaches with higher doses per fraction might overcome the problematic of late responding tissues [18]. Latest reduced treatment time schedules should maintain elevated biological effective doses (BED) without increasing either acute or late side effects for late-responding normal tissues, which limits therapeutic designs [18, 19]. An equilibrium between minimum normal surrounding tissue damage and efficient tumor local control must be considered [18]. Using higher doses, cell toxicity and death are typically more pronounced [18]. DNA is the most critical IR target, promoting intrinsic genomic instability mainly through the generation of DNA double-strand breaks (DSBs) [20]. DNA DSB repair is mediated through two major pathways, homologous recombination (HR) and non-homologous end joining (NHEJ) [20]. Changes in the normal cell division functioning affect DNA damage repair (DDR) processes [20]. The following subsections describe the major cellular mechanisms involved in PCa radioresistance.
Fig. 1.
Radiation-induced radiobiological molecular pathways in PCa. Ionizing radiation exposure leads to activation of pro-survival cell growth pathways, such as PI3K/Akt/mTOR, entailing efficient DNA DSB damage repair. Specifically, radiation-induced DNA-dependent protein kinases, γ-ATM and γ-H2AX accumulation, and activation of p53 and key factors involved in cell cycle progression, sustain cell growth and tumor proliferation. Furthermore, PTEN is reported to play a role in PCa radioresistance, sustaining cell cycle arrest due to Chk1 regulation in an Akt-dependent manner. All these changes induce PCa cell growth, proliferation, apoptosis evasion, and therapy resistance. This dynamic is supported by current knowledge of the classic R’s of radiobiology, including repair of DNA damage and aggressive cell repopulation, which improve overall tumor cell survival after radiation exposure. Conversely, reoxygenation of deeper layers and cell cycle phases redistribution allows greater therapeutic efficacy.: AKT, protein kinase B; AR, androgen receptor; AR-V, AR variant; ATM, ataxia telangiectasia mutated; ChK1/2, checkpoint kinase 1/2; CSC, cancer stem cells; HR, homologous recombination; mTOR, mechanistic target of rapamycin kinase; NED, neuroendocrine differentiation; NHEJ, non-homologous end joining; PI3K, phosphoinositide 3-kinase; PTEN, phosphatidylinositol 3,4,5-trisphosphate 3
DNA repair and apoptosis
The most accurate DNA repair pathway, HR, is activated during late S phase of the cell cycle, starting from the recruitment of several key proteins, such as autophosphorylated ataxia telangiectasia mutated (γ-ATM), DNA-dependent protein kinases, γ-H2AX, breast cancer gene 1/2 (BRCA1/2), poly(ADP-ribose) polymerase 1 (PARP-1), and RAD51 [21]. Defects in DDR pathways are commonly described as PCa drivers [21, 22]. Between 15 and 30% of PCa’s display DDR instability, involving the most common pathways such as mismatch repair (MMR), base-excision repair (BER), and NHEJ/HR for DSBs [23]. Specifically, DDR gene mutations were commonly found in metastatic castration-resistant PCa (CRPC) patients [24]. Impaired DNA damage repair may provide RT escape mechanisms of tumor cells. Specifically, RAD51 – Stat5a/b transcriptional regulation led to efficient DSBs DNA damage repair in PCa after IR exposure [25]. Targeting this axis significantly reduced survival [25]. Involvement of phosphoinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) pro-survival signaling pathway as well as overexpression of critical cell growth and cell cycle progression factors, including c-Myc and cyclin D1, have been implicated in PCa radioresponse’s regulation [26]. Additionally, activation of cell cycle checkpoint kinases Chk1 and Chk2 in a radioresistant PCa subpopulation was found to enhance cancer stemness features, increased invasion and epithelial-mesenchymal transition (EMT) [27].
In contrast, phosphatidylinositol 3,4,5-triphosphate 3-phosphatase (PTEN), proteins involved in the caspases cascade, and apoptotic proteins are key mediators in tumor cell death [20, 28]. Nonetheless, most of these proteins are mutated or epigenetically silenced during IR exposure, resulting in apoptosis evasion [28]. Indeed, disturbances in several steps of these signaling pathways were shown to augment tumor aggressiveness, reducing RT response [20, 28]. HMG-box transcription factor 1 (HBP1) and PTEN work as tumor suppressors allowing transcription inhibition [20, 29]. Positive correlation between HBP1 expression and RT efficacy was reported in a previous work [29]. PTEN is a key tumor suppressor gene (TSG) and negative Akt’s regulator found altered in PCa [20]. Intriguingly, although PTEN mutations are reported to contribute to tumor progression, PTEN phosphatase activity was suggested to be implicated in HR, DDR, and cell cycle arrest through Akt-dependent γ-CHK1 signaling regulation [20]. Additionally, tumor protein p53, another key dual-function protein, cell cycle and apoptosis regulator, undergoes alterations during radiation-induced DNA damage response in PCa [30, 31]. Specifically, p53 pathway upregulation was observed in PCa cells after IR exposure [30, 31]. Interestingly, p53 wild-type cells significantly reduced clonogenic capacity under RT exposure after E2F-1 transcription factor targeting [32]. These data were further supported and enriched with the combination of MDM2 knockdown, a negative regulator of p53, resulting in γ-p53 (serine 15) enhancement [33]. Otherwise, functional phosphorylated p53 was able to prolong G1-S and G2-M cell cycle arrest, giving cells sufficient time for DDR and thus contributing to tumor cell repopulation after RT [30].
Thus, the aforementioned alterations enable tumor cells to evade mechanisms of programmed cell death, promoting recovery and repopulation between RT fractions. Direct or indirect targeting these pathways and their downstream regulators was suggested to improve PCa radiosensitization [20, 28]. Of note, 20% of PCa patients display PTEN loss-of-function mutations [34]. Driver mutations in ETS transcription factor through gene fusion played a key role in PCa [35]. ERG-ETS family member increased PARP-1 activity resulting in less DNA damage and PCa radioresistance [35]. HR- and BER-defective PCa can benefit from targeted therapy with PARP inhibitors [34], such as Olaparib and veliparib, as supported by clinical trials. Nonetheless, only pre-clinical studies shown the role of these drugs as PCa cells radiosensitizers [35–39].
Hypoxia and PCa
Hypoxia is among the most relevant factors implicated in radioresistance and cell death escape [40, 41]. Considering the intrinsic heterogeneity of cell radiosensitivity, hypoxic foci are commonly found in both prostate hyperplasia and adenocarcinoma [42]. Theoretically, during fractionated RT, the deeper tumor layers usually farther from blood vessels become increasingly oxygenated [43]. However, for extremely hypoxic tumors with oxygen pressure (PO2) ≤ 1 mmHg, standard fractionated therapy was estimated to be successful in only ~ 15% of PCa patients [42]. Furthermore, hypofractionated schemes with reduced fractions might affect reoxygenation [43]. Therefore, the success rates achieved with hypofractionated schemes are not yet fully understood in PCa [42].
From a molecular point of view, hypoxia can activate a complex cellular signaling network in several tumors. The absence of oxygen was associated with hypoxia-inducible factors (HIFs) overexpression, triggering the activation of several downstream hypoxic responsive elements, such as vascular endothelial growth factor (VEGF), carbonic anhydrase IX (CAIX), and glucose transporter 1 (GLUT-1), allowing cells to adapt to the hypoxic microenvironment [44]. Severe hypoxia in the PCa niche during radiation exposure led to overall genomic instability (Fig. 1) [44]. DSBs are expected to appear after RT exposure, damaging DNA [45]. Interestingly, in hypoxic cells, along with reactive oxygen species (ROS) scavenging, the tumor microenvironment also it modulates DDR pathway [46–48]. Additionally, microenvironmental redox statement led to differential expression of NrF2 and their downstream targets allowing PCa radioresistance [49]. Conservative HR pathways and respective downstream targets were strongly deregulated both in chronic hypoxia and reoxygenation to refrain from radiation-induced cell’s death [47]. Two key HR genes, BRCA1 and RAD51, as well as, the MMR genes MLH1 and MSH2 were also downregulated in hypoxia [45, 47]. This genetic instability leads to errors through cell cycle checkpoints, maintaining tumor progression at a high mutational rate [47]. These include genetic aberrations such as point mutations or gene amplification [47]. Indeed, highly hypoxic PCa presents a stout reduction of PTEN transcription and increased mutational burden [50].
Furthermore, PI3K/Akt/mTOR and mitogen-activated protein kinase/extracellular regulated kinase (MAPK/ERK) are critical cell signaling pathways activated during hypoxia stabilization in PCa to regulate HIF synthesis [44]. VEGF expression was also reported to be induced after PI3K activation in a HIF-dependent manner and via Notch cascade signaling activation, even in oxygen-independent conditions, due to VHL, p53, and PTEN mutations or loss of function [44, 51]. Additionally, several key DDR-related genes, including ATM/ATR, Chk1/2, γ-H2AX, and Ku70/80, were upregulated in hypoxic cells, mainly through NHEJ repair pathway [47].
PCa stemness
PCa RT responsiveness was also regulated by a subpopulation of cells with stemness properties within tumor bulk [52]. Indeed, these cancer stem cells (CSCs), also known as tumor-initiating cells, are AR-independent and commonly maintained into the hypoxic tumor microenvironment [52–54]. These CD44+ and CD133+ cells persist after RT, promoting tumor progression and early biochemical recurrences [55]. Moreover, its avoided apoptosis and other radioresistant associated features (Fig. 1) [52]. The PCSCs specific-associated pluripotency genes, SOX2, OCT3/4, KL-F4, c-Myc, Nanog, and Snail were shown to be useful to identify recurrent patients after RT [27]. Indeed, these pluripotency-associated markers and EMT-associated molecules are induced by the HIF signaling network [56]. Furthermore, PI3K/Akt/mTOR and MAPK/ERK are commonly activated signaling pathways associated with PCSC growth and therapy evasion [57].
