Abstract
Background: Tumor-infiltrating lymphocytes (TILs) and chimeric antigen receptor (CAR) T-cell therapies have demonstrated promising, though limited, efficacy against melanoma. Methods: We designed a model system to explore the efficacy of dual specific T cells derived from melanoma patient TILs by transduction with a Her2-specific CAR. Results: Metastatic melanoma cells in our biobank constitutively expressed Her2 antigen. CAR-TIL produced greater amounts of IFN compared with parental TIL, when co-cultured with Her2 expressing tumor lines, including autologous melanoma tumor lines, although no consistent increase in cytotoxicity by TIL was afforded by expression of a CAR. Results of an in vivo study in NSG mice demonstrated tumor shrinkage when CAR-TILs were used in an adoptive cell therapy protocol. Conclusion: Potential limitations of transduced TIL in our study included limited proliferative potential and a terminally differentiated phenotype, which would need addressing in further work before consideration of clinical translation.
Keywords: autologous tumor, chimeric antigen receptor, Her2, immunotherapy, melanoma
Introduction
The anti-cancer potential of the human immune system has been recognized for over 100 years. It is only in the last decade, however, that these therapies have become standard of care for the treatment of patients with cancer. Checkpoint blockade immunotherapies (CBIs) are now used routinely in the management of patients with advanced melanoma, and the use of cell-based therapies has been expanding in many cancers, including melanoma.
Adoptive cell therapy (ACT) involves ex vivo expansion of anti-tumor lymphocytes followed by transfusion back into the patient, sometimes together with growth factors to promote T-cell survival and expansion. 1 Tumor-infiltrating lymphocytes (TILs) are a heterogeneous population capable of recognizing multiple tumor specific antigens but are often incapable of eradicating the tumor due to a combination of inhibiting signals from other immune cells, tumor cells and the surrounding tumor microenvironment.2,3 Ex vivo expansion of TILs allows optimization for cell growth away from the immunosuppressive tumor microenvironment. 4 ACT using TILs is a personalized therapy tailored specifically to each patient and their tumor, being human leukocyte antigen (HLA) specific, and has demonstrated durable disease control in patients with metastatic melanoma – even in patients heavily pre-treated with multiple forms of chemotherapy.1,5,6 ACT and has several advantages over vaccines and CBI, providing large numbers of T cells that can exhibit chemotaxis and home to tumor sites throughout the body, including the brain. 5 ACT requires only a small population of antigen-specific tumor cells that can be expanded to large numbers for treatment. 7 TIL therapy has been performed at multiple international centers with reproducible results.6,8–11
Alternative techniques in ACT include genetically modifying a patient’s peripheral blood T lymphocytes to express a transgenic T-cell receptor (TCR) or chimeric antigen receptor (CAR) targeted to a specific tumor antigen. CARs are an artificial receptor, genetically engineered to combine the extracellular antigen binding domain of an antibody with the intracellular signaling portion of TCR (CD3ζ) and one or more co-stimulatory domains (CD28 and/or 4-1BB). The highest clinical response rates have been shown with CAR targeting CD19, a protein expressed in most B-cell malignancies, with complete remission rates of 50–90%.12–14 To date, success using CAR technology in patients with solid malignancies has been limited.
CAR T cells offer advantages over TIL therapy. CAR T cells are obtained from peripheral blood samples and modified to target and destroy cells that express specific antigens. CAR T cells are not HLA restricted and do not require an antigen-presenting cell consort with additional co-stimulation to produce an effective T-cell response against antigens. CAR T cells function even at low levels of antigen expression; the avidity of cell-surface displayed CARs can be modified and is greater than the avidity of a bivalent antibody. CAR T cells have been shown to be effective in the treatment of some hematological malignancies with high rates of durable remission.15–18 Owing to the clinical success of CAR T cells, in 2017 the first agents were approved by the US Food and Drug Administration for refractory B-cell precursor acute lymphoblastic leukemia and refractory or relapsed large B-cell lymphoma.
Our study explores the novel concept of combining TIL and CAR therapies to generate dual-specific TILs. We hypothesized that combining TIL and CAR therapies would provide an opportunity to extend the degree and breadth of tumor antigen recognition and increase effector functions, which may enhance either therapy alone. We established a model system to test this proof of concept using TILs and transduced them to express a CAR directed against a common tumor antigen, Her2, expressed on tumor samples matching the autologous patient TIL. TILs derived from metastatic melanoma could be transduced with anti-Her2 CAR and demonstrated activity when CARs were stimulated. CAR-TILs had greater anti-tumor activity against Her2 expressing tumor cell lines, including autologous tumor, than non-Her2 expressing tumor lines. All patient samples analyzed from our tissue biobank inherently expressed Her2 antigen. In addition, we established a xenograft model using autologous melanoma tumors and TILs transduced with anti-Her2 CAR, which were used in an ACT protocol and tumor response measured.
Materials and methods
Generation of melanoma TILs
TILs and tumor specimens in the biobank were derived from patients at the Peter MacCallum Cancer Centre with metastatic melanoma identified prospectively in a multidisciplinary meeting and consented prior to surgery. Patients selected for inclusion in the biobank had in-transit, regional or oligometastatic disease and, in most cases, patients underwent surgical resection for curative intent. Some underwent resection after they demonstrated disease progression while receiving CBI. TILs were cultured from approximately 8 mm 3 tumor pieces in RPMI media and supplemented with 10% heat inactivated human AB serum filtered to 0.2 μm (Valley Biomedical, Winchester, VA, USA; HS1017), PenStrep (100 U/ml penicillin, 100 ug/ml streptomycin), GlutaMAX (2 mM L-glutamine ThermoFisher Scientific Inc.), gentamicin (10 μg/ml Amphotericin B (0.25 μg/ml; Fungizone, E.R. Squibb&Sons, L.L.C., Princeton, NJ, USA) and 6000 IU/ml of interleukin-2 (IL-2; Jiangsu Kingsley Pharmaceutical Co. Ltd, Yixing City, Jiangsu Province, China). When TIL culture numbers reached >10 × 107 they were frozen and stored in aliquots of 5 and 10 × 106 cells. When required, after thawing, T cells were rested overnight in IL-2-containing media (600 IU/ml) and then stimulated with Human T-Activator CD3/28 beads Dynabeads® (ThermoFisher Scientific, Catalogue # 11131D) in a 1:1 bead to T-cell ratio according to manufacturer instructions. IL-2 was maintained at concentration 600 IU/ml. Cells were incubated at 37°C 5% CO2. Five days after activation, T cells were transduced using supernatant from the packaging line PG13–anti-Her2 CD28/CD3ζ. T cells in culture were maintained in flasks in an upright position at a concentration of 1–2 × 106 cells/ml.
Transduction of TILs
Retrovirus encoding a CAR composed of an extracellular scFv–anti-human Her2 fused to the transmembrane and cytoplasmic domains of CD28 and the cytoplasmic domain of CD3ζ was obtained from the supernatant of the PG13 packaging line as previously described. 70 Four milliliters of retroviral supernatant was added to each well of RetroNectin® (Takara Bio)-coated (10 μg/ml) 6-well non-treated plates (Corning Costar, Fisher Scientific, Suwanee, GU, USA). After a 30-min spin of virus onto retronectin-coated plates (1200g), T cells were resuspended in 1 ml of additional retroviral-containing supernatant supplemented with IL-2 and added to the retronectin-coated plates to give a volume of 5 ml/well with T-cell concentration 2.5 × 106 per well. T cells were maintained in IL-2 containing media at 600 IU/ml. After a 60-min spin at 300g T cells were incubated overnight before the transduction process was repeated. Transduced T cells were selected using G418 (50 μg/ml) and analyzed by flow cytometry 5 days after selection. For control, T cells were transduced using this protocol using supernatant from a PG13 cell line producing an empty viral vector. CAR expression was detected using anti-Myc tag (2276; Cell Signaling Technology) with secondary antibody F(ab’)2 Donkey Anti-Mouse IgG PE (eBioscience). Following transduction, a second activation using rapid expansion protocol (REP) was utilized to increase T-cell numbers for experiments. T cells were rested overnight in IL-2-containing media (3000 IU/ml) and then stimulated with soluble anti-CD3 (OKT3; 30 ng/ml, Bioscience™) and 3000 IU/ml IL-2 in the presence of irradiated (50 Gy) allogeneic feeder peripheral blood mononuclear cells (PBMCs) in a ratio of 1:100. Flasks were incubated and the media was refreshed at days 3 and 6. TILs were assessed by flow cytometry on day 9 following second activation. The choice of TIL for phenotypic and functional characterization was largely dictated by the limited availability of patient material and matched TIL–tumour pairs. Patient TILs are used as listed in Table 1.
