Abstract
Introduction:
Keloids are pathological scars that are notorious for their chronic and relentless invasion into adjacent healthy skin, with commonly seen post-therapeutic recurrence after monotherapies.
Methods:
An English literature review on keloid pathophysiology was performed by searching the PubMed, Embase and Web of Science databases, to find out the up-to-date relevant articles. The level of evidence was evaluated based on the included studies with the highest level of evidence first.
Results:
Keloid morphology, signs, symptoms and the histopathological changes that occur in the local cells and extracellular matrix components are described. The theories on the pathophysiology of keloidogenesis that have been proposed to date are also covered; these include endocrinological, nutritional, vascular, and autoimmunological factors. In addition, we describe the local mechanical forces (and the mechanosignalling pathways by which these forces shape keloid cell activities) that promote keloid formation and determine the direction of invasion of keloids and the body sites that are prone to them.
Conclusion:
A better understanding of this pathological entity, particularly its mechanobiology, will aid the development of new diagnostic and therapeutic strategies for use in the clinic to prevent, reduce or even reverse the growth of this pathological scar.
Lay Summary
Keloids are skin scars that are famous for their chronic invasion into healthy skin, with commonly seen recurrence after surgeries. Cells such as lymphocytes, macrophages, mast cells and endothelial cells are involved in keloid growth. Particularly, endocrinological, nutritional, vascular, autoimmunological and mechanical factors actively take part in keloid progression.
Keywords: Keloid, mechanobiology, inflammation, endothelial cells, extracellular matrix, mechanosignalling pathway
Introduction
Keloids are commonly considered as pathological scars, and their prevalence ranges from 0.09% in the United Kingdom to 16% in the Congo. 1 In high-income countries, approximately 11 million patients with keloids were reported in 2000. 2 At present, there are no monotherapies that can resolve keloids completely. Moreover, post-therapeutic recurrence is common. Nevertheless, the ever-growing research on this chronic and refractory scar is helping to elucidate new therapeutic strategies. This review will focus on the clinical manifestations of keloids, including their signs and symptoms. The pathophysiological changes in the local cellular and extracellular matrix (ECM) components that occur during keloid growth will be discussed, along with the underlying molecular signalling pathways. Other hypotheses on the pathophysiology of keloid growth that have been proposed to date will also be described briefly.
Keloid morphology
Keloids often arise after non-severe trauma, such as chest hair folliculitis, mandibular border acne, scapular vaccination, ear piercing and caesarean section incision. Indeed, the patient often cannot recall the triggering event. As a result, the keloid is often ascribed to a ‘mosquito bite’ at the clinic. A notable feature of these scars is that they usually occur on specific body sites, namely, the anterior chest, mandibular border, scapula, earlobe and the suprapubic region. In contrast, they are rare on the scalp and the anterior lower leg, even in patients with severe keloids that cover much of their body. 3 This predilection for specific body sites relates closely to the degree of local mechanical stimuli on the skin in these regions. These stimuli include the hanging and stretching of the upper limbs and the friction that is imposed on the earlobes by jiggling earrings during daily body movements.
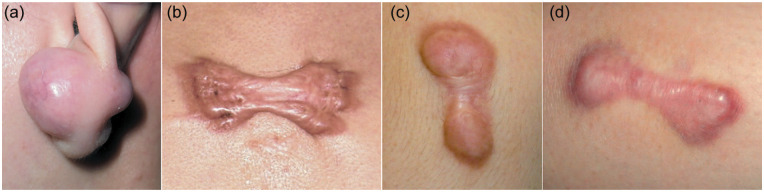
The notion that local mechanics promote keloid growth is supported by the fact that as keloids grow, they often adopt characteristic shapes on particular body regions. Specifically, keloids on the chest acquire symmetrical butterfly/crab’s claw shapes, whereas those on the upper arm/scapula and earlobe grow into dumbbell and isotropic ball shapes, respectively (Figure 1). These shapes reflect the predominant local distribution and balance of the mechanical skin tension on each body region. This tension places proinflammatory stress on certain areas of the scar, causing it to grow in the direction of the predominant stretching force. 3 Notably, if the mechanical balance on the skin is broken, the keloid shape will change accordingly. For example, when the symmetrical butterfly-shaped keloid on the anterior chest of a patient was accidentally bisected on the midline, the right-handed patient exhibited progression of the right half-butterfly and amelioration of the left half-butterfly over the next two years. This change reflects the more powerful vectorial stretching on the right side of the chest of this patient. 4
Figure 1.