CSCs are distinguished by a constant quiescent state of the cell cycle (G0/G1), contrasting with the rapid cell division required RT effectiveness. Of note, defects in DDR pathways have been often observed in PCSCs [55]. An imbalance between low levels of intracellular ROS-produced DNA damage and higher DDR efficiency through γ-H2AX, Ku70, and Ku80 was also described in PCSCs after RT [55]. Cell cycle and DDR defects implicate in PCSCs radioresistance. Remarkably, Chk1 Knockdown prevented G2-M radiation-induced cell cycle arrest, decreasing DDR, while inducing apoptosis in a CD133+/CD44+ subpopulation [58].
PCa neuroendocrine differentiation
NED is a frequent transitory event in CRPC associated with treatment failure [59, 60]. Currently, NED is accepted to be the most aggressive clinical variant form of PCa [59]. Approximately 1% of primary PCa is classified as NED tumors at diagnosis, whereas about 30% of advanced PCa patients display neuroendocrine transitory foci [61, 62]. This phenotype is characterized by the loss of characteristic markers of the prostate gland, such as AR and PSA [63]. Instead, AR-null PCa cells display increased NED markers’expression [64]. AR-dependent signaling is compensated in neuroendocrine PCa (NEPC) through enhanced activity of cell-sustaining growth factors, such as fibroblast growth factor and MAPK [64]. Consequently, as NEPC’s biology is not fully understood, effective treatment options are very limited. Nevertheless, cisplatin-based chemotherapeutic is routinely used to reduce tumor burden in these patients [62].
Radiation exposure was also reported to induce NEPC differentiation [65]. Additionally, NE-like cells transdifferentiation upon fractionated radiation exposure have been linked with the dynamic regulation of transcription factors, such as, AMP-response element binding protein (CREB) and activating transcription factor 2 (ATF2), at nuclear (active form) and cytoplasmatic location (inactive form), respectively [66]. Furthermore, CREB inhibition led to the blockage of IR-induced NED and radiosensitize PCa cells inducing cell death [67]. Similarly to PCSCs, NEPC cells are quiescent [68], also suggesting great ability to DDR after radiation exposure. Globally, NEPC cells are molecularly characterized by N-Myc overexpression, which drives the transcriptional enrichment of HR DDR and stress-related genes, such as BRCA1, PARP1/2, RecQ-mediated genome instability 2 (RMI2), and DNA topoisomerase II binding protein 1 (TOPBP1) [59]. Furthermore, TP53, PTEN, and retinoblastoma (RB1) loss of function were found in NEPC tumors [69]. This leads to blockage of pathways related to cell growth and proliferation inhibition, including interleukin 8-mediated IL-8/CXCR2/p53-pathway and further increasing aggressive properties of neuroendocrine malignancies [68]. Moreover, tumor plasticity plays a central role in NED due to the enhancement of EMT-induced pathways, with the activation of ZEB1/2, Snail, Slug, Twist, and N-cadherin [69]. These tumor cells have the ability to adapt, bypassing barriers, and becoming increasingly aggressive. Previous studies showed that both ADT and RT promote hypoxia-related CSC, strongly contributing to heterogeneous disease and consequently RT failure [70, 71].
Overall, lethal NEPC represents a hurdle to both RT/ADT therapy. Hence, the study of these patients’ epigenetic profile might allow for the identification of RT responders and non-responders markers, considering the previously mentioned molecular pathways involved in radioresistance.
Epigenetics in PCa: a brief overview
Epigenetic alterations induce reversible and heritable changes promoting differences in gene expression without changing DNA sequence [72]. They are generally classified as a constitutive hallmark of cancer, due to the combined action of oncogene activation and TSG knockdown [72]. DNA methylation, covalent histone-modifications, histone-variants and chromatin remodeling complexes are commonly accepted epigenetic mechanisms [72]. Nonetheless, PCa displays complex epigenetic landscape deregulation associated with changes in cell growth pathways and overall tumor progression (Fig. 2) [72].
Fig. 2.
Epigenetic landscape in PCa. Aberrant DNA methylation (A) and histone post-translational modifications (B, C) lead to overall PCa progression and aggressiveness due to uncontrolled gene transcription signature. Black filled circles represent methylated sites (A). ac, acetylation; APC, adenomatous polyposis coli; AR, androgen receptor; CBP, CREB-binding protein; CCND2, cyclin D2; CDNK2A, cyclin-dependent kinase inhibitor 2A; CpG, cytosine/guanine enriched sites; EZH2, enhancer of zeste homolog 2; GSTP1, glutathione S-transferase Pi 1; HDAC, histone deacetylase; HOXD3, homeobox protein Hox-D3; KDM, lysine demethylase; me, methylation; MGMT, O6-methylguanine-DNA methyltransferase; PCa, prostate cancer; PTGS2, prostaglandin-endoperoxide synthase 2; RARβ2, retinoic acid receptor beta 2; RASSF1A, Ras association domain family member 1; SIRT, sirtuin; TGS, tumor suppressor genes
DNA methylation
DNA methylation is the major epigenetic mechanism described in cancer [73]. Differential methylation levels among distal and genic regions are key dynamic drivers of PCa tumorigenesis and progression [74]. This process involves the addition of a methyl group to 5-methylcytosine (5mC), usually at the promoter region, in CpG-enriched islands, to promote gene silencing [72]. This reaction is catalyzed by DNA methyltransferases (DNMTs), including DNMT1, often associated with DNA methylation maintenance, and DNMT3a and DNMT3b, responsible for de novo methylation [72]. In contrast, DNA methylation is reversed by ten-eleven translocation (TET) enzymes [75]. In PCa, global DNA hypomethylation has been widely reported [76, 77]. Additionally, long interspersed nuclear element-1 (LINE-1) a gene frequently silenced in the normal human genome, is re-expressed in PCa cells [77]. Conversely, hypermethylated foci in specific TSG promoter regions were associated with gene silencing (Fig. 2A) [72]. DNMTs, particularly DNMT3a and DNMT3b, were highly expressed in PCa and associated with tumor progression [78]. Assessment of DNA- methylation based panels along with PSA screening demonstrated a high value for prediction and/or recurrence detection in PCa patients [79]. Adenomatous polyposis coli (APC) is a common transcriptionally repressed TSG associated with PCa progression [80]. Additionally, well-known specific TSGs and genomic stability regulators such as retinoic acid receptor beta2 (RARβ2), cyclin-dependent kinase inhibitor2A (CDNK2A), Ras association domain family member1A (RASSF1A), homeobox gene D3 (HOXD3), O6-methylguanine DNA methyltransferase (MGMT), cyclin2 (CCND2), prostaglandin-endoperoxide synthase 2 (PTGS2), and glutathione S-transferase Pi1 (GSTP1) were also commonly silenced by promoter methylation in PCa (Fig. 2A).
Chromatin remodeling modifiers
Histone post-translational modifications (PTMs) are enzymatic modifications of proteins. These reactions are written/established by histone methyltransferases (KMTs) and histone acetyltransferases (HATs) and erased/removed by histone demethylases (KDMs) and histone deacetylases (HDACs). Tumor plasticity and epigenetic dynamics may lead to tumor cells’ aggressive phenotypes, like those found in CSC markers, including, tumor growth and proliferation, metastasis, and resistance to therapy [81].
Histone methylation
Deregulation of KMTs and KDMs was observed in PCa cells and was associated with cancer cell proliferation [82]. These changes generally have a pleiotropic effect that can either lead to gene transcription-repression or -activation [72]. The KMT enhancer of zeste homolog 2 (EZH2) is strongly overexpressed in PCa cells, including aggressive NEPC cells, and is generally linked to transcriptional repression through trimethylation of lysine 27 on histone 3 (H3K27me3) [83]. A member of the Jumonji C-domain (JmjC) 2-oxoglutarate-dependent dioxygenase KDM superfamily, JMJD3/UTX/KDM6, that acts as a transcriptional activator is also deregulated in PCa (Fig. 2C) [84]. This tight balance between methylation and demethylation of histone 3 and the downstream impact in specific pro-tumorigenic or anti-neoplastic gene targets is a major challenge in translational and precision medicine research, meaning that specific epidrugs targeting either EZH2 or KDM6 can elicit antagonistic effects in tumor cells, depending on their context and environment [84]. In PCa samples, H3K27me3 mark was reported to be enriched in several specific promoter regions of TSGs, such as FBXO11, ING3, and RKIP, as well as other tumor regulatory genes [85]. In contrast, other studies found that KDM6 enhanced activity mediating transcriptional activation of specific target genes involved in key PCa carcinogenic pathways, including AR signaling [84]. Moreover, KMTs downregulation, namely SUV39H1 (KMT1A) results in lower levels of another repressive mark, H3K9me3, in PCa cells [86]. Increased expression of several KDMs including LSD1 (KDM1A), a lysine-specific demethylase, and JmjC-KDMs as JMJD1A/KDM3A, JMJD1B/KDM3B, JMJD2A/KDM4A, JMJD2C/KDM4C, JARID1B/KDM5B, and PHF8/KDM7B was also reported in PCa, resulting in higher PCa proliferation, migration, and invasion (Fig. 2C) [87].