Table 1.
The patient TIL number used in each experiment. The experiments in each of Figures 1–8 are listed, together with the patient number from which the TILs were derived.
| Experiment | Patient # included | |
|---|---|---|
| Figure 1 | Phenotypic screening | 10, 11, 15, 20 |
| Figure 2 | T-cell proliferation and differentiation | |
| (a) and (b): T-cell growth | 8, 9. 10, 11. 20, 22 | |
| (c): CTV measurements | 11, 20 | |
| (d) and (e): T-cell subtype | 11 | |
| Figure 3 | Transduction with CAR | |
| (a): CAR expression | 10 | |
| (b): CAR expression | 11 | |
| (c): CAR expression | 8, 9, 10, 11, 15, 20, 22 | |
| (d): #CAR per cell | 10, 11, 15 | |
| Figure 4 | Transduction maintained following REP | 10 |
| Figure 5 | Melanoma tumor Her2 antigen expression | |
| (a): S100 B expression | 10 | |
| (b): S100 B expression | 11 | |
| (c): melanoma tumor Her2 antigen expression | 10, 11, 34, 46 | |
| (d): Melanoma sorted for High Her2 antigen expression | 11, 15 | |
| (e): melanoma tumor Her2 antigen expression | 10, 11, 15, 34, 46 | |
| Figure 6 | IFNγ production and cell killing | |
| (a)–(f): IFNγ production | 11, 15, 20 | |
| (g)–(i): cell killing | 10, 11 | |
| Figure 7 | Transduction with CAR and cell phenotype | 10, 11 |
| Figure 8 | TIL activity in vivo | 10 |
CAR, chimeric antigen receptor; CTV, cell trace violet; REP, rapid expansion protocol; TIL, tumor-infiltrating lymphocyte.
Functional in vitro assessment
Cytokine measurement by enzyme-linked immunosorbent assay
The ability of TIL-CAR to secrete human interferon gamma (hIFN) in response to Her2 expressing tumor cells (24JKHer2, and autologous patient matched melanoma tumor cells) was assessed from overnight coculture of 1 × 106 T cells and 5 × 105 Her2 expressing cells/ml using T-cell media. Flat-bottom 96-well plates (Corning™ Costar™, NY, USA; product #3997) were coated with one row each of 50 μl of c-Myc tag (1:100), IgG2a (1:50) and OKT3 (1:500; 1 mg/ml) diluted in PBS and incubated at 4°C overnight. Cocultures were established and incubated overnight at 37°C at 5% CO2. The following day, plates were spun at 400 g for 4 min and supernatants aspirated and transferred to a 96-well U-bottom plate (Corning™ Costar™, NY, USA; product #3779) and cytokine production was measured using enzyme-linked immunosorbent assay (ELISA). For ELISA, flat-bottomed 96-well plates (Maxisorp, Nalgene Nunc International, Denmark) were prepared by coating with 50 μl of hIFNγ capture antibody (BD Biosciences), diluted 1:500 in PBS and incubated at 4°C overnight. Plates were then washed and blocked with 0.5% bovine serum albumin in PBS. Coculture supernatants were applied for 2 h at room temperature, followed by washing and then 100 μl of Biotin mouse anti-human IFNγ (diluted 1:500; BD Pharmingen, 0.5 mg/ml, clone 4S.B3, Cat.: 554550) was added to each well and incubated wrapped in foil at room temperature for 1 h. Binding of biotinylated antibody was detected using 1/500 dilution of streptavidin horseradish peroxidase conjugate (diluted 1:500; RPN1231V, GE Healthcare, Chicago, IL, USA). The reaction was stopped when developed by the addition of 1M HCl. Absorbance was analyzed using the VersaMax ELISA Microplate Reader (Molecular Devices, Sunnyvale, CA, USA) with Softmax Pro software (Molecular Devices, Sunnyvale, CA, USA). Results are expressed as percent of that obtained using anti-CD3, defined as maximal 100%.
Cellular cytotoxicity assay ( 51 Cr release assay)
Melanoma tumor cells from patients #10 and #11 were harvested and labeled with 100 mCi 51 Cr at 37°C in T-cell media before washing and seeding at 1 × 106/ml in triplicate wells of a 96-well round-bottomed plate. CTLs were added at various effector:target ratios and incubated at 37°C for 4 h. Chromium release was read using the Wallac Wizard 1470 (PerkinElmer), and the percentage of lysis calculated using the following formula: [(experimental counts/min – spontaneous counts/min)/(total counts/min – spontaneous counts/minute)]. Results are shown as the percentage of cytotoxicity (mean ± SEM) using pooled data from triplicate wells.
Generation of matched primary melanoma cell lines
All tumor tissue from patient samples was verified histologically and confirmed using H+E and standard immunohistochemistry (IHC) staining with a combination of Melan-A, HMB45, or S100B. Patients were deidentified and given sequential sample numbers designated #. Tumor and TILs derived from the same patient sample were designated by the same #number [e.g. melanoma tumor cells (Melanoma#10) is derived from the same patient sample as TIL#10]. Tumor pieces were finely minced and digested in a mixture of 1 mg/ml collagenase type IV (Sigma-Aldrich), 0.02 mg/ml DNase (Sigma-Aldrich) and gentamicin (10 μg/ml) and filtered through 0.2 μg filter. Primary melanoma cell lines were established in RPMI supplemented with 10% FCS, 2 mM glutamine, 0.1 mM non-essential amino acids, HEPES, 1 mM sodium pyruvate, and penicillin/streptomycin. Tumor cells were passaged through NSG mice and harvested when tumors reached a diameter of 100 mm2. Tumors #8, #9, #10 and #11 were passaged once, and Melanoma#15 was passaged twice. Melanoma tumors #34 and #46 were donated by Dr. Andreas Behren (Olivia Newton-John Cancer Research Institute, ON-JCRI, Heidelberg, VIC, Australia). They were HLA matched for Melanoma#20 in the Peter MacCallum Cancer Centre MEL-TIL biobank. Established melanoma lines were stained for S100B to verify their status as melanoma cells in culture.
Other cell lines
The mouse (C57BL/6) sarcoma cell line 24JK was donated to the laboratory by Dr. Patrick Hwu (National Institute of Health, Bethesda, MD, USA). The 24JK cell line was transduced with a retroviral vector encoding the human Her-2 antigen (24JK-Her2). Tumor lines were verified to be mycoplasma negative by Victorian Infectious Diseases Reference Laboratory (Melbourne, VIC, Australia) by polymerase chain reaction analysis. For in vivo experiments, the indicated number of cells were resuspended in PBS and injected subcutaneously in 100 μl volume. All cell lines were maintained in culture in a humidified incubator at 37°C at 5% CO2.
Treatment of tumor-bearing mice
Non-obese diabetic severe combined immunodeficiency IL2Rγnull (NOD/SCID Gamma; NSG) mice were bred in-house at the Peter MacCallum Cancer Centre. Mice were injected subcutaneously with 1 × 106 melanoma tumor cells (Patient #10). When tumors became established (>50 mm2), 1 × 107 TILs or dual specific CAR-TIL T cells derived from Patient 10 were administered via tail vein injection. Mice were also treated with 50,000 IU IL-2 delivered via intraperitoneal injection on days 0–3 after T-cell transfer.
Statistics
Statistical tests were performed as indicated in the figure legends with a p value less than 0.05 considered significant.
Study approvals
All animal studies were approved in advance by the Peter MacCallum Animal Experimentation Ethics Committee under Protocol E498. Animal experimentation was done in accordance with the recommendations of the Victorian Bureau of Animal Welfare, Department of Primary Industries and National Health and Medical Research Council of Australia, and recommendations to reduce the number of animals used and their suffering were followed. This project was conducted using the Human Melanoma Biobank at the Peter MacCallum Cancer Centre, Melbourne, Victoria. Studies using patient samples were approved by the Peter MacCallum Human Research Ethics Committee under Protocol 13/141. All patients provided informed consent, both written and verbal, to the use of their tissues and peripheral blood for experimentation.