Typical keloids on different areas of the body. Keloids adopt characteristic shapes on particular body regions. Specifically, keloids on the earlobe, chest and the upper arm/scapula commonly grow into isotropic balls (a), symmetrical butterfly/crab’s claw shapes (b) and dumbbell shapes (c, d).
Signs and symptoms of keloids
As keloids grow, they form a solid dermal mass with primary symptoms, such as bulkiness, erythema, itch and/or pain. These symptoms are particularly severe at the advancing edge of the keloid. The changes in colour and sensation are chronic and refractory, and their severity correlates with the speed with which the keloid invades the neighbouring skin. This growth always moves horizontally along the dermal plane and reflects the predominant direction of the mechanical stimuli on the skin. However, vertical keloid growth is confined to the cutaneous layer above the subcutaneous tissue.3,5
Keloid itchiness and pain mainly involve the border and centre of the scar, respectively. Other symptoms include higher warmth/heat pain thresholds and cold sensations; these symptoms probably reflect neuropathic sensory damage. Indeed, it has been shown that keloid skin exhibits abnormal small sensory fibre function, and the degree of this neuropathy correlates with the severity of itch. 6 Moreover, keloids exhibit substantial neuroinflammation, as shown by the increased levels of neuropeptides (e.g. substance P and the calcitonin gene-related peptide). 7 Animal models in which pathological scars are induced by the skin stretching ring also have high levels of these molecules in their scars. 8
The main secondary symptom of keloids is the presence of cutaneous fissures and infections. The solid texture of keloids and their uneven surface makes them comparatively difficult to clean. Consequently, they become contaminated easily. Moreover, the ever-increasing thickness and concurrent poor elasticity of keloids potentiate cutaneous fissures. Together with keloid contamination, this results in local infection in the form of hair folliculitis, furuncle or others. Such chronic and recurrent local infections can spur new keloid formation and/or promote keloid progression, thereby igniting a vicious cycle of pathology.
Keloids are not the only abnormally growing pathological scars; hypertrophic scars (HSs) also exhibit excessive scar growth. Indeed, both scar types are characterised by post-traumatic skin fibrosis and share several histological characteristics, namely, increased fibroblast numbers and the accumulation of ECM. However, the classical forms of these scars also differ in several clinical features, as described below:
(1) Etiological trigger: HSs have a history of being caused by local trauma, such as burns or surgical incisions. In contrast, the trauma that leads to keloids is often minor or cannot be recalled. Some keloids are caused by folliculitis, even though the patients do not realise it.
(2) Predilection sites: HSs usually do not show site preferences, whereas keloids tend to occur on body areas that are exposed to frequent mechanical stimuli (i.e. the anterior chest, scapula, earlobe and suprapubic region).
(3) Progression: HSs undergo temporary elevation within the confines of the original wound. In contrast, the progression of keloids is durable and results in continuous invasion into the adjacent normal skin and therefore beyond the original wound boundary.
(4) Contracture: HSs often demonstrate local pathological contracture. If the HS lies across a joint, such a local pathological contracture can result in functional disability. Such contractures are thought to be rarer in keloids. However, in our experience, patients with keloids can also develop joint contractures.
(5) Response to therapy: HSs respond well to excision and rarely exhibit postsurgical recurrence. In contrast, the same monotherapy in keloids (and even combined therapies) is frequently dogged by post-therapeutic recurrence.
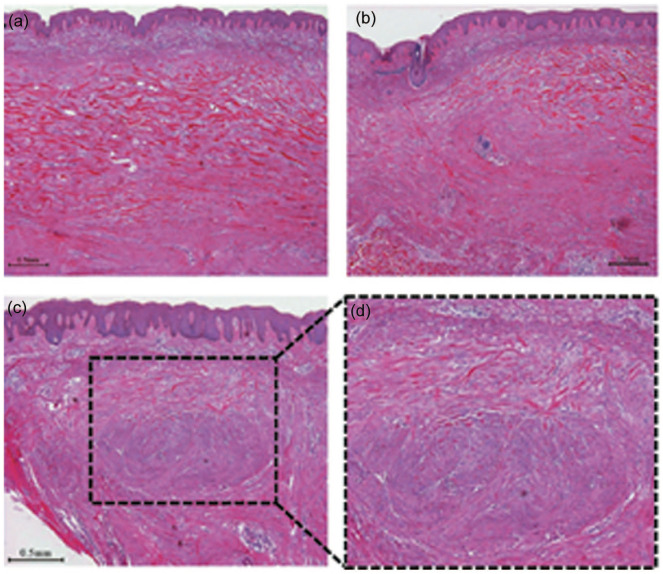
Given the different responses to various treatments, keloids and HSs should, if possible, be differentially diagnosed from each other. However, despite the clear demarcation between these scars at the theoretical level, clinicians (including experienced ones) can find it difficult to diagnose them. This reflects the fact that some of these lesions have intermediate clinical characteristics. Moreover, some keloids bear the traditional pathognomonic characteristics of keloids (hyalinised collagen) and HSs (dermal nodules); importantly, hyalinised collagen has been observed to initiate from within the dermal nodules (Figure 2). These findings have led us to view classical HSs and keloids as being successive stages of the same fibroproliferative skin disorder that are differentiated by the presence of differing degrees of inflammation. 9
Figure 2.