Histone acetylation
Histone acetylation is another widely studied histone modification in PCa. Unlike histone methylation, acetylation is usually associated with transcriptional activation due to DNA molecule relaxing by histone charge neutralization. Co-activator complex proteins with a conserved HAT domain, such as p300/CREB binding protein (CBP), play a key role in the progression of AR-dependent PCa cells through AR acetylation (Fig. 2D) [88]. Hyperacetylation of histone 3 lysine residues 9, 14, and 18 (H3K9ac, H3K14ac, H3K18ac) induces castration-resistant progression in PCa cells via p300 activity [89]. Additionally, higher levels of acetylated histone 4 lysine 16 (H4K16ac) in PCa cells was reported to induce transcriptional activation of pro-inflammatory genes, such as tumor necrosis factor alpha (TNF-α) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) [90].
Furthermore, HDACs are a large family of chromatin remodelers comprising four major classes (I, II, III, and IV) regulating both transcription factors and histone deacetylation [81]. Class III HDACs are a particular class of sirtuins (SIRTs) with a NAD+-dependent catalytic activity mechanism, whereas other classes are zinc-dependent. Histone deacetylation is a common PTM in PCa generally leading to transcriptional repression of specific genes [81]. HDACs’ overexpression, including HDAC1, HDAC2, and HDAC3, was tightly associated with PCa progression and aggressiveness (Fig. 2D) [91]. Specifically, HDAC1 activity induced Yan Yang 1(YY1)-mediated repression of XIAP-associated factor 1 (XAF1) in PCa cells [92]. Furthermore, HDAC2 was named a good candidate PCa prognostic biomarker [91]. The combined effect of both mechanisms, such as increased H3K27me3 and decreased H3K9ac, induced tumor suppressor TIMP3 inhibition, in PCa cells [93]. Increased HDAC activity was associated with ERG expression, which inhibits HATs activity in PCa cells [94]. Interestingly, HDAC6 (a class IIb HDAC) regulates AR protein stabilization in CRPC, through Hsp90 transcription factor substrate (Fig. 2D) [95]. Conversely, HDAC4 (a class IIa HDAC) an endogenous regulator, binds with AR, inhibiting its activity in AR-dependent PCa cells through SUMOylation, another epigenetic remodeling mechanism [96]. SIRTs are also overexpressed in PCa cells. Specifically, SIRT7 upregulation lead to PCa progression, increased cell migration, and invasion (Fig. 2D) [97]. These findings support the clinical value of using these targets as predictive biomarkers in advanced PCa patients. Further discussion about available drugs for these targets will be done in the next section of radiosensitizing strategies.
Radiation and epigenetic dynamics interactions
Although epigenetic regulation has been implicated in cellular radiation response control of PCa [98], convincing clinical evidence and validation of these findings are lacking. As discussed above, cancer cell death is the major goal in radiation-based therapy. Importantly, targeting DNA repair and cell cycle regulatory pathways might overcome PCa radioresistance [99]. Overall, radiation was demonstrated to induce chromosomal instability, mainly through specific methylation patterns and a wide range of histone PTMs [100]. Epigenomic modifications are involved in cell growth pathways and radioresistant signatures regulation. Concurrently, radiotherapeutic treatment was also found to cause epigenetic changes in PCa cells [101]. Notably, although global DNA hypomethylation has been considered a hallmark of most cancers (by contributing to general genomic instability), this is commonly accompanied by increased DNA methylation levels at specific CpG sites and gene promoters (namely those belonging to tumor suppressor genes), as observed after exposure to IR (Fig. 3A) [102–104]. These changes influenced the recruitment of DSB repair agents, such as γ-H2AX and BRCA1, for an efficient damage response in PC-3, a radioresistant PCa cell line (Fig. 3A) [102]. Additionally, PCSC growth was stimulated after RT exposure through specific epigenetic modulation [7]. Accumulation of H3K36me3 at aldehyde dehydrogenase 1A (ALDH1A1) promoter region, a CSC-related marker, along with EZH2 overexpression, associated with radiation-induced resistance in PCa (Fig. 3B) [7]. Accordingly, enhanced EZH2 activity cooperates with BRCA1, maintaining CSC signature and PCa radioresistance [105]. Furthermore, EZH2 sustained MEK/ERK signaling pathway activation during EMT, allowing PCa proliferation and invasiveness (Fig. 3B) [106].
Fig. 3.
Radiation-induced epigenetic reprograming in PCa. Epigenetic mechanism regulation plays a key role in PCa radiation response, contributing to cell cycle deregulation, active DNA damage repair, and apoptosis evasion. A Aberrant gene expression is mediated by high DNMT activity at specific CpG sites. This mechanism allows DNA damage repair efficacy and apoptosis evasion. B Histone post-translational modifications are able to modulate cell growth, CSC and EMT gene signature, and cell cycle deregulation. Uncontrolled PCa cell proliferation is maintained by an imbalance between repressive and activating markers. Black filled circles represent methylated sites. ALDH1A1, aldehyde dehydrogenase 1 family member A1; AR, androgen receptor; AKT, protein kinase B; BRCA1, breast cancer type 1; CSC, cancer stem cells; DNMT, DNA methyltransferase; EMT, epithelial-mesenchymal transition; ERK, extracellular regulated kinase; EZH2, enhancer of zeste homolog 2; HDAC, histone deacetylase; MEK, mitogen-activated protein kinase; mTOR, mechanistic target of rapamycin kinase; PI3K, phosphoinositide 3-kinase
As previously described, PI3K and androgens are key determinants of PCa signaling pathways, sustaining tumor growth after cell death-based treatments. Uncontrolled cell cycle proliferation and stimulated growth pathways are determined by epigenetic mechanisms [81]. Hence, increased androgen driven H3K4me2 levels activate PI3K signaling pathway, resulting in CRPC progression (Fig. 3B) [107]. Androgen-mediated effects were previously associated with a relaxed chromatin structure due to higher histone acetylation levels [108]. Both H3 and H4 acetylation activate Akt/mTOR, increasing PCa progression, and therapy resistance (Fig. 3B) [108]. Conversely, increased HDAC activity was linked to specific tumor suppressor inactivation in PCa (Fig. 3B) [91]. Moreover, several HDAC inhibitors (HDACi) were described as radiosensitizers, affecting both DSB repair machinery and cell cycle controlling pathways [109]. In terms of histone methylation, inactive KDM5D induced aberrant and faster cell division, activating DDR-related pathways in PCa, such as enhanced ATR kinase activity, conferring a more aggressive phenotype (Fig. 3B) [110]. Conversely, KDM5B overexpression led to a radioresistant phenotype in non-small cell lung cancer and PCa cell lines (Fig. 3B) [111]. Herein, clinical samples of non-small cell lung cancer showed lower RT response rates when higher levels of KDM5B were detected [111]. KDM3A, with substrate selectivity for H3K9me1/2, also induced aberrant DDR activation in radioresistant PCa [112]. Reversion of these epigenetic changes affected the recruitment of DSB repair molecules such as γ-H2AX and ATM, increasing tumor radiosensitivity [111]. Furthermore, a key regulatory cell cycle component, cyclin D2, is a candidate target of SMYD3 (KMT that specifically catalyzes transcriptional suppressive trimethylation of H4K20) [113]. This interplay, leads to deregulated cell cycle pathways and aberrant mitosis, resulting in uncontrolled PCa cell progression [113].
Strategies for radiosensitizing tumor cells with well-known epigenetic targeting drugs
The development of epigenetic compounds with radiosensitizing properties rationalizes the use of these drugs in a clinical context. Nevertheless, the demonstration of solid effectiveness of epigenetic-based radiosensitization in PCa is still lacking. Although a wide range of HDACi tested in several cancer models including PCa showed impressive anticancer properties, the specific effect of these epidrugs in cells after IR exposure remains elusive and do not progress after pre-clinical level. To date, only few clinical trials investigated the radiosensitizing effect of these inhibitors are summarized in Table 1. Remarkably, the major drawback of these studies are the toxicity effects. Additionally, larger randomized trials to corroborate the tolerability to the drugs and to evaluate the efficacy in the local control are needed. In fact, most of these clinical trials included a reduced number of participants who had completed the protocol. Furthermore, especially in relation to HDACi, to improve their current adoption into the clinical practice, it would be pertinent and helpful the development of newer, more specific HDACi or other epidrugs, as discussed previous. These inhibitors only target global histone acetylation and not specific lysine sites.
Table 1.