Results
Expansion of TILs is less than peripheral blood-derived T cells
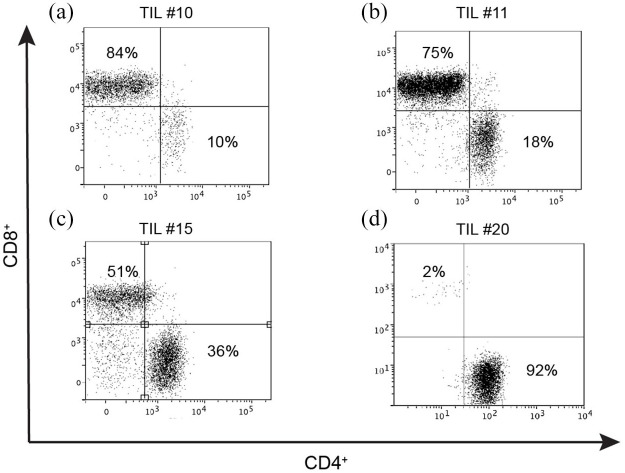
TILs were phenotypically diverse when considering CD4 and CD8 expression (Figure 1). In addition, proliferation rates were variable between patients (Figure 2). TIL proliferation rates and cell yield were consistently less than peripherally-derived patient T cells from autologous blood samples [Figure 2(a) and (b)]. TILs achieved a median fold increase of 3.3 times the original population (range: 1.4–12.5). In comparison, PBMCs achieved a median increase of 50.7 times the original population (range 41.7–114.6; see Table 2).
Figure 1.
TILs are phenotypically diverse. TILs were thawed from the biobank and activated using CD3/28 beads and IL-2 600 IU/ml in CM. IL-2 was replaced at 2–3 day intervals. Each flow cytometry plot demonstrates the cell phenotype for a different patient, designated by patient #number [(a) TIL#10, (b) TIL#11, (c) TIL#15, (d) TIL#20]. Cell populations were gated on morphology, viability, CD3+ and stained for CD4+ and CD8+.
CM, complete media; TIL, tumor-infiltrating lymphocyte.
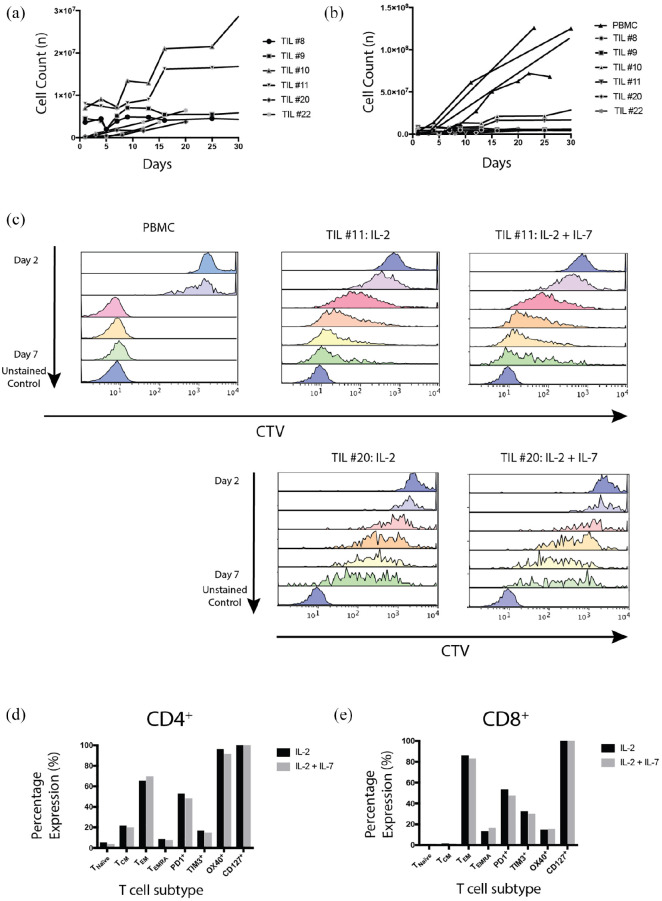
Figure 2.
TIL cultures had variable proliferation and terminal differentiation.
1 × 106 T cells were activated with CD3/28 Dynabeads and IL-2 (600 IU/ml). (a) TIL proliferation rates are demonstrated for individual patients designated by patient #number. (b) TIL proliferation rates are compared with allogeneic donor PBMCs (n = 4 PBMC cultures, 6 TIL cultures. p < 0.0001 by one-way analysis of variance). (c) TIL#11, #20 and allogeneic PBMCs were thawed, activated using CD3/28 beads, labeled with CTV and cultured in complete media with IL-2 (600 IU/ml). Cells were then analyzed for CTV fluorescence daily until day 7 for TIL or day 6 for PBMC. TIL#11 and #20 were cultured with IL-2 and IL-7 (10 ng/ml) for comparison. Cell proliferation, as seen from CTV dilution over time, was similar irrespective of whether IL-2 alone or IL-2+IL-7 was used in culture (results of a single experiment). (d) CD4+, and (e) CD8+, demonstrate TIL subtypes for TIL#11 with and without the addition of IL-7 to media. Cells were gated on morphology, viability, CD3+, CD4+ or CD8+. Naïve T cells: CD45RA+ CCR7+; TEMRA: CD45RA+ CCR7−; TCM: CD45RA− CCR7+; TEM: CD45RA− CCR7−. Similar proportions of cell subsets were observed in IL-2- and IL-2/IL-7-supplemented cultures (results from a single experiment).
CTV, cell trace violet; PBMC, peripheral blood mononuclear cell; TCM, central memory T cell; TEM, effector memory T cell; TEMRA, effector memory T cell expressing CD45RA; TIL, tumor-infiltrating lymphocyte.
Table 2.
PBMCs demonstrate greater growth potential compared with TILs. Demonstrates the minimum number of TILS (number at time of thaw) and the maximum number (peak of cell expansion) achieved for each TIL and PBMC in culture. The ratio represents the cell fold increase. PBMCs were from a normal allogeneic donor (PBMC#1), matched patient PMBCs from patient #10 (PBMC#2), and patient #15 (PBMC#3).
| Cells in culture | Number of T cells | Ratio of maximum:minimum | |
|---|---|---|---|
| Minimum | Maximum | ||
| TIL 8 | 3.60E+06 | 6.95E+06 | 1.93 |
| TIL 9 | 4.52E+06 | 6.50E+06 | 1.44 |
| TIL 10 | 7.00E+06 | 3.15E+07 | 4.50 |
| TIL 11 | 8.00E+06 | 1.72E+07 | 2.15 |
| TIL 20 | 3.00E+05 | 3.75E+06 | 12.50 |
| TIL 22 | 3.00E+05 | 3.65E+06 | 12.17 |
| PBMC #1 | 3.00E+06 | 1.25E+08 | 41.67 |
| PBMC #2 | 1.42E+06 | 7.20E+07 | 50.70 |
| PBMC #3 | 1.10E+06 | 1.26E+08 | 114.55 |
PBMC, peripheral blood mononuclear cell; TIL, tumor-infiltrating lymphocyte.
The standard protocol for generating TILs in our laboratory requires the addition of IL-2 to culture media. However, T-cell proliferation, survival and effector and memory subtypes are influenced by a complex orchestration of multiple cytokines. Cytokines known to enhance survival and proliferation of T lymphocytes include IL-2, IL-4, IL-7 and IL-15. 19 Although IL-4, IL-7 and IL-15 have demonstrated enhanced proliferation of T cells in vitro, IL-7 can be particularly beneficial in vivo.20,21 Owing to the potential for in vivo applications, we investigated the combined effect of IL-7 with IL-2 on the proliferation and phenotype of TILs. PBMCs demonstrated superior proliferation potential compared with TILs measured using dilution of cell trace violet fluorescence [Figure 2(c)]. The addition of IL-7 to media did not impact proliferation of TILs.
After 7 days a more extensive fluorescence-activated cell sorting (FACS) panel analysis was performed to examine subsets of CD4+ and CD8+ cells including naïve, central memory (TCM), effector memory (TEM) and TEM expressing CD45RA (TEMRA). The expression of signaling molecules including PD-1, OX-40 and T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) has featured prominently in the melanoma immunotherapy literature and they are potential targets for therapy. High PD-1 expression has been reported to be common in melanoma TIL populations and expression is associated with T-cell dysfunction, immune exhaustion and tumor progression.20,21 OX-40 is a costimulatory checkpoint protein and previous studies have shown that stimulation with OX-40L enhances T-cell proliferation and cytokine activity.22–25 TIM-3 plays a key role in inhibiting T-cell responses and expression of cytokines including IFNγ and TNFα. 26 High expression of these markers has been associated with regulatory T cells and immune exhaustion. 27 No significant alteration in T-cell subset expression was noted when IL-7 was added to the culture medium of TIL#11 [Figure 2(d) and (e)] despite CD127, an IL-7 receptor, being strongly expressed. PD-1 was expressed at moderately high levels in both CD8+ and CD4+ cell populations, and the predominant populations were well-differentiated TEM cells, suggesting previous antigen exposure. Combined, these results may indicate T-cell dysfunction, terminal differentiation that may reduce anti-neoplastic capacity.