A H&E-stained keloid sample showing that keloidal collagen can coexist with dermal nodules, that are characteristic of classical hypertrophic scars. (a) central area (40×); (b) peripheral area (40×); (c) coexistence of keloidal collagen and dermal nodules (40×). The area indicated by dashed lines is enlarged (d) (100×). The images are from Huang et al. 9 with permission. Scale bar = 0.5 mm. H&E, haematoxylin and eosin.
Keloid histopathology
Keloids are a good example of dermal fibrosis; the key cellular event is a massive increase in fibroblasts that takes place mainly in the reticular dermis. 10 However, the changes that contribute to keloid formation and progression are not confined to the increase in fibroblasts. The epidermal and dermal layers participate in keloid growth, along with other molecular and cellular players, including various ECM components (collagen, hyaluronan and fibronectin), lymphocytes, macrophages, mast cells and endothelial cells, as will be detailed below.
In terms of fibrosis, the dermis of keloids is highly expanded by multiple packed collagen bundles that exhibit a larger distance between collagen bundle centres than in normal skin and normal scars. 11 This feature reflects an imbalance in collagen synthesis and degradation. Additionally, keloids show expression of upregulated type I procollagen and unaltered type III procollagen. These changes result in a markedly elevated type I/III procollagen mRNA ratio (22.1), compared to that in normal skin (5.2). 12 Further, keloids exhibit less degradation of newly synthesised collagen polypeptides than in normal skin, which contributes to the extensive accumulation of collagen in keloids. 13 Additionally, the collagen fibres in keloids are often large, hyalinised, strongly eosinophilic, randomly oriented in swirls and vary in length. 14 Such hyalinised collagen can be detected in 55% of keloid specimens. If such keloidal collagen is not detected, keloids can also be identified by other features, such as the presence of non-fibrotic papillary dermis, the horizontal cellular fibrous bands in the upper reticular dermis and the presence of prominent fascia-like bands in the deeper portion of the scar. 15 Furthermore, some keloids have dermal nodules that are composed of focal aggregates of fibroblasts and randomly oriented collagen fibres, and these have less distinct borders than the nodules in HSs. 16 Compared to normal skin, keloids have higher levels of other ECM components, such as fibronectin, which is intensely distributed in the intercellular matrix, 17 and hyaluronan, which is mainly found in the thickened granular and spinous layers of the keloid epidermis. 18
Keloids have been observed to exhibit chronic inflammation that involves the presence of non-fibroblast cellular players, such as lymphocytes, macrophages and mast cells.