Epigenetic clinical trials with well-known epidrugs to overcome cancer radioresistance
| Drug | Clinical trials | Target | Cancer type | Patients | Doses | OS rates | Year of publication | References | Study ID |
|---|---|---|---|---|---|---|---|---|---|
| SAHA | Phase I | HDACi | Brain metastasis | 4 completed study protocol | 400 mg/day with 37.5 Gy (2.5 Gy/fr.) over 5 weeks | 36 weeks | 2014 | [119] | NCT01600742 |
| SAHA | Phase I | HDACi | NSCLC brain metastasis | 12 completed study protocol | 200–400 mg/day for 14 days with SD of SRS at day 3 | 13 months | 2017 | [120] | NCT00946673 |
| SAHA | Phase I/II | HDACi | Glioblastoma | Phase I: 15 Phase II: 107 | 300 or 400 mg/day with Std TMZ + RT | 55.1%, 15 months FU | 2018 | [121] | NCT00731731 |
| SAHA | Phase I | HDACi | Advanced head and neck SCC | 26 completed study protocol | 100–400 mg, 3 × weekly with concurrent CRT | 96.2% CR at 33.8 months FU | 2019 | [122] | NCT01064921 |
| VPA | Phase II | HDACi | Glioblastoma | 37 completed study protocol | 25 mg/kg oral divided in 2 dd concurrent with RT and TMZ | 97%, 86%, and 65% at 6, 12, and 24 months FU, respectively | 2015 | [123] | NIHMS686154 |
| VPA + Hydralazine | Phase III | HDACi + DNMTi | Stage III cervical cancer | 18 completed study protocol | 182 mg or 83 mg of hydralazine and 30 mg/kg VPA plus TCD of 85 Gy | NS | 2010 | [124] | NCT02446652 |
| LBH-589 | Phase I | HDACi | Stage III NSCLC | 9 with pRT | 20, 30, 45 mg twice/week, with pRT or rCRT | 9 months | 2015 | [125] | NA |
| LBH-589 | Phase I | HDACi | High grade gliomas | 12 completed study protocol | 10, 20, 30 mg/day, with 30–35 Gy (10 fr.) | 7.8; 6.1, and 16.1 for each drug concentration, respectively | 2016 | [126] | NCT01324635 |
| LBH-589 | Phase I | HDACi | HNC, PCa and Esophageal cancer | 7 completed study protocol | NS | NS | 2017 | NP | NCT00670553 |
CR, complete response; CRT, chemoradiotherapy; DNMTi, DNA methyltransferase inhibitors; FU, follow-up; Fr., fraction; HDACi, histone deacetylase inhibitors; HNC, Head and neck cancer; NS, not specified; OS, overall survival; RT, radiotherapy; TMZ, temozolomide; VPA, Valproic acid; pRT, palliative radiotherapy; PCa, prostate cancer; NA, not available; NP, not published; NS, not specified; NSCLC, non-small cell lung cancer; SCC, squamous cell carcinoma; SD, standard dose; SRS, stereotactic radiosurgery
Vorinostat, an FDA-approved HDACi (also known as SAHA) displays a potential radiosensitizer effect in several cancers (Fig. 4 and Additional file 1). Specifically, vorinostat improved radiosensitivity in three PCa cell lines with different radiation response patterns, both in normoxia and hypoxia conditions [114]. The radiosensitizer use of vorinostat decreased cell survival fraction and significantly increased G2/M cell fraction, along with specific HIF-1α and TP53 target downregulation [114]. Several clinical trials have reported considerable benefits of vorinostat in many cancer types subjected to IR (Table 1). Valproic acid (VPA), a common anti-epileptic drug, also a well-known HDACi, radiosensitize several cancers undergoing fractionated RT (Fig. 4 and Additional file 1). Low VPA concentrations (50 μM) sensitized PCa cells to IR through p53 acetylation stabilization and enhanced apoptosis (Fig. 4 and Additional file 1). Indeed, several reported studies corroborate these findings in other cancers (Fig. 4 and Additional file 1, Table 1). Trichostatin A (TSA), another HDACi, sensitized a PCSCs radioresistant fraction and depicted similar effect in other cancer models, showing increased radiation-induced DNA damage (Fig. 4). In the same vein, panobinostat (LBH-589), pan-HDACi, was able to increase PCa radiosensitivity as well as in other cancer types (Fig. 4 and Additional file 1, Table 1). The radiosensitizing effect of entinostat (MS-275) was studied in vitro in PCa and glioma cells (Fig. 4 and Additional file 1). Herein, along with irradiation, entinostat increased tumor cell apoptosis , as well as, radiosensitivity (Fig. 4 and Additional file 1). Although other well-known HDACi’s, such as FK228, CBHA, sodium butyrate, TMP195, and mocetinostat, were reported to exhibit a potential radiosensitizing effect by affecting DNA repair pathways (HR and NHEJ) in several malignancies, studies are lacking for PCa (Fig. 4 and Additional file 1).
Fig. 4.
Epigenetic targeting strategies to improve the clinical management of radioresistance. 59 pre-clinical studies have investigated a wide range of HDACi, DNMTi, and KDMi for radiosensitization purposes. Only 6 studies were conducted in PCa models. A further 8 clinical trials (Phase I, II, and III) are evaluating the use of HDACi and DNMTi in several cancer types in combination with conventional RT schemes. Overall, the use of these epidrugs results in cytotoxic effects promoting tumor cell death. DNMTi, DNA methyltransferase inhibitor; HDACi, histone deacetylase inhibitor; KDMi, histone lysine demethylase inhibitor. For additional information please access Additional file 1
DNA hypomethylating agents, DNMT inhibitors (DNMTi), also play a role in tumor radiosensitization. Specifically, the use of HDACi in combination with DNMTi showed a cumulative effect on cancer radiosensitization (Table 1, reporting combinatorial radiosensitization clinical trials in PCa and other cancers). 5-aza-2’-deoxycytidine (DAC) is the most commonly studied DNMTi (Fig. 4 and Additional file 1). Nonetheless, no reports are still available about PCa radiosensitization by these epidrugs (Fig. 4 and Additional file 1). Concerning histone methylation, JmjC-KDMs and LDS/KDM1A inhibitors are two well-known epigenetic drugs, with a limited number of studies about cancer radiosensitization. GSK-J4 is the most studied JmjC-KDM inhibitor, with KDM6/UTX specificity and cancer radiosensitivity effect (Fig. 4 and Additional file 1). Another JmjC-KDM inhibitor with higher specificity for KDM5 subfamily inhibition, JIB-04, was able to enhanced radiation response of lung squamous cell carcinoma cells [111]. Despite extensive knowledge of KDMs’ implication in hypoxia and tumor aggressiveness, as discussed in fifth section, few recent pre-clinical studies provided evidence that KDM activity inhibition may associate with tumor radiosensitization. Remarkably, LSD1/KDM1A has been highlighted as an important chromatin and gene transcriptional modulator in PCa [115]. LSD1 inhibitors are emerging as successful drugs with important role in PCa progression blockage, as well as, CRPC growth suppression [115]. However, there are a lack of approved inhibitors in radiation field with clinical validation. Of note, previous in vitro reports shown LSD1 transient recruitment to DNA damage allowing the activation of HR DNA repair machinery, such as 53BP1 and BRCA1 [116]. Herein, mechanistic studies with LSD1 knockdown significantly enhanced radiosensitivity [116]. Hence, LSD1 seems to be an interesting target to use in future as a tumor radiosensitizer, also for PCa. Additionally, BRD4, a bromodomain family-member with essential role as chromatin remodeler has been reported as a synergic interactor of LSD1 in CRPC [117]. Furthermore, BRD4 has a critical role for NHEJ DNA damage repair pathway and a low prognostic value for PCa patients submitted to RT [118]. In fact, according to the aforementioned clinical trials using epigenetic drugs, most of the inhibitors used are broad range effectors. The lack of specific targeting drugs is a critical drawback in the research field. Overall, further insights in the discovery of promising targetable epigenetic enzymes are urgently needed. For instance, as previously discussed in fifth section, KDM3A [112] and SMYD3 [113] were reported with a critical role as DNA damage and cell cycle mediators in PCa. The expression of these enzymes is commonly associated with poor PCa prognosis. However, until now there are no further validation with specific targeting epigenetic drugs.
Conclusions and future perspectives
Although PCa mostly constitutes a rather indolent malignancy, in a non-negligible percentage of cases can progress to highly aggressive disease. Advancements in translational research and innovative precision medicine techniques are urgently needed to determine the optimal clinical management based on intrinsic tumor biology. As many PCa patients are primarily treated with standard fractionated RT schemes, the development of novel adjuvant targeted strategies is imperative to overcome high recurrence rates of 20–40% in this subgroup of PCa patients. Epigenetic targeting might be the key to improving and extending survival of PCa patients. Indeed, epigenetically-inducible changes in DNA repair pathways and cell cycle play a critical role in response to IR. Cellular dynamics at epigenetic level led to overall tumor aggressiveness, increasing cell proliferation, invasion, and migration. Thus, the identification of specific key epigenetic targets and the use of epidrugs, such as inhibitors of DNMTs, HDACs, or KDMs, might radiosensitize tumor cells, increasing patients’ response to therapy and overall survival. Nevertheless, other studies are needed to further support these epidrugs as a novel treatment that should be used in combination with standard RT to improve PCa patients’ outcome.
Supplementary Information
Additional file 1. Complementary pre-clinical studies for tumor radiosensitization.