TILs can be transduced to express anti-Her2 CAR
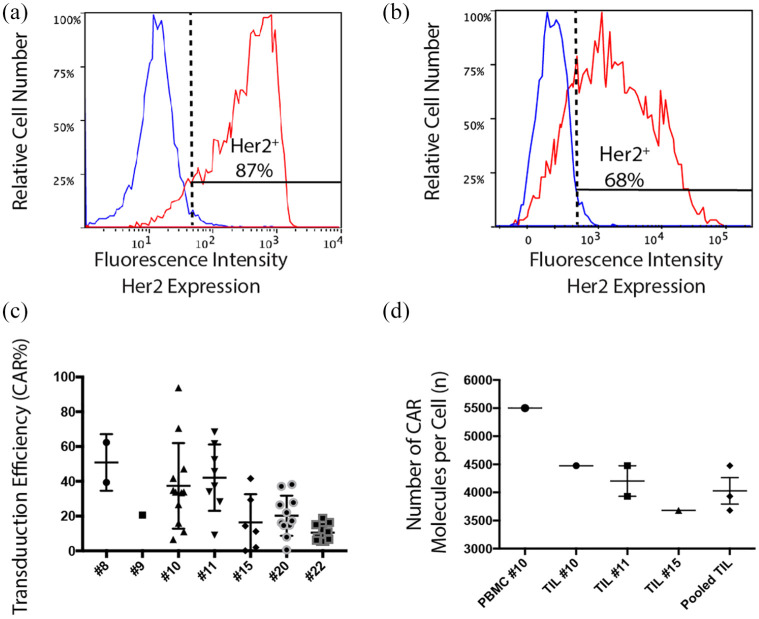
TILs from the melanoma biobank at the Peter MacCallum Cancer Centre were thawed, activated, and transduced with a CAR containing CD3ζ and CD28 signaling domains recognizing the human tumor-associated antigen Her2 (αHer2 CAR). Successful transduction was confirmed using flow cytometry, with peak transductions observed for TIL#10 and TIL#11 [Figure 3(a) and (b)]. TILs activated and cultured from our melanoma biobank were successfully transduced with a transduction efficiency between 2% and 94% (median 21.9%) [Figure 3(c)]. CAR receptor density ranged between 3683 and 4475/cell [Figure 3(d)]. Reasons for the wide variation in transduction frequency may include differences in proliferative rate between patient samples or variation in sample freezing/thawing process or changes in the titer of viral vector supernatant over time.
Figure 3.
TILs can be successfully transduced with anti-Her2 CAR.
TILs were thawed and activated using CD3/28 beads with IL-2 (600 IU/ml) and transduced with retroviral vector in the presence of retronectin and selected with G418 for expression of anti-He2-CAR. Non-transduced TILs underwent the transduction protocol in the presence of an empty viral vector. Cells were examined for CAR expression using flow cytometry. TIL-CAR were stained with c-Myc tag (red), with an isotype (IgG2A; blue). Cells are gated on morphology, viability, CD3+ and c-Myc tag staining. CAR expression was calculated based on <1% staining of the non-transduced CAR stained with c-Myc tag. (a) Maximal staining of transduction of TIL#10 and (b) TIL#11 transduced with anti-Her2 CAR. (c) CAR expression using flow cytometry shown for multiple transductions. Results are expressed with mean and SEM for each patient. (d) CAR density on transduced T cells using a proprietary bead standard kit (Simply Cellular Beads, BANGSLABS laboratories). CAR density on TILs was pooled and data shown ± SEM performed in triplicate wells.
CAR, chimeric antigen receptor; PBMC, peripheral blood mononuclear cell; TIL, tumor-infiltrating lymphocyte
Second activation of TILs using a rapid expansion protocol leads to significant population expansion
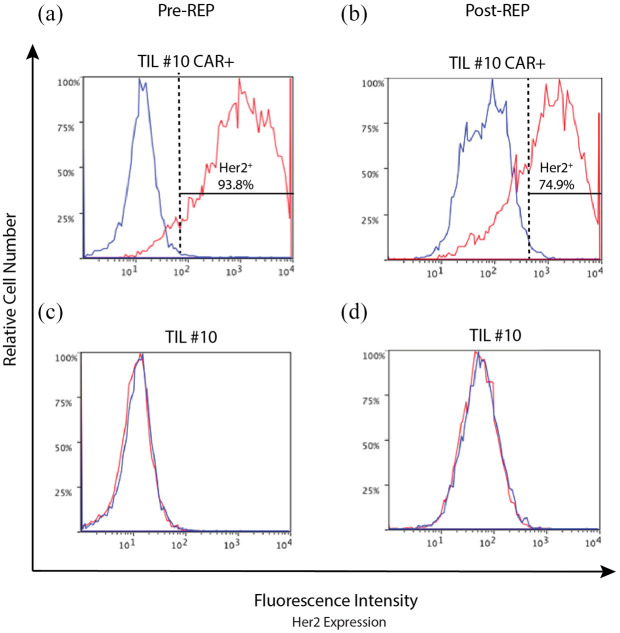
Transduction of TILs and culture enrichment for CAR-TILs using G418 resulted in low cell numbers, often limiting functional testing. Rapid expansion protocol (REP) is an established method for activating and proliferating TILs for ACT. 28 To counteract the low cell yield of TILs following transduction we hypothesized that a second activation of T cells using REP would result in greater cell numbers and facilitate functional studies. Using REP to induce a second activation of TILs following transduction resulted in significant expansion of TILs. Aliquots of 1 × 106 TILs were expanded to in excess of 100 × 106 cells over 12 days. Cells were confirmed to be viable and retained CAR expression following REP (Figure 4).
Figure 4.
Transduced TIL#10 retain CAR expression following REP.
TILS were thawed and activated using CD3/28 beads with IL-2 (600 × IU/ml). Five days following activation they were transduced with retroviral vector in the presence of retronectin and selected with G418 for expression of anti-He2-CAR. Non-transduced TILs underwent the transduction protocol in the presence of an empty viral vector to control for any changes induced by the process of transduction. Following confirmation of successful transduction and CAR expression using flow cytometry, to increase cell numbers for experiments, TILs underwent a second activation using REP. REP is a restimulation process utilizing soluble OKT3 and irradiated donor PBMCs. Cells were examined for CAR expression using flow cytometry. Data are shown for c-Myc tag (αHer2 CAR; red) with isotype control (blue). (a) TIL#10 expressed the CAR (92%) following transduction after CD3/28 bead activation and G418 selection. (b) Following further expansion, by REP, cells maintain CAR expression (73%). Control cells transduced with an empty viral vector maintain negative expression for CAR pre-REP (c) and post-REP (d). Cells are gated on cell morphology, viability, CD3+ and stained for c-Myc tag expression (CAR) or isotype (IgG2A; control).
CAR, chimeric antigen receptor; OKT3, anti-CD3; PBMC, peripheral blood mononuclear cell; REP, rapid expansion protocol; TIL, tumor-infiltrating lymphocyte.