Lymphocytes
Keloids have significantly increased numbers of CD3+ T lymphocytes and a significantly higher T-helper (CD4+):T-suppressor (CD8+) ratio compared to normal skin. 19 Moreover, T lymphocytes are more dominant in keloids than B lymphocytes, although there may be some discrepancy in reports regarding the frequency of B cells in keloids. One study reported that the perivascular region of the blood vessels in the upper dermis of keloids has CD20+ B lymphocytes, 20 whereas another study did not detect B lymphocytes in keloids. 19
Macrophages
Keloids associate with both the classical pro-inflammatory M1 (CD68+) and anti-inflammatory M2 (CD163+) types. Compared to normal skin, keloids have significantly higher numbers of M1 CD68+ macrophages, which are diffusely distributed within the keloid dermis. 19 However, keloid lesions also exhibit increased infiltration of M2 CD163+ macrophages. M2 cells represent the majority of the macrophage populations that infiltrate the intralesional and perilesional keloidal sites, 21 suggesting that keloid macrophages tend to exhibit M2 polarisation. This phenotype is supported by the greater expression of M2-associated genes (e.g. interleukin [IL]-10 and transforming growth factor [TGF]-β) in keloids compared to that of M1-associated genes (e.g. iNOS and IL-2). 22
Mast cells
Their numbers are significantly increased in keloids, 23 along with their activities, as indicated by the upregulation of the specific activation marker mast cell β-tryptase. 24 Moreover, keloids harbour mast cells that are rich in basophilic granules and degranulated mast cells with diffuse cytoplasmic staining. 20 Mast cells are frequently in direct cell contact with myofibroblasts in keloids, as shown by the intimate contact of the filopodia of the mast cells with the cell membranes of the myofibroblasts. 25
Endothelial cells and blood vessels they generate
At the morphological level, most of the blood vessels in keloids appear to be partially occluded. This is due to either hypertrophy of their endothelial lining or the deposition of a homogenous perivascular matrix and fine collagen fibrils. 20 At the quantitative level, keloid patients have significantly higher numbers of circulating endothelial progenitor cells (i.e. CD45-CD34+CD133+VEGFR2+ cells) than healthy controls. 26 At the functional level, keloids differ from normal skin controls in two endothelial function indices, i.e. keloids have greater baseline brachial artery diameters and smaller endothelium-dependent vasodilatory responses. These findings suggest that endothelial dysfunction may participate in keloid growth. 27 We hypothesise that this endothelial dysfunction may become pathogenic in the context of provocative genetic mutations, systemic conditions and/or local conditions that promote and thereby aggravate local inflammation during keloid pathogenesis. 28
Theories on the pathophysiology underlying keloidogenesis
It is well known that keloid formation is associated with specific endocrinological, nutritional, vascular and autoimmunological characteristics. Some of these are described in detail below.
Endocrinological characteristics
These include excessive sebaceous secretions in adolescents and at body sites with active sebaceous secretions that are particularly prone to keloid formation. 29
Nutritional characteristics
These include an imbalance between omega-6 and omega-3 essential fatty acid intake. Keloid patients have higher levels of dietary linoleic acid and arachidonic acid, and lower levels of α-linolenic acid, eicosapentaenoic acid and docosahexaenoic acid than recommended. 30
Vascular characteristics
Keloid patients with hypertension have more severe keloids than normotensive patients. 31 The ability of antihypertension pharmaceuticals to improve keloids, 32 and the histological and clinical vascular abnormalities described above further support the notion that vascular (endothelial) dysfunction contributes to keloid growth. 33
Autoimmunological characteristics
Keloid patients, but not HS patients or healthy controls, have antinuclear antibodies (in the eluates) that mainly target the fibroblasts in keloids. 34 The possibility that immunological autoreactivity may promote keloid growth is further supported by the fact that keloid patients have higher levels of circulating IgG complexes than healthy controls. 35 As the evidence for some of these hypotheses remains tentative, further investigations are warranted.
Another theory about the pathophysiology underlying keloid formation relates to local mechanics and the mechanosignalling pathways that transduce these activating forces to culprit cells in keloids, such as fibroblasts/myofibroblasts. Studies have shown a close association between local skin mechanics and keloid development. 36 This finding has led to considerable research in scar mechanobiology, which has greatly increased our understanding of how external mechanical stimuli are sensed by cells and converted to biochemical signals, directing scarring responses. Several signalling pathways have been found to participate in keloid growth, including the TGF-β/Smad, integrin, and calcium ion, as well as the potential mitogen-activated protein kinase (MAPK) and G protein, Wnt/β-catenin, tumour necrosis factor-α (TNF-α)/nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) and IL signalling pathways. 37 During keloid progression, these cellular mechanosignalling pathways interact actively with the ECM and/or crosstalk extensively with the hypoxia, inflammation and angiogenesis pathways. 37 Delineating these molecular changes during keloid invasion is likely to help us to better understand the mechanisms that underlie keloid development. This will aid the identification of potential biomarkers for keloid invasion and the development of pharmaceutical targets for use in keloid therapy.