Abbreviations
- RT
Radiotherapy
- PCa
Prostate cancer
- PSA
Prostate specific antigen
- EBRT
External beam radiation therapy
- HRPC
High risk prostate cancer
- IR
Ionizing radiation
- PCSC
Prostate cancer stem cells
- NED
Neuroendocrine differentiation
- IMRT
Intensity modulated radiotherapy
- VMAT
Volumetric-modulated arc therapy
- IGRT
Image-guided radiotherapy
- bDFS
Biochemical disease-free survival
- ADT
Androgen deprivation therapy
- DSB
Double strand breaks
- DDR
DNA damage repair
- y-ATM
Autophosphorylated ataxia telangiectasia mutated
- BRCA
Breast cancer gene 1/2
- PARP
Poly(ADP-ribose) polymerase 1
- BER
Base excision repair
- NHEJ
Non homologous end-joining
- HR
Homologous recombination
- CRPC
Castration resistant prostate cancer
- EMT
Epithelial mesenchymal transition
- HIF
Hypoxia-inducible factor
- VEGF
Vascular endothelial growth factor
- CAIX
Carbonic anhydrase IX
- GLUT-1
Glucose transporter 1
- ROS
Reactive oxygen species
- PTEN
Phosphatidylinositol 3,4,5-triphosphate 3-phosphatase
- CSC
Cancer stem cells
- NEPC
Neuroendocrine prostate cancer
- lncRNA
Long non-coding RNA
- miRNA
Micro RNA
- RMI2
RecQ-mediated genome instability 2
- TOPBP1
DNA topoisomerase II binding protein 1
- DNMT
DNA methyltransferase
- LINE-1
Long interspersed nuclear element-1
- TET
Ten-eleven translocation
- APC
Adenomatous polyposis coli
- TSG
Tumor supressor gene
- RARβ2
Retinoic acid receptor beta2
- RASSF1A
Ras association domain family member1A
- CDNK2A
Cyclin-dependent kinase inhibitor2A
- HOXD3
Homeobox gene D3
- MGMT
O6-methylguanine DNA methyltransferase
- PTGS2
Prostaglandin-endoperoxide synthase 2
- CCND2
Cyclin2
- GSTP1
Glutathione S-transferase Pi1
- KMT
Lysine methyltransferase
- KDM
Lysine demethylase
- EZH2
Enhancer of zeste homolog 2
- JmjC
Jumonji c domain
- CBP
P300/CREB binding protein
- HAT
Histone acetyltransferase
- TNF
Tumor necrosis factor
- NF-kB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- SIRT
Sirtuins
- XAF1
XIAP-associated factor 1
- YY1
Yan Yang 1
- PTM
Post translational modifications
- IR
Ionizing radiation
- HDACi
HDAC inhibitors
- DNMTi
DNMT inhibitors
Authors' contributions
CM-S and RB conceptualized the paper. CM-S collected, analysed the information and elaborated the figures. CM-S, RB, FC, SC, CJ and LA drafted and revised the paper. All authors read and approved the final manuscript.
Funding
This work was supported by AIRC-17217; Campania Regional Government Technology Platform Lotta alle Patologie Oncologiche: iCURE-B21C17000030007; “Epigenetic Hallmarks of Multiple Sclerosis” (acronym Epi-MS) (id:415, Merit Ranking Area ERC LS) in VALERE 2019 Program; V:ALERE 2020 – Progetto competitivo “CIRCE” in risposta al bando D.R. n. 138 del 17/02/2020 Program; Campania Regional Government FASE2: IDEAL:Identificazione e caratterizzazione di nuovi approcci terapeutici contro il cancro CUP B63D18000560007; MIUR, Proof of Concept-EPICUREPOC01_00043-B64I19000290008; P.O.R. CAMPANIA FSE 2014/2020 ASSE III-B27D18001070006.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have declared no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Catarina Macedo-Silva, Email: anacatarina.macedodasilva@unicampania.it, Email: ana.catarina.macedo.silva@ipoporto.min-saude.pt.
Rosaria Benedetti, Email: rosaria.benedetti@unicampania.it.
Fortunato Ciardiello, Email: fortunato.ciardiello@unicampania.it.
Salvatore Cappabianca, Email: salvatore.cappabianca@unicampania.it.
Carmen Jerónimo, Email: carmenjeronimo@ipoporto.min-saude.pt, Email: cljeronimo@icbas.up.pt.
Lucia Altucci, Email: lucia.altucci@unicampania.it.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Morgan SC, Hoffman K, Loblaw DA, Buyyounouski MK, Patton C, Barocas D, et al. Hypofractionated radiation therapy for localized prostate cancer: executive summary of an ASTRO, ASCO, and AUA evidence-based guideline. Pract Radiat Oncol. 2018;8(6):354–360. doi: 10.1016/j.prro.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Khuntia D, Reddy CA, Mahadevan A, Klein EA, Kupelian PA. Recurrence-free survival rates after external-beam radiotherapy for patients with clinical T1–T3 prostate carcinoma in the prostate-specific antigen era: what should we expect? Cancer. 2004;100(6):1283–1292. doi: 10.1002/cncr.20093. [DOI] [PubMed] [Google Scholar]
- 5.Nickoloff JA, Boss MK, Allen CP, LaRue SM. Translational research in radiation-induced DNA damage signaling and repair. Transl Cancer Res. 2017;6(Suppl 5):S875–S891. doi: 10.21037/tcr.2017.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDermott N, Meunier A, Mooney B, Nortey G, Hernandez C, Hurley S, et al. Fractionated radiation exposure amplifies the radioresistant nature of prostate cancer cells. Sci Rep. 2016;6:34796. doi: 10.1038/srep34796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peitzsch C, Cojoc M, Hein L, Kurth I, Mäbert K, Trautmann F, et al. An epigenetic reprogramming strategy to resensitize radioresistant prostate cancer cells. Cancer Res. 2016;76(9):2637–2651. doi: 10.1158/0008-5472.CAN-15-2116. [DOI] [PubMed] [Google Scholar]
- 8.Fowler JF. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol. 2005;44(3):265–276. doi: 10.1080/02841860410002824. [DOI] [PubMed] [Google Scholar]
- 9.Dasu A, Toma-Dasu I. Prostate alpha/beta revisited—an analysis of clinical results from 14 168 patients. Acta Oncol. 2012;51(8):963–974. doi: 10.3109/0284186X.2012.719635. [DOI] [PubMed] [Google Scholar]
- 10.Cho LC, Timmerman R, Kavanagh B. Hypofractionated external-beam radiotherapy for prostate cancer. Prostate Cancer. 2013;2013:103547. doi: 10.1155/2013/103547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow JCL, Jiang R. Dose-volume and radiobiological dependence on the calculation grid size in prostate VMAT planning. Med Dosim. 2018;43(4):383–389. doi: 10.1016/j.meddos.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Alongi F, Fiorentino A, De Bari B. SBRT and extreme hypofractionation: A new era in prostate cancer treatments? Rep Pract Oncol Radiother. 2015;20(6):411–416. doi: 10.1016/j.rpor.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Bari B, Fiorentino A, Arcangeli S, Franco P, D'Angelillo RM, Alongi F. From radiobiology to technology: what is changing in radiotherapy for prostate cancer. Expert Rev Anticancer Ther. 2014;14(5):553–564. doi: 10.1586/14737140.2014.883282. [DOI] [PubMed] [Google Scholar]
- 14.Zelefsky MJ, Pei X, Chou JF, Schechter M, Kollmeier M, Cox B, et al. Dose escalation for prostate cancer radiotherapy: predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur Urol. 2011;60(6):1133–1139. doi: 10.1016/j.eururo.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrie C, Magné N, Burban-Provost P, Sargos P, Latorzeff I, Lagrange JL, et al. Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): a 112-month follow-up of a phase 3, randomised trial. Lancet Oncol. 2019;20(12):1740–1749. doi: 10.1016/S1470-2045(19)30486-3. [DOI] [PubMed] [Google Scholar]
- 16.Vale CL, Burdett S, Rydzewska LHM, Albiges L, Clarke NW, Fisher D, et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. Lancet Oncol. 2016;17(2):243–256. doi: 10.1016/S1470-2045(15)00489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pajonk F, Vlashi E, McBride WH. Radiation resistance of cancer stem cells: the 4 R's of radiobiology revisited. Stem Cells. 2010;28(4):639–648. doi: 10.1002/stem.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray KJ, Sibson NR, Kiltie AE. Treatment of breast and prostate cancer by hypofractionated radiotherapy: potential risks and benefits. Clin Oncol (R Coll Radiol) 2015;27(7):420–426. doi: 10.1016/j.clon.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo W, Sun YC, Bi JQ, He XY, Xiao L. Hypofractionated radiotherapy versus conventional radiotherapy in patients with intermediate- to high-risk localized prostate cancer: a meta-analysis of randomized controlled trials. BMC Cancer. 2019;19(1):1063. doi: 10.1186/s12885-019-6285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansour WY, Tennstedt P, Volquardsen J, Oing C, Kluth M, Hube-Magg C, et al. Loss of PTEN-assisted G2/M checkpoint impedes homologous recombination repair and enhances radio-curability and PARP inhibitor treatment response in prostate cancer. Sci Rep. 2018;8(1):3947. doi: 10.1038/s41598-018-22289-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Felice F, Tombolini V, Marampon F, Musella A, Marchetti C. Defective DNA repair mechanisms in prostate cancer: impact of olaparib. Drug Des Devel Ther. 2017;11:547–552. doi: 10.2147/DDDT.S110264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberti C. Prostate cancer: radioresistance molecular target-related markers and foreseeable modalities of radiosensitization. Eur Rev Med Pharmacol Sci. 2014;18(16):2275–2282. [PubMed] [Google Scholar]
- 23.Warner EW, Yip SM, Chi KN, Wyatt AW. DNA repair defects in prostate cancer: impact for screening, prognostication and treatment. BJU Int. 2019;123(5):769–776. doi: 10.1111/bju.14576. [DOI] [PubMed] [Google Scholar]
- 24.Frank S, Nelson P, Vasioukhin V. Recent advances in prostate cancer research: large-scale genomic analyses reveal novel driver mutations and DNA repair defects. F1000Res. 2018;7. [DOI] [PMC free article] [PubMed]