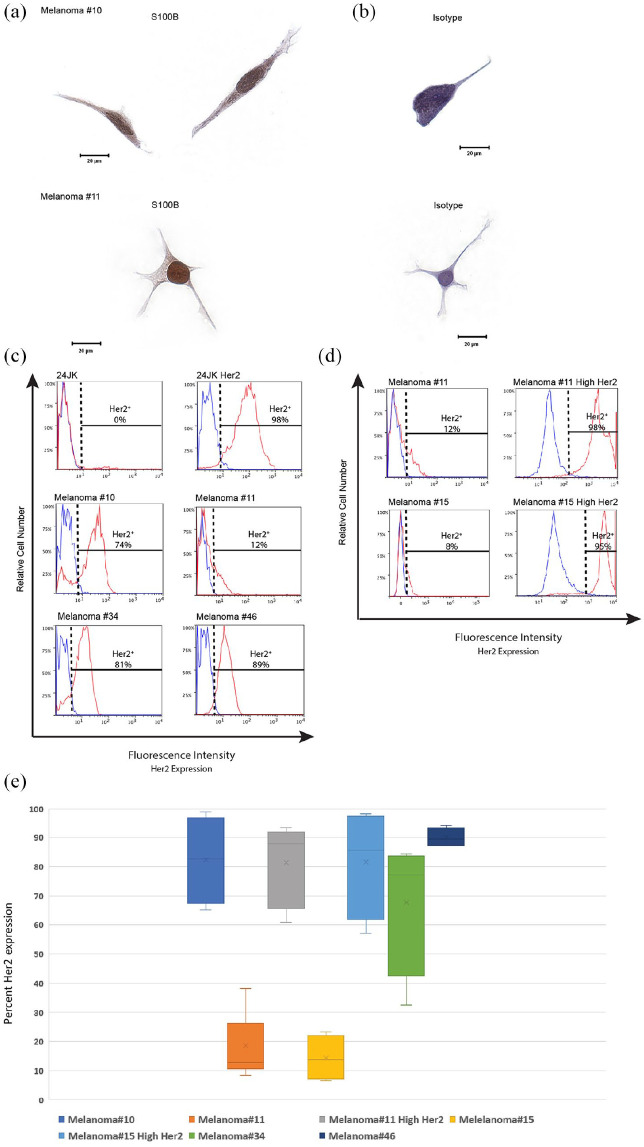
Melanoma tumor cell lines constitutively express Her2 antigen
Despite progressive growth being one of the hallmarks of tumors in vivo, growth of human tumor cell lines in vitro is often unsuccessful and unpredictable. 29 Human fibroblasts are more easily established in vitro and can be a major contaminant of tumor lines in culture. 30 S100B is a highly sensitive IHC marker commonly employed for diagnosis of melanoma and is used to exclude stromal contaminants. 31 Prior to Her2 antigen testing, tumor cells were confirmed positive for S100B by IHC [Figure 5(a)]. In addition, all tumors at the time of specimen resection were confirmed by histopathology to be metastatic melanoma. For CAR T cells to incite a cellular response the target antigen must be expressed on the surface of tumor cells. Tumor lines from patient cell lines in our biobank were found to constitutively express Her2 antigen [median expression: 48.5% range: 12–89%; Figure 5(b)]. Her2 is not an antigen commonly known to be expressed in melanoma and reports in the literature regarding Her2 antigen expression in melanoma are conflicting.32–35 Two melanoma tumor lines with low autologous baseline Her2 expression were transduced for Her2 expression and sorted to create additional tumor lines with high Her2 antigen expression to explore the impact of matched patient tumor lines with increased expression of Her2 [Figure 5(c)].
Figure 5.
Melanoma tumor cells constitutively express Her2 antigen.
(a-b) Melanoma tumor cells #10 and #11 were cultured onto slides and fixed using formaldehyde and stained for S100B. Images were taken using an Olympus BX51 Microscope at ×40 magnification and captured using SpotFire software. Single representative cells are presented stained with either an S100B-specific antibody (a) or isotype control (b). (c) Tumor lines were examined for Her2 antigen by flow cytometry. Cells were gated on morphology, viability and Her2 antigen expression. Cells that express Her2 antigen (red) are demonstrated against an isotype for Her2 (blue). 24JK tumor lines negative and positive for Her2 expression were used as additional controls. (d) Cell lines of melanoma tumors #11 and #15 were transduced using lipofectamine transduction protocols and sorted for Her2 expression. Control lines with 24JK parental and Her2 expressing tumor lines are shown. Melanoma tumor cell lines from patients #11 and #15 are shown before transduction (Melanoma#) and after transduction and sorting (Melanoma# High Her2). Her2 expressing cells are demonstrated in red and isotype staining is demonstrated in blue. (e) Box plot demonstrating range of Her2 antigen expression for each patient cell line (n = ⩾4 observations per patient).
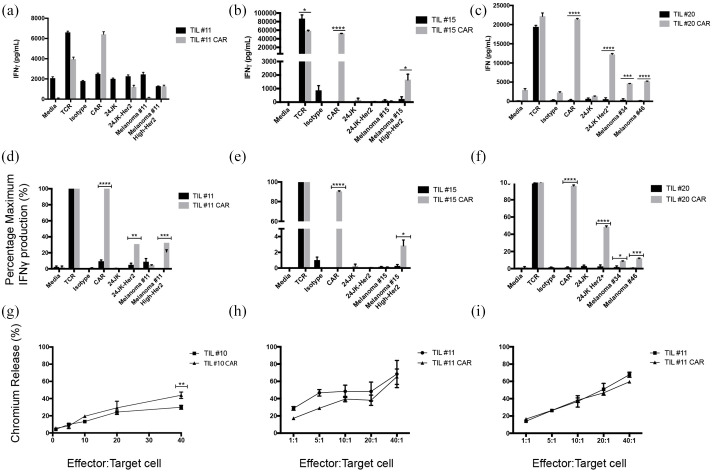
CAR-TILs demonstrate increased activity against Her2 expressing tumor lines
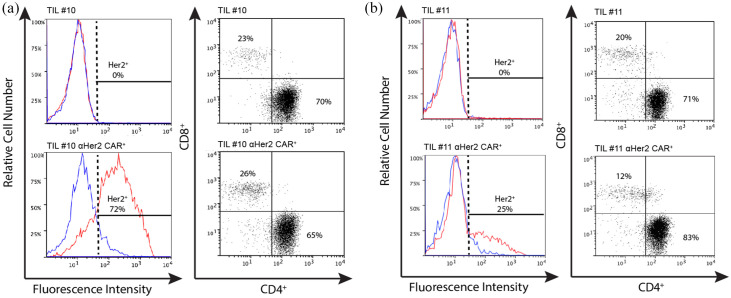
Activity of CAR-TILs co-cultured against autologous or HLA matched melanoma tumor cell lines was investigated using ELISA to measure cytokine production, and cytotoxic killing capacity measured using chromium ( 51 Cr) release assays. CAR-TILS produced greater amounts of IFNγ than non-transduced TILS when CAR was stimulated and co-cultured with Her2 expressing tumor lines [Figure 6(a)–(f)] with the exception of TIL#15 against 24JK-Her2. Cytotoxic activity demonstrated increasing chromium release as effector:target ratios were increased. However, there was no consistent increase in TIL cytotoxicity mediated by the CAR, with only TIL#10 demonstrating a small increase in cytotoxicity against an autologous tumor cell line [Figure 6(g)–(i)]. Although detailed longitudinal analyses of TIL characteristics and function throughout their culture and restimulation was not performed, the variation in function may reflect TIL transduction efficiency and differences in αHer2 CAR expression between patient cells (Figure 7). In addition, CAR-TIL#10 and #11 were predominantly CD4+ (70–83%), which may explain functional assay results with moderate cytokine production but limited cytotoxic capacity. The reason for the predominance of CD4+ cells is not clear, but could have been due to the conditions of stimulation, as has been observed previously. 36
Figure 6.
TILs transduced with anti-Her2 CAR respond against autologous melanoma tumor cell lines.
(a)–(f) IFNγ production of TILs transduced with anti-Her2 CAR compared with non-transduced TILs. (a)–(c) Raw data values. (a) TIL#11 co-cultured with a panel of targets, including both parental (Melanoma#11) and high expressing Her2 tumor lines (Melanoma#11 High Her2); (b) TIL#15 co-cultured with a panel of targets, including parental (Melanoma#15) and high expressing Her2 tumor lines (Melanoma#15 High-Her2); (c) TIL#20 co-cultured with a panel of targets, including HLA matched melanoma tumor lines. Data are presented as mean ± SEM performed in triplicate. (d)–(f) Normalized values of data from panels (a)–(c); we defined T-cell responses to CD3 ligation as “maximal” (100%) and presented responses to other stimuli as a proportion of maximum. (g)–(i) Cytotoxic activity measured using 51Cr release assay. (g) TIL#10 transduced with anti-Her2 CAR were compared with TILs transduced with an empty viral vector control against autologous tumor cell targets. (h) TIL#11 (TIL-CAR versus TIL) compared against parental tumor cell line (mean Her2 antigen expression 11%) and (i) TIL#11 against autologous tumor lines transduced for high expression of Her2 antigen (mean Her2 antigen expression 98%).
Statistical significance was determined using unpaired Student’s t-test of triplicate wells from a single experiment (*p ⩽ 0.05, **p ⩽ 0.01, ***p ⩽ 0.001, ****p ⩽ 0.0001).
CAR, chimeric antigen receptor; TIL, tumor-infiltrating lymphocyte; TCR, transgenic T-cell receptor
Figure 7.
Comparing cell lines for transduction efficiency and phenotype.
Transduction efficiency comparing TILs transduced with an empty vector (control) and with anti-Her2 CAR (Her2 CAR) with corresponding cell phenotype. (a) CAR expression for TIL#10 transduced with the empty vector (control) and Her2 CAR (shown in red) and isotype (shown in blue). The phenotypes TIL#10 are shown. (b) CAR expression for TIL#11 transduced with empty vector (control) and Her2 CAR. Cell phenotype of TIL#11 is shown. Cells are gated on morphology, viability, CD3+, CD4+ and CD8+.