Conclusion
In summary, keloids are pathological scars that show chronic and relentless invasion into the adjacent healthy skin. They are characterised by the profound accumulation of fibroblasts and by changes in various ECM components, other cell ingredients and molecular signalling pathways. A better understanding of this pathological entity will aid the development of new diagnostic and therapeutic strategies, for use in the clinic, that can prevent, reduce or even reverse the growth of this pathological scar.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Chenyu Huang  https://orcid.org/0000-0001-9521-5650
https://orcid.org/0000-0001-9521-5650
Rei Ogawa  https://orcid.org/0000-0003-3658-555X
https://orcid.org/0000-0003-3658-555X
References
- 1. Louw L. Keloids in rural black South Africans. Part 1: general overview and essential fatty acid hypotheses for keloid formation and prevention. Prostaglandins Leukot Essent Fatty Acids 2000; 63: 237–245. [DOI] [PubMed] [Google Scholar]
- 2. Bayat A, McGrouther DA, Ferguson MWJ. Skin scarring. BMJ 2003; 326: 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ogawa R, Okai K, Tokumura F, et al. The relationship between skin stretching/contraction and pathologic scarring: the important role of mechanical forces in keloid generation. Wound Repair Regen 2012; 20: 149–157. [DOI] [PubMed] [Google Scholar]
- 4. Huang C, Liu L, You Z, et al. Keloid progression: a stiffness gap hypothesis. Int Wound J 2017; 14: 764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang C, Liu L, You Z, et al. Managing keloid scars: From radiation therapy to actual and potential drug deliveries. Int Wound J 2019; 16: 852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee SS, Yosipovitch G, Chan YH, et al. Pruritus, pain, and small nerve fiber function in keloids: a controlled study. J Am Acad Dermatol 2004; 51: 1002–1006. [DOI] [PubMed] [Google Scholar]
- 7. Akaishi S, Ogawa R, Hyakusoku H. Keloid and hypertrophic scar: neurogenic inflammation hypotheses. Med Hypotheses 2008; 71: 32–38. [DOI] [PubMed] [Google Scholar]
- 8. Chin MS, Lancerotto L, Helm DL, et al. Analysis of neuropeptides in stretched skin. Plast Reconstr Surg 2009; 124: 102–113. [DOI] [PubMed] [Google Scholar]
- 9. Huang C, Akaishi S, Hyakusoku H, et al. Are keloid and hypertrophic scar different forms of the same disorder? A fibroproliferative skin disorder hypothesis based on keloid findings. Int Wound J 2014; 11: 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci 2017; 18: 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verhaegen PDHM, van Zuijlen PPM, Pennings NM, et al. Differences in collagen architecture between keloid, hypertrophic scar, normotrophic scar, and normal skin: An objective histopathological analysis. Wound Repair Regen 2009; 17: 649–656. [DOI] [PubMed] [Google Scholar]
- 12. Uitto J, Perejda AJ, Abergel RP, et al. Altered steady-state ratio of type I/III procollagen mRNAs correlates with selectively increased type I procollagen biosynthesis in cultured keloid fibroblasts. Proc Natl Acad Sci U S A 1985; 82: 5935–5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abergel RP, Pizzurro D, Meeker CA, et al. Biochemical composition of the connective tissue in keloids and analysis of collagen metabolism in keloid fibroblast cultures. J Invest Dermatol 1985; 84: 384–390. [DOI] [PubMed] [Google Scholar]
- 14. Rockwell WB, Cohen IK, Ehrlich HP. Keloids and hypertrophic scars: a comprehensive review. Plast Reconstr Surg 1989; 84: 827–837. [DOI] [PubMed] [Google Scholar]
- 15. Lee JYY, Yang CC, Chao SC, et al. Histopathological differential diagnosis of keloid and hypertrophic scar. Am J Dermatopathol 2004; 26: 379–384. [DOI] [PubMed] [Google Scholar]
- 16. Clark JA, Turner ML, Howard L, et al. Description of familial keloids in five pedigrees: evidence for autosomal dominant inheritance and phenotypic heterogeneity. BMC Dermatol 2009; 9: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kischer CW, Hendrix MJ. Fibronectin (FN) in hypertrophic scars and keloids. Cell Tissue Res 1983; 231: 29–37. [DOI] [PubMed] [Google Scholar]