- 25.Maranto C, Udhane V, Hoang DT, Gu L, Alexeev V, Malas K, et al. STAT5A/B blockade sensitizes prostate cancer to radiation through inhibition of RAD51 and DNA repair. Clin Cancer Res. 2018;24(8):1917–1931. doi: 10.1158/1078-0432.CCR-17-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casimiro MC, Di Sante G, Ju X, Li Z, Chen K, Crosariol M, et al. Cyclin D1 promotes androgen-dependent DNA damage repair in prostate cancer cells. Cancer Res. 2016;76(2):329–338. doi: 10.1158/0008-5472.CAN-15-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, et al. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013;4:e875. doi: 10.1038/cddis.2013.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gravina GL, Marampon F, Sherris D, Vittorini F, Di Cesare E, Tombolini V, et al. Torc1/Torc2 inhibitor, Palomid 529, enhances radiation response modulating CRM1-mediated survivin function and delaying DNA repair in prostate cancer models. Prostate. 2014;74(8):852–868. doi: 10.1002/pros.22804. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Wang Y, Yu Y, Xu L, Zhang Y, Yu S, et al. Transcription factor HBP1 enhances radiosensitivity by inducing apoptosis in prostate cancer cell lines. Anal Cell Pathol (Amst) 2016;2016:7015659. doi: 10.1155/2016/7015659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(4):928–942. doi: 10.1016/j.ijrobp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Keam SP, Caramia F, Gamell C, Paul PJ, Arnau GM, Neeson PJ, et al. The transcriptional landscape of radiation-treated human prostate cancer: analysis of a prospective tissue cohort. Int J Radiat Oncol Biol Phys. 2018;100(1):188–198. doi: 10.1016/j.ijrobp.2017.09.037. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen KH, Hachem P, Khor LY, Salem N, Hunt KK, Calkins PR, et al. Adenoviral-E2F-1 radiosensitizes p53wild-type and p53null human prostate cancer cells. Int J Radiat Oncol Biol Phys. 2005;63(1):238–246. doi: 10.1016/j.ijrobp.2005.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Udayakumar TS, Hachem P, Ahmed MM, Agrawal S, Pollack A. Antisense MDM2 enhances E2F1-induced apoptosis and the combination sensitizes androgen-sensitive [corrected] and androgen-insensitive [corrected] prostate cancer cells to radiation. Mol Cancer Res. 2008;6(11):1742–1754. doi: 10.1158/1541-7786.MCR-08-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamel D, Gray C, Walia JS, Kumar V. PARP inhibitor drugs in the treatment of breast, ovarian, prostate and pancreatic cancers: an update of clinical trials. Curr Drug Targets. 2018;19(1):21–37. doi: 10.2174/1389450118666170711151518. [DOI] [PubMed] [Google Scholar]
- 35.Han S, Brenner JC, Sabolch A, Jackson W, Speers C, Wilder-Romans K, et al. Targeted radiosensitization of ETS fusion-positive prostate cancer through PARP1 inhibition. Neoplasia. 2013;15(10):1207–1217. doi: 10.1593/neo.131604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van de Ven AL, Tangutoori S, Baldwin P, Qiao J, Gharagouzloo C, Seitzer N, et al. Nanoformulation of olaparib amplifies PARP inhibition and sensitizes PTEN/TP53-deficient prostate cancer to radiation. Mol Cancer Ther. 2017;16(7):1279–1289. doi: 10.1158/1535-7163.MCT-16-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oing C, Tennstedt P, Simon R, Volquardsen J, Borgmann K, Bokemeyer C, et al. BCL2-overexpressing prostate cancer cells rely on PARP1-dependent end-joining and are sensitive to combined PARP inhibitor and radiation therapy. Cancer Lett. 2018;423:60–70. doi: 10.1016/j.canlet.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 38.Barreto-Andrade JC, Efimova EV, Mauceri HJ, Beckett MA, Sutton HG, Darga TE, et al. Response of human prostate cancer cells and tumors to combining PARP inhibition with ionizing radiation. Mol Cancer Ther. 2011;10(7):1185–1193. doi: 10.1158/1535-7163.MCT-11-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gani C, Coackley C, Kumareswaran R, Schütze C, Krause M, Zafarana G, et al. In vivo studies of the PARP inhibitor, AZD-2281, in combination with fractionated radiotherapy: An exploration of the therapeutic ratio. Radiother Oncol. 2015;116(3):486–494. doi: 10.1016/j.radonc.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Hennessey D, Martin LM, Atzberger A, Lynch TH, Hollywood D, Marignol L. Exposure to hypoxia following irradiation increases radioresistance in prostate cancer cells. Urol Oncol. 2013;31(7):1106–1116. doi: 10.1016/j.urolonc.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Horsman MR, Overgaard J. The impact of hypoxia and its modification of the outcome of radiotherapy. J Radiat Res. 2016;57(Suppl 1):i90–i98. doi: 10.1093/jrr/rrw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nahum AE, Movsas B, Horwitz EM, Stobbe CC, Chapman JD. Incorporating clinical measurements of hypoxia into tumor local control modeling of prostate cancer: implications for the alpha/beta ratio. Int J Radiat Oncol Biol Phys. 2003;57(2):391–401. doi: 10.1016/S0360-3016(03)00534-0. [DOI] [PubMed] [Google Scholar]
- 43.Carlson DJ, Keall PJ, Loo BW, Jr, Chen ZJ, Brown JM. Hypofractionation results in reduced tumor cell kill compared to conventional fractionation for tumors with regions of hypoxia. Int J Radiat Oncol Biol Phys. 2011;79(4):1188–1195. doi: 10.1016/j.ijrobp.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang J, Xia C, Cao ZX, Zheng JZ, Reed E, Jiang BH. Apigenin inhibits VEGF and HIF-1 expression via PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 2005;19(3):342–353. doi: 10.1096/fj.04-2175com. [DOI] [PubMed] [Google Scholar]
- 45.Meng AX, Jalali F, Cuddihy A, Chan N, Bindra RS, Glazer PM, et al. Hypoxia down-regulates DNA double strand break repair gene expression in prostate cancer cells. Radiother Oncol. 2005;76(2):168–176. doi: 10.1016/j.radonc.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 46.Chan N, Milosevic M, Bristow RG. Tumor hypoxia, DNA repair and prostate cancer progression: new targets and new therapies. Future Oncol. 2007;3(3):329–341. doi: 10.2217/14796694.3.3.329. [DOI] [PubMed] [Google Scholar]
- 47.Bindra RS, Crosby ME, Glazer PM. Regulation of DNA repair in hypoxic cancer cells. Cancer Metastasis Rev. 2007;26(2):249–260. doi: 10.1007/s10555-007-9061-3. [DOI] [PubMed] [Google Scholar]
- 48.Kim W, Lee S, Seo D, Kim D, Kim K, Kim E, et al. Cellular stress responses in radiotherapy. Cells. 2019;8(9):1105. doi: 10.3390/cells8091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jayakumar S, Kunwar A, Sandur SK, Pandey BN, Chaubey RC. Differential response of DU145 and PC3 prostate cancer cells to ionizing radiation: role of reactive oxygen species, GSH and Nrf2 in radiosensitivity. Biochim Biophys Acta. 2014;1840(1):485–494. doi: 10.1016/j.bbagen.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Bhandari V, Hoey C, Liu LY, Lalonde E, Ray J, Livingstone J, et al. Molecular landmarks of tumor hypoxia across cancer types. Nat Genet. 2019;51(2):308–318. doi: 10.1038/s41588-018-0318-2. [DOI] [PubMed] [Google Scholar]
- 51.Marignol L, Rivera-Figueroa K, Lynch T, Hollywood D. Hypoxia, notch signalling, and prostate cancer. Nat Rev Urol. 2013;10(7):405–413. doi: 10.1038/nrurol.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho YM, Kim YS, Kang MJ, Farrar WL, Hurt EM. Long-term recovery of irradiated prostate cancer increases cancer stem cells. Prostate. 2012;72(16):1746–1756. doi: 10.1002/pros.22527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma Y, Liang D, Liu J, Axcrona K, Kvalheim G, Stokke T, et al. Prostate cancer cell lines under hypoxia exhibit greater stem-like properties. PLoS ONE. 2011;6(12):e29170. doi: 10.1371/journal.pone.0029170. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Civenni G, Albino D, Shinde D, Vázquez R, Merulla J, Kokanovic A, et al. Transcriptional reprogramming and novel therapeutic approaches for targeting prostate cancer stem cells. Front Oncol. 2019;9:385. doi: 10.3389/fonc.2019.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim YS, Kang MJ, Cho YM. Low production of reactive oxygen species and high DNA repair: mechanism of radioresistance of prostate cancer stem cells. Anticancer Res. 2013;33(10):4469–4474. [PubMed] [Google Scholar]
- 56.Mimeault M, Batra SK. Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer- and metastasis-initiating cells. J Cell Mol Med. 2013;17(1):30–54. doi: 10.1111/jcmm.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kyjacova L, Hubackova S, Krejcikova K, Strauss R, Hanzlikova H, Dzijak R, et al. Radiotherapy-induced plasticity of prostate cancer mobilizes stem-like non-adherent, Erk signaling-dependent cells. Cell Death Differ. 2015;22(6):898–911. doi: 10.1038/cdd.2014.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X, Ma Z, Xiao Z, Liu H, Dou Z, Feng X, et al. Chk1 knockdown confers radiosensitization in prostate cancer stem cells. Oncol Rep. 2012;28(6):2247–2254. doi: 10.3892/or.2012.2068. [DOI] [PubMed] [Google Scholar]
- 59.Thompson TC, Liu B, Li L. N-MYC regulation of DNA damage response in neuroendocrine prostate cancer: mechanistic insight and novel combination therapy approaches. Oncoscience. 2018;5(11–12):273–275. doi: 10.18632/oncoscience.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krauss DJ, Hayek S, Amin M, Ye H, Kestin LL, Zadora S, et al. Prognostic significance of neuroendocrine differentiation in patients with Gleason score 8–10 prostate cancer treated with primary radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81(3):e119–e125. doi: 10.1016/j.ijrobp.2010.12.064. [DOI] [PubMed] [Google Scholar]