CAR, chimeric antigen receptor; TIL, tumor-infiltrating lymphocyte.
Adoptive transfer of CAR-TILs inhibits tumor growth in mice
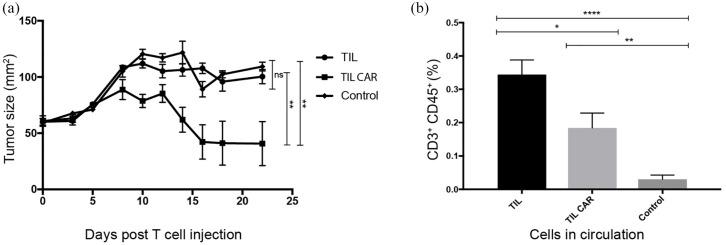
An ACT model to test our proof of concept was designed using patient matched TILs and tumor cells in a melanoma xenograft model using non-obese diabetic severe combined immunodeficiency IL2Rγnull (NOD/Shi-SCID Gamma; NSG) mice. These mice have severe immunodeficiency in both adaptive and innate immune systems. They have been shown to readily accept grafts from some human melanoma specimens and these tumors may metastasize, mimicking natural tumor biology in humans. 37 We hypothesized that mice treated with CAR-TILs would demonstrate greater tumor inhibition compared with mice treated with parental (non-transduced) TIL. We chose matched TIL#10/Melanoma#10 for the in vivo model as this was the tumor–TIL pair that demonstrated the greatest activity against autologous tumor cells from our samples with sufficient residual stock in our biobank for testing.
When tumors were established, ACT was performed via intravenous injection into the main tail vein. IL-2 injections (50,000 IU/dose) were delivered twice daily for five doses to the peritoneal cavity of each mouse to facilitate T-cell engraftment. Median tumor size at the time of commencing ACT was 61 mm2 (range 46–82 mm2). A reduction of tumor size was observed when mice were treated with TILs transduced with anti-Her2 CAR (mean tumor area 40.7 mm2 ± 19.6 SEM at day 22, n = 7) compared with parental TILs (mean tumor area 100.4 mm2 ± 6.2 SEM, n = 7; p = 0.0028) and controls (mean tumor area 109.4 mm2 ± 3.9 SEM, n = 9; p = 0.0026) [Figure 8(a)]. Tumors of mice in the control group were of similar size to those that received parental TILs (p = 0.7605). After 18 days, four of seven tumors demonstrated complete response in the group that received TIL transduced with anti-Her2 CAR. No mice treated with non-transduced TILs achieved a complete tumor response.
Figure 8.
ACT using TIL transduced with anti-Her2 CAR inhibited tumor growth in mice.
(a) ACT using 1 × 107 TILs transduced with anti-Her2 CAR or non-transduced TILs was delivered into NSG mice bearing established (>50 mm2) xenografts of an autologous patient melanoma tumor cell line. A control (untreated group) was included for comparison. Time is measured from the day ACT was performed (day 0). Treated mice also received five intraperitoneal injections of IL-2 (50,000 IU/injection per mouse) over 3 days. Tumor growth was significantly inhibited by ACT using CAR-transduced T cells. Results are from a single experiment, with seven mice per treatment group and nine mice for control group. Statistical significance was determined using Student’s t-test. (b) A greater proportion of non-transduced TILs was detected in the peripheral circulation following ACT. A peripheral blood sample was drawn from each of the mice on day 3 (n = 7–9) following delivery of ACT and analyzed for human T cells using flow cytometry. Mice received TILs (parental) or TILs transduced with anti-Her2 CAR. Significantly greater numbers of circulating TILs were detected in the peripheral circulation of mice following intravenous injection compared with controls. Cells were gated on morphology, viability, CD3+ and CD45+.
Statistical significance was determined using Student’s t-test (*p ⩽ 0.05, **p ⩽ 0.01, ****p ⩽ 0.0001, ns: not significant).
ACT, adoptive cell therapy; CAR, chimeric antigen receptor; TIL, tumor-infiltrating lymphocyte.
On day 3 following ACT, mice were bled and FACS was performed on these samples to assess for the presence of circulating T cells. CAR-TILs were detectable circulating in peripheral blood. A small but statistically significant reduced fraction of human TIL was identified in circulation from mice treated with TILs transduced with anti-Her2 CAR compared with non-transduced TILs [p = 0.024; Figure 8(b)].
Discussion
T-cell engineering is an exciting new development in the oncological armamentarium. Here we demonstrate that Her2 antigen can be constitutively expressed on metastatic cutaneous melanoma cell lines. TILs cultured from these melanoma tumors can be isolated and transduced to express a Her2 CAR and produce measurable response in vitro and in an in vivo mouse model. This report is the first to explore this concept of combining the two ACT techniques of TIL and CAR therapy.
Her2/neu antigen is a transmembrane tyrosine kinase receptor and is a member of the epidermal growth receptor family. Overexpression is associated with chronic stimulation of signal transduction pathways and is often correlated with poor clinical outcomes, even when expressed at low levels. 38 Her2 overexpression has been best studied in breast cancer, but has also been detected in ovarian, endometrial, salivary gland, gastric, bladder and pancreatic cancers.39–44 Although tumor cells lines in our biobank were found to constitutively express some level of Her2, it is generally not considered a good antigenic target for melanoma. We used it in our studies as a model system due to the availability of the CAR and associated materials. Further development of the approach may be better achieved targeting a more relevant melanoma cell-surface antigen, such as GD2. 45 The clinical impact of Her2 expression in melanoma is unknown. In the few papers where it has been explored its expression is variable: 2.5–75% of melanoma tumor specimens.32,33,46–50 The inconsistency of Her2 expression in melanoma reported in the literature may be attributed to methods of antigen detection. We used flow cytometry to examine for Her2 antigen expression. All tumor lines tested from our melanoma biobank were Her2 positive (range: 12–89%). Two other studies have explored Her2 expression in melanoma by flow cytometry.33,35 A recent study by Forsberg et al. 51 demonstrates that CAR T cells against Her2 can eradicate uveal and cutaneous melanoma cells in vitro and in vivo. Our study confirms variable Her2 expression in melanoma, and Her2 CAR T cells are effective in mediating cancer cell death. Her2 CAR T cells demonstrated deep or complete regression of all tumors in hIL2-NOG mice. Results from their study and ours suggest that Her2 may be an overlooked targetable antigen in melanoma treatment.
CAR T cells have been shown to be effective in the treatment of some hematological malignancies with high rates of durable remission observed.16–18 Antigen targeting in solid tumors using CAR technology has been less successful. One of the major hurdles of this therapy is the antigenic heterogeneity of solid tumors. This increases the complexity of antigen targeting and therapeutic efficiency and hostile tumor microenvironments.52–54 Interplay within the immunosuppressive tumor microenvironment includes cytokine secretion, T-cell trafficking to the tumor and inhibitory signals from other cells (including T regulatory cells, myeloid-derived suppressor cells) may also impair CAR T-cell killing efficacy.55–58 In our study we used TILs and transduced them to target a known tumor antigen (Her2). The principal advantage of TILs is the presence of a tumor-specific T-cell population capable of recognizing a heterogeneous range of tumor-specific antigens. 59 Antigens yet to be identified likely account for >90% of tumor-specific TILs and are thought to result from epitopes of mutant self-proteins (e.g. signaling and housekeeping genes).60,61 TIL diversity complements tumor mutation load, and is a predictor of successful ACT. 62 TIL may have chemotactic properties that facilitate T-cell homing to the tumor. 63 The combination of TIL and CAR has the potential to augment the immune response by targeting multiple tumor neoantigens not limited by CAR T-cell targets, facilitate T-cell activation and reduce tumor induced anergy. Other strategies described to improve CAR therapy outcomes include modifying T cells to express tumor specific chemokine receptors, combination with anti-angiogenic agents, and reducing Treg cells using preconditioning lymphodepleting chemotherapy. The combination of CAR T cells with checkpoint blockade with anti-CTLA-4 or PD-1 inhibitors is being trialed [ClinicalTrials.gov identifiers: NCT01993719 and NCT02652455]. Superior CAR efficiency has been demonstrated using the gene editing technique of CRISPR/Cas-9 targeting PD-1. 64 CAR technology is being explored in cells other than lymphocytes, including natural killer (NK) cells. 63–65
Transduction of TILs to increase expression of receptors, cytokines or other molecules creates “dual-specific” TILs. In the literature to date a single trial has reported successful transduction of TILs using a gene encoding IL-12. 65 Investigators performed a dose escalation trial in 33 patients with metastatic melanoma of autologous TILs transduced with a gene encoding a single-chain IL-12 driven by a nuclear factor of the activated T cells promotor (NFAT.IL12). Between cell doses of 0.3–3 × 109, 63% of patients demonstrated objective clinical responses, but response was short lived and complicated by high levels of toxicity attributed to high serum levels of IL-12. Our study demonstrates that TILs can be successfully transduced using anti-Her2 CAR, although transduction frequencies can vary, and a restimulation step in the presence of antibiotic selection was necessary for generating high frequencies of CAR-expressing TILs. CAR expression in TILs was capable of enabling small improvements in cytokine production, but limited increases to cytotoxic activity, against Her2 expressing tumor lines compared with non-transduced TIL. During this process several challenges were encountered which may have influenced the outcomes and efficacy of these experiments. These include the low proliferation rates, duration of TILs in culture, T-cell subtypes, transduction efficiency and the intensive labor and resources required to perform this combined technique.