- 18. Bertheim U, Hellström S. The distribution of hyaluronan in human skin and mature, hypertrophic and keloid scars. Br J Plast Surg 1994; 47: 483–489. [DOI] [PubMed] [Google Scholar]
- 19. Boyce DE, Ciampolini J, Ruge F, et al. Inflammatory-cell subpopulations in keloid scars. Br J Plast Surg 2001; 54: 511–516. [DOI] [PubMed] [Google Scholar]
- 20. Shaker SA, Ayuob NN, Hajrah NH. Cell talk: a phenomenon observed in the keloid scar by immunohistochemical study. Appl Immunohistochem Mol Morphol 2011; 19: 153–159. [DOI] [PubMed] [Google Scholar]
- 21. Bagabir R, Byers RJ, Chaudhry IH, et al. Site-specific immunophenotyping of keloid disease demonstrates immune upregulation and the presence of lymphoid aggregates. Br J Dermatol 2012; 167: 1053–1066. [DOI] [PubMed] [Google Scholar]
- 22. Jin Q, Gui L, Niu F, et al. Macrophages in keloid are potent at promoting the differentiation and function of regulatory T cells. Exp Cell Res 2018; 362: 472–476. [DOI] [PubMed] [Google Scholar]
- 23. Ghazawi FM, Zargham R, Gilardino MS, et al. Insights into the pathophysiology of hypertrophic scars and keloids: how do they differ? Adv Skin Wound Care 2018; 31: 582–595. [DOI] [PubMed] [Google Scholar]
- 24. Ong CT, Khoo YT, Mukhopadhyay A, et al. Comparative proteomic analysis between normal skin and keloid scar. Br J Dermatol 2010; 162: 1302–1315. [DOI] [PubMed] [Google Scholar]
- 25. Lee YS, Vijayasingam S. Mast cells and myofibroblasts in keloid: a light microscopic, immunohistochemical and ultrastructural study. Ann Acad Med Singapore 1995; 24: 902–905. [PubMed] [Google Scholar]
- 26. Zhang GY, Wu LC, Liao T, et al. Altered circulating endothelial progenitor cells in patients with keloid. Clin Exp Dermatol 2016; 41: 152–155. [DOI] [PubMed] [Google Scholar]
- 27. Ziyrek M, Şahin S, Acar Z, et al. The relationship between proliferative scars and endothelial function in surgically revascularized patients. Balkan Med J 2015; 32: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ogawa R, Akaishi S. Endothelial dysfunction may play a key role in keloid and hypertrophic scar pathogenesis - Keloids and hypertrophic scars may be vascular disorders. Med Hypotheses 2016; 96: 51–60. [DOI] [PubMed] [Google Scholar]
- 29. Fong EP, Bay BH. Keloids - the sebum hypothesis revisited. Med Hypotheses 2002; 58: 264–269. [DOI] [PubMed] [Google Scholar]
- 30. Louw L, Dannhauser A. Keloids in rural black South Africans. Part 2: dietary fatty acid intake and total phospholipid fatty acid profile in the blood of keloid patients. Prostaglandins Leukot Essent Fatty Acids 2000; 63: 247–253. [DOI] [PubMed] [Google Scholar]
- 31. Arima J, Huang C, Rosner B, et al. Hypertension: A systemic key to understanding local keloid severity. Wound Repair Regen 2015; 23: 213–221. [DOI] [PubMed] [Google Scholar]
- 32. Huang C, Ogawa R. Pharmacological treatment for keloids. Expert Opin Pharmacother 2013; 14: 2087–2100. [DOI] [PubMed] [Google Scholar]
- 33. Huang C, Liu L, You Z, et al. Endothelial dysfunction and mechanobiology in pathological cutaneous scarring: Lessons learned from soft tissue fibrosis. Br J Dermatol 2017; 177: 1248–1255. [DOI] [PubMed] [Google Scholar]
- 34. De Limpens AM, Cormane RH. Studies on the immunologic aspects of keloids and hypertrophic scars. Arch Dermatol Res 1982; 274: 259–266. [DOI] [PubMed] [Google Scholar]
- 35. Kazeem AA. The immunological aspects of keloid tumor formation. J Surg Oncol 1988; 38: 16–18. [DOI] [PubMed] [Google Scholar]
- 36. Ogawa R, Akaishi S, Huang C, et al. Clinical applications of basic research that shows reducing skin tension could prevent and treat abnormal scarring: the importance of fascial/subcutaneous tensile reduction sutures and flap surgery for keloid and hypertrophic scar reconstruction. J Nippon Med Sch 2011; 78: 68–76. [DOI] [PubMed] [Google Scholar]
- 37. Huang C, Akaishi S, Ogawa R. Mechanosignaling pathways in cutaneous scarring. Arch Dermatol Res 2012; 304: 589–597. [DOI] [PubMed] [Google Scholar]
References
How to cite this article
- Huang C, Ogawa R. Keloidal pathophysiology: Current notions. Scars, Burns & Healing, Volume 6, 2020. DOI: 10.1177/2059513118980320. [DOI] [PMC free article] [PubMed] [Google Scholar]