- 61.Patel GK, Chugh N, Tripathi M. Neuroendocrine differentiation of prostate cancer-an intriguing example of tumor evolution at play. Cancers. 2019;11(10):1405. doi: 10.3390/cancers11101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta K, Gupta S. Neuroendocrine differentiation in prostate cancer: key epigenetic players. Transl Cancer Res. 2017;6(Suppl 1):S104–S108. doi: 10.21037/tcr.2017.01.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Conteduca V, Oromendia C, Eng KW, Bareja R, Sigouros M, Molina A, et al. Clinical features of neuroendocrine prostate cancer. Eur J Cancer. 2019;121:7–18. doi: 10.1016/j.ejca.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bluemn EG, Coleman IM, Lucas JM, Coleman RT, Hernandez-Lopez S, Tharakan R, et al. Androgen receptor pathway-independent prostate cancer is sustained through FGF signaling. Cancer Cell. 2017;32(4):474–89.e6. doi: 10.1016/j.ccell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu CD, Choo R, Huang J. Neuroendocrine differentiation in prostate cancer: a mechanism of radioresistance and treatment failure. Front Oncol. 2015;5:90. doi: 10.3389/fonc.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Deng X, Liu H, Huang J, Cheng L, Keller ET, Parsons SJ, et al. Ionizing radiation induces prostate cancer neuroendocrine differentiation through interplay of CREB and ATF2: implications for disease progression. Cancer Res. 2008;68(23):9663–9670. doi: 10.1158/0008-5472.CAN-08-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suarez CD, Deng X, Hu CD. Targeting CREB inhibits radiation-induced neuroendocrine differentiation and increases radiation-induced cell death in prostate cancer cells. Am J Cancer Res. 2014;4(6):850–861. [PMC free article] [PubMed] [Google Scholar]
- 68.Huang YH, Zhang YQ, Huang JT. Neuroendocrine cells of prostate cancer: biologic functions and molecular mechanisms. Asian J Androl. 2019;21(3):291–295. doi: 10.4103/aja.aja_128_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Soundararajan R, Paranjape AN, Maity S, Aparicio A, Mani SA. EMT, stemness and tumor plasticity in aggressive variant neuroendocrine prostate cancers. Biochim Biophys Acta Rev Cancer. 2018;1870(2):229–238. doi: 10.1016/j.bbcan.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schulz A, Meyer F, Dubrovska A, Borgmann K. Cancer stem cells and radioresistance: DNA repair and beyond. Cancers (Basel) 2019;11(6):862. doi: 10.3390/cancers11060862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saga R, Matsuya Y, Takahashi R, Hasegawa K, Date H, Hosokawa Y. Analysis of the high-dose-range radioresistance of prostate cancer cells, including cancer stem cells, based on a stochastic model. J Radiat Res. 2019;60(3):298–307. doi: 10.1093/jrr/rrz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31(1):27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Valente MJ, Henrique R, Costa VL, Jeronimo C, Carvalho F, Bastos ML, et al. A rapid and simple procedure for the establishment of human normal and cancer renal primary cell cultures from surgical specimens. PLoS ONE. 2011;6(5):e19337. doi: 10.1371/journal.pone.0019337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Jadhav RR, Liu J, Wilson D, Chen Y, Thompson IM, et al. Roles of distal and genic methylation in the development of prostate tumorigenesis revealed by genome-wide DNA methylation analysis. Sci Rep. 2016;6:22051. doi: 10.1038/srep22051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rasmussen KD, Helin K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016;30(7):733–750. doi: 10.1101/gad.276568.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zelic R, Fiano V, Grasso C, Zugna D, Pettersson A, Gillio-Tos A, et al. Global DNA hypomethylation in prostate cancer development and progression: a systematic review. Prostate Cancer Prostatic Dis. 2015;18(1):1–12. doi: 10.1038/pcan.2014.45. [DOI] [PubMed] [Google Scholar]
- 77.Fiano V, Zugna D, Grasso C, Trevisan M, Delsedime L, Molinaro L, et al. LINE-1 methylation status in prostate cancer and non-neoplastic tissue adjacent to tumor in association with mortality. Epigenetics. 2017;12(1):11–18. doi: 10.1080/15592294.2016.1261786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gravina GL, Ranieri G, Muzi P, Marampon F, Mancini A, Di Pasquale B, et al. Increased levels of DNA methyltransferases are associated with the tumorigenic capacity of prostate cancer cells. Oncol Rep. 2013;29(3):1189–1195. doi: 10.3892/or.2012.2192. [DOI] [PubMed] [Google Scholar]
- 79.Bakavicius A, Daniunaite K, Zukauskaite K, Barisiene M, Jarmalaite S, Jankevicius F. Urinary DNA methylation biomarkers for prediction of prostate cancer upgrading and upstaging. Clin Epigenetics. 2019;11(1):115. doi: 10.1186/s13148-019-0716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Y, Li J, Yu X, Li S, Zhang X, Mo Z, et al. APC gene hypermethylation and prostate cancer: a systematic review and meta-analysis. Eur J Hum Genet. 2013;21(9):929–935. doi: 10.1038/ejhg.2012.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Audia JE, Campbell RM. Histone modifications and cancer. Cold Spring Harb Perspect Biol. 2016;8(4):a019521. doi: 10.1101/cshperspect.a019521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cimadamore A, Gasparrini S, Scarpelli M, Doria A, Mazzucchelli R, Massari F, et al. Epigenetic Modifications and Modulators in Prostate Cancer. Crit Rev Oncog. 2017;22(5–6):439–450. doi: 10.1615/CritRevOncog.2017020964. [DOI] [PubMed] [Google Scholar]
- 83.Ngollo M, Lebert A, Daures M, Judes G, Rifai K, Dubois L, et al. Global analysis of H3K27me3 as an epigenetic marker in prostate cancer progression. BMC Cancer. 2017;17(1):261. doi: 10.1186/s12885-017-3256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Daures M, Idrissou M, Judes G, Rifaï K, Penault-Llorca F, Bignon YJ, et al. A new metabolic gene signature in prostate cancer regulated by JMJD3 and EZH2. Oncotarget. 2018;9(34):23413–23425. doi: 10.18632/oncotarget.25182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ren G, Baritaki S, Marathe H, Feng J, Park S, Beach S, et al. Polycomb protein EZH2 regulates tumor invasion via the transcriptional repression of the metastasis suppressor RKIP in breast and prostate cancer. Cancer Res. 2012;72(12):3091–3104. doi: 10.1158/0008-5472.CAN-11-3546. [DOI] [PubMed] [Google Scholar]
- 86.Tamgue O, Lei M. Triptolide promotes senescence of prostate cancer cells through histone methylation and heterochromatin formation. Asian Pac J Cancer Prev. 2017;18(9):2519–2526. doi: 10.22034/APJCP.2017.18.9.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Crea F, Sun L, Mai A, Chiang YT, Farrar WL, Danesi R, et al. The emerging role of histone lysine demethylases in prostate cancer. Mol Cancer. 2012;11:52. doi: 10.1186/1476-4598-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Choi KC, Park S, Lim BJ, Seong AR, Lee YH, Shiota M, et al. Procyanidin B3, an inhibitor of histone acetyltransferase, enhances the action of antagonist for prostate cancer cells via inhibition of p300-dependent acetylation of androgen receptor. Biochem J. 2011;433(1):235–244. doi: 10.1042/BJ20100980. [DOI] [PubMed] [Google Scholar]
- 89.Lee JH, Yang B, Lindahl AJ, Damaschke N, Boersma MD, Huang W, et al. Identifying dysregulated epigenetic enzyme activity in castrate-resistant prostate cancer development. ACS Chem Biol. 2017;12(11):2804–2814. doi: 10.1021/acschembio.6b01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.denDekker AD, Davis FM, Joshi AD, Wolf SJ, Allen R, Lipinski J, et al. TNF-α regulates diabetic macrophage function through the histone acetyltransferase MOF. JCI Insight. 2020;5(5):e132306. doi: 10.1172/jci.insight.132306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weichert W, Röske A, Gekeler V, Beckers T, Stephan C, Jung K, et al. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br J Cancer. 2008;98(3):604–610. doi: 10.1038/sj.bjc.6604199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Camacho-Moctezuma B, Quevedo-Castillo M, Melendez-Zajgla J, Aquino-Jarquin G, Martinez-Ruiz GU. YY1 negatively regulates the XAF1 gene expression in prostate cancer. Biochem Biophys Res Commun. 2019;508(3):973–979. doi: 10.1016/j.bbrc.2018.12.056. [DOI] [PubMed] [Google Scholar]
- 93.Shinojima T, Yu Q, Huang SK, Li M, Mizuno R, Liu ET, et al. Heterogeneous epigenetic regulation of TIMP3 in prostate cancer. Epigenetics. 2012;7(11):1279–1289. doi: 10.4161/epi.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fortson WS, Kayarthodi S, Fujimura Y, Xu H, Matthews R, Grizzle WE, et al. Histone deacetylase inhibitors, valproic acid and trichostatin-A induce apoptosis and affect acetylation status of p53 in ERG-positive prostate cancer cells. Int J Oncol. 2011;39(1):111–119. doi: 10.3892/ijo.2011.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ai J, Wang Z. HDAC6 Regulation of Androgen Signaling in Prostate Cancer. 2013. p. 443–59.