TIL samples in our biobank are a limited resource and hence the number of repetitions of experiments was restricted. We were not able to establish tumor cells from all tissue samples and hence in our biobank there were unmatched TIL and tumor pairs. We had viable matched autologous TIL–tumor tissue pairs for patient samples #10, #11 and #15. In addition, this study faced significant challenges in T-cell proliferation and expansion. TILs were activated using CD3/28 beads and the transduction process was initiated after 5 days in culture. At the conclusion of the transduction protocol the culture was enriched for TIL-CAR using G418. Transduction rates were variable and often low prior to selection. Transduction efficiency may have been influenced by the properties of the cells undergoing transduction, cytokine composition, viral vector and the transduction conditions.66–68 T cells maintained in prolonged culture have been demonstrated to have reduced anti-tumor activity with alterations in cytokine secretion, effector cell function and potency. 69 We believe the limited function of transduced TILs demonstrated may be due to exhaustion of these cultures and limited cytotoxicity influenced by cell phenotype. It would be of interest in future to determine whether cytokines other than IFNγ were secreted. The predominance of CD4+ cells in cultures may have limited the cytotoxic capacity observed against autologous tumor cells. Tumor response was seen in our pilot in vivo experiment. Repeated experiments with additional matched TIL–tumor pairs and PBMCs transduced with anti-Her2 CAR to compare tumor response would be an interesting comparison for future studies.
In conclusion, TILs from patients with metastatic melanoma can be successfully isolated and transduced using anti-Her2 CAR. Although CAR-TIL show activity against Her2 expressing tumor lines, including activity against parental Her2 expressing melanoma tumor, the improvements are modest and require significant technical expertise, time and resources. TILs used in our study were found to be in an advanced differentiation state, had lower proliferative capacity and may be potentially exhausted owing to the length of exposure and duration in culture. Although combining TIL and CAR is a novel and interesting concept, and shows marginal improvements in TIL activity, it may have limited translational capacity using current protocols. Future directions for this and other projects include the optimization of the transduction process to improve efficiency, preserve cell count and viability, and minimize time in culture.
Footnotes
Author contributions: JKM, DG and MHK designed the experiments, collected and analyzed data, and wrote the manuscript. JKM, MAH, PP, JAW and LG performed the experiments and analyzed data. DG collected patient materials. JKM, MHK, PKD, PJN and DG provided intellectual input and prepared the manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Health and Medical Research Council (NHMRC) of Australia (1103352 and 1132373). PKD was supported by an NHMRC Principal Research Fellowship.
ORCID iD: Michael H. Kershaw  https://orcid.org/0000-0002-2697-487X
https://orcid.org/0000-0002-2697-487X
Contributor Information
Jane K. Mills, Cancer Immunology Program, Peter MacCallum Cancer Centre, Melbourne, Australia
Melissa A. Henderson, Cancer Immunology Program, Peter MacCallum Cancer Centre, Melbourne, Australia
Lauren Giuffrida, Cancer Immunology Program, Peter MacCallum Cancer Centre, Melbourne, Australia.
Pasquale Petrone, Cancer Immunology Program, Peter MacCallum Cancer Centre, Melbourne, Australia.
Jennifer A. Westwood, Cancer Immunology Program, Peter MacCallum Cancer Centre, Melbourne, Australia
Phillip K. Darcy, Cancer Immunology Program, Peter MacCallum Cancer Centre, Melbourne, Australia Sir Peter MacCallum Department of Oncology, University of Melbourne, Parkville, Australia.
Paul J. Neeson, Cancer Immunology Program, Peter MacCallum Cancer Centre, Melbourne, Australia Sir Peter MacCallum Department of Oncology, University of Melbourne, Parkville, Australia.
Michael H. Kershaw, Cancer Immunology Program, Peter MacCallum Cancer Centre, Melbourne, Australia Sir Peter MacCallum Department of Oncology, University of Melbourne, Parkville, Australia.
David E. Gyorki, Department of Cancer Surgery, Peter MacCallum Cancer Centre, 305 Grattan Street, Melbourne, Victoria 3000, Australia; Sir Peter MacCallum Department of Oncology, University of Melbourne, Parkville, Australia; Department of Surgery, St Vincent’s Hospital, University of Melbourne, Fitzroy, Australia.
References
- 1. Rosenberg SA, Yang JC, Sherry RM, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res 2011; 17: 4550–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hadrup S, Donia M, Thor Straten P. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron 2013; 6: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009; 114: 1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Calcinotto A, Filipazzi P, Grioni M, et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res 2012; 72: 2746–2756. [DOI] [PubMed] [Google Scholar]
- 5. Hong JJ, Rosenberg SA, Dudley ME, et al. Successful treatment of melanoma brain metastases with adoptive cell therapy. Clin Cancer Res 2010; 16: 4892–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goff SL, Dudley ME, Citrin DE, et al. Randomized, prospective evaluation comparing intensity of lymphodepletion before adoptive transfer of tumor-infiltrating lymphocytes for patients with metastatic melanoma. J Clin Oncol 2016; 34: 2389–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosenberg SA, Restifo NP, Yang JC, et al. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 2008; 8: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Besser MJ, Shapira-Frommer R, Itzhaki O, et al. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin Cancer Res 2013; 19: 4792–4800. [DOI] [PubMed] [Google Scholar]
- 9. Andersen R, Donia M, Ellebaek E, et al. Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL2 regimen. Clin Cancer Res 2016; 22: 3734–3745. [DOI] [PubMed] [Google Scholar]
- 10. Ellebaek E, Iversen TZ, Junker N, et al. Adoptive cell therapy with autologous tumor infiltrating lymphocytes and low-dose interleukin-2 in metastatic melanoma patients. J Transl Med 2012; 10: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zippel DB, Besser M, Shapira R, et al. Adoptive cell therapy with autologous tumor-infiltrating lymphocytes and high-dose interleukin-2 for metastatic melanoma: the surgeon’s perspective. Exp Ther Med 2012; 3: 898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grupp SA, Maude SL, Shaw PA, et al. Durable remissions in children with relapsed/refractory ALL treated with T cells engineered with a CD19-targeted chimeric antigen receptor (CTL019). Blood 2015; 126: 681. [Google Scholar]
- 13. Locke FL, Neelapu SS, Bartlett NL, et al. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol Ther 2017; 25: 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Turtle CJ, Hay KA, Hanafi LA, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol 2017; 35: 3010–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013; 5: 177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017; 377: 2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol 2002; 2: 547–556. [DOI] [PubMed] [Google Scholar]
- 20. Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439: 682–687. [DOI] [PubMed] [Google Scholar]
- 21. Inozume T, Hanada K, Wang QJ, et al. Selection of CD8+PD-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T cells. J Immunother 2010; 33: 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roszik J, Markovits E, Dobosz P, et al. TNFSF4 (OX40L) expression and survival in locally advanced and metastatic melanoma. Cancer Immunol Immunother 2019; 9: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buchan SL, Rogel A, Al-Shamkhani A. The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy. Blood 2018; 131: 39–48. [DOI] [PubMed] [Google Scholar]
- 24. Andarini S, Kikuchi T, Nukiwa M, et al. Adenovirus vector-mediated in vivo gene transfer of OX40 ligand to tumor cells enhances antitumor immunity of tumor-bearing hosts. Cancer Res 2004; 64: 3281–3287. [DOI] [PubMed] [Google Scholar]