- 96.Yang Y, Tse AK, Li P, Ma Q, Xiang S, Nicosia SV, et al. Inhibition of androgen receptor activity by histone deacetylase 4 through receptor SUMOylation. Oncogene. 2011;30(19):2207–2218. doi: 10.1038/onc.2010.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haider R, Massa F, Kaminski L, Clavel S, Djabari Z, Robert G, et al. Sirtuin 7: a new marker of aggressiveness in prostate cancer. Oncotarget. 2017;8(44):77309–77316. doi: 10.18632/oncotarget.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim JG, Park MT, Heo K, Yang KM, Yi JM. Epigenetics meets radiation biology as a new approach in cancer treatment. Int J Mol Sci. 2013;14(7):15059–15073. doi: 10.3390/ijms140715059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oronsky B, Scicinski J, Kim MM, Cabrales P, Salacz ME, Carter CA, et al. Turning on the radio: epigenetic inhibitors as potential radiopriming agents. Biomolecules. 2016;6(3):32. doi: 10.3390/biom6030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Friedl AA, Mazurek B, Seiler DM. Radiation-induced alterations in histone modification patterns and their potential impact on short-term radiation effects. Front Oncol. 2012;2:117. doi: 10.3389/fonc.2012.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stone L. Prostate cancer: radiotherapy induces epigenetic changes. Nat Rev Urol. 2016;13(5):241. doi: 10.1038/nrurol.2016.68. [DOI] [PubMed] [Google Scholar]
- 102.Sutton LP, Jeffreys SA, Phillips JL, Taberlay PC, Holloway AF, Ambrose M, et al. DNA methylation changes following DNA damage in prostate cancer cells. Epigenetics. 2019;14(10):989–1002. doi: 10.1080/15592294.2019.1629231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu X, Wang Y, Tan L, Fu X. The pivotal role of DNA methylation in the radio-sensitivity of tumor radiotherapy. Cancer Med. 2018;7(8):3812–3819. doi: 10.1002/cam4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Armstrong CA, Jones GD, Anderson R, Iyer P, Narayanan D, Sandhu J, et al. DNMTs are required for delayed genome instability caused by radiation. Epigenetics. 2012;7(8):892–902. doi: 10.4161/epi.21094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gorodetska I, Lukiyanchuk V, Peitzsch C, Kozeretska I, Dubrovska A. BRCA1 and EZH2 cooperate in regulation of prostate cancer stem cell phenotype. Int J Cancer. 2019;145(11):2974–2985. doi: 10.1002/ijc.32323. [DOI] [PubMed] [Google Scholar]
- 106.Nolan KD, Franco OE, Hance MW, Hayward SW, Isaacs JS. Tumor-secreted Hsp90 subverts polycomb function to drive prostate tumor growth and invasion. J Biol Chem. 2015;290(13):8271–8282. doi: 10.1074/jbc.M115.637496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pang J, Yang YW, Huang Y, Yang J, Zhang H, Chen R, et al. P110β inhibition reduces histone H3K4 di-methylation in prostate cancer. Prostate. 2017;77(3):299–308. doi: 10.1002/pros.23271. [DOI] [PubMed] [Google Scholar]
- 108.Makarević J, Tawanaie N, Juengel E, Reiter M, Mani J, Tsaur I, et al. Cross-communication between histone H3 and H4 acetylation and Akt-mTOR signalling in prostate cancer cells. J Cell Mol Med. 2014;18(7):1460–1466. doi: 10.1111/jcmm.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abbas A, Gupta S. The role of histone deacetylases in prostate cancer. Epigenetics. 2008;3(6):300–309. doi: 10.4161/epi.3.6.7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Komura K, Yoshikawa Y, Shimamura T, Chakraborty G, Gerke TA, Hinohara K, et al. ATR inhibition controls aggressive prostate tumors deficient in Y-linked histone demethylase KDM5D. J Clin Investig. 2018;128(7):2979–2995. doi: 10.1172/JCI96769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bayo J, Tran TA, Wang L, Peña-Llopis S, Das AK, Martinez ED. Jumonji inhibitors overcome radioresistance in cancer through changes in H3K4 methylation at double-strand breaks. Cell Rep. 2018;25(4):1040–50.e5. doi: 10.1016/j.celrep.2018.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fan L, Xu S, Zhang F, Cui X, Fazli L, Gleave M, et al. Histone demethylase JMJD1A promotes expression of DNA repair factors and radio-resistance of prostate cancer cells. Cell Death Dis. 2020;11(4):214. doi: 10.1038/s41419-020-2405-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vieira FQ, Costa-Pinheiro P, Almeida-Rios D, Graça I, Monteiro-Reis S, Simões-Sousa S, et al. SMYD3 contributes to a more aggressive phenotype of prostate cancer and targets Cyclin D2 through H4K20me3. Oncotarget. 2015;6(15):13644–13657. doi: 10.18632/oncotarget.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jonsson M, Ragnum HB, Julin CH, Yeramian A, Clancy T, Frikstad KM, et al. Hypoxia-independent gene expression signature associated with radiosensitisation of prostate cancer cell lines by histone deacetylase inhibition. Br J Cancer. 2016;115(8):929–939. doi: 10.1038/bjc.2016.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gao S, Chen S, Han D, Wang Z, Li M, Han W, et al. Chromatin binding of FOXA1 is promoted by LSD1-mediated demethylation in prostate cancer. Nat Genet. 2020;52(10):1011–1017. doi: 10.1038/s41588-020-0681-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mosammaparast N, Kim H, Laurent B, Zhao Y, Lim HJ, Majid MC, et al. The histone demethylase LSD1/KDM1A promotes the DNA damage response. J Cell Biol. 2013;203(3):457–470. doi: 10.1083/jcb.201302092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang J, Yu Q, Qiu Z, Dai T, Wang S, Yang X, et al. The combined effect of epigenetic inhibitors for LSD1 and BRD4 alters prostate cancer growth and invasion. Aging (Albany NY) 2020;12(1):397–415. doi: 10.18632/aging.102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li X, Baek G, Ramanand SG, Sharp A, Gao Y, Yuan W, et al. BRD4 promotes DNA repair and mediates the formation of TMPRSS2-ERG gene rearrangements in prostate cancer. Cell Rep. 2018;22(3):796–808. doi: 10.1016/j.celrep.2017.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shi W, Lawrence YR, Choy H, Werner-Wasik M, Andrews DW, Evans JJ, et al. Vorinostat as a radiosensitizer for brain metastasis: a phase I clinical trial. J Neurooncol. 2014;118(2):313–319. doi: 10.1007/s11060-014-1433-2. [DOI] [PubMed] [Google Scholar]
- 120.Choi CYH, Wakelee HA, Neal JW, Pinder-Schenck MC, Yu HM, Chang SD, et al. Vorinostat and concurrent stereotactic radiosurgery for non-small cell lung cancer brain metastases: a phase 1 dose escalation trial. Int J Radiat Oncol Biol Phys. 2017;99(1):16–21. doi: 10.1016/j.ijrobp.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 121.Galanis E, Anderson SK, Miller CR, Sarkaria JN, Jaeckle K, Buckner JC, et al. Phase I/II trial of vorinostat combined with temozolomide and radiation therapy for newly diagnosed glioblastoma: results of Alliance N0874/ABTC 02. Neuro Oncol. 2018;20(4):546–556. doi: 10.1093/neuonc/nox161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Teknos TN, Grecula J, Agrawal A, Old MO, Ozer E, Carrau R, et al. A phase 1 trial of Vorinostat in combination with concurrent chemoradiation therapy in the treatment of advanced staged head and neck squamous cell carcinoma. Invest New Drugs. 2019;37(4):702–710. doi: 10.1007/s10637-018-0696-4. [DOI] [PubMed] [Google Scholar]
- 123.Krauze AV, Myrehaug SD, Chang MG, Holdford DJ, Smith S, Shih J, et al. A phase 2 study of concurrent radiation therapy, temozolomide, and the histone deacetylase inhibitor valproic acid for patients with glioblastoma. Int J Radiat Oncol Biol Phys. 2015;92(5):986–992. doi: 10.1016/j.ijrobp.2015.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Candelaria M, Cetina L, Pérez-Cárdenas E, de la Cruz-Hernández E, González-Fierro A, Trejo-Becerril C, et al. Epigenetic therapy and cisplatin chemoradiation in FIGO stage IIIB cervical cancer. Eur J Gynaecol Oncol. 2010;31(4):386–391. [PubMed] [Google Scholar]
- 125.Takhar HS, Singhal N, Gowda R, Penniment M, Takhar P, Brown MP. Phase I study evaluating the safety and efficacy of oral panobinostat in combination with radiotherapy or chemoradiotherapy in patients with inoperable stage III non-small-cell lung cancer. Anticancer Drugs. 2015;26(10):1069–1077. doi: 10.1097/CAD.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 126.Shi W, Palmer JD, Werner-Wasik M, Andrews DW, Evans JJ, Glass J, et al. Phase I trial of panobinostat and fractionated stereotactic re-irradiation therapy for recurrent high grade gliomas. J Neurooncol. 2016;127(3):535–539. doi: 10.1007/s11060-016-2059-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Complementary pre-clinical studies for tumor radiosensitization.
Data Availability Statement
Not applicable.