- 25. Shin C-A, Cho HW, Shin AR, et al. Co-expression of CD40L with CD70 or OX40L increases B-cell viability and antitumor efficacy. Oncotarget 2016; 7: 46173–46186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev 2017; 276: 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Montler R, Bell RB, Thalhofer C, et al. OX40, PD-1 and CTLA-4 are selectively expressed on tumor-infiltrating T cells in head and neck cancer. Clin Trans Immunol 2016; 5: e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dudley ME, Wunderlich JR, Shelton TE, et al. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother 2003; 26: 332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turin I, Schiavo R, Maestri M, et al. In vitro efficient expansion of tumor cells deriving from different types of human tumor samples. Med Sci 2014; 2: 70–81. [Google Scholar]
- 30. Giard DJ, Aaronson SA, Todaro GJ, et al. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst 1973; 51: 1417–1423. [DOI] [PubMed] [Google Scholar]
- 31. Weinstein D, Leininger J, Hamby C, et al. Diagnostic and prognostic biomarkers in melanoma. J Clin Aesthet Dermatol 2014; 7: 13–24. [PMC free article] [PubMed] [Google Scholar]
- 32. Kluger HM, DiVito K, Berger AJ, et al. Her2/neu is not a commonly expressed therapeutic target in melanoma – a large cohort tissue microarray study. Melanoma Res 2004; 14: 207–210. [DOI] [PubMed] [Google Scholar]
- 33. Inman JL, Kute T, White W, et al. Absence of HER2 overexpression in metastatic malignant melanoma. J Surg Oncol 2003; 84: 82–88. [DOI] [PubMed] [Google Scholar]
- 34. Persons DL, Arber DA, Sosman JA, et al. Amplification and overexpression of HER-2/neu are uncommon in advanced stage melanoma. Anticancer 2000; 20: 1965–1968. [PubMed] [Google Scholar]
- 35. Ma J, Han H, Liu D, et al. HER2 as a promising target for cytotoxicity T cells in human melanoma therapy. PLoS One 2013; 8: e73261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gargett T, Brown MP. Different cytokine and stimulation conditions influence the expansion and immune phenotype of third-generation chimeric antigen receptor T cells specific for tumor antigen GD2. Cytotherapy 2015; 17: 487–495. [DOI] [PubMed] [Google Scholar]
- 37. Quintana E, Piskounova E, Shackleton M, et al. Human melanoma metastasis in NSG mice correlates with clinical outcome in patients. Sci Transl Med 2012; 4: 159ra149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Slichenmyer WJ, Fry DW. Anticancer therapy targeting the erbB family of receptor tyrosine kinases. Semin Oncol 2001; 28: 67–79. [DOI] [PubMed] [Google Scholar]
- 39. Hansel DE, Ashfaq R, Rahman A, et al. A subset of pancreatic adenocarcinomas demonstrates coamplification of topoisomerase IIalpha and HER2/neu: use of immunolabeling and multicolor FISH for potential patient screening and treatment. Am J Clin Pathol 2005; 123: 28–35. [PubMed] [Google Scholar]
- 40. Marín AP, Arranz EE, Sánchez AR, et al. Role of anti-Her-2 therapy in bladder carcinoma. J Cancer Res Clin Oncol 2010; 136: 1915–1920. [DOI] [PubMed] [Google Scholar]
- 41. Glisson B, Colevas AD, Haddad R, et al. HER2 expression in salivary gland carcinomas: dependence on histological subtype. Clin Cancer Res 2004; 10: 944–946. [DOI] [PubMed] [Google Scholar]
- 42. Pagni F, Zannella S, Ronchi S, et al. HER2 status of gastric carcinoma and corresponding lymph node metastasis. Pathol Oncol Res 2013; 19: 103–109. [DOI] [PubMed] [Google Scholar]
- 43. Jørgensen JT, Hersom M. HER2 as a prognostic marker in gastric cancer - a systematic analysis of data from the literature. J Cancer 2012; 3: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gilcrease MZ, Woodward WA, Nicolas MM, et al. Even low-level HER2 expression may be associated with worse outcome in node-positive breast cancer. Am J Surg Pathol 2009; 33: 759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yvon E, Del Vecchio M, Savoldo B, et al. Immunotherapy of metastatic melanoma using genetically engineered GD2-specific T cells. Clin Cancer Res 2009; 15: 5852–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Persons DL, Arber DA, Sosman JA, et al. Amplification and overexpression of HER-2/neu are uncommon in advanced stage melanoma. Anticancer Res 2000; 20: 1965–1968. [PubMed] [Google Scholar]
- 47. Schraml P, Kononen J, Bubendorf L, et al. Tissue microarrays for gene amplification surveys in many different tumor types. Clin Cancer Res 1999; 5: 1966–1975. [PubMed] [Google Scholar]
- 48. Natali PG, Nicotra MR, Digiesi G, et al. Expression of gp185HER-2 in human cutaneous melanoma: implications for experimental immunotherapeutics. Int J Cancer 1994; 56: 341–346. [DOI] [PubMed] [Google Scholar]
- 49. Bodey B, Siegel SE, Luck JV, et al. Immunophenotypic characterization of human primary and metastatic melanoma infiltrating leukocytes. Anticancer Res1996; 16: 3439–3446. [PubMed] [Google Scholar]
- 50. Eliopoulos P, Mohammed MQ, Henry K, et al. Overexpression of HER-2 in thick melanoma. Melanoma Res 2002; 12: 139–145. [DOI] [PubMed] [Google Scholar]
- 51. Forsberg EMV, Lindberg MF, Jespersen H, et al. HER2 CAR-T cells eradicate uveal melanoma and T-cell therapy-resistant human melanoma in IL2 transgenic NOD/SCID IL2 receptor knockout mice. Cancer Res 2019; 79: 899–904. [DOI] [PubMed] [Google Scholar]
- 52. Zhang B-L, Qin DY, Mo ZM, et al. Hurdles of CAR-T cell-based cancer immunotherapy directed against solid tumors. Sci China Life Sci 2016; 59: 340–348. [DOI] [PubMed] [Google Scholar]
- 53. Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature 2013; 501: 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sun X-X, Yu Q. Intra-tumor heterogeneity of cancer cells and its implications for cancer treatment. Acta Pharmacologica Sinica 2015; 36: 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Peske JD, Woods AB, Engelhard VH. Control of CD8 T-Cell infiltration into tumors by vasculature and microenvironment. Adv Cancer Res 2015; 128: 263–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang L, Yu Z, Muranski P, et al. Inhibition of TGF-β signaling in genetically engineered tumor antigen-reactive T cells significantly enhances tumor treatment efficacy. Gene Ther 2013; 20: 575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Beyer M, Schultze JL. Regulatory T cells: major players in the tumor microenvironment. Curr Pharm Des 2009; 15: 1879–1892. [DOI] [PubMed] [Google Scholar]
- 58. Lu T, Ramakrishnan R, Altiok S, et al. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest 2011; 121: 4015–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Prickett TD, Crystal JS, Cohen CJ, et al. Durable complete response from metastatic melanoma after transfer of autologous T cells recognizing 10 mutated tumor antigens. Cancer Immunol Res 2016; 4: 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Matsushita H, Vesely MD, Koboldt DC, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 2012; 482: 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cohen CJ, Gartner JJ, Horovitz-Fried M, et al. Isolation of neoantigen-specific T cells from tumor and peripheral lymphocytes. J Clin Invest 2015; 125: 3981–3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lauss M, Donia M, Harbst K, et al. Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma. Nat Commun 2017; 8: 1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mukaida N, Baba T. Chemokines in tumor development and progression. Exp Cell Res 2012; 318: 95–102. [DOI] [PubMed] [Google Scholar]
- 64. Rupp LJ, Schumann K, Roybal KT, et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep 2017; 7: 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang L, Morgan RA, Beane JD, et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin Cancer Res 2015; 21: 2278–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shayakhmetov DM, Papayannopoulou T, Stamatoyannopoulos G, et al. Efficient gene transfer into human CD34+ cells by a retargeted adenovirus vector. J Virol 2000; 74: 2567–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Essand M, Loskog AS. Genetically engineered T cells for the treatment of cancer. J Intern Med 2013; 273: 166–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lee H-J, Lee YS, Kim HS, et al. Retronectin enhances lentivirus-mediated gene delivery into hematopoietic progenitor cells. Biologicals 2009; 37: 203–209. [DOI] [PubMed] [Google Scholar]
- 69. Sussman JJ, Parihar R, Winstead K, et al. Prolonged culture of vaccine-primed lymphocytes results in decreased antitumor killing and change in cytokine secretion. Cancer Res 2004; 64: 9124–9130. [DOI] [PubMed] [Google Scholar]
- 70. Westwood JA, Smyth MJ, Teng MW, et al. Adoptive transfer of T cells modified with a humanized chimeric receptor gene inhibits growth of Lewis-Y-expressing tumors in mice. Proc Natl Acad Sci U S A 2005; 102: 19051–19056. [DOI] [PMC free article] [PubMed] [Google Scholar]